Introduction
Milk components have been recognized as functional
foods, with positive health effects (1). Milk proteins are available as whole
milk proteins, caseinates, and whey proteins. Whey protein is a
high quality protein that contains higher amounts of essential
amino acids in comparison with other sources of protein, such as
egg and soy (2).
Whey, a by-product of cheese manufacturing, has been
considered for a long time a waste product. Its discharge to the
environment and the improper management and exploitation causes
important environmental problems (3). The discovery of whey as a functional
food with nutritional applications, raised its status to co-product
in cheese production (2). The
composition of whey depends mainly on the type of cheese and milk,
on the lactation phase and on its processing. Whey is a mixture of
proteins, such as α-lactalbumin, β-galactoglobulin, serum albumin,
immunoglobulins, lactoferrin, and galactoperoxidase with various
chemical, physical and functional properties (4,5).
Whey protein, not only plays an important role in nutrition, as it
is a rich and balance source of amino acids, but in some cases also
exhibits specific physiological activity in vivo (6). Furthermore, whey protein exhibits
antioxidant activity probably due to its rich content in cysteines,
which promotes glutathione (GSH) synthesis, an important
intracellular antioxidant (2). It
is therefore a potentially important tool for the treatment of
oxidative stress-associated diseases.
In previous studies of our laboratory, we examined
the possible antioxidant activity of sheep/goat whey protein in
vitro. Firstly, it was found that sheep/goat whey protein has
the ability to neutralize free radicals and consequently protect
biomolecules from oxidative damage (7). Furthermore, sheep/goat whey protein
exerts a protective effect against oxidative stress in muscle
(C2C12) and endothelial (Ea.hy926) cells (7,8).
Finally, in Ea.hy926 cells, it has been found that a number of
antioxidant enzymes were activated through the nuclear factor
E2-related factor 2 (Nrf2)-antioxidant response element (ARE)
pathway. In C2C12 cells, activation of the antioxidant enzymes was
not dependent on the Nrf2-ARE pathway (9). In an in vivo study in
athletes, the combination of whey proteins with carbohydrates
reduced lipid peroxidation and exerted anti-inflammatory action
(10,11).
Considering the above beneficial effects of
sheep/goat whey protein against oxidative stress and the growing
need for biofunctional foods with health claims, it would be of
great interest to study the effects of sheep/goat whey protein on a
living organism at blood and tissue level. Notably, the studies
concerning the biological activity of sheep/goat whey protein are
limited and the majority concerns bovine whey protein. Thus, the
aim of the present study is to evaluate the antioxidant activity of
sheep/goat whey protein in vivo. Specifically, it will
determine a number of redox status markers in blood and tissues of
rats after the administration of sheep/goat whey protein.
Materials and methods
Chemicals
Hydrogen peroxide (H2O2) 30%,
5,5′-dithiobis (2-nitrobenzoic acid) (DTNB),
2,4-dinitrophenylhydrazine (DNPH), 2,2-diphenyl-1-picryl hydrazyl
(DPPH), urea, sodium sulfate and Bradford reagent were purchased
from Sigma-Aldrich (St. Louis, MO, USA). Sodium citrate was
purchased from Merck KGaA (Darmstadt, Germany). Protease inhibitor
coctail tablets were supplied from Roche Diagnostics (Indianapolis,
IN, USA). Phosphate-buffered saline (PBS) was purchased from Gibco;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Materials
Sheep/goat whey protein was obtained from the
Hellenic Protein S.A (Athens, Greece) and its content was 80/100 g.
In Table I, the nutritional
content and the profile fraction of sheep/goat whey protein are
presented.
 | Table I.Nutritional content and profile
fraction of sheep/goat whey protein. |
Table I.
Nutritional content and profile
fraction of sheep/goat whey protein.
| Nutritional content
(per 100 g). |
|---|
|
|---|
| Items | Sheep/goat whey
protein |
|---|
| Energy | 396 kcal/1678
kJ |
| Proteins | 80 g |
| Carbohydrates | 10 g |
| Fats | 4
g |
| Sodium | 157 mg |
| Potassium | 397 mg |
| Calcium | 415 mg |
| Phosphorus | 319 mg |
| Magnesium | 73
mg |
|
| Profile fraction
(per 100 g). |
|
|
| Protein
mixture | Sheep/goat whey
protein |
|
|
β-lactoglobulin | 47 g |
| α-lactalbumin | 14 g |
|
Glycomacropeptide | 13 g |
| Serum albumin | 3
g |
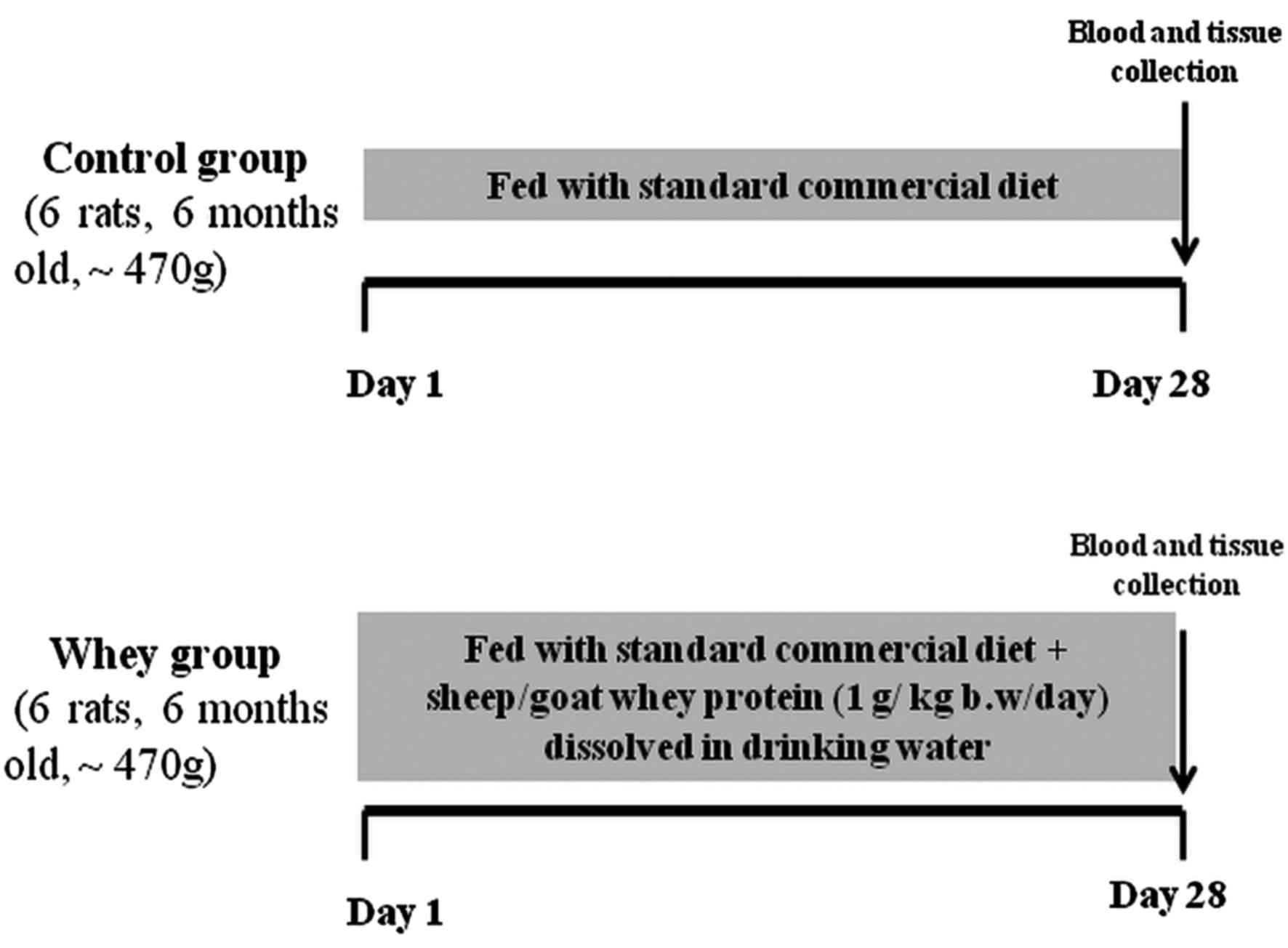
Experimental animals
Twelve male Wistar rats (6-month-old) with an
average body weight of 470 g were used in the experiments under the
frame of an animal experiment performed in the animal facilities of
the Veterinary Medicine School of Aristotle University of
Thessaloniki and licenced by the National Veterinary Administration
authorities. All the animals received human care according to the
Helsinki Declaration and national standards. Animals were housed in
individual cages in a room with controlled temperature (20–22°C)
and humidity conditions and a 12-h light/dark cycle.
Experimental design
Animals were divided into 2 groups (6 rats/group)
and were maintained on their respective diet for 28 days as
follows: Control group (n=6); fed with standard commercial diet
(corn, soybean meal, barley, bran, milk paste, molasses) purchased
from Viozois S.A. (Ioannina, Greece), and the experimental group
(n=6); fed with standard commercial diet plus sheep/goat whey
protein (1 g/kg body weight/day) dissolved in drinking water. The
animals were observed daily for general health and body weight was
measured at the beginning and at the end of the experimental
period. At the end of the treatment period, the animals were
anaesthetized with diethyl ether and blood samples were drawn by
cardiac puncture. The animals were euthanized by decapitation under
deep anaesthesia. Tissues from liver, spleen, pancreas, brain,
heart, quadriceps muscle, lung, small intestine and kidney were
excised, snap-frozen in liquid nitrogen and stored at −80°C until
analysis. The experimental design is described in Fig. 1.
Blood preparation
Blood samples were centrifuged immediately at 1,370
× g for 10 min at 4°C and the plasma was collected and used for the
measurement of total antioxidant capacity (TAC), thiobarbituric
reactive substances (TBARS) and protein carbonyls. The packed
erythrocytes were lysed with distilled water (1:1 v/v), inverted
vigorously, centrifuged at 4,000 × g for 15 min at 4°C and the
erythrocyte lysate was collected for the measurement of GSH and
catalase (CAT) activity. Plasma and erythrocyte lysate were stored
at −20°C until analysis.
Preparation of tissue homogenates
Tissue samples were thawed at 37°C and homogenized
in PBS (0.01 M, pH 7.4) containing a cocktail of protease
inhibitors (Complete™ mini protease inhibitors). Brief sonication
(3×10 sec) on ice followed for better homogenization. The
homogenate was then centrifuged at 10,000 × g for 15 min at 4°C,
the supernatant was collected and the protein concentration was
measured using the Bradford method. Samples were stored at −80°C
until biochemical analysis.
Assays
GSH was measured according to a slightly modified
method of Reddy et al (12), as described by Spanidis et
al (13). A total of 20 µl of
erythrocyte lysate or 400 µg of tissue homogenate was processed
with 5% trichloroacetic acid (TCA), mixed with 660 µl of 67 mM
sodium potassium phosphate (pH 8) and 330 µl of 1 mM DTNB. The
samples were incubated in the dark at room temperature for 15 min
and the absorbance was read at 412 nm. GSH concentration was
calculated on the basis of a calibration curve made using
commercial standards.
CAT activity in erythrocytes and the rate of
H2O2 decomposition were determined as
previously described by Spanidis et al (13), a slightly modified method of Aebi
(14). Briefly, 4 µl of
erythrocyte lysate (diluted 1:10) or 400 µg of tissue homogenate
were added to 2,991 µl of 67 mM sodium potassium phosphate (pH 7.4)
and the samples were incubated at 37°C for 10 min. Five microliters
of 30% H2O2 were added to the samples and the
change in absorbance was immediately read at 240 nm for 130 sec.
Calculation of CAT activity was based on the molar extinction
coefficient of H2O2.
The determination of TAC was based on the method of
Janaszewska and Bartosz (15) with
slight modifications as previously described by Spanidis et
al (13). Briefly, 20 µl of
plasma or 400 µg of tissue homogenate were added to 480 µl of 10 mM
sodium potassium phosphate (pH 7.4) and 500 µl of 0.1 mM
DPPH• free radical and the samples were incubated in the
dark for 60 min at room temperature. The samples were centrifuged
at 20,000 × g for 3 min and the absorbance was read at 520 nm. TAC
was presented as mmol of DPPH• reduced to
2,2-diphenyl-1-picrylhydrazine (DPPH:H) by the antioxidants of
plasma.
For TBARS determination, a slightly modified assay
of Keles et al (16) was
used as per the protocol used in Spanidis et al (13). According to this method, 100 µl of
plasma or 400 µg of tissue homogenate was mixed with 500 µl of 35%
TCA and 500 µl of Tris-HCl (200 mM, pH 7.4). Incubation for 10 min
at room temperature followed. Then, 1 ml of 2 M
Na2SO4 and 55 mM thiobarbituric acid solution
was added and the samples were incubated at 95°C for 45 min. The
samples were cooled on ice for 5 min, followed by the addition of 1
ml of 70% TCA and then the samples were vortexed. The samples were
centrifuged at 15,000 × g for 3 min and the absorbance of the
supernatant was read at 530 nm. Calculation of TBARS concentration
was based on the molar extinction coefficient of
malondialdehyde.
Protein carbonyl determination was based on a
slightly modified method of Patsoukis et al (17), as previously described by Spanidis
et al (13). Briefly, 50 µl
of 20% TCA were added to 50 µl of plasma or to 400 µg of tissue
homogenate and this mixture was incubated in an ice bath for 15 min
and centrifuged at 15,000 × g for 5 min at 4°C. The supernatant was
discarded and 500 µl of 10 mM DNPH (in 2.5 N HCl) for the sample,
or 500 µl of 2.5 N HCl for the blank, were added in the pellet. The
samples were incubated in the dark at room temperature for 1 h,
with vortexing every 15 min, followed by centrifugation at 15,000 ×
g for 5 min at 4°C. The supernatant was discarded and 1 ml of 10%
TCA was added, vortexed and centrifuged at 15,000 × g for 5 min at
4°C. The supernatant was again discarded and 1 ml of ethanol-ethyl
acetate (1:1 v/v) was added, vortexed and centrifuged at 15,000 × g
for 5 min at 4°C. This step was repeated twice. The supernatant was
discarded and 1 ml of 5 M urea (pH 2.3) was added, vortexed and
incubated at 37°C for 15 min. The samples were centrifuged at
15,000 × g for 3 min at 4°C and the absorbance was read at 375 nm.
Calculation of protein carbonyl concentration was based on the
molar extinction coefficient of DNPH.
Statistical analysis
For the statistical analysis, one-way ANOVA followed
by Tukey's test was applied to compare the means between the two
groups (n=6 per group). Differences were considered significant at
P<0.05 with α level set at 0.025. The results are expressed as
mean ± SEM. Statistical analyses were performed using the SPSS
software (version 14.0; SPSS, Inc., Chicago, IL, USA).
Results
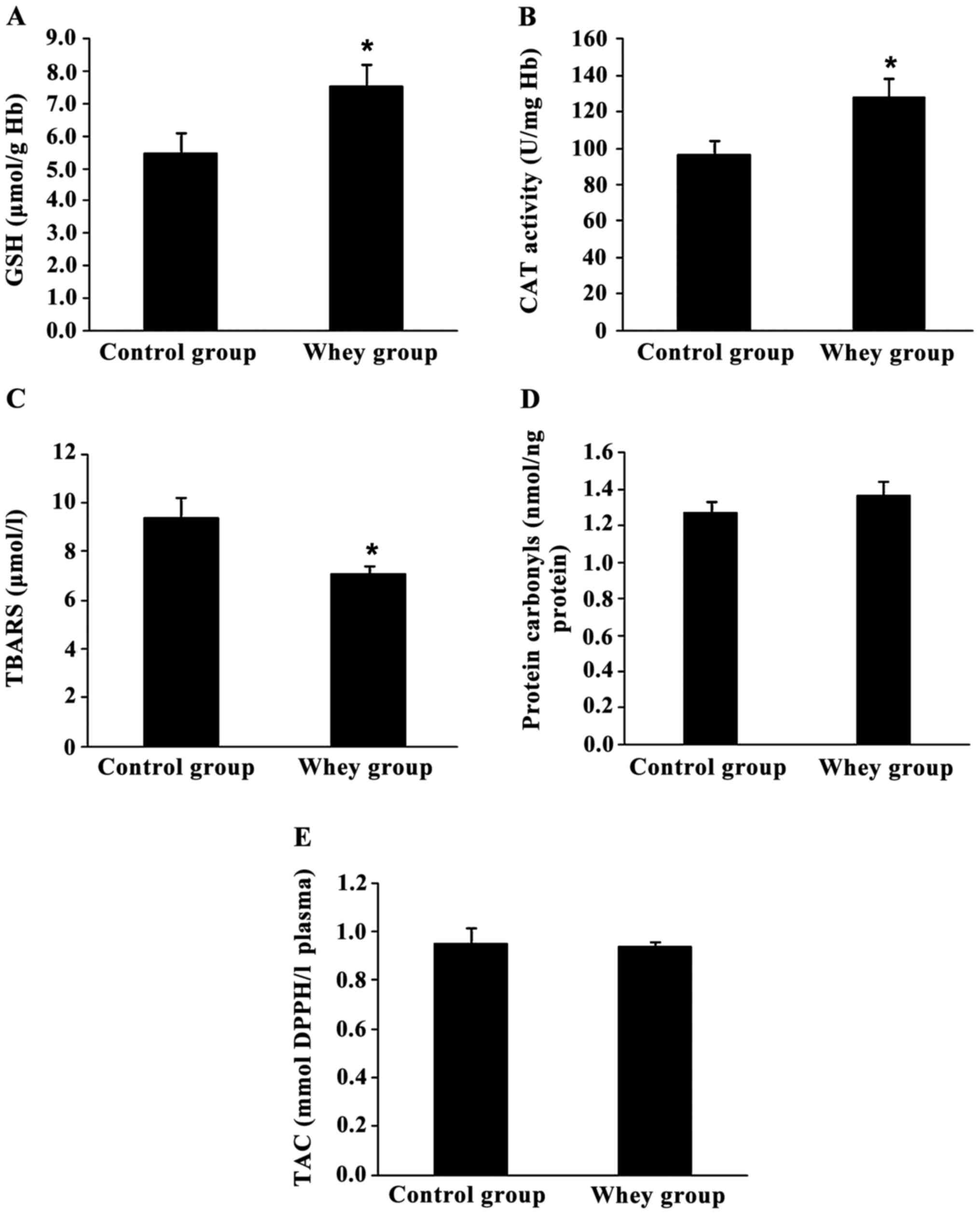
Assessment of redox status markers in rats' blood.
Fig. 2 shows the effects of
sheep/goat whey protein on the redox status of rats in blood.
Oxidative stress markers measured in blood showed that sheep/goat
whey protein improved the redox status of rats.
Specifically, GSH levels and CAT activity in
erythrocyte lysate were increased significantly by 37.6% (P=0.047)
and 32.2% (P=0.041), respectively, in the sheep/goat whey protein
group compared to the control group (Fig. 2A and B).
TBARS levels in plasma were significantly decreased
by 24.5% (P=0.033) in the sheep/goat whey protein group compared to
the control group (Fig. 2C).
Finally, there was not any significant change in
protein carbonyl levels and TAC in plasma between the sheep/goat
whey protein and control groups (Fig.
2D and E).
Assessment of redox status markers in tissues of
rats. The administration of sheep/goat whey protein increased
significantly the GSH levels in small intestine, quadriceps muscle,
pancreas and lung by 37.8% (P=0.032), 34.7% (P=0.044), 78%
(P=0.013) and 172% (P=0.004), respectively, compared to the control
group (Fig. 3A and B).
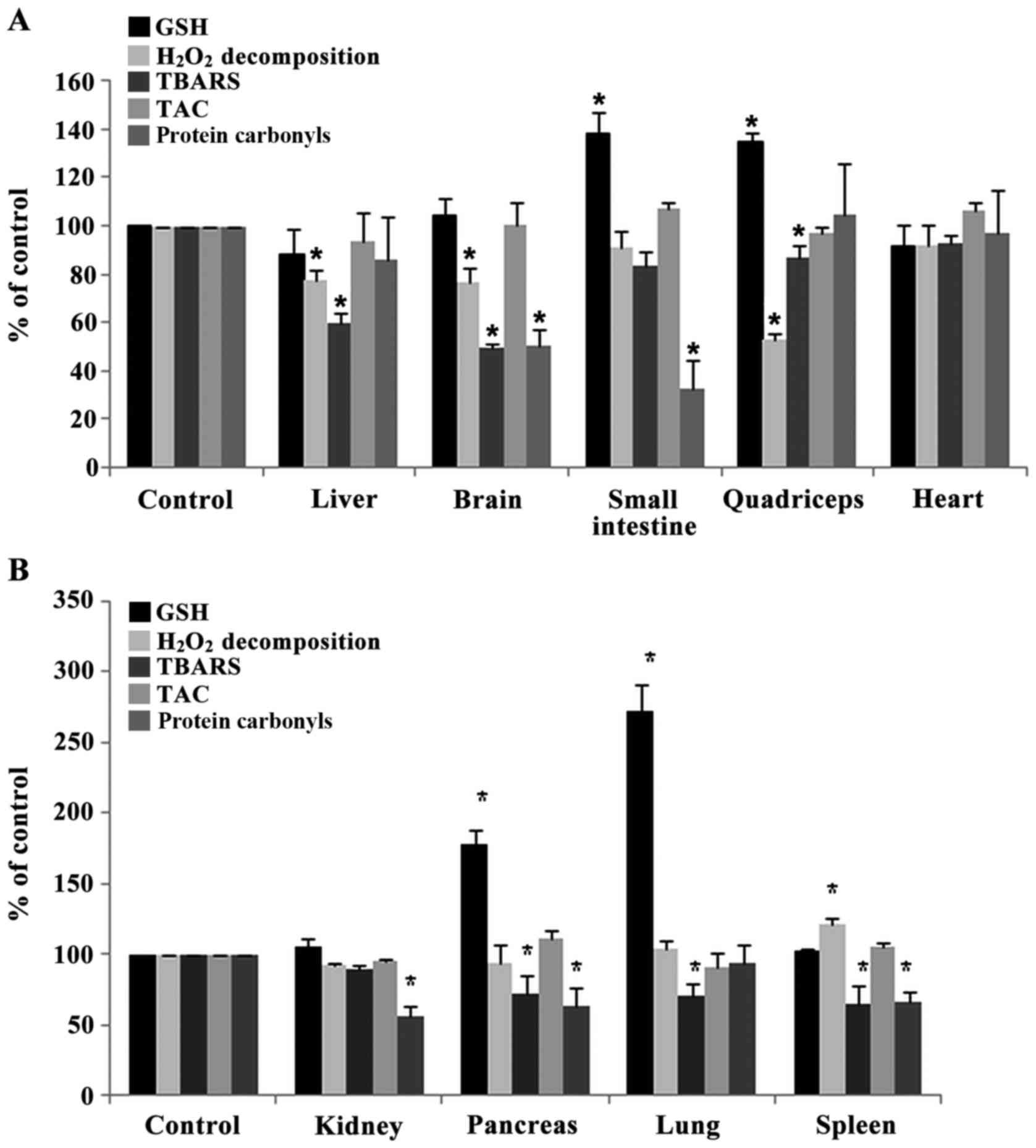 | Figure 3.Effects of sheep/goat whey protein on
redox status markers of tissues. (A) GSH,
H2O2 decomposition, TBARS, TAC, protein
carbonyls levels in liver, brain, small intestine, quadriceps and
heart of rats in the control and whey groups. (B) GSH,
H2O2 decomposition, TBARS, TAC, protein
carbonyls levels in kidney, pancreas, lung and spleen of rats in
control and whey group. *P<0.05; statistically significant
compared to control group. Results are presented as mean ± SEM.
GSH, glutathione; TBARS, thiobarbituric reactive substances; TAC,
total antioxidant capacity. |
The rate of H2O2 decomposition
was decreased significantly in liver, brain and quadriceps muscle
by 22.7% (P=0.012), 23.5% (P=0.042), 47.2% (P=0.048) respectively
in sheep/goat whey protein group compared to control group
(Fig. 3A). In spleen, the rate of
H2O2 decomposition was increased
significantly by 21.3% (P=0.009) in sheep/goat WP group compared to
control group (Fig. 3B).
TBARS levels were decreased significantly in liver,
brain, quadriceps muscle, pancreas, lung and spleen by 40.5%
(P=0.006), 50.6% (P=0.000), 12.9% (P=0.005), 27.6% (P=0.031), 28.9%
(P=0.031) and 36% (P=0.017), respectively, in the sheep/goat whey
protein group compared to the control group (Fig.3A and B).
Regarding protein carbonyl levels, there was a
significant decrease in brain, small intestine, kidney, pancreas
and spleen by 50% (P=0.002), 67.7% (P=0.032), 44% (P=0.009), 37.3%
(P=0.045) and 33.7% (P=0.018), respectively, in the sheep/goat whey
protein group compared to the control group (Fig. 3A and B).
Finally, TAC levels were not affected by the
administration of sheep/goat whey protein in any tissue (Fig. 3A and B).
Discussion
In recent years, there has been an increased
interest regarding the intake of natural products in order to
prevent oxidative damage caused by free radicals (18–22).
When the production of free radicals is increased to an extent that
cannot be coped by the organisms' antioxidant mechanisms, oxidative
stress is induced (23). This
results in irreversible damage, not only to cellular structure and
function, but also to vital organs. Exposure to high levels of free
radicals can cause lipid and protein oxidation and also damage to
DNA (24,25). Oxidative damage of biomolecules
(lipids, proteins, DNA) is one of the main factors in the process
of aging and in various diseases such as carcinogenesis,
cardiovascular diseases, neurodegenerative diseases,
atherosclerosis, diabetes and asthma (26–29).
The discovery of whey as a functional food increased
its status as a co-product in cheese production (2). Whey protein is a rich and balanced
source of sulfur-containing amino acids (cysteine, methionine).
These amino acids enhance antioxidant defense either by acting
directly as reducing agents or as precursors of the intracellular
formation of GSH (30).
In the present in vivo study, we evaluated
the effects of sheep/goat whey protein on the redox status of rats.
Specifically, we estimated the levels of five redox status markers
(GSH, CAT, TBARS, protein carbonyls, TAC) in blood and tissues. GSH
is the most rich non-protein source of thiol (SH) in cells. GSH is
important to a variety of processes, including the detoxification
of xenobiotics, maintenance of the -SH level of proteins and
scavenging of hydroperoxides and free radicals (31). CAT is an antioxidant enzyme that is
present in every cell type and especially in peroxisomes and
catalyzes the conversion of H2O2 to water and
molecular oxygen (32). TBARS and
protein carbonyls are markers of lipid and protein oxidation,
respectively. Finally, TAC is referred to the capability of the
plasma components to scavenge reactive species and is an indicator
of the overall antioxidant capacity of plasma.
Specifically, it was found that GSH levels were
significantly increased in erythrocytes, small intestine,
quadriceps muscle, pancreas and lung tissues compared to the
control. According to the literature, it has also been found that
whey protein enhances antioxidant capacity in rats through GSH
synthesis. In particular, whey protein enhanced GSH synthesis in
erythrocytes in rats by 31% (33).
Other findings showed that treatment with whey protein enhanced
total liver GSH content in rats (34). GSH is the most important
antioxidant in cells and offers protection against oxidative damage
caused by free radicals, peroxides and heavy metals. The increased
levels of GSH may be attributed to the rich content of whey in
cysteine and methionine. These amino acids are precursors for the
intracellular conversion of GSH (30). Whey may also increase GSH levels
through modulation of the two key enzymes that are involved in its
biosynthesis; glutamate cysteine ligase (GCL) and glutathione
synthetase (GS). In previous studies, it has been shown that
sheep/goat whey protein increased GCL expression in C2C12 muscle
cells (9). A possible mechanism,
through which the expression of these enzymes is regulated, may be
the cytosolic system Nrf2-Kelch-like ECH-associated protein 1
(Keap1). In endothelial cells it has been found that the expression
of a number of antioxidant enzymes is regulated through Nrf2-Keap1
pathway (9). Under normal
conditions, Nrf2 is located in the cytoplasm anchored by Keap1
resulting in its ubiquitination and proteasome degradation. The
activation of Nrf2 is regulated by two signaling pathways: i)
Oxidation of Keap1s' cysteine residues; and/or ii) activation of a
number of protein kinases that induce phosphorylation of Nrf2. Both
facilitate the disassociation from Keap1 and its translocation in
the nucleus. The nuclear Nrf2 binds to the ARE and induces the
activation of a number of antioxidant enzymes or phase II
metabolism enzymes (35–38). It has been found that whey protein
can activate c-Jun N-terminal kinases (JNKs) and extracellular
signal-regulated kinases (ERKs) (39). Thus, sheep/goat whey protein may
induce the phosphorylation of Nrf2, disassociation from Keap1,
translocation in the nucleus and finally activation of antioxidant
enzymes.
In erythrocytes, the conversion of
H2O2 to H2O and O2 is
based on CAT activity (40) while
in tissues, the decomposition of H2O2 to
H2O and O2 is attributed, not only to CAT
activity, but also to other enzymes such as glutathione peroxidase
(GPx) and peroxiredoxins (41). In
erythrocytes, it was found that CAT activity was increased
significantly compared to control. In liver, brain and quadriceps
muscle tissues, the rate of H2O2
decomposition was decreased compared to the control group while in
spleen tissue the decomposition rate was increased compared to the
control. While we would expect sheep/goat whey protein to increase
the rate of H2O2 decomposition, in some
tissues a reduction is observed. This could be probably attributed
to the activation of a different number of antioxidant enzymes and
especially GSH-associated enzymes. Haraguchi et al (34) has shown that whey protein decreased
liver CAT activity in rats. In previous studies, we have shown that
CAT expression and activity was increased in C2C12 muscle and
Ea.hy926 endothelial cells (9).
Concerning the effects of sheep/goat whey protein on
lipid peroxidation in plasma, it has been found that TBARS was
significantly decreased compared to the control group. The decrease
in lipid peroxidation in plasma can be attributed to the respective
increase in CAT activity and GSH synthesis. Concerning the tissues,
we have observed that TBARS levels were significantly decreased in
liver, brain, quadriceps muscle, pancreas, lung and spleen compared
to the control group. Depending on the tissue, the decrease could
be attributed either to GSH molecule and CAT enzyme or to the
activation of a different number of antioxidant enzymes [GPx,
glutathione-s-transferase (GST)]. GSH reduces
H2O2 and organic peroxides through a
GPx-catalyzed reaction, neutralizes hydroxyl radical
(OH•) and therefore prevents lipid peroxidation
(42). The OH• is one
of the most reactive and dominant ROS that can initiate lipid
peroxidation. In biological systems, OH• is formed by
Fenton-Haber-Weiss reactions where free iron (Fe2+)
reacts with H2O2 (43). Another enzyme that may prevent
lipid peroxidation is GST through the conjugation of electrophilic
compounds to GSH, leading to their elimination from the body
(44). It has been found that
sheep/goat whey protein increases GST activity in C2C12 muscle and
Ea.hy926 endothelial cells (9).
Thus, GSH and CAT can offer protection against lipid damage and its
subsequent detrimental effects (destruction of the integrity of
cell membranes, cell death).
Protein damage can cause loss of enzyme function,
alter cellular activities and can also cause changes in the type
and level of cellular proteins (45,46).
Oxidation of proteins and amino acids is accompanied by increases
in the levels of protein carbonyl groups (47,48).
The results of the present study have shown that protein carbonyls
were decreased in brain, small intestine, kidney, pancreas and
spleen tissue compared to the control group. It is suggested that
OH• can also cause damage to proteins by reducing their
disulfide bonds, resulting in their unfolding or misfolding
(49). Thus, sheep/goat whey
protein can protect both from protein and lipid peroxidation either
by activating a number of antioxidant molecules and enzymes or by
scavenging OH• (7–9).
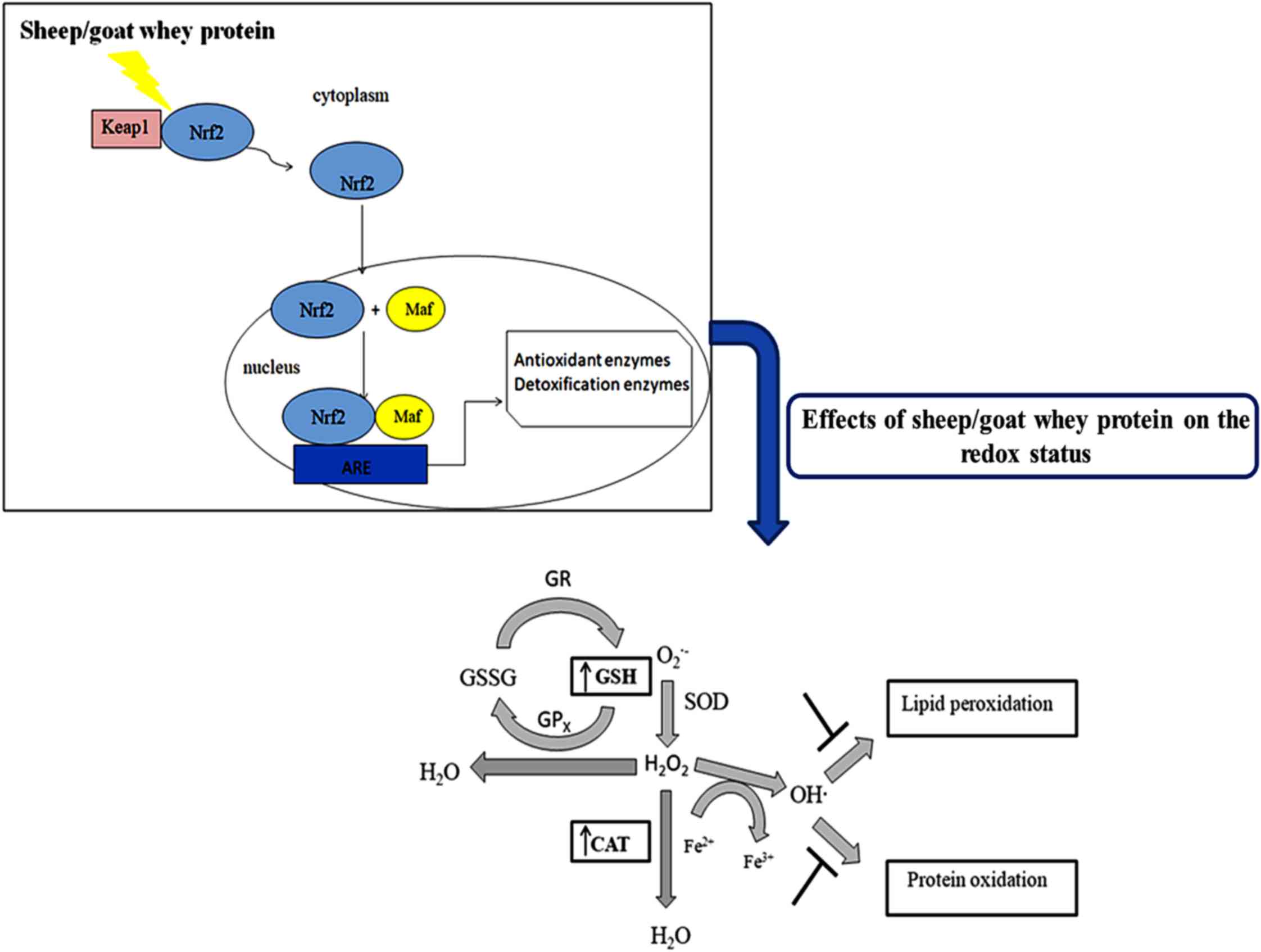
In conclusion, the findings of the present study
have shown that sheep/goat whey protein protects against the
detrimental effects of oxidative stress and enhances the
antioxidant defense mechanisms in blood and tissues (Fig. 4). The results have shown a
tissue-specific effect of sheep/goat whey protein. Taking this
finding into account, we could lead to nutritional intervention
strategies in order to prevent various oxidative stress-associated
diseases. Considering the above beneficial effects of sheep/goat
whey protein, the investigation of its molecular mechanism of
action in order to be incorporated as a bioactive ingredient into
food products would be of particular interest and importance. This
could give additional value to sheep/goat whey protein contributing
on one hand to its profitable utilization and on the other hand to
addressing the environmental problems caused by its uncontrolled
disposal.
Acknowledgements
I would like to thank PhD candidate Sotiria Makri
and Dr Ioannis Kafantaris for their technical assistance.
Funding
The present study was supported in part by IKY
Fellowships funded by the action ‘Enhancement of Post-Doctoral
Researchers’ from the resources of the European Program
‘Development of Human Resources, Education and Life-Long Learning’
co-funded by the European Social Fund.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EK designed the experiment, analyzed and interpreted
the data concerning the redox status. DS was involved in the design
of the experiment. AMT and DAS contributed to the evaluation of the
results and to the writing of the manuscript. IT provided the
animal facility, took care of the animals and collected the blood
and the tissues. DK was the supervisor of experiment, involved in
the draft of the manuscript and gave the final approval of the
manuscript to be published. All the authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The animals received human care according to the
Helsinki Declaration and national standards.
Consent for publication
Not applicable.
Competing interests
Demetrios A. Spandidos is the Editor-in-Chief for
the journal, but had no personal involvement in the reviewing
process, or any influence in terms of adjudicating on the final
decision, for this article.
Glossary
Abbreviations
Abbreviations:
|
ARE
|
antioxidant response element
|
|
CAT
|
catalase
|
|
DNPH
|
2,4-dinitrophenylhydrazine
|
|
DPPH
|
2,2-diphenyl-1-picryl hydrazyl
|
|
DTNB
|
5,5′-dithiobis (2-nitrobenzoic
acid)
|
|
ERKs
|
extracellular signal-regulated
kinases
|
|
GCL
|
glutamate cysteine ligase
|
|
GPx
|
glutathione peroxidase
|
|
GS
|
glutathione synthetase
|
|
GSH
|
glutathione
|
|
GST
|
glutathione-s-transferase
|
|
H2O2
|
hydrogen peroxide
|
|
HCL
|
hydrochloride
|
|
JNKs
|
c-Jun N-terminal kinases
|
|
Keap1
|
Kelch-like ECH-associated protein
1
|
|
Nrf2
|
nuclear factor E2-related factor 2
|
|
OH·
|
hydroxyl radical
|
|
PBS
|
phosphate-buffered saline
|
|
TAC
|
total antioxidant capacity
|
|
TBARS
|
thiobarbituric reactive substances
|
|
TAC
|
trichloroacetic acid
|
|
Tris
|
trishydroxymethylaminomethane
|
References
|
1
|
Gill HS, Rutherfurd-Markwick KJ and Cross
ML: Bovine milk: A unique source of immunomodulatory ingredients
for functional foods. Functional Foods II - Claims and Evidence.
Saltmarsh M and Buttriss J: Royal Society of Chemistry Press. 248.
Cambridge: pp. 82–90. 2000
|
|
2
|
Walzem RL, Dillard CJ and German JB: Whey
components: Millennia of evolution create functionalities for
mammalian nutrition: What we know and what we may be overlooking.
Crit Rev Food Sci Nutr. 42:353–375. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smithers GW: Whey and whey proteins - from
‘gutter-to-gold’. Int Dairy J. 18:695–704. 2008. View Article : Google Scholar
|
|
4
|
Farrell HM Jr, Jimenez-Flores R, Bleck GT,
Brown EM, Butler JE, Creamer LK, Hicks CL, Hollar CM, Ng-Kwai-Hang
KF and Swaisgood HE: Nomenclature of the proteins of cows' milk -
sixth revision. J Dairy Sci. 87:1641–1674. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mollea C, Marmo L and Bosco F:
Valorisation of cheese whey, a by-product from the dairy industry.
Food Industry. Muzzalupo I: InTech549–588. 2013.
|
|
6
|
Marshall K: Therapeutic applications of
whey protein. Altern Med Rev. 9:136–156. 2004.PubMed/NCBI
|
|
7
|
Kerasioti E, Stagos D, Priftis A,
Aivazidis S, Tsatsakis AM, Hayes AW and Kouretas D: Antioxidant
effects of whey protein on muscle C2C12 cells. Food Chem.
155:271–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kerasioti E, Stagos D, Georgatzi V, Bregou
E, Priftis A, Kafantaris I and Kouretas D: Antioxidant effects of
sheep whey protein on endothelial cells. Oxid Med Cell Lonqev 2016.
65857372016.
|
|
9
|
Kerasioti E, Stagos D, Tzimi A and
Kouretas D: Increase in antioxidant activity by sheep/goat whey
protein through nuclear factor-like 2 (Nrf2) is cell type
dependent. Food Chem Toxicol. 97:47–56. 2016b. View Article : Google Scholar
|
|
10
|
Kerasioti E, Kiskini A, Veskoukis A,
Jamurtas A, Tsitsimpikou C, Tsatsakis AM, Koutedakis Y, Stagos D,
Kouretas D and Karathanos V: Effect of a special
carbohydrate-protein cake on oxidative stress markers after
exhaustive cycling in humans. Food Chem Toxicol. 50:2805–2810.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kerasioti E, Stagos D, Jamurtas A, Kiskini
A, Koutedakis Y, Goutzourelas N, Pournaras S, Tsatsakis AM and
Kouretas D: Anti-inflammatory effects of a special
carbohydrate-whey protein cake after exhaustive cycling in humans.
Food Chem Toxicol. 61:42–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reddy YN, Murthy SV, Krishna DR and
Prabhakar MC: Role of free radicals and antioxidants in
tuberculosis patients. Indian J Tuberc. 51:213–218. 2004.
|
|
13
|
Spanidis Y, Mpesios A, Stagos D,
Goutzourelas N, Bar-Or D, Karapetsa M, Zakynthinos E, Spandidos DA,
Tsatsakis AM, Leon G and Kouretas D: Assessment of the redox status
in patients with metabolic syndrome and type 2 diabetes reveals
great variations. Exp Ther Med. 11:895–903. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aebi H: Catalase in vitro. Methods
Enzymol. 105:121–126. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Janaszewska A and Bartosz G: Assay of
total antioxidant capacity: Comparison of four methods as applied
to human blood plasma. Scand J Clin Lab Invest. 62:231–236. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Keles MS, Taysi S, Sen N, Aksoy H and
Akçay F: Effect of corticosteroid therapy on serum and CSF
malondialdehyde and antioxidant proteins in multiple sclerosis. Can
J Neurol Sci. 28:141–143. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patsoukis N, Zervoudakis G, Panagopoulos
NT, Georgiou CD, Angelatou F and Matsokis NA: Thiol redox state
(TRS) and oxidative stress in the mouse hippocampus after
pentylenetetrazol-induced epileptic seizure. Neurosci Lett.
357:83–86. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goutzourelas N, Stagos D, Demertzis N,
Mavridou P, Karterolioti H, Georgadakis S, Kerasioti E, Aligiannis
N, Skaltsounis L, Statiri A, et al: Effects of polyphenolic grape
extract on the oxidative status of muscle and endothelial cells.
Hum Exp Toxicol. 33:1099–1112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goutzourelas N, Stagos D, Housmekeridou A,
Karapouliou C, Kerasioti E, Aligiannis N, Skaltsounis AL, Spandidos
DA, Tsatsakis AM and Kouretas D: Grape pomace extract exerts
antioxidant effects through an increase in GCS levels and GST
activity in muscle and endothelial cells. Int J Mol Med.
36:433–441. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Priftis A, Stagos D, Konstantinopoulos K,
Tsitsimpikou C, Spandidos DA, Tsatsakis AM, Tzatzarakis MN and
Kouretas D: Comparison of antioxidant activity between green and
roasted coffee beans using molecular methods. Mol Med Rep.
12:7293–7302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kouka P, Priftis A, Stagos D, Angelis A,
Stathopoulos P, Xinos N, Skaltsounis AL, Mamoulakis C, Tsatsakis
AM, Spandidos DA and Kouretas D: Assessment of the antioxidant
activity of an olive oil total polyphenolic fraction and
hydroxytyrosol from a Greek Olea europea variety in endothelial
cells and myoblasts. Int J Mol Med. 40:703–712. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Makri S, Kafantaris I, Stagos D,
Chamokeridou T, Petrotos K, Gerasopoulos K, Mpesios A, Goutzourelas
N, Kokkas S, Goulas P, et al: Novel feed including bioactive
compounds from winery wastes improved broilers' redox status in
blood and tissues of vital organs. Food Chem Toxicol. 102:24–31.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li YR: Free Radical Biomedicine:
Principles, Clinical Correlations, and Methodologies. Bentham
Science Publishers; Blacksburg, VA: 2012, View Article : Google Scholar
|
|
24
|
Sahiner UM, Birben E, Erzurum S, Sackesen
C and Kalayci O: Oxidative stress in asthma. World Allergy Organ J.
4:151–158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nikitovic D, Corsini E, Kouretas D,
Tsatsakis A and Tzanakakis G: ROS-major mediators of extracellular
matrix remodeling during tumor progression. Food Chem Toxicol.
61:178–186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jenner P: Oxidative stress in Parkinson's
disease. Ann Neurol. 53 Suppl 3:S26–S36, discussion S36-S38. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Honda K, Casadesus G, Petersen RB, Perry G
and Smith MA: Oxidative stress and redox-active iron in Alzheimer's
disease. Ann N Y Acad Sci. 1012:179–182. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dut R, Dizdar EA, Birben E, Sackesen C,
Soyer OU, Besler T and Kalayci O: Oxidative stress and its
determinants in the airways of children with asthma. Allergy.
63:1605–1609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Uchida K: Role of reactive aldehyde in
cardiovascular diseases. Free Radic Biol Med. 28:1685–1696. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shoveller AK, Stoll B, Ball RO and Burrin
DG: Nutritional and functional importance of intestinal sulfur
amino acid metabolism. J Nutr. 135:1609–1612. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu SC: Regulation of glutathione
synthesis. Mol Aspects Med. 30:42–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Weydert CJ and Cullen JJ: Measurement of
superoxide dismutase, catalase and glutathione peroxidase in
cultured cells and tissue. Nat Protoc. 5:51–66. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim J, Paik HD, Yoon YC and Park E: Whey
protein inhibits iron overload-induced oxidative stress in rats. J
Nutr Sci Vitaminol (Tokyo). 59:198–205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Haraguchi FK, Silva ME, Neves LX, dos
Santos RC and Pedrosa ML: Whey protein precludes lipid and protein
oxidation and improves body weight gain in resistance-exercised
rats. Eur J Nutr. 50:331–339. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kelly VP, Ellis EM, Manson MM, Chanas SA,
Moffat GJ, McLeod R, Judah DJ, Neal GE and Hayes JD:
Chemoprevention of aflatoxin B1 hepatocarcinogenesis by coumarin, a
natural benzopyrone that is a potent inducer of aflatoxin
B1-aldehyde reductase, the glutathione S-transferase A5 and P1
subunits, and NAD(P)H:quinone oxidoreductase in rat liver. Cancer
Res. 60:957–969. 2000.PubMed/NCBI
|
|
36
|
Li J, Lee JM and Johnson JA: Microarray
analysis reveals an antioxidant responsive element-driven gene set
involved in conferring protection from an oxidative stress-induced
apoptosis in IMR-32 cells. J Biol Chem. 277:388–394. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Talalay P, Dinkova-Kostova AT and
Holtzclaw WD: Importance of phase 2 gene regulation in protection
against electrophile and reactive oxygen toxicity and
carcinogenesis. Adv Enzyme Regul. 43:121–134. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kwak MK, Wakabayashi N, Itoh K, Motohashi
H, Yamamoto M and Kensler TW: Modulation of gene expression by
cancer chemopreventive dithiolethiones through the Keap1-Nrf2
pathway. Identification of novel gene clusters for cell survival. J
Biol Chem. 278:8135–8145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tsuji-Naito K and Jack RW: Concentrated
bovine milk whey active proteins facilitate osteogenesis through
activation of the JNK-ATF4 pathway. Biosci Biotechnol Biochem.
76:1150–1154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Scott MD, Lubin BH, Zuo L and Kuypers FA:
Erythrocyte defense against hydrogen peroxide: Preeminent
importance of catalase. J Lab Clin Med. 118:7–16. 1991.PubMed/NCBI
|
|
41
|
Halliwell B, Clement MV and Long LH:
Hydrogen peroxide in the human body. FEBS Lett. 486:10–13. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tabet F and Touyz RM: Reactive oxygen
species, oxidative stress, and vascular biology in hypertension.
Comprehensive Hypertension. Elsevier; pp. 337–347. 2007, View Article : Google Scholar
|
|
43
|
Ayala A, Muñoz MF and Argüelles S: Lipid
peroxidation: Production, metabolism, and signaling mechanisms of
malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev.
2014:3604382014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sherratt PJ and Hayes JD: Glutathione
S-transferases. Enzyme Systems that Metabolise Drugs and Other
Xenobiotics. Ioannides C: John Wiley & Sons Ltd.; Hoboken, NJ:
pp. 319–352. 2002, View Article : Google Scholar
|
|
45
|
Grune T, Reinheckel T and Davies KJA:
Degradation of oxidized proteins in mammalian cells. FASEB J.
11:526–534. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Halliwell B and Gutteridge JMC: Chapter 2.
In: Free radicals in biology and medicine. 3rd. Oxford Science
Publications; 1999
|
|
47
|
Renke J, Popadiuk S, Korzon M, Bugajczyk B
and Wozniak M: Protein carbonyl groups' content as a useful
clinical marker of antioxidant barrier impairment in plasma of
children with juvenile chronic arthritis. Free Radic Biol Med.
29:101–104. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Levine RL: Carbonyl modified proteins in
cellular regulation, aging, and disease. Free Radic Biol Med.
32:790–796. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lipinski B: Hydroxyl radical and its
scavengers in health and disease. Oxid Med Cell Longev.
2011:8096962011. View Article : Google Scholar : PubMed/NCBI
|