Introduction
Osteoporosis (OP) is a disease characterized by
structural deteriorationist, low bone mass, and porous bone
associated with higher fracture risk, significantly influencing
expectancy and quality of life in humans. Osteoblasts (OB) play
pivotal roles in mediation of formation, resorption and remodeling
of bones (1). OB and osteoclast
(OC) are units of bone remodeling and healthy of bone is dependent
on balance and interaction between them (1). The imbalance caused by reduction of
OB differentiation and increased differentiation of OC were the
major reasons of OP (1). OP was
increased year by year with aging population and per-capita life
expectancy, especially for postmenopausal womenfolk (2). In the past decades, researches showed
that estrogen deficiency and immune and inflammatory responses are
the main factors inducing OP (3,4).
However, recent investigations indicated that oxidative stress can
stimulate bone marrow OC differentiation and inhibit OB
differentiation, thereby promoting the development of OP (5,6).
Therefore, as a risk factor for the development of OP, oxidative
stress has received more attention (6,7). And
the focus of pathologic mechanism of OP was gradually shifted from
estrogen-centric to aging and oxidative stress (8).
Oxidative stress is a highly reactive molecule in
living organisms, such as reactive nitrogen radicals (RNS) and
reactive oxygen species (ROS) (9,10).
High production of them would lead to oxidative damage to
bimolecular, which further induces tissues and organs damaged
(10). ROS is closely related with
biomedical and pathogenic bases of variety diseases and
pathological processes. Oxidative stress levels in the body depend
on the balance between ROS and antioxidant defense system in
vivo (10).
Investigations showed that oxidative stress can
inhibit bone marrow stromal cell line (M2-1 OB4) and OB progenitor
cell line (MC3T3-E1) differentiation into OB, and inhibit OB
mineralization and induce its apoptosis (11). Studies revealed that
H2O2 in human bone marrow stromal cells
(HBMSCs) increased level of ROS through activation of c-Jun
N-terminal kinase (JNK) and nuclear factor κB (NF-κB) Pathway,
which result in reduction of glutathione, activation of caspases 3,
9 and 8, and finally inducing apoptosis of HBMSCs (12).
ROS is capable of regulating the osteoclastic bone
resorption from bone cells (13).
Superoxide produced by OC may be directly involved in the
degradation of bone tissue, and inhibiting production or
utilization of ROS and bone resorption (13). Researches showed that production of
ROS and OC differentiation were completely inhibited by blocking
nicotinamide adenine dinucleotide phosphate oxidase 1 (Noxl) in OC
precursor cells (14). In other
words, ROS can stimulate the growth and differentiation of OC,
while OC, in turn, increases the production of ROS, and if the
body's antioxidant defense mechanisms impaired, it will form a
vicious circle, leading to increased bone resorption and OP.
Currently, there are two kinds of drugs in the
treatment of OP: The bone resorption inhibitor such as calcium,
vitamin D, estrogen (15), the
other is bone enhancers, such as fluoride and parathyroid hormone
(16). Antioxidant treatment is a
new and effective approach to the prevention and treatment of OP
(17).
OB is the main functional cell of bone formation in
bone metabolic processes, and its proliferation and differentiation
were affected by multiple signaling pathways, including ER,
BMP-2/Smads, OPG/RANKL, Wnt and forkhead box O (FoxO)1/β-catenin
signaling pathways (18,19). FoxO transcription factor family is
a community of transcription factors with conservative Fork domain,
including four family members: FoxO1 (Fkhr), FoxO3 (Fkhrl 1), FoxO4
(Afx) and FoxO6 (20,21). Investigators reported that among
four family members of FoxOs, only the FoxO1 is the transcription
factor that promotes OB proliferation and is necessary to maintain
redox balance and control bone formation (22). FoxOs activities are regulated by
multiple signaling pathways, including insulin,
phosphatidylinositol-3-kinase/serine threonine kinase (PI3K/Akt),
which mainly involving regulation of
phosphorylation/dephosphorylation of specific amino acid sites
(23). After phosphorylation of
specific amino acid sites, FoxOs were transferred from the nucleus
to the cytoplasm, where transcriptional activity of FoxOs and its
functions are inhibited (23).
Therefore, the nuclear translocation of FoxOs determines the
regulation of transcription of target genes.
Silent information regulator type-1 [sirtuin
(SIRT)1] is a NAD+-dependent deacetylase (24). Because of the substrate diversity
of SIRT1, it has a wide range of physiological functions, including
genome stability, cell survival, apoptosis, inflammation and
metabolism (24,25). Among them, SIRT1 and apoptosis are
closely related. It has been proved that SIRT1 can not only
deacetylate histone and regulate the transcriptional activity of
transcription factors in preadipocyte differentiation (26), but also can deacetylate lipids to
produce key transcription factors (27). In addition, SIRT1 can promote the
osteogenic differentiation of mesenchymal stem cells (MSCs) and
inhibit the adipogenic differentiation of MSCs (28). However, the molecular mechanism of
SIRT1 regulation of osteogenic differentiation of MSCs remains
unclear, and further elucidation is needed. Nuclear localization of
FoxO1 is regulated by SIRT1 deacetylase (29).
In summary, the aim of this study is to investigate
the inhibition efficiency of overexpressing SIRT1 on apoptosis of
OB cell induced by H2O2. Our results revealed
that upregulated SIRT1 can inhibit apoptosis of OB cell induced by
H2O2 though FoxO1/β-catenin pathway.
Materials and methods
Cell culture
MC3T3-E1 cells, derived from newborn mice calvaria,
were purchased from Chinese Academy of Sciences Cell Bank
(Shanghai, China) and cultured in DMEM containing 10% fetal bovine
serum (FBS) and antibiotics, at 37°C under a humidified atmosphere
with 5% CO2 and 95% air, as previously described. Cells
were grown to about 70–80% confluence and used for following
experiments.
Cell transfection and experiments
Exponentially growing cells at 1×105 were
seeded to 6-well plates and cultured at 37°C and 5% CO2
for 24 h. After cells were grown to about 80% subconfluence, medium
was discarded and replaced by serum-free DMEM-F12 medium overnight.
According to the instructions, Lipofectamine 2000 10 µl was diluted
with 250 µl serum-free DMEM-F12 and incubated for 5 min at room
temperature. Serum-free DMEM-F12 medium (250 µl) were added into
two groups of centrifuge tubes. The blank control plasmid and SIRT1
overexpression plasmid were constructed by Shanghai Jike Gene
Chemical Technology Co., Ltd. (Shanghai, China) and 4 mg of Sirtl
overexpression plasmid and blank control plasmid were then added
into the two groups, respectively. Plasmids were added to
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) diluted solution and incubated at room
temperature for 20 min. The DNA-liposome mixtures obtained were
added to 6-well plates, respectively. After incubation for 6 h,
culture medium was replaced by normal culture medium to continue
cultivation. After transfection for 48 h, transfection efficiency
was assessed by western blot and RT-PCR assays. Cells used in this
study were divided into four groups as follows: Normal cells
(untransfected, control), normal cells with
H2O2 treatment (H2O2),
negative transfection cells with H2O2
treatment (H2O2 + NT), and positive
transfection cells with H2O2 treatment
(H2O2 + SIRT1). All cells were then cultured
in complete DMEM and harvested at 24, 48, and 72 h.
CCK-8 assay
Cell viability was determined by CCK-8 assay as
described previously (30).
Briefly, after treatment, cells harvested at 24, 48 and 72 h were
seeded on 96-well plates at 1×104 and 10 µl of CCK-8
(Dojindo Laboratories, Kumamoto, Japan) was added into the cell
solution. The absorption of cell solution was measured at 450
nm.
ROS determination by flow cytometer
assay
After treatment for 24, 48, and 72 h as described
above, cells collected from all groups were washed with PBS and
detached using a trypsin solution (Sigma-Aldrich, St. Louis, MO,
USA) containing 0.05% trypsin and 0.02% EDTA for 2 min at 37°C, and
then cells were resuspended within 1 ml Hanks' Balanced Salt
Solution (HBSS; Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China). Subsequently, cells were incubated in 20 µM
dichlorofluorscein-diacetate (DCFH-DA) (Sigma-Aldrich) solution
[dissolved in dimethylsulfoxide (DMSO)] at 37°C for 1 h. After
cells were washed with PBS, ROS production in cells was detected
using flow cytometry.
Apoptosis determination by flow
cytometer assay
After treatments for 24, 48, and 72 h as described
above, cells in all groups were detached using a trypsin solution
containing 0.05% trypsin and 0.02% EDTA for 2 min at 37°C. When
cells were observed to be round and float, 10% serum medium was
added to stop digestion. Cells were then centrifuged at 1,000 rpm
for 4 min. After washed with PBS, cells were placed into diluted
binding buffer (500 µl). Then FITC-labeled Annexin V and 5 µl PI
(BioDesign, Quakertown, PA, USA) were added, respectively. After
incubation at room temperature for 10 min, apoptosis was finally
measured using flow cytometer. Annexin V-FITC positive and PI
negative represent early apoptotic cells and late apoptotic cells
were expressed as Annexin V-FITC positive and PI positive.
Western blot assay
Cultured cells from all groups were homogenized in a
100 µl regular lysis buffer (Cell Signaling Technology, Inc.,
Danvers, MA, USA) containing a protease and phosphatase inhibitor
cocktail (Roche Diagnostics, Basel, Switzerland). Quantifications
of total protein extracts were performed by BCA Protein Assay kit
(Thermo Fisher Scientific, Inc.). Proteins was isolated by 10%
sodium dodecyl sulphate-polyacrylamide gel and then transferred to
immobilon-P-polyvinylidene fluoride (PVDF) membrane (Millipore,
Bedford, MA, USA). Membranes were then blocked in 5% non-fat dry
milk in TBST (20 mM Tris-HCl pH 7.2, 137 mM NaCl, 0.1% Tween-20)
for 1 h at room temperature and incubated with appropriate
monoclonal antibody [FoxO1, 1:1,000, ab60270; β-catenin, 1:5,000,
ab32572; growth arrest and DNA damage inducible protein 45
(Gadd45), 1:100, ab76664; Bim, 1:1,000, ab32158; peroxisome
proliferator activated receptor (PPAR)-γ, 1:500, ab45036; GAPDH,
1:1,000, ab8245; all from Abcam, Cambridge, UK] at 4°C overnight.
Membranes were then washed with PBS and incubated with horseradish
peroxidase (HRP)-conjugated secondary antibodies (1:2,000 dilution,
DAKO) for 1 h. Each sample was performed in triplicate and GAPDH
expression was used to normalize the sample values. Proteins were
then determined by enhanced chemiluminescence system (Amersham
Pharmacia Biotech, Piscataway, NJ, USA). Band intensity was
measured using ImageJ (National Institutes of Health, Bethesda, MD,
USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA was extracted from cells using the RNeasy
mini kit (Qiagen, Valencia, CA, USA) according to manufacturer's
instructions. Total RNA (1 µg) was converted to cDNA using the
Omniscript™ Reverse Transcriptase kit (Qiagen).
Real-time PCR was performed using a SYBR-Green PCR master mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and determined
on an ABI PRISM 7700 Sequence Detection system (Applied Biosystem;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. The PCR products were confirmed by melting curve
analysis and garose gel electrophoresis. Data were normalized to
GAPDH and were calculated using delta-delta cycle threshold (CT)
method. All primer sequences are listed as follows: SIRT1 forward,
5′GGGTTTCTGTCTCCTGTGGGA3′ and reverse, 5′GCTTGAGGGTCTGGGAGGTC3′;
Bax forward, 5′TCATGGGCTGGACACTGGAC3′ and reverse,
5′CACAGTCCAAGGCAGTGGGA3′; Bcl-2 forward, 5′GCCTGAGAGCAACCCAATGC3′
and reverse, 5′CGGAGGGTCAGATGGACCAC3′; FoxO1 forward,
5′GTCTACGCTGCCCAGTCTGT3′ and reverse, 5′TGTTTGGCGGTGCAAACGAA3′;
β-catenin forward, 5′GGATACGGCCAGGATGCCTT3′ and reverse,
5′CCAGATCAGGCAGCCCATCA3′; Gadd45a forward, 5′ATTACGGTCGGCGTGTACGA3′
and reverse, 5′ACATCCCGGTCGTCGTCTTC3′; Bim forward,
5′GTTTCCCTTGCCTCCTCGGT3′ and reverse, 5′CAGCAGGCTGCAATTGTCCA3′;
PPAR-γ forward, 5′GCTGAACGTGAAGCCCATCG3′ and reverse,
5′GGCGAACAGCTGAGAGGACT3′; and GAPDH forward,
5′AAGGCTGTGGGCAAGGTCAT3′ and reverse, 5′CGTCAGATCCACGACGGACA3′.
Data analysis
Multiple groups are compared by one-way ANOVA
followed by post hoc Tukey's comparison test. Data was carried out
using Student's t-test for paired data and P<0.05 was considered
as a statistically significant.
Results
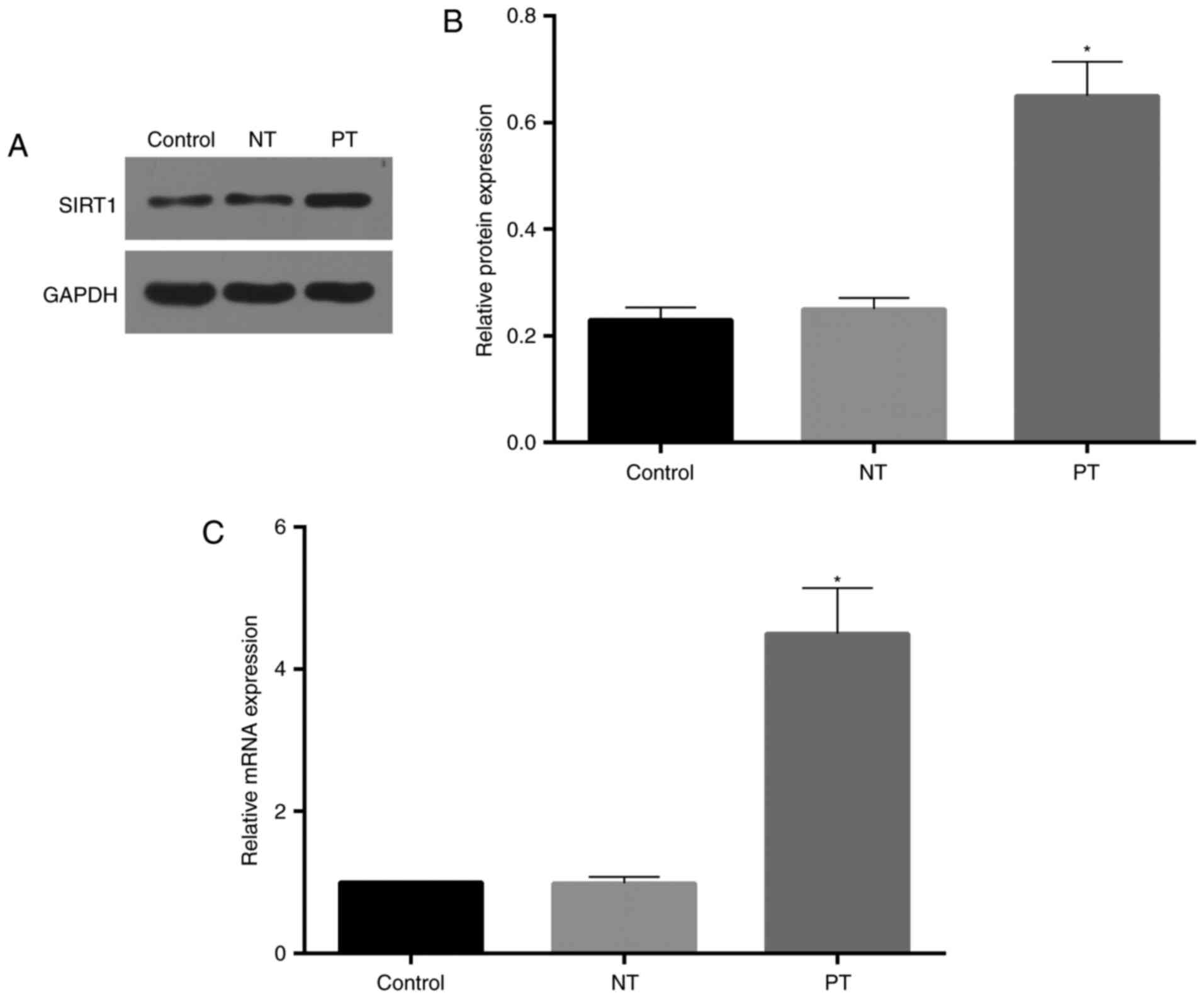
Western blot assay showed high
efficiency of cell transfection
To evaluate the transfection effectiveness, we
tested the expression levels of both mRNA and protein of SIRT1.
After transfection for 48 h, cells were harvested to determine the
expression efficiency of SIRT1. As showed in Fig. 1, expression of SIRT1 mRNA and
protein were upregulated compared to the control (untransfected)
and negative transfection.
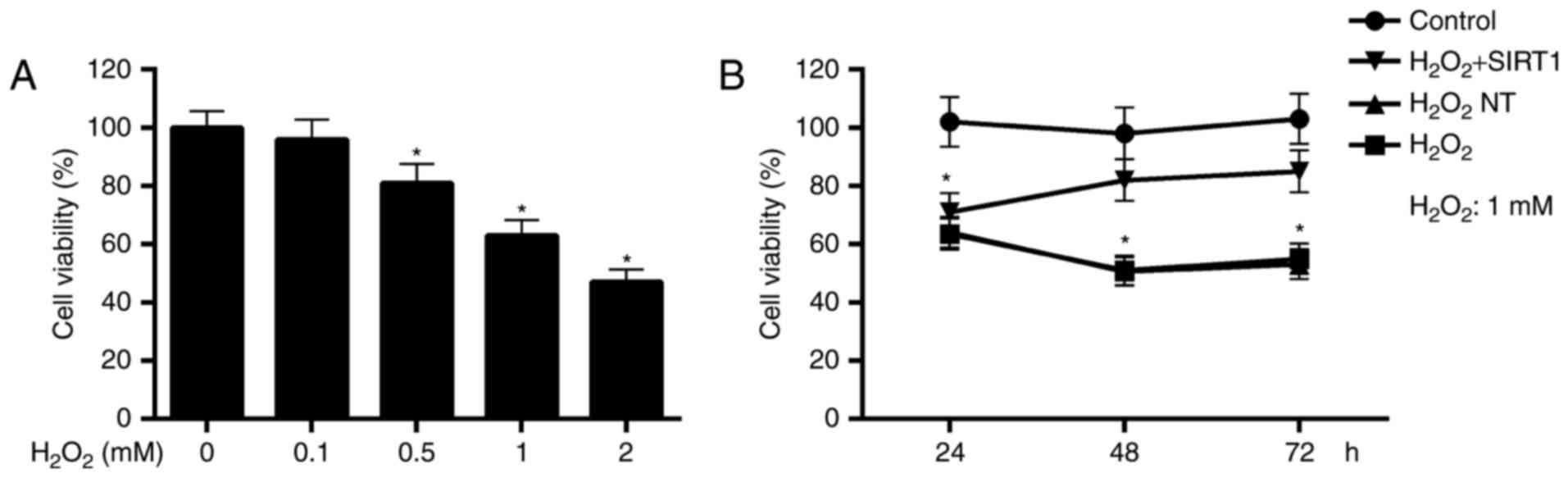
Cell viability determination revealed
that SIRT1 overexpression protected cells against
H2O2-induced damage
H2O2 is one of main forms of
ROS that is relatively more stable in vivo and membrane
permeable (31). Cell viability
assay performed by CCK-8 showed that the cytotoxicity of
H2O2 increased in a dosage dependent manner.
Compared to the control, cell viabilities were significantly
reduced in cells with 0.5, 1, 2 mM H2O2
treatment for 24 h (Fig. 2A).
To explore the protection effect of SIRT1
overexpression on cells against oxidative damage, we compared the
cell viabilities for normal cells, negative transfected cells and
positive transfected cells in the present of
H2O2with normal cells without
H2O2. As showed in Fig. 2B, cell viabilities in
H2O2 NT and H2O2 group
were significantly reduced in a time dependent manner, compared to
control. While, cell viabilities in H2O2 +
SIRT1 group were increased from 24 to 72 h and up to no significant
difference levels at 48 and 72 h with respect to control group.
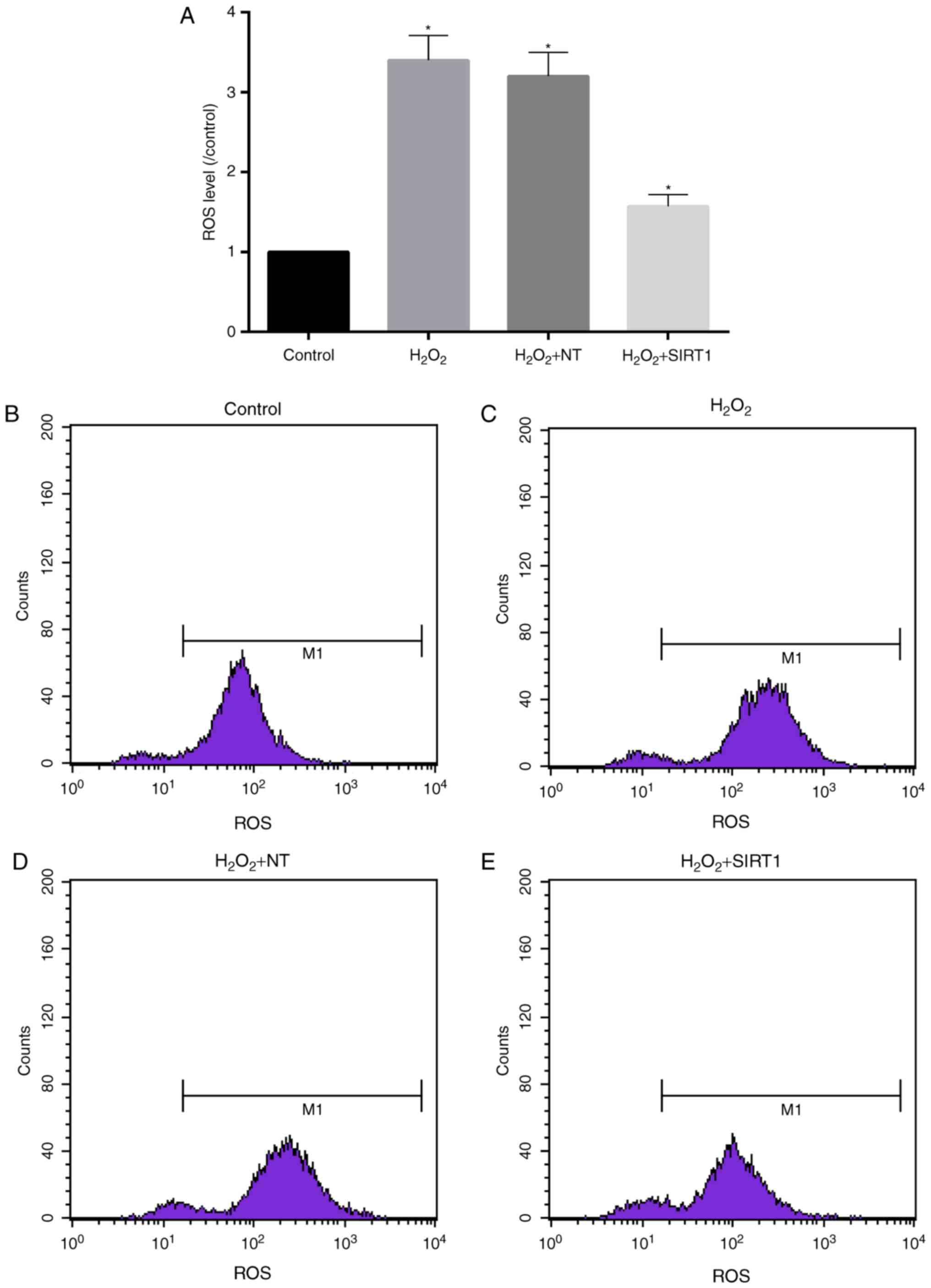
High levels of ROS induced by
H2O2 was reduced in cells with upregulated
SIRT1 expression
ROS is capable of regulating the osteoclastic bone
resorption from bone cells (13).
Superoxide produced by OC may be directly involved in the
degradation of bone tissue, and inhibiting production or
utilization of ROS and bone resorption (13). Flow cytometer assay revealed that
ROS levels in cells were significantly increased after cells
treated with 1 mM H2O2 for 48 h. As showed in
Fig. 3A-E, ROS levels
H2O2 NT and H2O2 group
were more 3-fold of control. While in H2O2 +
SIRT1 group, ROS levels were only half of that in
H2O2 NT and H2O2 group.
However, ROS levels in H2O2 + SIRT1 group was
significant higher than that in control group.
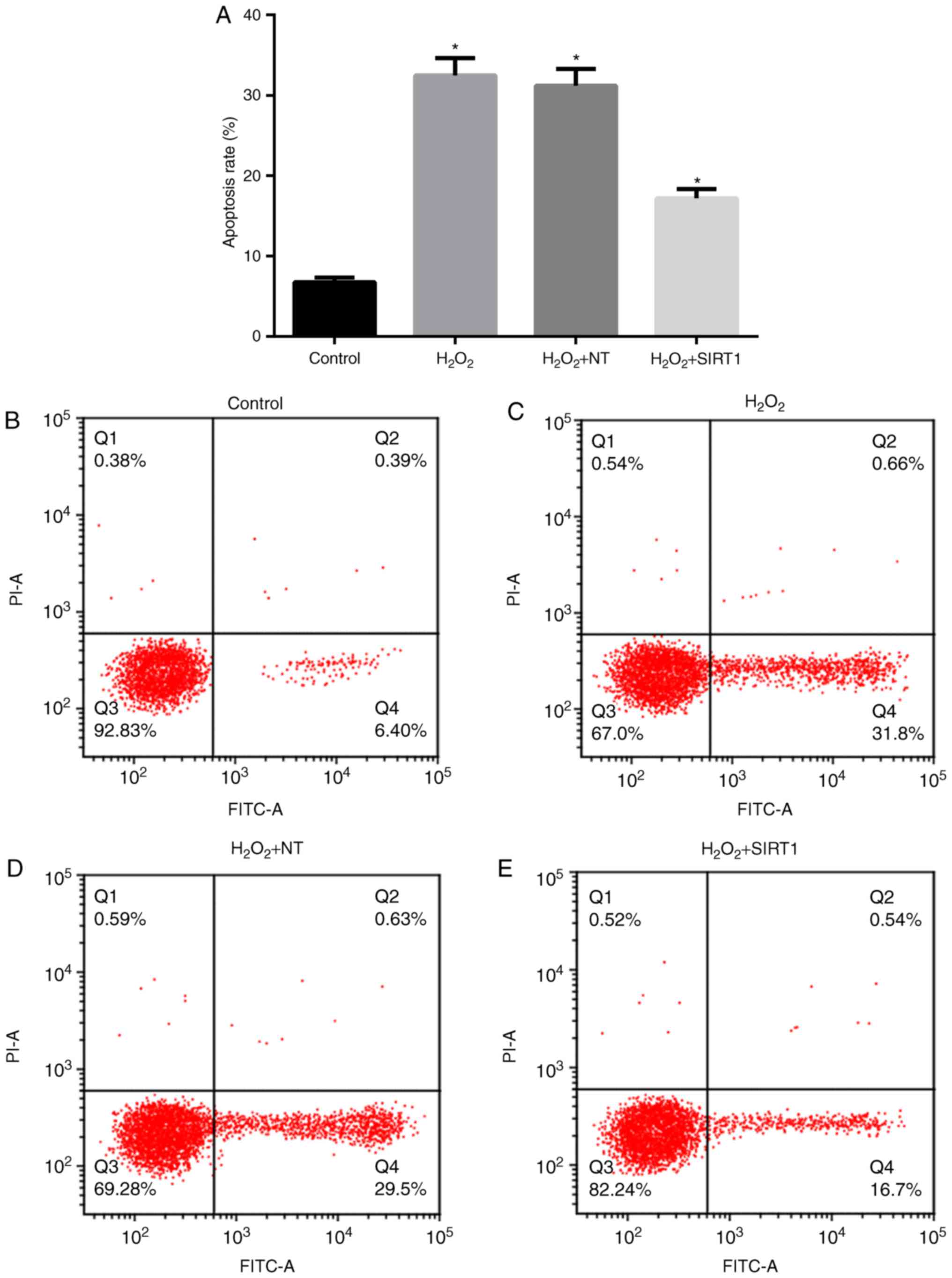
SIRT1 overexpression reduced the
apoptosis rates induced by H2O2
To further assess cytotoxicity of
H2O2 on MC3T3-E1, we tested the apoptosis
rates induced by H2O2 under different SIRT1
expression levels. Apoptosis rates were determined in cells in the
present of H2O2 (Fig. 4). The results showed that after
treatment with 1 mM H2O2 for 48 h, apoptosis
rates in H2O2 NT and
H2O2 group were increased to approximately
32%, which is significantly higher than control. However, apoptosis
rate in H2O2 + SIRT1 group was <20%, which
is one half of that in H2O2 NT and
H2O2 group and more than 2-fold of that in
control.
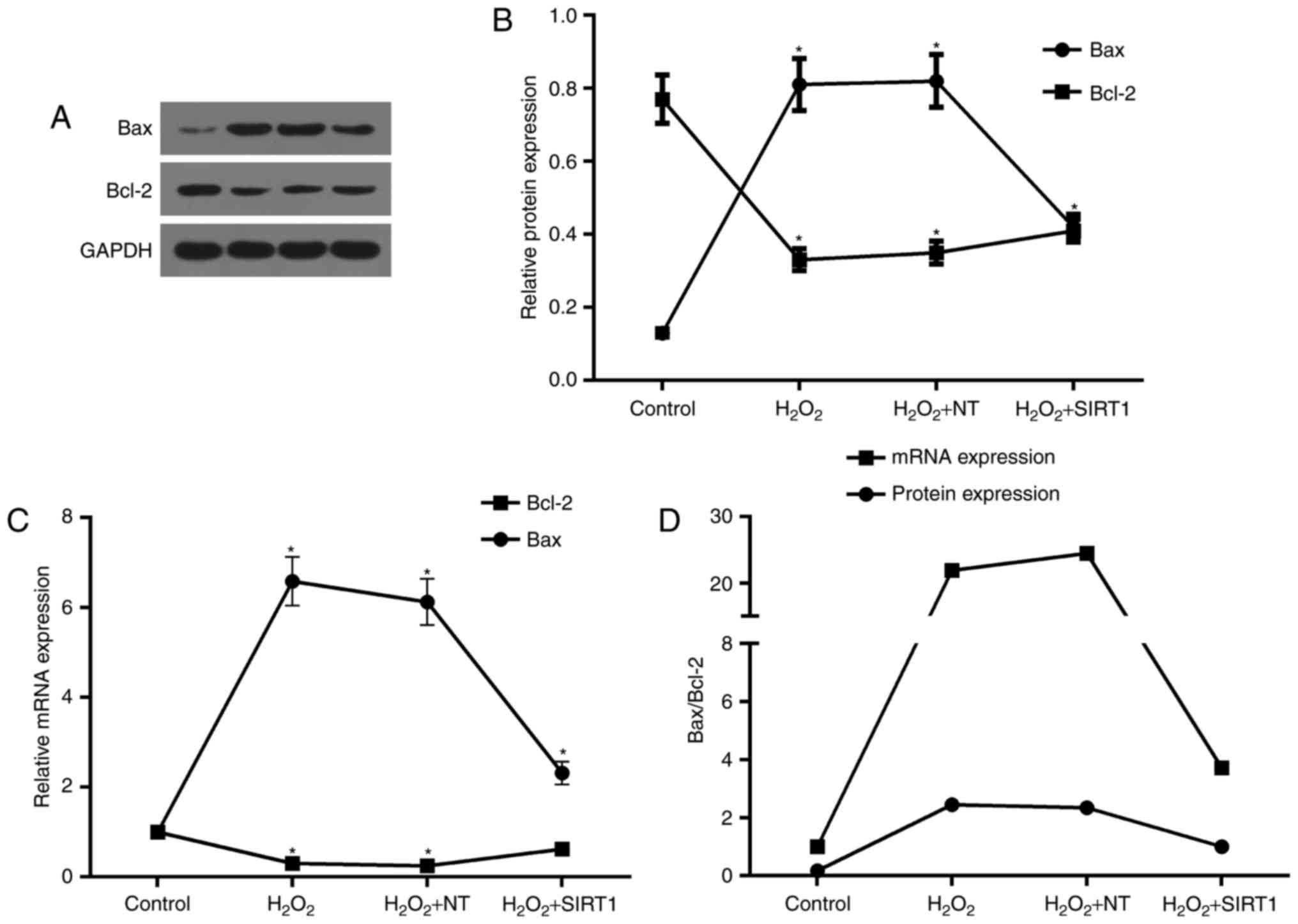
Overexpression of SIRT1 reduced the
apoptosis induced by H2O2
Based on pro-apoptosis effect induced by
H2O2, we further explored the evidences on
apoptosis-related genes. Bax and Bcl-2 are closely associated with
apoptosis, and changes in their levels and ratios are key
indicators of apoptosis and surviving. We hereby investigated the
effect of SIRT1 overexpression on cells apoptosis induced by
H2O2. After cells from all groups incubated
in 1 mM H2O2 for 48 h, the expression levels
of Bax and Bcl-2 were significantly changed. As showed in Fig. 5A-C, Bax expression was upregulated
in both translation and transcription levels, compared to control,
while Bcl-2 expression levels was downregulated in both protein and
mRNA. However, expression levels of Bax and Bcl-2 protein and mRNA
were reduced and increased in cell from H2O2
+ SIRT1 group compared to cells from H2O2 NT
and H2O2 group, respectively. The ratios of
Bax and Bcl-2 were also significantly changed due to the changes of
them. As showed in Fig. 5D,
compared to control, the ratios of Bax and Bcl-2 for both protein
and mRNA were increased in cells from H2O2 NT
and H2O2 group after cells with 1 mM
H2O2 treatment for 48 h. While in cells with
SIRT1 overexpression (H2O2 + SIRT1 group),
these ratios were decreased, compared to H2O2
NT and H2O2 group. Comparing protein
expression with mRNA expression, the more significant changes in
mRNA ratios of Bax and Bcl-2 were observed (Fig. 5D).
SIRT1 overexpression simulated
downregulation of FoxO1/β-catenin pathway and its downstream
genes
Our previous results showed that SIRT1
overexpression reduced apoptosis and ROS levels. Here, to clarify
the molecular mechanism of these processes, we determined the
expression of the FoxO1/β-catenin pathway and its downstream genes,
which are associated with proliferation and differentiation of OB.
As showed in Fig. 6A and B,
protein expression levels of FoxO1 and β-catenin and their
downstream genes (Gadd45, PPAR-γ, Bim) were increased in
H2O2 NT and H2O2 group,
compared to control. However, a reduction of expression for all
these was observed in H2O2 + SIRT1 group
compared to H2O2 NT and
H2O2 group.
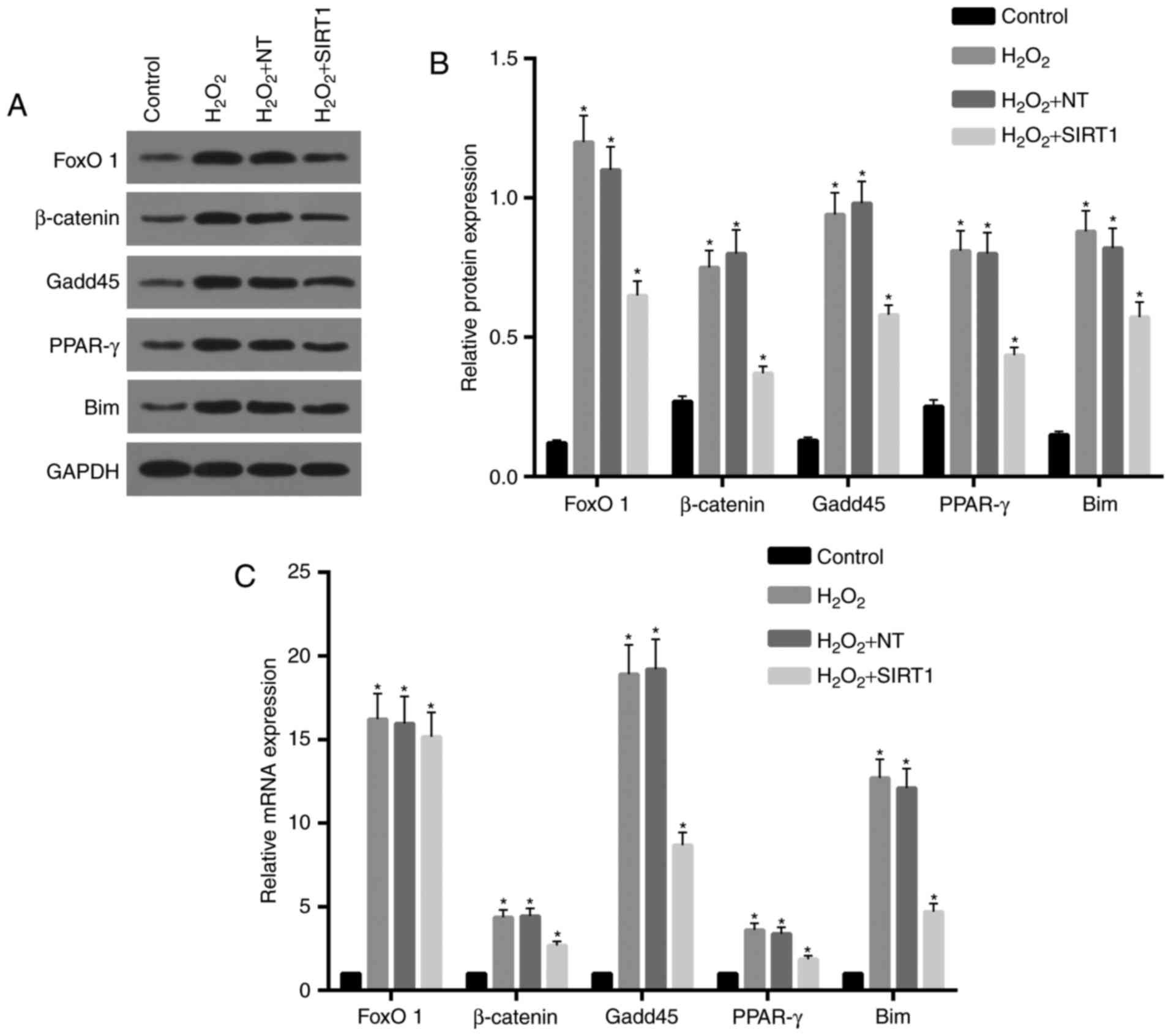 | Figure 6.Expression of FoxO1/β-catenin pathway
and its downstream genes were downregulated by SIRT1
overexpression. (A and B) Western blot assay was performed and the
results showed that protein levels of FoxO1, β-catenin, Gadd45,
PPAR-γ and Bim in H2O2 NT and
H2O2 group were significantly increased
compared to control. Protein levels for these genes in
H2O2 + SIRT1 group were reduced compared to
H2O2 NT and H2O2 group.
(C) Real-time RT-PCR assay showed that mRNA levels of FoxO1,
β-catenin, Gadd45, PPAR-γ and Bim in H2O2 NT
and H2O2 group were significantly increased
compared to control. Except for FoxO1, expression levels of
β-catenin, Gadd45, PPAR-γ and Bim mRNA in
H2O2 + SIRT1 group were lower than that of
H2O2 NT and H2O2 group.
*P<0.05, significantly different from control group. FoxO1,
forkhead box O1; SIRT1, sirtuin 1; Gadd45, growth arrest and DNA
damage inducible protein 45; PPAR-γ, peroxisome proliferator
activated receptor-γ. |
Fig. 6C shows the
relative expression levels of mRNA for FoxO1 and β-catenin and
their downstream genes. Expression levels of FoxO1, Gadd45 and Bim
mRNA inH2O2 NT and H2O2
group were apparently increased and approximately 16-fold of that
of control. Compared to the control, expression levels of β-catenin
and PPAR-γ in H2O2 NT and
H2O2 group were also increased and nearly
4-fold of control. There were no significant differences in
expression levels of FoxO1 mRNA in groups from
H2O2 + SIRT1, H2O2 NT
and H2O2. Expression levels of β-catenin,
Gadd45, PPAR-γ and Bim mRNA in H2O2 + SIRT1
group were decreased compared to H2O2 NT and
H2O2 group.
Discussion
Metabolism of the skeletal system is a homeostasis
process of forming new bone by OBs and absorption of old bone by
OCs (32). The process of OB
differentiation and proliferation are regulated by multiple
signaling pathways and a variety of factors, such as hyperglycemia
or oxidative stress (16,32). Recent years studies showed that
FoxO1 is an important regulatory factor and plays an important role
in the skeletal system (22,23).
In addition, in response to hyperglycemia or oxygen stress, FOXO1
are acetylated and localized to the promyelocytic leukemia (PML)
nuclear bodies, thereby blocking the ubiquitination of FOXO1 and
its transcriptional activity (22,33).
Subsequently, the acetylated FOXO1 protein is deacetylated in the
PML nuclear bodies mediated by SIRT1, inducing FOXO1-dependent
transcriptional processes and rapid degradation of FOXO1 (22).
Therefore, in present study, we explored the role of
SIRT1 and FOXO1 in the inhibition of apoptosis in OB induced by
H2O2 (34,35).
Our results showed that cell viabilities were inhibited after
treatments with H2O2 and increased in cells
with SIRT1 overexpression. In addition, a reduction of increased
ROS level induced by H2O2 treatment was
observed in cells with SIRT1 overexpression. Together with these
results, we suggested that SIRT1 overexpression can increase cell
viability by reducing the oxidative stress. Similar researches
showed that SIRT1 expression is strongly associated with oxidative
stress in mouse myocardium (36).
Moderate overexpression of SIRT1 can induce the expression of
important antioxidant enzymes, such as catalase, preventing
oxidative stress (37). Studies
showed that specific overexpression of SIRT1 in the heart tissues
delays senescence and prevents oxidative stress; moderate
overexpression of SIRT1 (about 3–8-fold) can be effective in
preventing drug-induced cardiac stress and apoptosis (37,38).
In contrast, oxidative stress and apoptosis were increased in cells
with high levels of SIRT1 expression (approximately 12-fold),
eventually leading to myocardial infarction and death (38). In this study, both oxidative stress
and apoptosis were reduced in cells with moderate SIRT1 expression
(approximately 3-fold of control). The ratio of Bax and Bcl-2
expression level was decreased in cells with overexpression of
SIRT1, which confirmed the observation of decreasing of
apoptosis.
To clarify the role FOXO1 and its downstream genes
in the reduction of apoptosis of OB induced by moderate
overexpression of SIRT1, we investigated the expression of
downstream genes mediated by FOXO1, such as β-catenin, Gadd45,
PPAR-γ and Bim. Our results revealed that the expression level of
these genes was all increased in cells with
H2O2 treatment, while it was decreased in
cells with additional treatment of moderate overexpression of
SIRT1. These results suggested that downregulated expression of
β-catenin, Gadd45, PPAR-γ and Bim was induced by SIRT1 via reducing
the level of FOXO1 protein. Investigations showed that the
interaction of β-catenin and FOXO1 can prevent oxidative damage in
cancer cell lines by regulating and controlling the expression of
specific target genes (22). It
has also been found that under oxidative stress, the competitive
binding of FoxOs to β-catenin in OBs reduces the binding of the
latter to the T-cell specific transcription factor (TCF). It's well
known that SIRT1 can not only deacetylate histone and regulate the
transcriptional activity of transcription factors in preadipocyte
differentiation (26). Extensive
studies had shown that SIRT1 can directly deacetylate FOXO family
transcription factors such as FoxO1, FOXO3a and FOXO4 (22,23,33).
In addition, studies had shown that subcellular localization of
β-catenin is also affected by SIRT1 via deacetylating it (39,40).
It had been found that PPAR-γ is mainly expressed in
adipocytes and its precursor cells, which mediate the
differentiation and proliferation of adipocytes (41). It is also expressed in OBs and
plays an important role in maintaining bone metabolism balance
(42). OBs and bone marrow
adipocytes are differentiated by the same precursor, MSCs, which
can be transformed into each other under certain conditions
(18,22,32).
Differentiation of MSCs into OB requires OB-specific transcription
factors, namely nuclear protein-binding factor al (cbfa1), which
activates OB-specific gene expression such as osteocalcin gene,
type I collagen fiber gene, and osteopontin gene (43). PPAR-γ inhibits the expression of
cbfal and reduces the expression of the above genes, thereby
inhibiting the differentiation of MSCs into OB. On the contrary, it
promotes the differentiation of MSCs into adipocytes (41). Transcription of PPAR-γ was
simulated by FoxO1 in cells with H2O2
treatment and inhibited in cells with overexpression of SIRT1.
These results may be explained in two ways. On one hand, SIRT1
overexpression induced ubiquitination of FOXO1 and inhibited
β-catenin and FOXO1 pathway way, eventually leading downregulation
of Gadd45, PPAR-γ and Bim. On the other hand, investigation showed
that the transcriptional activation of PPAR-γ was also directly
inhibited by SIRT1 through recruiting the nuclear receptor
co-repressors (NCoR and SMRT) to the promoter region of PPAR-γ
(41,44).
Gadd45 is a cell cycle-related protein that plays an
important role in cell cycle regulation, apoptosis and repair of
DNA damage. Increased expression of Gadd45 is necessary for cell
cycle arrest and repair of DNA damage (45). The abnormality or dysfunction of
Gadd45 expression may lead to abnormal DNA repair and repair
pathways mediated by p53, which cannot normally respond to DNA
damage, inhibit abnormal cell proliferation and control the
regulation of cell cycle, resulting in damage accumulation and
malignant transformation and even formation of tumor (45,46).
Our studies showed that the expression level of Gadd45 was
increased in cells treated with H2O2, where
Gadd45 may protect cells from oxidative damage by inducing cell
cycle arrest and repair of DNA damage. However, our results
revealed that an increased apoptosis was observed in cells treated
with H2O2. Although Gadd45 is essential for
maintaining genomic stability, studies had shown that apoptosis of
epithelial cells is inhibited by Gadd45 knockdown by UV
irradiation, suggesting that Gadd45 can promote apoptosis (45). The regulation of Gadd45 protein on
apoptosis is still controversial, which may be related to cell
types and the environmental stimuli inducing apoptosis (46).
The Bcl-2 family is an important regulator of the
apoptotic pathway, including the anti-apoptotic Bcl-2 subfamily and
pro-apoptotic Bax and BH3 subfamily (also known as BH3-only
protein) (47,48). Bim (Bcl-2 interacting mediator of
cell death) is one of the BH3-only proteins, which was considered
to be a molecule that promotes apoptosis in recent years (49). Previous studies had shown that Bim
is one of the downstream target genes of FoxOs (49,50).
Here, our results showed that Bim expression was significantly
increased in cells treated with H2O2 and
inhibited in cells with SIRT1 overexpression, suggesting that SIRT1
inhibits Bim expression by mediating FoxOs. Bim can interact with
Bcl-2/Bax and then activate Bax-induced mitochondrial pathway
(47,49). It had been found that Bim is a very
important factor in the process of oxidative stress-induced
apoptosis, both in vitro and in vivo in oxidative
stress models (50). Therefore, we
suggested that apoptosis induced by H2O2 was
mediated by SIRT1/FoxOs/Bim pathway.
In conclusion, moderate SIRT1 overexpression can
increase cell viability by reducing the oxidative stress, which was
involved the expression of specific target genes of β-catenin and
FOXO1 pathway, such as Gadd45, PPAR-γ and Bim. FOXO1 and β-catenin
pathway was inhibited by SIRT1. In addition, PPAR-γ, an inhibitor
of differentiation of MSCs into OB, was also directly inhibited by
SIRT1, suggesting that SIRT1 probably promote differentiation of
MSCs into OB. However, there is a major limitation that we did not
further investigate the deacetylation effect on FoxO1 and
β-catenin, which were proved in others studies.
References
|
1
|
Taichman RS and Hauschka PV: Effects of
interleukin-1 beta and tumor necrosis factor-alpha on osteoblastic
expression of osteocalcin and mineralized extracellular matrix
in vitro. Inflammation. 16:587–601. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Papapoulos S, Roux C, Bone HG, Dakin P,
Czerwiński E, Frey D, Kendler D, Lewiecki EM, Malouf J, Mellström
D, et al: FRI0289 denosumab treatment in postmenopausal women with
osteoporosis for up to 9 years: Results through year 6 of the
freedom extension. Ann Rheum Dis. 74:529–530. 2015. View Article : Google Scholar
|
|
3
|
Yang N, Wang G, Hu C, Shi Y, Liao L, Shi
S, Cai Y, Cheng S, Wang X, Liu Y, et al: Tumor necrosis factor α
suppresses the mesenchymal stem cell osteogenesis promoter miR-21
in estrogen deficiency-induced osteoporosis. J Bone Miner Res.
28:559–573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jochems C, Islander U, Erlandsson M,
Verdrengh M, Ohlsson C and Carlsten H: Osteoporosis in experimental
postmenopausal polyarthritis: The relative contributions of
estrogen deficiency and inflammation. Arthritis Res Ther.
7:R837–R843. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Muruganandan S and Sinal CJ: The impact of
bone marrow adipocytes on osteoblast and osteoclast
differentiation. IUBMB Life. Mar 17–2014.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheon YH, Kim JY, Baek JM, Ahn SJ, Jun HY,
Erkhembaatar M, Kim MS, Lee MS and Oh J: WHI-131 promotes
osteoblast differentiation and prevents osteoclast formation and
resorption in mice. J Bone Miner Res. 31:403–415. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sánchez-Rodríguez MA, Ruiz-Ramos M,
Correa-Muñoz E and Mendoza-Núñez VM: Oxidative stress as a risk
factor for osteoporosis in elderly Mexicans as characterized by
antioxidant enzymes. BMC Musculoskelet Disord. 8:1242007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Manolagas SC: From estrogen-centric to
aging and oxidative stress: A revised perspective of the
pathogenesis of osteoporosis. Endocr Rev. 31:266–300. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fubini B and Hubbard A: Reactive oxygen
species (ROS) and reactive nitrogen species (RNS) generation by
silica in inflammation and fibrosis. Free Radical Biol Med.
34:1507–1516. 2003. View Article : Google Scholar
|
|
10
|
Mossman BT: Introduction to serial reviews
on the role of reactive oxygen and nitrogen species (ROS/RNS) in
lung injury and diseases. Free Radical Biol Med. 34:1115–1116.
2003. View Article : Google Scholar
|
|
11
|
Singha UK, Jiang Y, Yu S, Luo M, Lu Y,
Zhang J and Xiao G: Rapamycin inhibits osteoblast proliferation and
differentiation in MC3T3-E1 cells and primary mouse bone marrow
stromal cells. J Cell Biochem. 103:434–446. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Byun CH, Koh JM, Kim DK, Park SI, Lee KU
and Kim GS: Alpha-lipoic acid inhibits TNF-alpha-induced apoptosis
in human bone marrow stromal cells. J Bone Miner Res. 20:1125–1135.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Halleen JM, Räisänen S, Salo JJ, Reddy SV,
Roodman GD, Hentunen TA, Lehenkari PP, Kaija H, Vihko P and
Väänänen HK: Intracellular fragmentation of bone resorption
products by reactive oxygen species generated by osteoclastic
tartrate-resistant acid phosphates. J Biol Chem. 274:22907–22910.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee SH, Kim JK and Jang HD: Genistein
inhibits osteoclastic differentiation of RAW 264.7 cells via
regulation of ROS production and scavenging. Int J Mol Sci.
15:10605–10621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Uchiyama Y, Higuchi Y, Takeda S, Masaki T,
Shira-Ishi A, Sato K, Kubodera N, Ikeda K and Ogata E: ED-71, a
vitamin D analog, is a more potent inhibitor of bone resorption
than alfacalcidol in an estrogen-deficient rat model of
osteoporosis. Bone. 30:582–588. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wronski TJ, Ratkus AM, Thomsen JS, Vulcan
Q and Mosekilde L: Sequential treatment with basic fibroblast
growth factor and parathyroid hormone restores lost cancellous bone
mass and strength in the proximal tibia of aged ovariectomized
rats. J Bone Miner Res. 16:1399–1407. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De França NA, Camargo MB, Lazaretti-Castro
M and Martini LA: Antioxidant intake and bone status in a
cross-sectional study of Brazilian women with osteoporosis. Nutr
Health. 22:133–142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim MB, Song Y and Hwang JK: Kirenol
stimulates osteoblast differentiation through activation of the BMP
and Wnt/β-catenin signaling pathways in MC3T3-E1 cells.
Fitoterapia. 98:59–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujita KI and Janz S: Attenuation of WNT
signaling by DKK-1 and −2 regulates BMP2-induced osteoblast
differentiation and expression of OPG, RANKL and M-CSF. Mol Cancer.
6:712007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yen K, Narasimhan SD and Tissenbaum HA:
DAF-16/Forkhead box O transcription factor: Many paths to a single
Fork(head) in the road. Antioxid Redox Sign. 14:623–634. 2011.
View Article : Google Scholar
|
|
21
|
Ouyang W, Beckett O, Flavell RA and Li MO:
An essential role of the forkhead-box transcription factor foxo1 in
control of T cell homeostasis and tolerance. Immunity. 30:358–371.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rached MT, Kode A, Xu L, Yoshikawa Y, Paik
JH, Depinho RA and Kousteni S: FoxO1 is a positive regulator of
bone formation by favoring protein synthesis and resistance to
oxidative stress in osteoblasts. Cell Metab. 11:147–160. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paik JH, Kollipara R, Chu G, Ji H, Xiao Y,
Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, et al: FoxOs are
lineage-restricted redundant tumor suppressors and regulate
endothelial cell homeostasis. Cell. 128:309–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Borra MT, O'Neill FJ, Jackson MD, Marshall
B, Verdin E, Foltz KR and Denu JM: Conserved enzymatic production
and biological effect of O-acetyl-ADP-ribose by silent information
regulator 2-like NAD+-dependent deacetylases. J Biol Chem.
277:12632–12641. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wojcik M, Mac-Marcjanek K and Wozniak LA:
Physiological and pathophysiological functions of SIRT1. Mini-Rev
Med Chem. 9:386–394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bai L, Pang WJ, Yang YJ and Yang GS:
Modulation of Sirt1 by resveratrol and nicotinamide alters
proliferation and differentiation of pig preadipocytes. Mol Cell
Biochem. 307:129–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mader I, Wabitsch M, Debatin KM,
Fischer-Posovszky P and Fulda S: Identification of a novel
proapoptotic function of resveratrol in fat cells:
SIRT1-independent sensitization to TRAIL-induced apoptosis. FASEB
J. 24:1997–2009. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shakibaei M, Shayan P, Busch F, Aldinger
C, Buhrmann C, Lueders C and Mobasheri A: Resveratrol mediated
modulation of Sirt-1/Runx2 promotes osteogenic differentiation of
mesenchymal stem cells: Potential role of Runx2 deacetylation. PLoS
One. 7:e357122012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hallows WC, Albaugh BC and Denu JM: Where
in the cell is SIRT3? Functional localization of an NAD+-dependent
protein deacetylase. BIOCHEM J. 411:e11–e13. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao Y, Feng G, Wang Y, Yue Y and Zhao W:
Regulation of apoptosis by long non-coding RNA HIF1A-AS1 in VSMCs:
Implications for TAA pathogenesis. Int J Clin Exp Pathol.
7:7643–7652. 2014.PubMed/NCBI
|
|
31
|
Park WH: The effect of MAPK inhibitors and
ROS modulators on cell growth and death of
H2O2-treated HeLa cells. Mol Med Rep. 8:557–564.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suzin J, Szubert M and Kowalczyk-Amico K:
Osteoporosis - A frequent problem of postmenopausal woman.
Menopausal Review. 6:320–323. 2009.
|
|
33
|
Kitamura YI, Kitamura T, Kruse JP, Raum
JC, Stein R, Gu W and Accili D: FoxO1 protects against pancreatic
beta cell failure through NeuroD and MafA induction. Cell Metab.
2:153–163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang Y, Hou H, Haller EM, Nicosia SV and
Bai W: Suppression of FOXO1 activity by FHL2 through SIRT1-mediated
deacetylation. EMBO J. 24:1021–1032. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chae HD and Broxmeyer HE: SIRT1 deficiency
downregulates PTEN/JNK/FOXO1 pathway to block reactive oxygen
species-induced apoptosis in mouse embryonic stem cells. Stem Cells
Dev. 20:1277–1285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang C, Feng Y, Qu S, Wei X, Zhu H, Luo
Q, Liu M, Chen G and Xiao X: Resveratrol attenuates
doxorubicin-induced cardiomyocyte apoptosis in mice through
SIRT1-mediated deacetylation of p53. Cardiovasc Res. 90:538–545.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Alcendor RR, Gao S, Zhai P, Zablocki D,
Holle E, Yu X, Tian B, Wagner T, Vatner SF and Sadoshima J: Sirt1
regulates aging and resistance to oxidative stress in the heart.
Circ Res. 100:1512–1521. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Calvanese V and Fraga MF: SirT1 brings
stemness closer to cancer and aging. Aging (Albany NY). 3:162–167.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Firestein R, Blander G, Michan S,
Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S,
de Cabo R, Fuchs C, et al: The SIRT1 deacetylase suppresses
intestinal tumorigenesis and colon cancer growth. PLoS One.
3:e20202008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Simic P, Zainabadi K, Bell E, Sykes DB,
Saez B, Lotinun S, Baron R, Scadden D, Schipani E and Guarente L:
SIRT1 regulates differentiation of mesenchymal stem cells by
deacetylating β-catenin. EMBO Mol Med. 5:430–440. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ikarashi N, Tajima M, Suzuki K, Toda T,
Ito K, Ochiai W and Sugiyama K: Inhibition of preadipocyte
differentiation and lipid accumulation by Orengedokuto treatment of
3T3-L1 cultures. Phytother Res. 26:91–100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Viccica G, Francucci CM and Marcocci C:
The role of PPARγ for the osteoblastic differentiation. J
Endocrinol Invest. 33 7 Suppl:S9–S12. 2010.
|
|
43
|
Xiao G, Jiang D, Ge C, Zhao Z, Lai Y,
Boules H, Phimphilai M, Yang X, Karsenty G and Franceschi RT:
Cooperative interactions between activating transcription factor 4
and Runx2/Cbfa1 stimulate osteoblast-specific osteocalcin gene
expression. J Biol Chem. 280:30689–30696. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Picard F, Kurtev M, Chung N, Topark-Ngarm
A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW and
Guarente L: Sirt1 promotes fat mobilization in white adipocytes by
repressing PPAR-gamma. Nature. 429:771–776. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Patra S, Mascarenhas R, Maliyakkal N and
Aranjani JM: Protocatechualdehyde induces apoptosis in human
non-small-cell lung cancer cells by up regulation of growth arrest
and DNA damage-inducible (GADD) genes. Mol Biol. 2:1132013.
View Article : Google Scholar
|
|
46
|
Hassumani DO: Expression of growth arrest
and DNA damage protein 45-alpha (gadd45-alpha) and the
CCAAT/enhancer binding protein-delta (C/EBP-delta) in fishes
exposed to heat and hypoxia. Thesis, Portland State University.
Dissertations and Theses. paper 943. 2013.
|
|
47
|
Senft D, Weber A, Saathoff F, Berking C,
Heppt MV, Kammerbauer C, Rothenfusser S, Kellner S, Kurgyis Z,
Besch R and Häcker G: In non-transformed cells Bak activates upon
loss of anti-apoptotic Bcl-XL and Mcl-1 but in the absence of
active BH3-only proteins. Cell Death Dis. 6:e19962015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang L, Fang Y, Xu XF and Jin DY:
Moscatilin induces apoptosis of pancreatic cancer cells via
reactive oxygen species and the JNK/SAPK pathway. Mol Med Rep.
15:1195–1203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Marani M, Tenev T, Hancock D, Downward J
and Lemoine NR: Identification of novel isoforms of the BH3 domain
protein bim which directly activate bax to trigger apoptosis. Mol
Cell Biol. 22:3577–3589. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Al-Mubarak B, Soriano FX and Hardingham
GE: Synaptic NMDAR activity suppresses FOXO1 expression via a
cis-acting FOXO binding site: FOXO1 is a FOXO target gene. Channels
(Austin). 3:233–238. 2009. View Article : Google Scholar : PubMed/NCBI
|