Introduction
Gastric cancer is curable if it is diagnosed at
early stages, however, most patients are diagnosed at a late stage
when the current cancer regimen is limited (1–3).
Although surgery combined with chemotherapies has been used to
treat advanced gastric cancer, the overall 5-year survival rate is
less than 24% (3,4). Over the past decades, numerous
studies have been focusing on the molecular mechanisms of gastric
cancer. Alterations of certain gene expression and genetic
variation have been discovered in gastric cancer (5). However, the exact molecular
mechanisms underlying gastric cancer initiation and progression is
still not well understood, which prevents the development of
personalized and suitable cancer therapy strategies. Therefore, it
is critical to discover reliable molecular biomarkers for gastric
cancer, which can be used for prognosis and therapeutic
targets.
We found that cyclin-dependent kinase 10 (CDK10,
also called PISSLRE) is one of the targets with potential
prognostic impact and great therapeutic promise. In human
nasopharyngeal carcinoma, CDK10 gene promoter is often
hypermethylated resulting in its silence, while restoration of
CDK10 expression may strongly suppress the malignant behaviors of
cancer cells (6). Our previous
report revealed that CDK10 expression levels are significantly
decreased in breast cancer and correlated with patient survival
rates (7,8).
CDKs currently hold great promise for anti-tumor
therapeutics and are been considered as potential molecular targets
for cancer therapy. CDK10, a cdc2-related kinase, is essential for
cell cycle progression from the G2 to M phase (9–11).
In breast cancer, the resistance to endocrine therapies is
determined by CDK10, the silencing of which leads to cancer cell
reliance lost upon estrogen signaling (12,13).
The expression of CDK10 is observed in various
normal tissues, whereas it is often decreased or absent in cancers
(7,12,14,15).
Furthermore, overexpression of CDK10 increases the sensitivity of
hepatocellular carcinoma and biliary tract cancer cells to
chemotherapy (14,15). Together with our reports, these
encouraging findings suggest that CDK10 may be a tumor suppressor
candidate, which contributes to prognosis as well as offers new
personalized therapeutic strategies for cancers. To the best of our
knowledge, the expression pattern, clinical relevance and
biological functions of CDK10 in gastric cancer are not well
understood. To this aim, we firstly evaluated CDK10 expression
status and its clinical significance in gastric cancer. We then
determined the tumor suppressing functions of CDK10 with respect to
cancer cell proliferation, migration and invasion.
Materials and methods
Patients and tissue samples
We randomly collected patient samples from the
Cancer Hospital of Shantou University Medical College. 128
formalin-fixed paraffin-embedded (FFPE) specimens were obtained
from patients with gastric cancer undergoing curative surgery
between 2000 and 2005 (median age, 61 years; range, 35–84 years).
The median follow-up period was 37 months (range, 1–80 months) from
the date of surgery. Additionally, cancer tissues (n=20) and
their matching noncancerous tissues (n=20), for immunoblot
assays, were immediately snap frozen in liquid nitrogen and kept at
−80°C until further use. Of note, they were harvested from another
independent cohort of patients with gastric cancer who underwent
surgery at the same institution between May 2010 and July 2012.
Tumor grade and stage were classified according to
the International Union against Cancer (UICC)/American Joint
Committee on Cancer (AJCC) pathologic tumor-node-metastasis (TNM)
classification, 7th edition (2010). Table I demonstrates the clinicopathologic
characteristics for these patients. No patient was found to have
undergone preoperative radiotherapy or chemotherapy. The current
study conformed to the ethical guidelines of the Declaration of
Helsinki and was approved by the Institution Review Board (no.
04-470) of the Cancer Hospital of Shantou University Medical
College. Written informed consent was obtained from all patients
before sample collection. All samples were coded and data was
stored anonymously.
 | Table I.Correlation of CDK10 expression with
clinicopathological parameters. |
Table I.
Correlation of CDK10 expression with
clinicopathological parameters.
|
|
| CDK10 expression |
|
|---|
|
|
|
|
|
|---|
| Variable | No. of patients | Negative, n (%) | Positive, n (%) | P-value |
|---|
| Age |
|
|
|
|
| ≤60
years | 63 | 37 (58.7) | 26 (41.3) |
0.077 |
| >60
years | 65 | 28 (43.1) | 37 (56.9) |
|
| Gender |
|
|
|
|
| Male | 106 | 51 (48.1) | 55 (51.9) |
0.185 |
|
Female | 22 | 14 (63.6) | 8
(36.4) |
|
| Tumor size |
|
|
|
|
| ≤5
cm | 64 | 29 (45.3) | 35 (54.7) |
0.216 |
| >5
cm | 64 | 36 (56.3) | 28 (43.7) |
|
| Histological
type |
|
|
|
|
|
Intestinal | 62 | 35 (56.5) | 27 (43.5) |
0.578 |
|
Diffuse | 50 | 22 (44.0) | 28 (56.0) |
|
|
Mixed | 16 | 8
(50.0) | 8
(50.0) |
|
| Differentiation |
|
|
|
|
|
Well/moderate | 51 | 18 (35.3) | 33 (64.7) |
0.004 |
| Poor | 77 | 47 (61.0) | 30 (39.0) |
|
| Stage of tumors |
|
|
|
|
|
I/II | 43 | 11 (25.6) | 32 (74.4) | <0.001 |
|
III/IV | 85 | 54 (63.5) | 31 (36.5) |
|
| Invasive depth |
|
|
|
|
|
T1/T2 |
9 | 3
(33.3) | 6
(66.7) |
0.278 |
|
T3/T4 | 119 | 62 (52.1) | 57 (47.9) |
|
| Lymph node
metastasis |
|
|
|
|
|
N0/N1 | 41 | 11 (26.8) | 30 (73.2) | <0.001 |
|
N2/N3 | 87 | 54 (62.1) | 33 (37.9) |
|
| Distant
metastasis |
|
|
|
|
| M0 | 100 | 45 (45.0) | 55 (55.0) |
0.013 |
| M1 | 28 | 20 (71.4) | 8
(28.6) |
|
Immunoblot analysis
CDK10 protein levels were examined by immunoblot
analysis as described previously (6–8,16,17).
60 µg proteins in RIPA lysis buffer were separated by SDS-PAGE and
transferred to a PVDF membrane. After one hour incubation in
blocking buffer (Tris-buffered saline with 0.1% Tween and 5% nonfat
dry milk), the membrane was then incubated with a rabbit polyclonal
antibody against CDK10 (catalog no. ab72710; dilution, 1:500;
Abcam, Cambridge, MA, USA), followed by a horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG;
catalog no. sc-2004; dilution, 1:2,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA). Signals were visualized using an enhanced
chemiluminescence kit (GE Healthcare Life Sciences, Little
Chalfont, UK) as described by the manufacturer. Anti-GAPDH
monoclonal antibody (catalog no. ab9485; dilution, 1:2,500; Abcam)
was used to assure equal loading of protein. Quantification of the
intensity of CDK10 in the immunoblot was conducted using the
Bio-Rad Quantity One quantitation software (20), with the ratio between the tumors
and the paired noncancerous tissues identified as being less than
two folds, suggesting decreased CDK10 expression.
Immunohistochemistry and staining
evaluation
We performed immunohistochemical staining to detect
CDK10 expression using a standard EnVision complex method as
described previous (7,14,17).
4-µm sections were cut from FFPE specimens and then processed with
deparaffinization and rehydration. The endogenous peroxidase
activity was blocked with 0.3% hydrogen peroxide for 30 min at room
temperature. After antigen retrieval, tissue sections were
incubated with a rabbit polyclonal anti-CDK10 antibody (catalog no.
ab72710; dilution, 1:50; Abcam), after which immunohistochemical
staining was conducted by an EnVision antibody complex
(anti-Mouse/Rabbit) method using an EnVision™ Detection
kit (ZSGB-BIO, Beijing, China) and 3,3′-diaminobenzidine as the
chromogen substrate. As a negative control, the primary antibody
was replaced by a normal rabbit IgG.
The staining evaluation was carried out as follows
(7): Ten 400× microscopic fields
per slide were random selected and evaluated by two independent
pathologists. CDK10 immunostaining was determined using a
semi-quantitative approach combining the percentage of positive
cells and the staining intensity. The mean percentage of stained
cells was scored as follows: 0, 0%; 1, 1–25%; 2, 26–50%; 3, 51–75%;
and 4, 76–100%. The staining intensity was classified as follows:
0, Absent; 1, weak staining; 2, moderate staining; and 3, strong
staining. We summed up the intensity and extent scores as the final
staining score. For the purpose of statistical evaluation, we
grouped the tumor samples with a final staining score of <3 into
negative CDK10 expression and those with scores ≥3 into positive
CDK10 expression.
Cell culture and transfection
Two established gastric cancer cell lines (HGC-27
and SGC-7901) were cultured as described previously (6,16). A
recombinant CDK10-expressing plasmid (pcDNA3.1-CDK10) and a
pre-designed validated siRNA targeting CDK10
(5′-GUCCCAGUAAAGCCAAUGATT-3′ and 5′-UCAUUGGCUUUACUGGGACTT3′) were
prepared as described previously (6). Cell transfection was conducted using
Lipofectamine 2000 reagent (GE Healthcare Life Sciences, Little
Chalfont, UK) according to the manufacturer's instructions. Cells
were harvested 48 h post-transfection and used for subsequent
experiments described below. The expression levels of CDK10 after
transfection was confirmed by immunoblot analysis.
Cell proliferation and colony
formation assays
The cell proliferative and cologenic capacities were
measured using a 3-(4,5-dimethylthiazol-2-yl)-2
5-diphenyl-2H-tetrazolium bromide (MTT) colorimetric assay and a
colony formation (soft agar culture) assay, respectively, according
to standard methods described before (6,16,18).
Each experiment was conducted three times in replicates of six
wells.
Wound healing and invasion assays
The cell motility and invasive capacities were
measured using a wound healing assay and a Matrigel invasion
chamber assay, respectively, according to standard methods
described before (6,16,18).
Each experiment was conducted in triplicate wells and repeated
three times.
Statistical analyses
Statistical analyses were performed using the SPSS
statistical software package (version 17.0; SPSS, Inc., Chicago,
IL, USA). A Student's t test was used to evaluate the statistical
significance of differences in numerical data. The Pearson
χ2 test or Fisher's exact test was used to analyze the
correlations between CDK10 expression and clinicopathologic
parameters, and the Spearman's rank method was used to obtain the
correlation coefficient between variables. Overall survival (OS)
rates were generated using the Kaplan-Meier method with log rank
test. The prognostic significance of clinicopathological variables
was determined by univariate and multivariate regression analysis
with the Cox hazards model. P<0.05 (two-tailed) was considered
to indicate a statistically significant difference.
Results
Decreased CDK10 protein levels in
gastric cancer
Giving that evidenced function of CDK10 as a
potential tumor suppressor frequent silenced during tumorigenesis
(5–7), we first determined whether CDK10
expression was downregulated in tumors vs. noncancerous tissues.
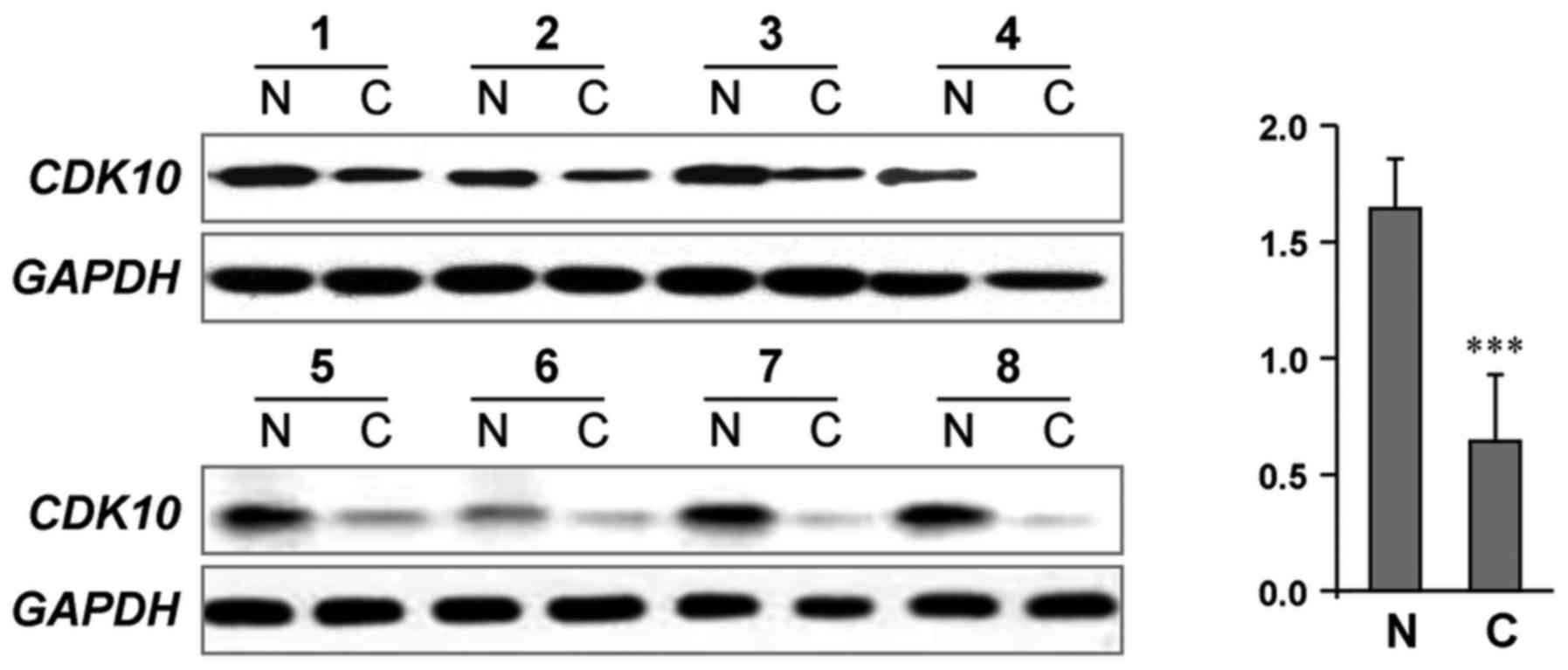
Fig. 1 shows the representative
results in a cohort of gastric cancer specimen (n=20) by
immunoblot analysis. We found that 85% (17/20) of cancer tissues
had lower CDK10 protein levels compared to their matching adjacent
noncancerous tissues (P<0.001).
Correlation of CDK10 expression with
clinicopathological parameters
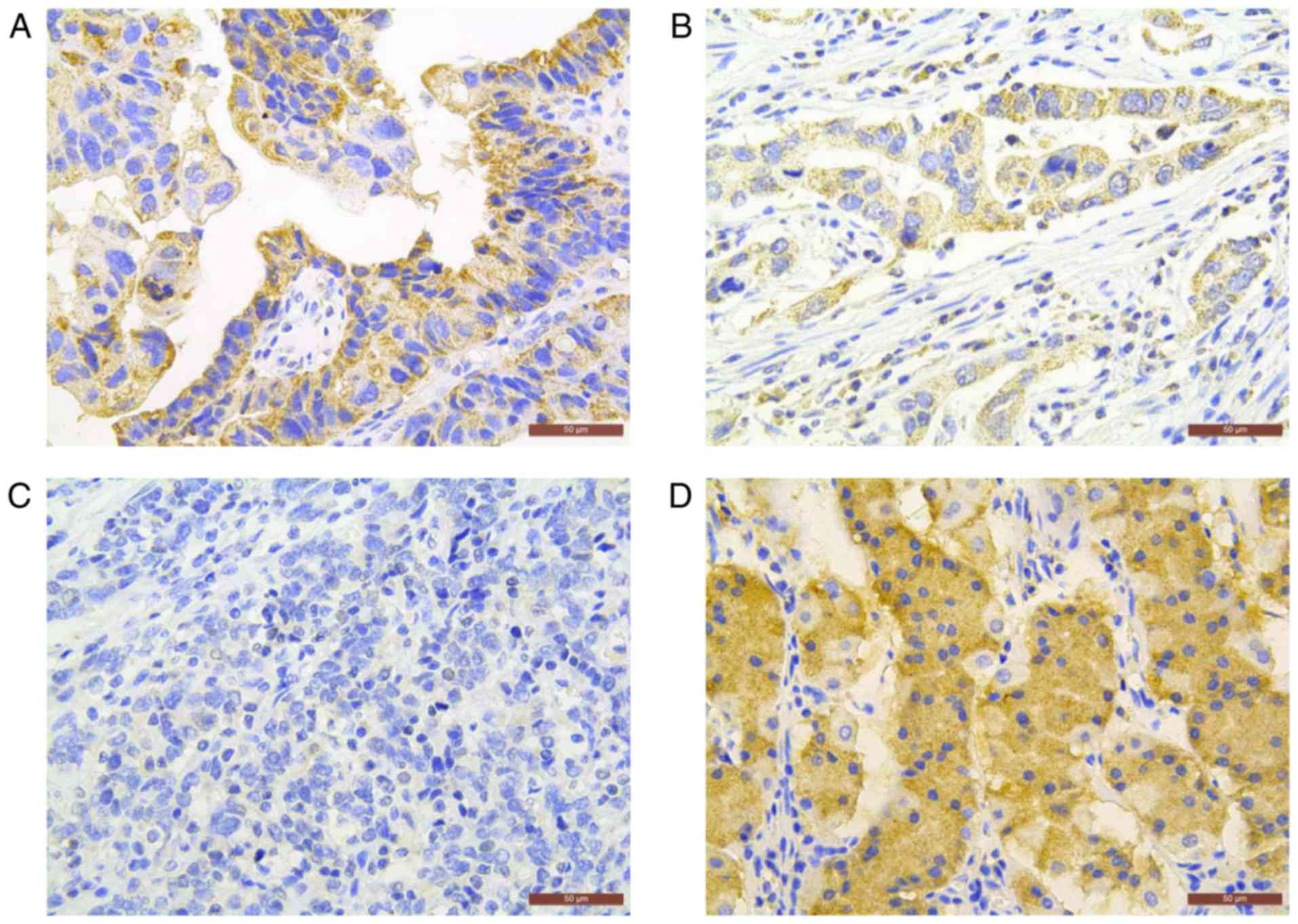
To verify the above observation, we next determined
the expression profile and localization of CDK10 protein in 168
gastric cancer specimens by utilizing immunohistochemistry.
Positive CDK10 immunostaining was observed primarily in the
cytoplasm of neoplastic cells in 50.8% (65/128) of primary gastric
tumors tested (Fig. 2). To better
understand the significance of CDK10 expression in gastric cancer,
we further assessed the association between CDK10 expression and
the clinicopathological parameters. We found that negative CDK10
expression was associated with advanced tumor stage (stage III/IV
vs. stage I/II; P<0.001), frequent lymph node metastasis (N2/N3
vs. N0/N1; P<0.001), distant metastasis (P=0.013) and tumor
differentiation (poor vs. well/moderate; P=0.004) (Table I). No significant relationship was
observed between CDK10 expression and the other clinicopathologic
factors such as age, gender, tumor size and invasive depth.
Spearman correlation analysis revealed CDK10 expression levels were
correlated with clinical tumor staging (r=−0.375;
P<0.001), lymph node metastasis (r=−0.349; P<0.001),
distant metastasis (r=−0.239; P=0.002) and tumor
differentiation (r=−0.288; P<0.001). Together, these data
demonstrate that a lack of CDK10 expression may correlate with
malignant properties mainly relevant to lymph node metastasis and
tumor advancement. These observations provide solid evidence that
dysregulation of CDK10 expression may contribute to gastric cancer
progression.
Association between CDK10 expression
and patient survival
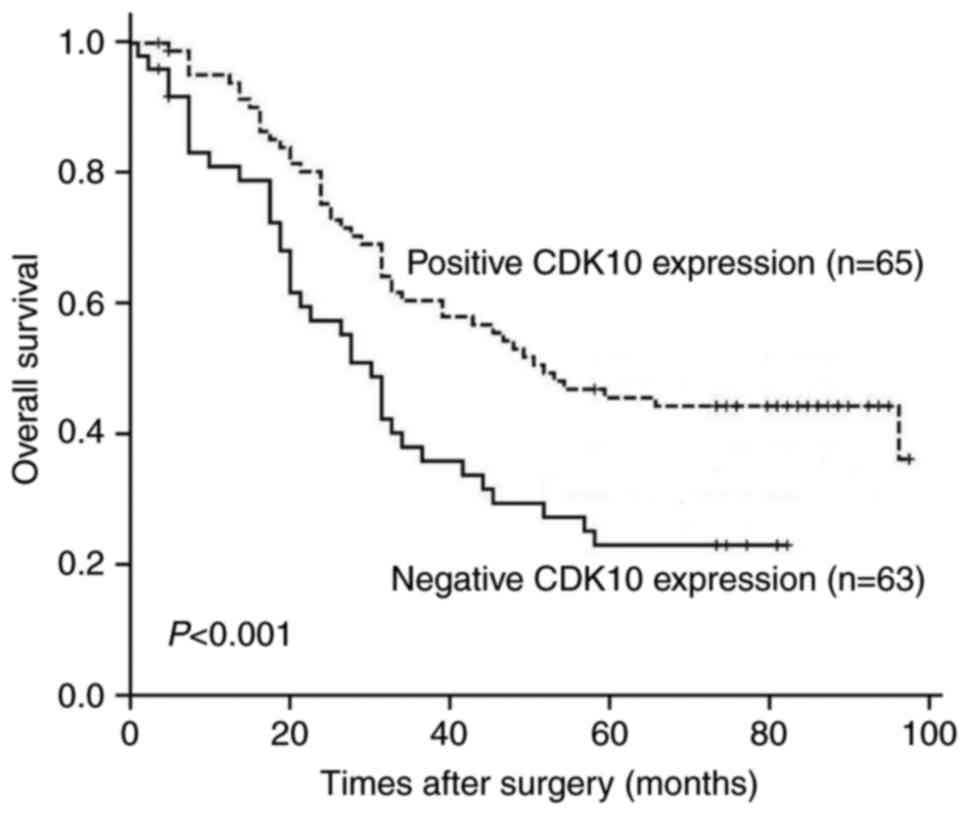
Kaplan-Meier survival analyses were performed to
investigate the prognostic impacts of CDK10 expression on the
outcome of patients with gastric cancer. We found that the OS rate
of patients with tumors that expressed CDK10 was higher than that
of patients with tumors did not express CDK10 (P<0.001)
(Fig. 3). Further, we examined
whether CDK10 expression may be served as a prognostic factor for
gastric cancer patients by utilizing univariate and multivariate
analyses. On univariate analysis, CDK10 expression (P<0.001),
patient age (P=0.039), tumor size (P<0.001), histological type
(P=0.001), stage of tumors (P=0.001) and lymph node metastasis
(P<0.001) were significantly correlated with an unfavorable OS
(Table II). After adjusting the
prognostic factors that achieved significance in univariate
analysis, CDK10 expression (P=0.045), histological type (P=0.004)
and tumor size (P=0.001) maintained independent significance for
predicting the prognosis of gastric cancer patients.
 | Table II.Univariate and multivariate Cox
proportional hazards models showing variables that affect overall
survival in patients with gastric cancer. |
Table II.
Univariate and multivariate Cox
proportional hazards models showing variables that affect overall
survival in patients with gastric cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (years) |
|
|
|
|
| >60
vs. ≤60 | 0.557
(0.319–0.971) |
0.039 | 0.686
(0.390–1.205) | 0.190 |
| Gender |
|
|
|
|
| Male
vs. Female | 0.730
(0.492–1.258) |
0.247 | – | – |
| Tumor size
(cm) |
|
|
|
|
| >5
vs. ≤5 | 3.188
(1.964–5.175) | <0.001 | 2.660
(1.588–4.457) | 0.001 |
| Histological
type |
|
|
|
|
| Poor
vs. Well/moderate | 2.676
(1.593–4.495) |
0.001 | 2.185
(1.276–3.740) | 0.004 |
|
Differentiation |
|
|
|
|
|
Intestinal vs. Diffuse | 0.580
(0.509–1.117) |
0.183 | – | – |
| Stage of
tumors |
|
|
|
|
| III/IV
vs. I/II | 2.353
(1.417–4.107) |
0.001 | 1.246
(0.677–2.291) | 0.480 |
| Invasive depth |
|
|
|
|
| T3/T4
vs. T1/T2 | 2.840
(0.89229.040) |
0.770 | – | – |
| Lymph node
metastasis |
|
|
|
|
|
Positive vs. Negative | 2.344
(1.482–3.706) | <0.001 | 1.355
(0.825–2.225) |
0.229 |
| Distant
metastasis |
|
|
|
|
|
Positive vs. Negative | 1.931
(0.98023.805) |
0.057 | – | – |
| CDK10
expression |
|
|
|
|
|
Negative vs. Positive | 3.188
(1.964–5.175) | <0.001 | 1.604
(1.011–2.543) | 0.045 |
Ectopic CDK10 expression suppresses
gastric cancer cell growth and invasion
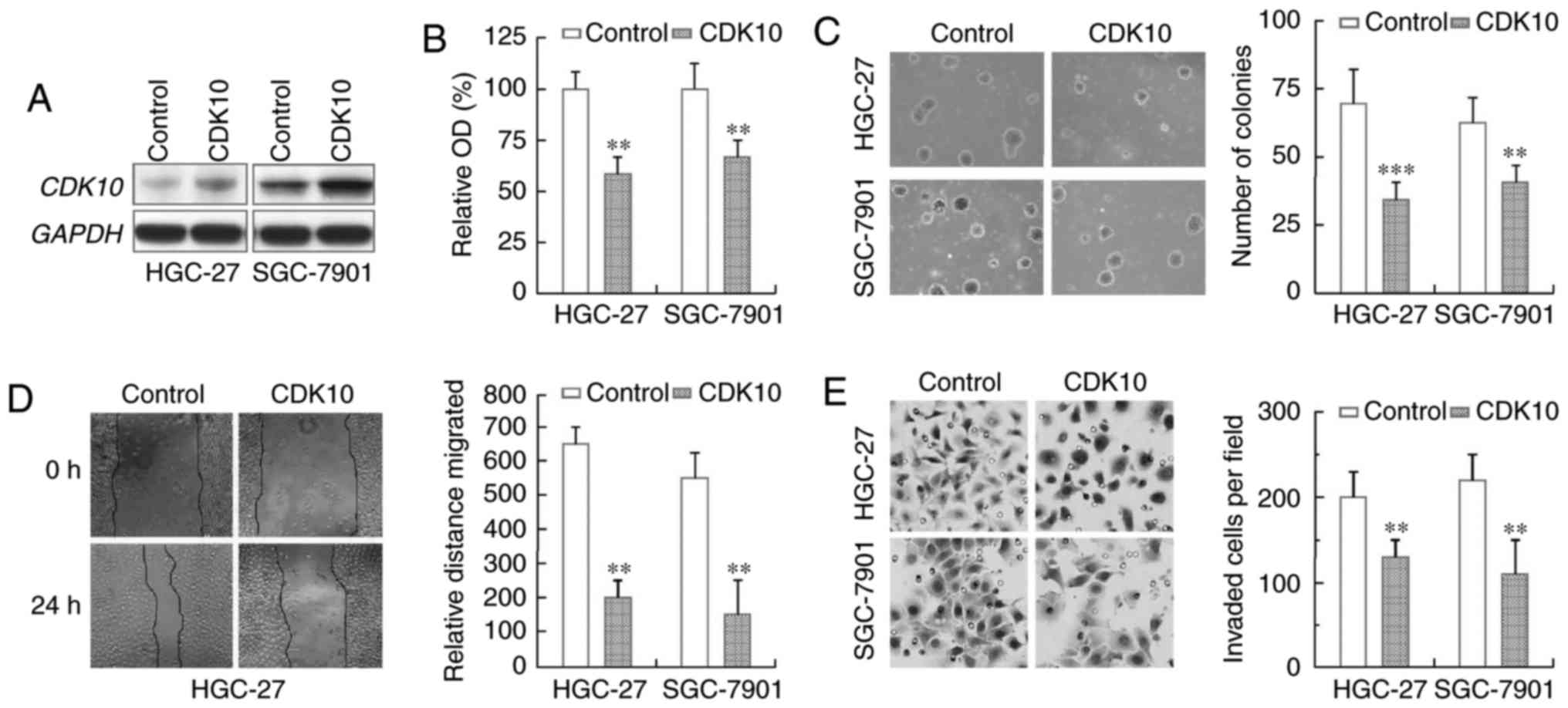
To confirm that CDK10 functions as a tumor
suppressor in gastric cancer pathogenesis and progression, we
examined the effect of CDK10 on cell proliferation by transfecting
a CDK10 expression construct into gastric cancer cell lines HGC-27
and SGC-7901. Overexpression of CDK10 was achieved in these two
cell lines (Fig. 4A). As evidenced
by MTT assays, we found that proliferation rates of these cell
lines with CDK10 overexpression were significantly reduced in
comparison to the empty vector-transfected control cells (Fig. 4B). Moreover, HGC-27 and SGC-7901
cells exogenously expressing CDK10 produced fewer colonies than
their respective control cells (Fig.
4C).
We subsequently investigated whether CDK10 could
regulate the metastasis capacities of gastric cancer cells.
Confluent HGC-27 and SGC-7901 cells with or without ectopic CDK10
expression were scratched and cell motility was determined. By
doing so, we found that ectopic CDK10 expression, in both gastric
cell lines, significantly delayed closure of wound area compared to
the empty vector controls (Fig.
4D). Next, we examined the invasive ability of these gastric
cell lines using a Matrigel assay. As shown in Fig. 4E, CDK10 overexpression let to a
significant decrease in invasiveness of both cell lines, as
compared with their own controls. All these observations clearly
demonstrated that CDK10 expression restoration inhibits the
migration and invasion of gastric cancer cells.
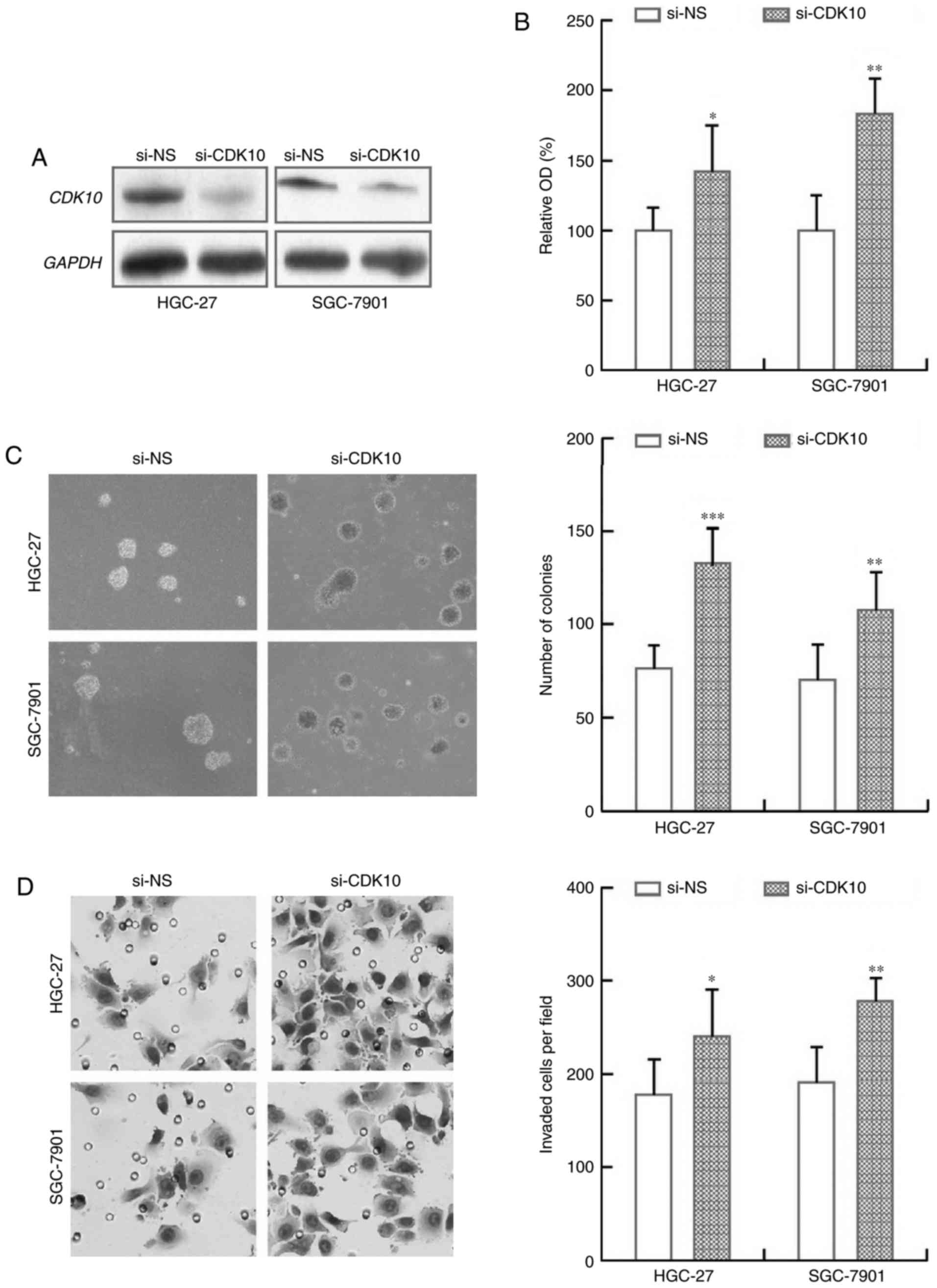
Knockdown of CDK10 expression promotes
gastric cancer cell growth and invasion
We further assessed the biological effects of
silencing CDK10 expression on gastric cancer cells. The efficiency
of CDK10 knockdown using a pre-design and validated siRNA was
tested by immunoblot analysis (Fig.
5A). We observed that HGC-27 and SGC-7901 cells with CDK10
knockdown exhibited a consistent and significant increase in cell
proliferation and colony formation, as compared to those cells
transfected with non-specific control siRNA (Fig. 5B and C). Further, the inhibitory
effect of CDK10 on cell invasion was confirmed using a Matrigel
assay. The results showed that HGC-27 and SGC-7901 cells with CDK10
knockdown showed a significant increase in cell invasive capacity
compare to their respective control cells (Fig. 5D). Together, these findings clearly
demonstrate that knockdown of CDK10 promotes the proliferation and
the migration-invasion of gastric cancer cells.
Discussion
We were encouraged to study the CDK10 gene because
it is frequently listed in the genes with their expression
correlated with cancer patients survival rate (7,8,12).
Our present study is to understand the relationship between CDK10
gene expression profiles and cancer outcomes, and discover new
biomarkers for diagnosis and prognosis, as well as new therapeutic
targets. In our study, we provided the comprehensive analysis of
CDK10 in 128 samples from primary gastric cancer patients and
investigated the biological function of CDK10 in gastric cancer
cells. Our data not only demonstrate that CDK10 expression is
down-regulated in gastric cancers and highly associated with
patient survival, consistent with our recent study of CDK10 in
breast cancer (7), but also show
the correlation of CDK10 expression with gastric cancer malignancy
further supported by our biological studies. Therefore, our study
emphasizes the critical role of CDK10 as a prognostic biomarker for
gastric cancer with great therapeutic promise.
Although multiple CDK proteins, particularly those
with great therapeutic promise, have been evaluated for prognosis
and cancer therapy (19–21), studies on CDK10 have so far only
focused on several cancer types. Our current analysis in gastric
cancer documents that a connection exists between negative CDK10
expression with severe tumor progression phenotype, including
advanced tumor stage, lymph node and distant metastasis, and poor
tumor differentiation. These results are similar to the earlier
reports on biliary tract cancer and hepatocellular carcinoma, as
well as our more recent report in breast cancer (7,14,15).
However, in our study we failed to discover that CDK expression was
significantly correlated with tumor invasion depth. One of
possibilities may be due to the actual composition of samples in
which 93.0% (119/128) were T3/T4 samples, whereas T1/T2 samples
were barely 7.0% (9/128). In addition, our data suggests that the
expression of CDK10 may serve as an independent prognostic
biomarker for the favorable OS, consistent with our report on
breast cancer (7). Overall, these
observations indicate that CDK10 may be an important factor in
regulating gastric cancer development and progression.
CDK10, a member of the Cdc2-related kinase, can
identify and activate cyclin M (22). Silencing of CDK10 increases the
activation of MAPK pathway drove by ETS2, which confers tamoxifen
resistance to breast cancer cells (12,13).
To tease out the function of CDK10 in gastric cancer, its
expression was restored in gastric cancer cells and its influence
on cell growth and migration-invasion were assessed.
Over-expression of CDK10 led to a significant decrease in cell
growth and migration-invasion activities in vitro; and vice
versa, silencing of CDK10 enhanced gastric cancer cell
proliferation, migration and invasion. These observations are
concordant with the previous reports on other cancer types
(14,15), and also support our results in
immunohistochemistry analysis in which loss of CDK10 expression was
closely correlated with frequent lymph node metastasis and advanced
tumor stage. Our data suggest that the CDK10 expression may
influence and directly correlate with aggressive behavior of
gastric cancer cells.
Although our study has some limitations to
generalize the results, to the best of our knowledge, our data
provided the first prognostic evidence that CDK10 may function as a
tumor suppressor in gastric cancer. In our future study, we will
increase the sample number and perform more detailed analyses to
validate the clinical value of CDK10 for gastric cancer therapy.
Additionally, the precise signaling pathways and molecular
mechanism underlying CDK10 involvement in the tumorigenesis and
progression of gastric cancer deserves further exploration.
Taken together, our data suggest that silencing of
CDK10 is a molecular hallmark of gastric cancer progression.
Down-regulation of CDK10 is correlated with aggressive cancer
characteristics mainly relevant to metastasis and poor overall
survival. We conclude that CDK10 may serve as an independent marker
for gastric cancer prognosis that holds great promise to against
this malignancy.
Acknowledgements
This study was supported in part by the Science and
Technology Planning Project of Henan Province, China (142102310464)
and the Key Research Foundation of Higher Education of Henan
Province, China (15B320003). No additional external funding
received for this study. The funders had no role in study design,
data collection and analysis, decision to publish, or preparation
of the manuscript.
References
|
1
|
Crew KD and Neugut AI: Epidemiology of
gastric cancer. World J Gastroenterol. 12:354–362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoo CH, Noh SH, Shin DW, Choi SH and Min
JS: Recurrence following curative resection for gastric carcinoma.
Br J Surg. 87:236–242. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen CN, Lin JJ, Chen JJ, Lee PH, Yang CY,
Kuo ML, Chang KJ and Hsieh FJ: Gene expression profile predicts
patient survival of gastric cancer after surgical resection. J Clin
Oncol. 23:7286–7295. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jian P, Yanfang T, Zhuan Z, Jian W,
Xueming Z and Jian N: MMP28 (epilysin) as a novel promoter of
invasion and metastasis in gastric cancer. BMC Cancer. 11:2002011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
You Y, Yang W, Wang Z, Zhu H, Li H, Lin C
and Ran Y: Promoter hypermethylation contributes to the frequent
suppression of the CDK10 gene in human nasopharyngeal carcinomas.
Cell Oncol (Dordr). 36:323–331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
You Y, Li H, Qin X, Zhang Y, Song W, Ran Y
and Gao F: Decreased CDK10 expression correlates with lymph node
metastasis and predicts poor outcome in breast cancer patients-a
short report. Cell Oncol (Dordr). 38:485–491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hong CQ, Zhang F, You YJ, Qiu WL, Giuliano
AE, Cui XJ, Zhang GJ and Cui YK: Elevated C1orf63 expression is
correlated with CDK10 and predicts better outcome for advanced
breast cancers: A retrospective study. BMC Cancer. 15:5482015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Graña X, Claudio PP, De Luca A, Sang N and
Giordano A: PISSLRE, a human novel CDC2-related protein kinase.
Oncogene. 9:2097–2103. 1994.PubMed/NCBI
|
|
10
|
Brambilla R and Draetta G: Molecular
cloning of PISSLRE, a novel putative member of the cdk family of
protein serine/threonine kinases. Oncogene. 9:3037–3041.
1994.PubMed/NCBI
|
|
11
|
Li S, MacLachlan TK, De Luca A, Claudio
PP, Condorelli G and Giordano A: The cdc-2-related kinase, PISSLRE,
is essential for cell growth and acts in G2 phase of the cell
cycle. Cancer Res. 55:3992–3995. 1995.PubMed/NCBI
|
|
12
|
Iorns E, Turner NC, Elliott R, Syed N,
Garrone O, Gasco M, Tutt AN, Crook T, Lord CJ and Ashworth A:
Identification of CDK10 as an important determinant of resistance
to endocrine therapy for breast cancer. Cancer Cell. 13:91–104.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khanal P, Yun HJ, Lim SC, Ahn SG, Yoon HE,
Kang KW, Hong R and Choi HS: Proyl isomerase Pin1 facilitates
ubiquitin-mediated degradation of cyclin-dependent kinase 10 to
induce tamoxifen resistance in breast cancer cells. Oncogene.
31:3845–3856. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu JH, Zhong XY, Zhang WG, Wang ZD, Dong
Q, Tai S, Li H and Cui YF: CDK10 functions as a tumor suppressor
gene and regulates survivability of biliary tract cancer cells.
Oncol Rep. 27:1266–1276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhong XY, Xu XX, Yu JH, Jiang GX, Yu Y,
Tai S, Wang ZD and Cui YF: Clinical and biological significance of
Cdk10 in hepatocellular carcinoma. Gene. 498:68–74. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
You Y, Yang W, Qin X, Wang F, Li H, Lin C,
Li W, Gu C, Zhang Y and Ran Y: ECRG4 acts as a tumor suppressor and
as a determinant of chemotherapy resistance in human nasopharyngeal
carcinoma. Cell Oncol (Dordr). 38:205–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
You Y, Li H, Qin X, Ran Y and Wang F:
Down-regulated ECRG4 expression in breast cancer and its
correlation with tumor progression and poor prognosis-A short
Report. Cell Oncol (Dordr). 39:89–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin C, Xin S, Qin X, Li H, Lin L and You
Y: Zoledronic acid suppresses metastasis of esophageal squamous
cell carcinoma cells through upregulating the tight junction
protein occludin. Cytotechnology. 68:1233–1241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mahale S, Bharate SB, Manda S, Joshi P,
Jenkins PR, Vishwakarma RA and Chaudhuri B: Antitumour potential of
BPT: A dual inhibitor of cdk4 and tubulin polymerization. Cell
Death Dis. 6:e17432015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
VanArsdale T, Boshoff C, Arndt KT and
Abraham RT: Molecular pathways: Targeting the cyclin D-CDK4/6 axis
for cancer treatment. Clin Cancer Res. 21:2905–2910. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng Z, Xu S, Liu M, Zeng YX and Kang T:
Chk1 inhibitor Gö6976 enhances the sensitivity of nasopharyngeal
carcinoma cells to radiotherapy and chemotherapy in vitro and in
vivo. Cancer Lett. 297:190–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guen VJ, Gamble C, Flajolet M, Unger S,
Thollet A, Ferandin Y, Superti-Furga A, Cohen PA, Meijer L and
Colas P: CDK10/cyclin M is a protein kinase that controls ETS2
degradation and is deficient in STAR syndrome. Proc Natl Acad Sci
USA. 110:pp. 19525–19530. 2013; View Article : Google Scholar : PubMed/NCBI
|