Introduction
Colorectal cancer (CRC) has a substantial effect on
human health; it is the third most prevalent type of cancer
worldwide, causing an estimated 693,900 deaths in 2012 (1,2).
Despite the development of new therapeutic approaches and
treatments for CRC in recent decades, there has been little change
in the overall mortality rate (3,4). CRC
development involves a multistep process, which can be due to
genetic or environmental factors, leading to mutations in a series
of molecules associated with cancer cell proliferation, apoptosis,
and differentiation (5,6). Owing to the rising popularity of
molecular therapies, various studies have investigated the
molecular pathogenesis of CRC by analysing the molecular
abnormalities in CRC progression (7–9).
Long non-coding RNAs (lncRNAs) are a subset of RNAs
first identified in eukaryotes. They have a transcript length of
200–100,000 nt and lack a complete functional open reading frame
(ORF). Rarely, they may encode a short functional peptide and are
located in the nucleus or cytoplasm (10,11).
Recently, more focus has been placed on lncRNAs for their effect on
biological cell behaviour, especially in tumour cells. Increasing
number of studies have revealed that lncRNAs are abnormally
expressed in many different cancers, such as gastric cancer
(12), cervical cancer (13), non-small cell lung cancer (NSCLC)
(14), and CRC (15). These abnormally expressed lncRNAs
have been used as biomarkers for cancer therapies and
diagnoses.
The lncRNA plasmacytoma variant translocation 1
(PVT1) is located on chr8q24.21 and is 1,716 nt in length.
The gene region of PVT1 contains the myelocytomatosis
(myc) oncogene; the MYC protein can result in the
accumulation of PVT1 in primary human cancers (16). Emerging evidence indicates that
PVT1 is associated with tumourigenesis in various cancers,
including gastric cancer (17),
NSCLC (18), and hepatocellular
cancer (19); however, the
specific effects of PVT1 on the proliferation, invasion, and
metastasis of CRC are still poorly understood. In the present
study, we first demonstrated that PVT1 is overexpressed in
CRC tissues and cell lines. We then determined that CRC patients
with high PVT1 expression showed poor overall survival (OS),
by analysing Gene Expression Omnibus (GEO) datasets. PVT1
knockdown was also shown to suppress the proliferation, invasion,
and metastasis of CRC cells in vitro. These results suggest
that PVT1 plays a significant role in CRC tumourigenesis and
tumour progression.
Materials and methods
Bioinformatics analysis
All microarray expression dates, containing primary
CRC data and their correlated clinic data, were deposited in the
GEO database: GSE9348 (20),
GSE23878 (21), GSE22598 (22), and GSE17536 (23) (Affymetrix Human Genome U133 Plus
2.0 platform) and GSE50760 (24)
(Illumina HiSeq 2000 platform). GSE9348 has 70 primary CRC samples
and 12 normal colon samples; GSE23878 has 35 primary CRC samples
and 24 normal colon samples; GSE22598 contains 17 pairs of CRC and
adjacent non-tumour tissues; GSE17536 is divided into the low
PVT1 expression group (n=83) and high PVT1 expression
group (n=60); GSE50760 has 17 metastasis CRC samples and 37
non-metastasis CRC samples.
Cell culture and transfection
The human colorectal cancer cell lines used in this
study were obtained from the American Type Culture Collection
(ATCC; Manassas, VA, USA). HCT116 cells were maintained in DMEM
(Dulbecco's modified Eagle's medium) with 10% fetal bovine serum
(FBS; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), and other cell lines (SW480, HT29, NCM460, SW620, CaCO2) were
cultured in RPMI-1640 media (Invitrogen; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS. HCT116, SW480, HT29, NCM460, SW620
and CaCO2 are all human colorectal cancer cell lines, while NCM460
is a normal colonic epithelial cell line from the tissue of a
patient with gastric cancer. Transfection was conducted with. When
cell densities were approximately 60%, 50 nM short interfering RNA
(siRNA) oligos were transfected by Lipofectamine 3000 (Invitrogen,
USA). The sequences of the PVT1 targeting siRNAs were as follows:
PVT1-si-1: 5′-CUGGACCUUAUGGCUCCA-3′; PVT1-si-2:
5′-CACUGAGGCUACUGCAUCU-3′; sequences of non-target scramble
controls were provided by RiboBio (Guangzhou, China).
Reverse transcriptase-quantitative
polymerase chain reaction (RT-qPCR)
Tissue RNA isolation and amplification were
performed as described previously (25). RNA was isolated from the cells,
using Trizol reagent (Invitrogen, The Netherlands). For the
RT-qPCR, RNA was reverse transcribed to cDNA, using a Revert Aid
First Strand cDNA Synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc.,). RT-qPCR was performed using a SYBR_Premix ExTaq
II kit (Takara Biotechnology Co., Ltd., Dalian, China) in the CFX96
Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) to determine the relative expression of target
genes. The following program was used for qPCR: 95°C for 30 sec
followed by 40 cycles of 95°C for 5 sec and then 60°C for 30 sec.
The sequences of RT-qPCR primers were as follows: PVT1 forward
5′-TTCAGCACTCTGGACGGACTT-3′, reverse 5′-TATGGCATGGGCAGGGTAG-3′;
human cyclin D1 forward 5′-TCGTTGCCCTCTGTGCCACA-3′, reverse
5′-GCAGTCCGGGTCACACTTGA-3′; human E-cadherin forward
5′-TGAAGCCCCCATCTTTGTGC-3′, reverse 5′-GGCTGTGTACGTGCTGTTCT-3;
human vimentin forward 5′-TGAAGCCAATTGCAGGAGGAGA-3′, reverse
5′-TCTTGGCAGCCACACTTTCAT-3′; human cyclin-dependent kinase 4 (CDK4)
forward 5′-TTGGTGTCGGTGCCTATGGG-3′, reverse
5′-CCATCAGCCGGACAACATTGGG-3′; human GAPDH forward
5′-AACGGATTTGGTCGTATTGG-3′, reverse 5′-TTGATTTTGGAGGGATCTCG-3′.
Western blotting
Cell lysis, cell lysate electrophoresis, and target
protein visualisation were performed as described previously
(25). Firstly, the cells were
resuspended in lysis buffer [1% Nonidet P-40, 50 mM Tris-HCl, pH
7.5, 50 mM NaF, 2 mM EDTA, 400 mM NaCl, 10% glycerol plus Complete
protease inhibitor mixture (Merck KGaA, Darmstadt, Germany)]. Then,
50 µg of cell lysates were separated by 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto Nitrocellulose membrane (Bio-Rad Laboratories,
Inc.). After the membranes were blocked in Tris-buffered
saline/Tween-20 (25 mM Tris-HCl, 150 mM NaCl, pH 7.5 and 0.05%
Tween-20) with 5% defatted milk for 1 h at 37°C, the membranes were
incubated overnight at 4°C with the primary antibodies, including
E-cadherin (cat. no. 3195, 1:1,000; Cell Signaling Technology,
Inc., Danvers, MA, USA), vimentin (cat. no. 5741, 1:500; Cell
Signaling Technology, Inc.), cyclin D1 (cat. no. 2978, 1:1,000;
Cell Signaling Technology, Inc.), CDK4 (cat. no. 12790, 1:500; Cell
Signaling Technology, Inc.), and GAPDH (cat. no. 5174, 1:1,000;
Cell Signaling Technology, Inc.). After washing with TBST, the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibodies (Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) and visualized using the ECL detection system. Densitometric
analysis of immunodetected bands was performed using Image Analysis
software (Bio-Rad Laboratories, Inc.).
Cell proliferation assay
The transfected cells were seeded in 96-well
flat-bottom plates at a density of 2×103 cells/well in
200 ul of medium, and cultured for the CCK-8 (Dojin Laboratories,
Kumamoto, Japan), with the operating steps carried out as described
previously (26).
Tumour cell clone-formation assay
The tumour cell clone-formation assay was carried
out as described previously (26).
Briefly, 1×103 cells were seeded into each well of a
6-well culture plate and incubated for 14 days, after which the
cell colonies were stained with haematoxylin. Then, the clone
formation efficiency was calculated. Each experiment was repeated
three times independently.
Matrigel invasion assays
Colorectal cell invasiveness was determined in a
24-well transwell plate (8 µp pore size; Costar), as described
previously (26). Briefly,
5×104 cells were placed in the upper chamber of each
insert coated with 200 mg/ml of Matrigel (BD Biosciences, Franklin
Lakes, NJ, USA). After 48 h, the invaded cells were stained with
haematoxylin and counted. Each experiment was repeated three times
independently.
Statistical analysis
All statistical analyses were carried out using SPSS
version 18.0 (SPSS, Inc., Chicago, IL, USA) and presented with
Graphpad prism software (GraphPad Software, Inc., La Jolla, CA,
USA). Data are expressed as the mean ± standard error of the mean.
Differences between two independent groups were tested using
Student's t-test and analysis of variance was performed for
comparisons among multiple groups. OS was calculated using the
Kaplan-Meier method, and the results of the analysis were
considered significant in a log-rank test if P<0.05.
Results
PVT1 is upregulated in CRC tissues and
cell lines
To determine the expression of PVT1 in CRC
tissues, we first analysed three previously published datasets
(nos. GSE9348, GSE23878, and GSE22598), using Affymetrix HG_U133
Plus 2 arrays to identify dysregulated lncRNAs in CRC tissues. We
found that the lncRNA PVT1 was significantly up regulated in
GSE9348, GSE23878, and GSE22598 (P<0.001, Fig. 1A-C). Next, PVT1 expression
was also determined by RT-qPCR in five CRC cell lines (SW480, HT29,
Caco-2, HCT116, and SW620), and the normal colon mucosal cell line
NCM460. The results showed that PVT1 expression was higher
in the CRC cell lines than in NCM460 (P<0.05, Fig. 1D), and that PVT1 expression
was the highest in HCT116 cells. Therefore, we selected HCT116
cells for further studies.
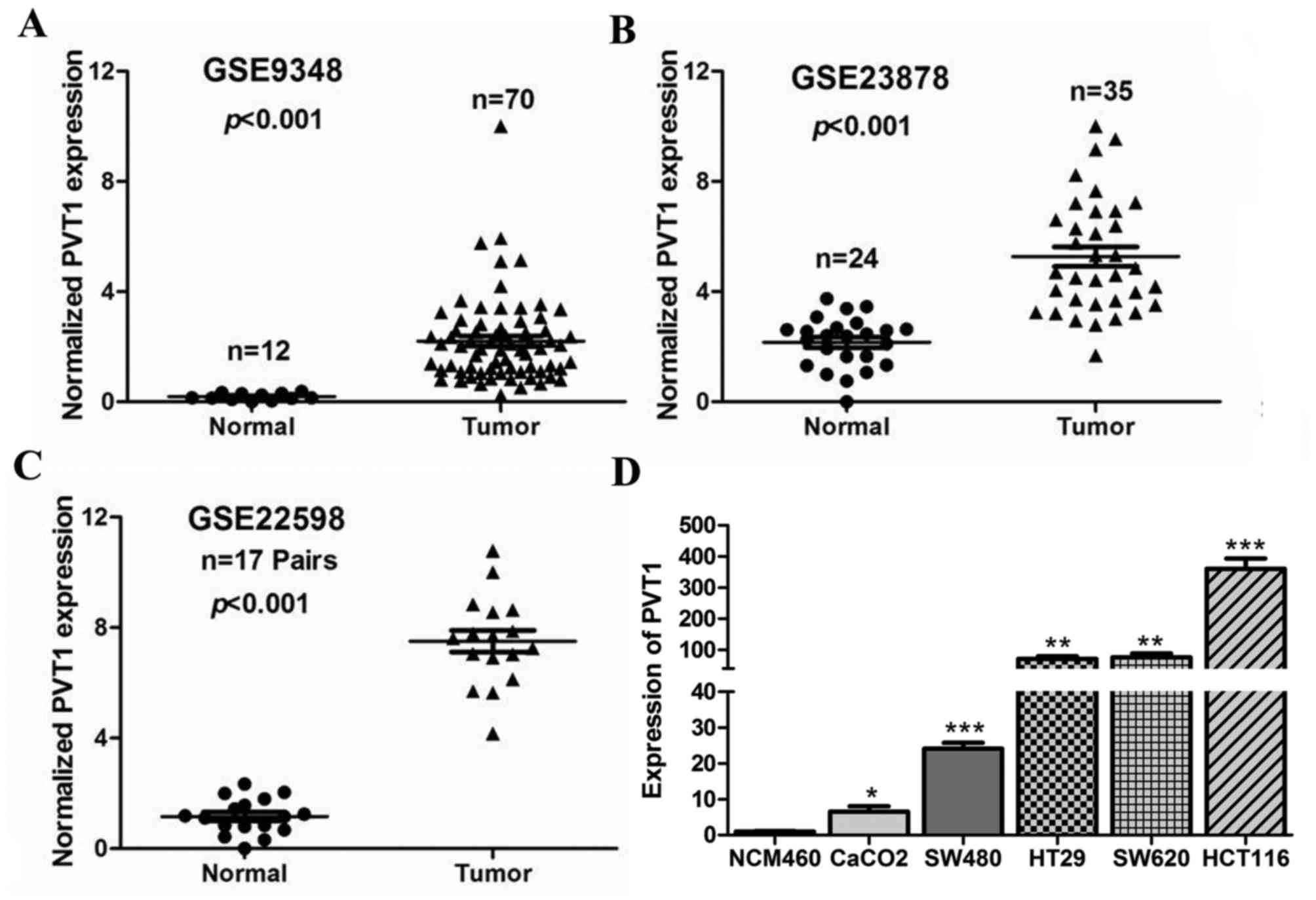 | Figure 1.PVT1 is highly expressed in
CRC tissues and cell lines. PVT1 expression, as measured by
Affymetrix microarray, was upregulated in CRC tissues compared with
that in normal colon mucosal tissues in (A) #GSE9348
(containing 12 normal colorectal tissues and 70 CRC tissue
biopsies), (B) #GSE23878 (containing 24 normal
colorectal tissues and 35 CRC tissue biopsies) and (C)
#GSE22598 (containing 17 pairs of CRC tissues and
corresponding normal colorectal tissues) from the GEO database. (D)
PVT1 expression significantly increased in CRC cell lines
(SW480, HT29, Caco-2, HCT116, and SW620) compared with that in
NCM460, a normal colon mucosal cell line. Data are shown as mean ±
standard error of the mean. *P<0.05, **P<0.01, ***P<0.001
vs. NCM460 cells. PVT1, plasmacytoma variant translocation
1; GEO, Gene Expression Omnibus; CRC, colorectal cancer. |
Upregulation of PVT1 predicts a poor
prognosis and could be regarded as an independent predictor for OS
in CRC
We next assessed the correlation between PVT1
expression and distant metastasis in CRC tissues. We analysed a
previously published dataset (no. GSE50760), using Illumina HiSeq
2000 arrays, to identify dysregulated lncRNAs in CRC. We found that
the higher expression of PVT1 was significantly correlated
with CRC distant metastasis (P<0.001, Fig. 2A).
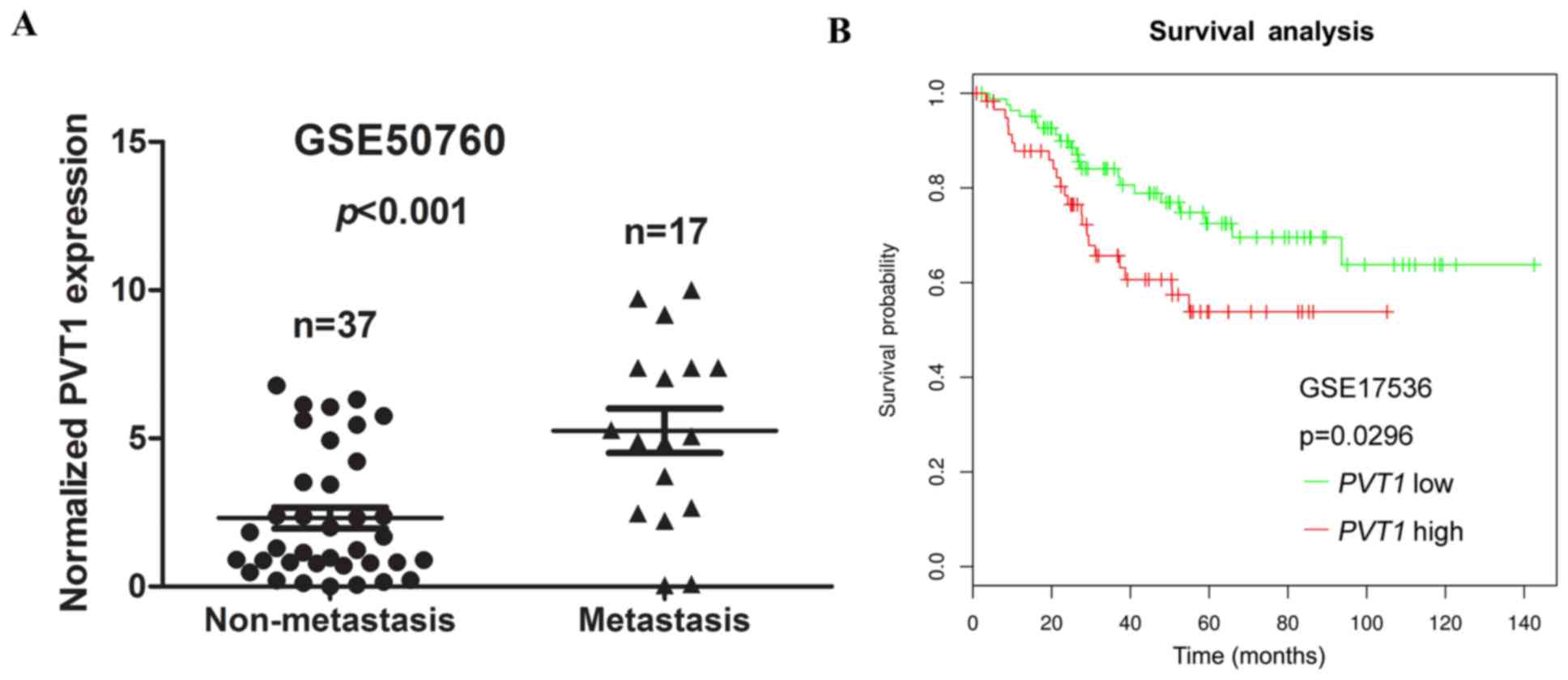 | Figure 2.Association between PVT1
expression and clinicopathological features. (A) Relative
expression of PVT1 in non-metastasis and metastasis human
CRC tissues were obtained from the GEO database (no. GSE50760,
non-metastasis, n=37; metastasis, n=17). (B) SynTarget database was
used to analyse the clinic impact of PVT1 expression
patterns on CRC patient's survival in a CRC specimen expression
profile dataset (no. GSE17536, the specimen of GSE17536 was divided
into two groups, using the SynTarget database: Group 1, low
expression of PVT1, n=83; group 2, high expression of
PVT1, n=60). PVT1, plasmacytoma variant translocation
1; GEO, Gene Expression Omnibus; CRC, colorectal cancer. |
To assess the prognostic value of PVT1
expression in CRC patients, the SynTarget database (27,28)-a database for survival analyses in
cancer-was used to analyse the clinical impact of PVT1
expression patterns on the survival of CRC patients in a CRC
specimen expression profile dataset (no. GSE17536). The results
revealed that PVT1 expression showed a negative correlation
with the OS of CRC patients (P=0.0296, Fig. 2B). Collectively, these data
indicate that high PVT1 expression is an independent risk
factor for CRC patients.
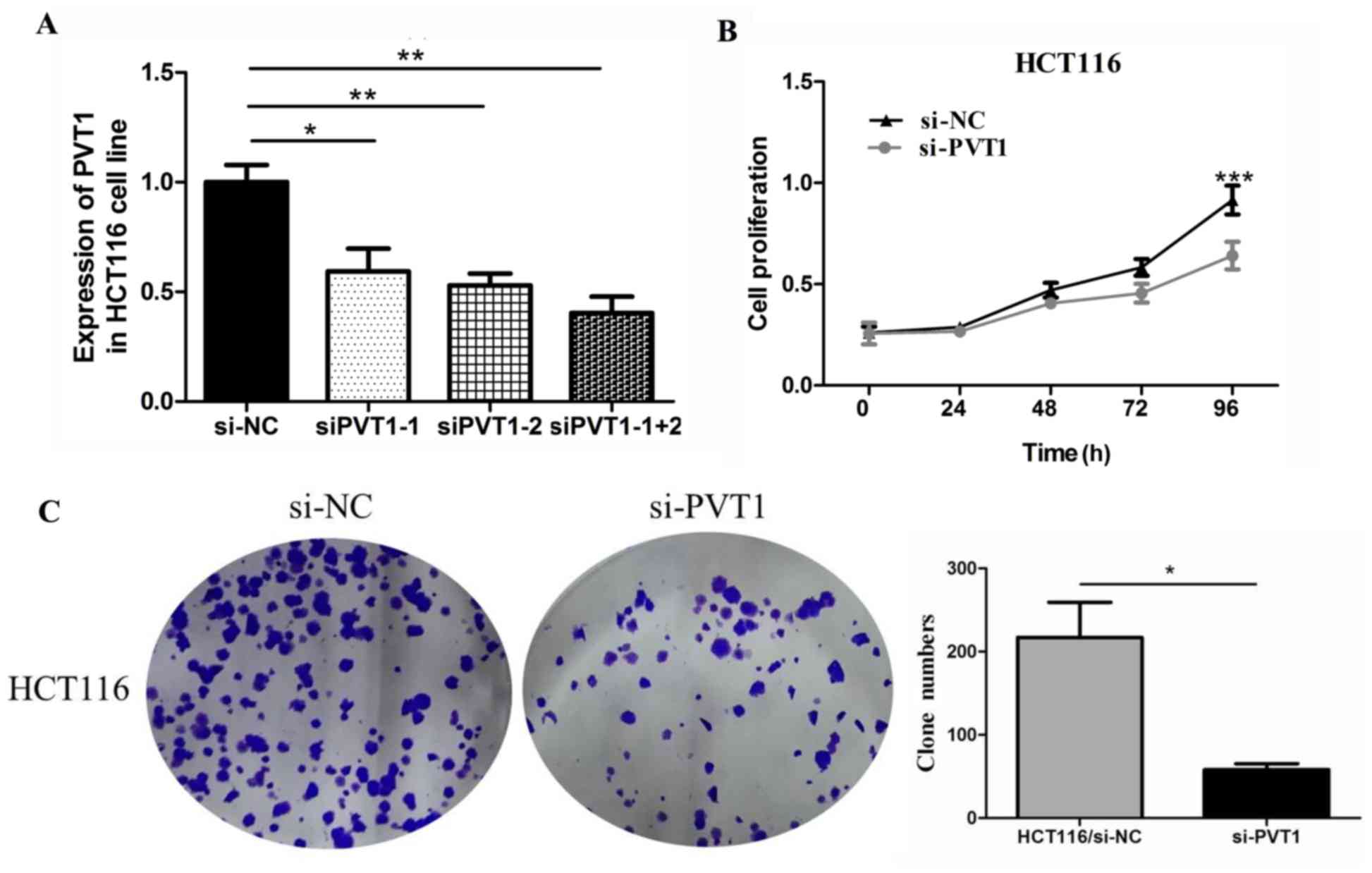
Knockdown of PVT1 inhibits cell
proliferation and invasion in CRC
To verify the function of PVT1 in colon
cancer cells, we first measured the efficiency of the siRNA
si-PVT1. Compared with the siPVT1-1 and
siPVT1-2 groups, the siPVT1-1+2 group showed the highest
efficiency in HCT116 cells (Fig.
3A). Therefore, we chose siPVT1-1+2 for in vivo
knockdown of PVT1 expression to assess the biological
function of PVT1 in CRC tissues. We investigated the effect
of PVT1 knockdown on CRC cell proliferation by performing
CCK-8 proliferation assays. PVT1 knockdown expression
significantly inhibited HCT116 cell proliferation compared to that
of the control group in the 96 h (P<0.001, Fig. 3B). PVT1 knockdown also
inhibited HCT116 cell clone formation compared to that of the
control group (P<0.05, Fig.
3C).
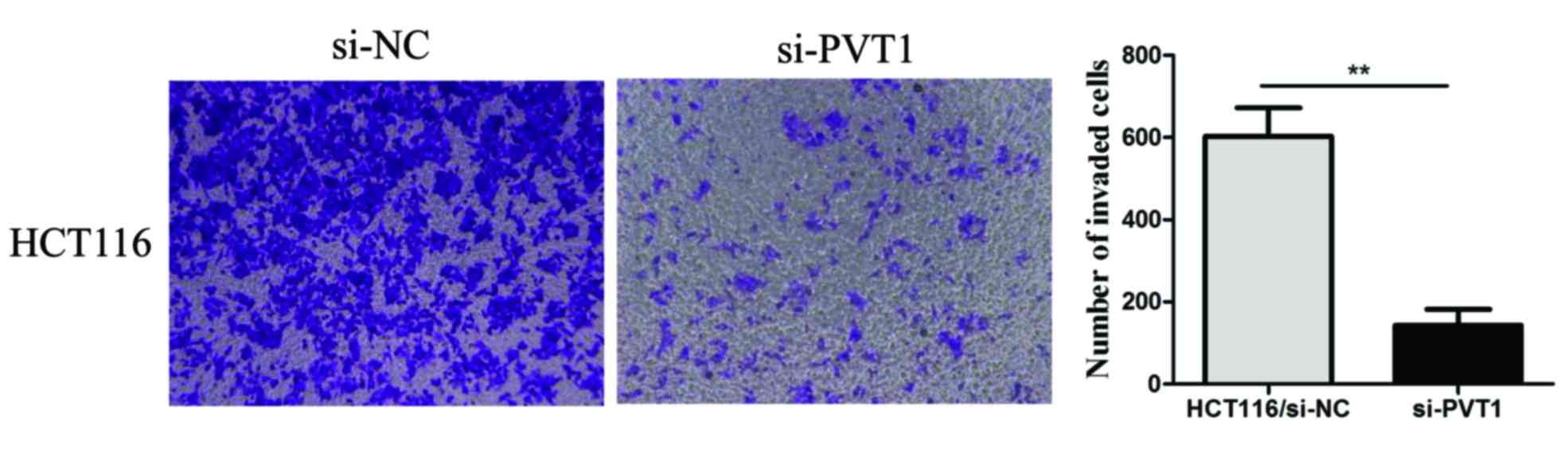
We also explored the effect of PVT1 knockdown
on CRC cell invasion. A transwell invasion assay was performed to
assess the effect of PVT1 on the invasiveness of CRC cells.
PVT1 knockdown significantly inhibited HCT116 cell invasion
compared to that of the control group (P<0.01, Fig. 4). These results demonstrated that
PVT1 knockdown suppressed the proliferation, invasion, and
metastasis of CRC cells in vitro.
Knockdown of PVT1 suppresses
proliferation and EMT markers in CRC
To confirm that PVT1 knockdown suppresses the
proliferation, invasion, and metastasis of CRC cells in
vitro, RT-qPCR and western blotting were used to assess the
mRNA and protein level of the epithelial marker E-cadherin,
mesenchymal markers vimentin, and proliferation markers cyclin D1
and CDK4 in HCT116 cell lines. PVT1 knockdown significantly
decreased vimentin and enhanced E-cadherin expression (P<0.05,
Fig. 5A-B), thereby inhibiting the
progression of EMT. Meanwhile, PVT1 knockdown significantly
inhibited cyclin D1 and CDK4 (P<0.01, Fig. 5C and D). This indicates that
PVT1 regulates proliferation and EMT markers expression in
CRC cell lines.
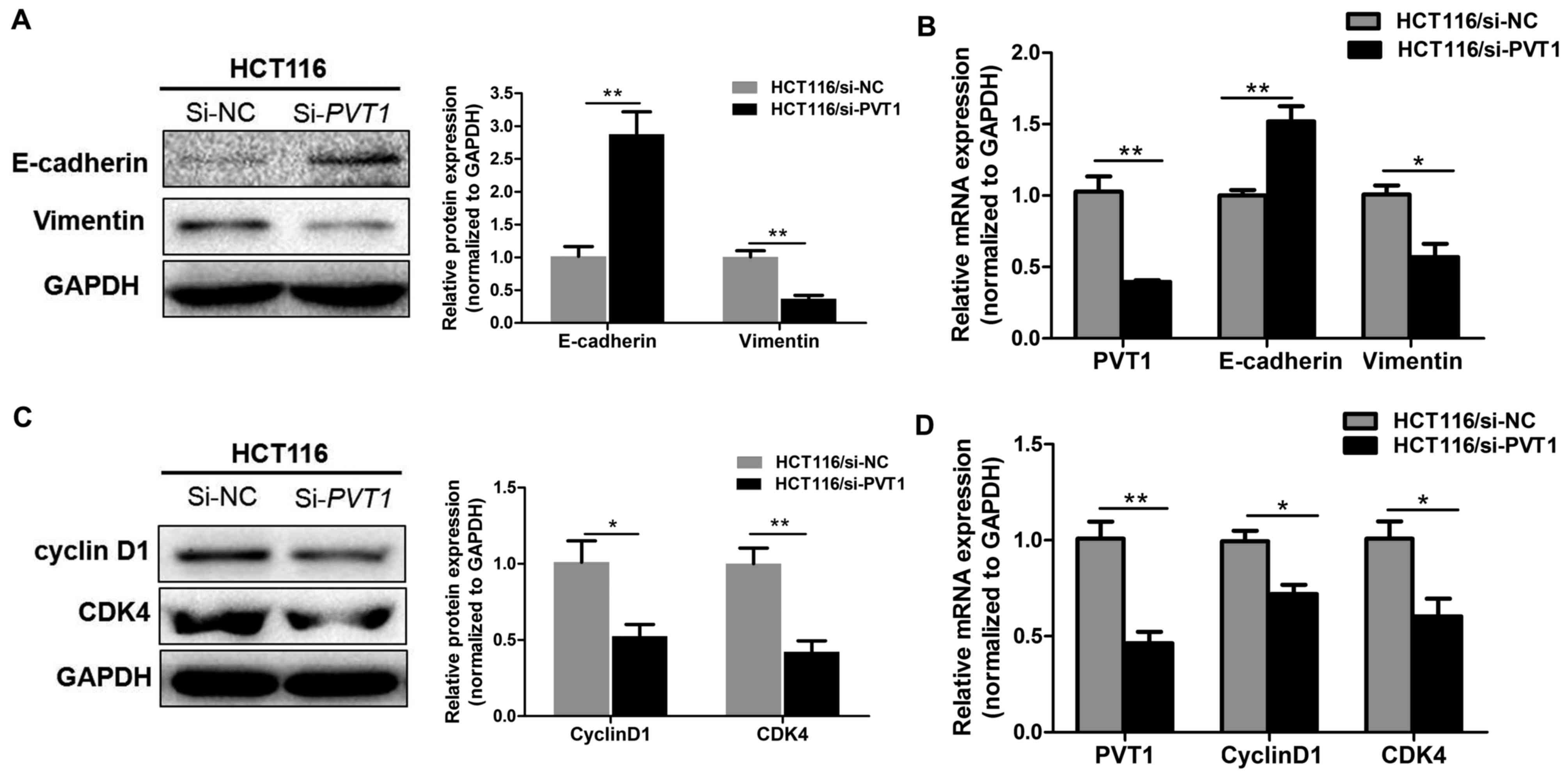 | Figure 5.Knockdown of PVT1 suppresses
proliferation and EMT markers in CRC. (A) Protein and (B) mRNA
expressions of E-cadherin and vimentin in CRC cells were analysed
by western blot analysis and RT-qPCR, respectively, following
transfection with si-NC or si-PVT1 for 48 h in HCT116 cells.
(C) Protein and (D) mRNA expression of cyclin D1 and CDK4 in CRC
cells were analysed by western blot analysis and RT-qPCR following
transfection with si-NC or si-PVT1 for 48 h in HCT116 cells.
Data are shown as the mean ± standard error of the mean.
*P<0.05, **P<0.01. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; PVT1,
plasmacytoma variant translocation 1; CRC, colorectal cancer; NC,
negative control; si, short interfering; EMT,
epithelial-mesenchymal transition; CDK4, cyclin dependent kinase 4;
si, small interfering. |
Discussion
CRC is one of the most common causes of
cancer-associated mortality worldwide (29), especially in developed countries.
It wasestimated in 2015 that there were 777,987 new cases and
352,589 deaths from CRC in developed countries (30). Surgery is currently the primary
method of treatment for CRC, along with adjuvant radio-chemotherapy
treatments. Although substantial progress has been made in the
diagnosis and treatment of CRC, it retains a high morbidity and
mortality rate owing to frequent recurrence and metastasis after
treatment. Therefore, the treatment of CRC requires a novel
therapeutic target to better control recurrence and metastasis.
LncRNAs are emerging as pivotal regulators in
various biological processes. They are modulators of gene
expression at the epigenetic, transcriptional, and
post-transcriptional levels (31,32),
controlling the fate of cellular processes including cell
proliferation, apoptosis, and differentiation (33). Recent studies have revealed that
disrupting or disabling lncRNA expression strongly correlates with
a decrease in the incidence and development of malignant tumours,
because lncRNAs have roles in cancer cell proliferation, the
epithelial-mesenchymal transition (EMT), and drug resistance
(34,35). Because lncRNAs are easier to
extract, can be detected with higher specificity and sensitivity,
and exist steadily in the blood and tissue (36), they have great potential to be a
novel biomarker for cancer diagnosis, predicting recurrence, and
chemosensitivity. Several lncRNAs have been shown to be
differentially expressed in CRC and indicators of a poor prognosis,
including MEG3 (37),
GAS5 (38), MALAT1
(39), TUG1 (40), HOTAIR (41), and PVT1 (42).
The lncRNA PVT1 is reported to be
overexpressed in many diseases, including several cancers.
PVT1 overexpression has recently been identified as an
independent predictor for OS in various human cancers, such as
gastric cancer (17), NSCLC
(18), and hepatocellular cancer
(19). However, there has been
insufficient research on the association of PVT1 expression
with the OS of CRC patients. Takahashi et al (42) demonstrated that the location of
PVT1 was similar to that of MYC, which were mapped to
chromosome 8q24. They also showed that 8q24 copy-number
amplification promoted MYC and PVT1
expression-prognostic indicators for CRC in patients. Similarly, Li
et al (43) reported that
the higher levels of AFAP1-AS1, MALAT1, H19, HOXA-AS2, BCAR4
or PVT1 in CRC tissues might predict the poor prognosis of
CRC patients. In our study, we aimed to explore this lncRNA,
whichhas the potential to be developed into a novel biomarker for
CRC diagnosis and prognosis. We reported that PVT1
expression was significantly higher in CRC tissues than in normal
colon mucosal tissues by GEO database analysis. Furthermore,
multivariate analysis showed that CRC patients with PVT1
overexpression had a poorer OS time, which indicates that
overexpression of PVT1 may be an independent indicator of
poor prognosis in CRC patients.
Evidence strongly suggests that PVT1 plays a
critical role in the development and progression of cancer by
regulating cancer cell proliferation, metastasis, cell cycle,
apoptosis, stemness, and drug resistance (44). Huang et al (45) demonstrated that PVT1 was
overexpressed in small cell lung cancer (SCLC) tissues, and that
knocking down PVT1 expression with siRNA significantly
suppressed SCLC cell migration and invasion in vitro.
Additionally, Kong et al (17) revealed that upregulation of
PVT1 promotes cell proliferation in gastric cancer by
epigenetically regulating p15 and p16. Shen et al (46) also showed that PVT1 could
decrease miR-195 expression by enhancing histone H3K27me3 in
the miR-195 promoter region and by direct sponging of
miR-195 to modulate EMT and chemo-resistance in cervical
cancer cells. However, the effects of PVT1 on CRC
proliferation, invasion, and metastasis are poorly understood. Guo
et al (47) reported that
PVT1 may be a new oncogene co-amplified with c-Myc in
CRC tissues and functionally correlated with the proliferation and
apoptosis of CRC cells. Our results demonstrated that inhibition of
PVT1 suppressed CRC cell proliferation, invasion, and
metastasis in HCT116 cell lines, which was associated with
decreased vimentin, cyclin D1, and CDK4 expression, but enhanced
E-cadherin expression. This indicates that PVT1 contributes to the
regulation of proliferation and EMT marker expression in CRC cell
lines.
In summary, the results presented in this study
indicate that PVT1 expression is upregulated in CRC
patients, and that patients with high PVT1 expression show
poor OS. Multivariate analysis indicated that high PVT1
expression is an independent risk factor for CRC patients. We also
demonstrated that PVT1 expression mediates the
proliferation, invasion, and metastasis of CRC cells. PVT1
knockdown significantly suppressed the proliferative and invasive
capabilities of CRC cells. However, our study exists two
limitations: 1) our study was limited by the use of only one CRC
cell line; 2) our study was not further investigate the regulatory
mechanism underlying PVT1's promotion of the proliferation,
invasion, and metastasis of CRC cells. Taken together, our study
demonstrated the oncogene role of PVT1 in tumour progression
of CRC, and shows potential as a target for development of novel
CRC therapies after further investigation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the Gene Expression Omnibus datasets
(ncbi.nlm.nih.gov/gds/).
Authors' contributions
CW and XS developed the concept and designed the
study. CW, XZ and CP collected the data. CW, XZ and CP analysed and
interpreted the data. All authors contributed to the writing of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen C, Wang L, Liao Q, Huang Y, Ye H,
Chen F, Xu L, Ye M and Duan S: Hypermethylation of EDNRB promoter
contributes to the risk of colorectal cancer. Diagn Pathol.
8:1992013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sunkara V and Hebert JR: The colorectal
cancer mortality-to-incidence ratio as an indicator of global
cancer screening and care. Cancer. 121:1563–1569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rupnarain C, Dlamini Z, Naicker S and
Bhoola K: Colon cancer: Genomics and apoptotic events. Biol Chem.
385:449–464. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ou C, Sun Z, Li S, Li G, Li X and Ma J:
Dual roles of yes-associated protein (YAP) in colorectal cancer.
Oncotarget. 8:75727–75741. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Edwards BK, Ward E, Kohler BA, Eheman C,
Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I,
Seeff LC, et al: Annual report to the nation on the status of
cancer, 1975–2006, featuring colorectal cancer trends and impact of
interventions (risk factors, screening and treatment) to reduce
future rates. Cancer. 116:544–573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Colussi D, Brandi G, Bazzoli F and
Ricciardiello L: Molecular pathways involved in colorectal cancer:
Implications for disease behavior and prevention. Int J Mol Sci.
14:16365–16385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vaiopoulos AG, Athanasoula K and
Papavassiliou AG: Epigenetic modifications in colorectal cancer:
Molecular insights and therapeutic challenges. Biochim Biophys
Acta. 1842:971–980. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guttman M and Rinn JL: Modular regulatory
principles of large non-coding RNAs. Nature. 482:339–346. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bazzini AA, Johnstone TG, Christiano R,
Mackowiak SD, Obermayer B, Fleming ES, Vejnar CE, Lee MT, Rajewsky
N, Walther TC and Giraldez AJ: Identification of small ORFs in
vertebrates using ribosome footprinting and evolutionary
conservation. EMBO J. 33:981–993. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song H, Sun W, Ye G, Ding X, Liu Z, Zhang
S, Xia T, Xiao B, Xi Y and Guo J: Long non-coding RNA expression
profile in human gastric cancer and its clinical significances. J
Transl Med. 11:2252013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shang C, Zhu W, Liu T, Wang W, Huang G,
Huang J, Zhao P, Zhao Y and Yao S: Characterization of long
non-coding RNA expression profiles in lymph node metastasis of
early-stage cervical cancer. Oncol Rep. 35:3185–3197. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang Y, Li H, Hou S, Hu B, Liu J and Wang
J: The noncoding RNA expression profile and the effect of lncRNA
AK126698 on cisplatin resistance in non-small-cell lung cancer
cell. PLoS One. 8:e653092013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu H, Wu R, Chen M, Li D, Dai J, Zhang Y,
Gao K, Yu J, Hu G, Guo Y, et al: Comprehensive analysis of
differentially expressed profiles of lncRNAs and construction of
miR-133b mediated ceRNA network in colorectal cancer. Oncotarget.
8:21095–21105. 2017.PubMed/NCBI
|
|
16
|
Tseng YY, Moriarity BS, Gong W, Akiyama R,
Tiwari A, Kawakami H, Ronning P, Reuland B, Guenther K, Beadnell
TC, et al: PVT1 dependence in cancer with MYC copy-number increase.
Nature. 512:82–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kong R, Zhang EB, Yin DD, You LH, Xu TP,
Chen WM, Xia R, Wan L, Sun M, Wang ZX, et al: Long noncoding RNA
PVT1 indicates a poor prognosis of gastric cancer and promotes cell
proliferation through epigenetically regulating p15 and p16. Mol
Cancer. 14:822015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui D, Yu CH, Liu M, Xia QQ, Zhang YF and
Jiang WL: Long non-coding RNA PVT1 as a novel biomarker for
diagnosis and prognosis of non-small cell lung cancer. Tumour Biol.
37:4127–4134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang F, Yuan JH, Wang SB, Yang F, Yuan SX,
Ye C, Yang N, Zhou WP, Li WL, Li W and Sun SH: Oncofetal long
noncoding RNA PVT1 promotes proliferation and stem cell-like
property of hepatocellular carcinoma cells by stabilizing NOP2.
Hepatology. 60:1278–1290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hong Y, Downey T, Eu KW, Koh PK and Cheah
PY: A ‘metastasis-prone’ signature for early-stage mismatch-repair
proficient sporadic colorectal cancer patients and its implications
for possible therapeutics. Clin Exp Metastasis. 27:83–90. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Uddin S, Ahmed M, Hussain A, Abubaker J,
Al-Sanea N, AbdulJabbar A, Ashari LH, Alhomoud S, Al-Dayel F, Jehan
Z, et al: Genome-wide expression analysis of Middle Eastern
colorectal cancer reveals FOXM1 as a novel target for cancer
therapy. Am J Pathol. 178:537–547. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okazaki S, Ishikawa T, Iida S, Ishiguro M,
Kobayashi H, Higuchi T, Enomoto M, Mogushi K, Mizushima H, Tanaka
H, et al: Clinical significance of UNC5B expression in colorectal
cancer. Int J Oncol. 40:209–216. 2012.PubMed/NCBI
|
|
23
|
Smith JJ, Deane NG, Wu F, Merchant NB,
Zhang B, Jiang A, Lu P, Johnson JC, Schmidt C, Bailey CE, et al:
Experimentally derived metastasis gene expression profile predicts
recurrence and death in patients with colon cancer.
Gastroenterology. 138:958–968. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim SK, Kim SY, Kim JH, Roh SA, Cho DH,
Kim YS and Kim JC: A nineteen gene-based risk score classifier
predicts prognosis of colorectal cancer patients. Mol Oncol.
8:1653–1666. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ou C, Sun Z, Zhang H, Xiong W, Ma J, Zhou
M, Lu J, Zeng Z, Bo X, Chen P, et al: SPLUNC1 reduces the
inflammatory response of nasopharyngeal carcinoma cells infected
with the EB virus by inhibiting the TLR9/NF-κB pathway. Oncol Rep.
33:2779–2788. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ou C, Sun Z, Li X, Li X, Ren W, Qin Z,
Zhang X, Yuan W, Wang J, Yu W, et al: MiR-590-5p, a
density-sensitive microRNA, inhibits tumorigenesis by targeting
YAP1 in colorectal cancer. Cancer Lett. 399:53–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Antonov AV: BioProfiling.de: Analytical
web portal for high-throughput cell biology. Nucleic Acids Res.
39:W323–W327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amelio I, Tsvetkov PO, Knight RA, Lisitsa
A, Melino G and Antonov AV: SynTarget: An online tool to test the
synergetic effect of genes on survival outcome in cancer. Cell
Death Differ. 23:9122016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bray F, Ren JS, Masuyer E and Ferlay J:
Global estimates of cancer prevalence for 27 sites in the adult
population in 2008. Int J Cancer. 132:1133–1145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He X, Ou C, Xiao Y, Han Q, Li H and Zhou
S: LncRNAs: Key players and novel insights into diabetes mellitus.
Oncotarget. 8:71325–71341. 2017.PubMed/NCBI
|
|
33
|
Ou C and Li G: Long non-coding RNA TUG1: A
novel therapeutic target in small cell lung cancer. J Thorac Dis.
9:E644–E645. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Prensner JR and Chinnaiyan AM: The
emergence of lncRNAs in cancer biology. Cancer Discov. 1:391–407.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ou C and Li G: Exosome-transmitted
lncARSR: A novel therapeutic target in renal cancer. Transl Cancer
Res. 6:656–657. 2017. View Article : Google Scholar
|
|
37
|
Yin DD, Liu ZJ, Zhang E, Kong R, Zhang ZH
and Guo RH: Decreased expression of long noncoding RNA MEG3 affects
cell proliferation and predicts a poor prognosis in patients with
colorectal cancer. Tumour Biol. 36:4851–4859. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yin D, He X, Zhang E, Kong R, De W and
Zhang Z: Long noncoding RNA GAS5 affects cell proliferation and
predicts a poor prognosis in patients with colorectal cancer. Med
Oncol. 31:2532014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zheng HT, Shi DB, Wang YW, Li XX, Xu Y,
Tripathi P, Gu WL, Cai GX and Cai SJ: High expression of lncRNA
MALAT1 suggests a biomarker of poor prognosis in colorectal cancer.
Int J Clin Exp Pathol. 7:3174–3181. 2014.PubMed/NCBI
|
|
40
|
Sun J, Ding C, Yang Z, Liu T, Zhang X,
Zhao C and Wang J: The long non-coding RNA TUG1 indicates a poor
prognosis for colorectal cancer and promotes metastasis by
affecting epithelial-mesenchymal transition. J Transl Med.
14:422016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Svoboda M, Slyskova J, Schneiderova M,
Makovicky P, Bielik L, Levy M, Lipska L, Hemmelova B, Kala Z,
Protivankova M, et al: HOTAIR long non-coding RNA is a negative
prognostic factor not only in primary tumors, but also in the blood
of colorectal cancer patients. Carcinogenesis. 35:1510–1515. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Takahashi Y, Sawada G, Kurashige J, Uchi
R, Matsumura T, Ueo H, Takano Y, Eguchi H, Sudo T, Sugimachi K, et
al: Amplification of PVT-1 is involved in poor prognosis via
apoptosis inhibition in colorectal cancers. Br J Cancer.
110:164–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li Q, Dai Y, Wang F and Hou S:
Differentially expressed long non-coding RNAs and the prognostic
potential in colorectal cancer. Neoplasma. 63:977–983. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Colombo T, Farina L, Macino G and Paci P:
PVT1: A rising star among oncogenic long noncoding RNAs. Biomed Res
Int. 2015:3042082015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang C, Liu S, Wang H, Zhang Z, Yang Q
and Gao F: LncRNA PVT1 overexpression is a poor prognostic
biomarker and regulates migration and invasion in small cell lung
cancer. Am J Transl Res. 8:5025–5034. 2016.PubMed/NCBI
|
|
46
|
Shen CJ, Cheng YM and Wang CL: LncRNA PVT1
epigenetically silences miR-195 and modulates EMT and
chemoresistance in cervical cancer cells. J Drug Target.
25:637–644. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guo K, Yao J, Yu Q, Li Z, Huang H, Cheng
J, Wang Z and Zhu Y: The expression pattern of long non-coding RNA
PVT1 in tumor tissues and in extracellular vesicles of colorectal
cancer correlates with cancer progression. Tumour Biol.
39:10104283176991222017. View Article : Google Scholar : PubMed/NCBI
|