Introduction
Breast cancer is a common malignant tumor in women
and its incidence increases every year; it has therefore become a
serious threat to women's health (1,2). In
recent years, there has been marked progress in the diagnosis and
treatment of breast cancer; however, patient mortality has not been
significantly improved (3,4). Currently, Triple Negative Breast
Cancer (TNBC) with all negative estrogen receptor/progesterone
receptor/human epidermal growth factor receptor-2 has become a
growing concern for clinicians (5). TNBCs account for ~15–26% of breast
cancer cases (5,6). Due to its phenotype and
characteristics, TNBC is a more aggressive type of cancer and is
easy to metastasize, which reduces the number of effective target
sites for treatment (7,8). The survival rate of TNBC is markedly
lower than that of other types of breast cancer (9,10).
As such, TNBC has become a key area of research in recent
years.
In the clinic, previous studies have revealed that
the effect of anti-angiogenesis treatment in some patients was
quite poor and was much less successful than it was expected to be
(11–13). As a result, it has been suggested
that there may be a novel mode of circulation in tumor blood
supply. Vasculogenic mimicry (VM) was first observed in high
invasive uveal melanoma (14).
Following further research, VM has been confirmed in invasive
breast cancer (15), ovarian
cancer (16), glioma (17), liver cancer and gastrointestinal
stromal tumors (18). VM is
closely associated with tumor growth, invasion, metastasis and
prognosis of patients. Patients with VM often have shorter survival
periods and a poorer prognosis (19–21).
VM enables tumor cells to simulate endothelial cell function by
reconstruction and forming a unique circulation conduit structure
(20). Due to VM, traditional
anti-angiogenesis treatment cannot completely block the blood
supply to tumors (19,21). However, its formation and
regulatory mechanism are still unclear and require further research
in TNBCs.
Epithelial-mesenchymal transition (EMT) provides a
novel reasoning to explain the mechanism of VM formation in
epithelial tumors. EMT causes epithelial cells to lose their
differentiated phenotype and specific epithelial cell features
including cell adhesion, top-basal polarity and poor athletic
ability; however, epithelial cells also obtain some mesenchymal
cell phenotypes such as an enhanced moving metastasis ability and a
strong anti-apoptosis ability (22). When epithelial cells undergo EMT,
epithelial cell markers including E-cadherin, desmoplakin, tight
junction protein and cytokeratin are downregulated, and mesenchymal
cell markers including vimentin, fibronectin, N-Cadherin and
α-smooth muscle actin are upregulated (23). The formation of VM is very similar
to the EMT process. In colon cancer samples with VM, it was
observed that Zinc finger E-box binding homeobox (ZEB1) and
vimentin expression were upregulated, and E-cadherin expression was
downregulated. Following ZEB1 knockdown, the formation of VM was
inhibited and the cell phenotype was recovered (24), indicating that the EMT process may
be regulated by ZEB1 and that it may serve an important role in VM
formation. Therefore, the transcription factor ZEB1 may also be
closely associated with VM formation in breast cancer via the
regulation of EMT. Furthermore, vascular endothelial growth factor
(VEGF) serves an important role in the growth of tumor blood
vessels. Fetal liver kinase 1 [flk-1; also known as VEGF receptor 2
(VEGFR-2)] is an important VEGF receptor that is primarily
expressed in endothelial cells (25).
The aim of the present study was to examine whether
decreasing the expression of ZEB1 in turn inhibited VM, via flk-1,
and the EMT process in TNBC.
Materials and methods
Cell culture
The human breast cancer cell line MDA-MB-231
(American Type Culture Collection, Manassas, VA, USA) was cultured
in RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 4 mM L-glutamine and 1% penicillin-streptomycin
(Beyotime Institute of Biotechnology, Haimen, China) at 37°C in 5%
CO2. Cells (5×105) were seeded into 6-well
plates. At 80% confluence, 1 µM Semaxanib (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was added to the cells for 1 h
preincubation to inhibit flk-1. Following the addition of 1 ml cell
suspension to cell culture plates and culture for 72 h, the ability
of cells to form a canal structure (tube) was periodically observed
under an inverted microscope (Olympus IX70; Olympus Corporation,
Tokyo, Japan).
ZEB1 gene silencing
The non-effective scrambled-small hairpin (sh)RNA
plasmid (cat. no. TR30021) and ZEB1 shRNA plasmid (cat. no.
TL301174) were purchased from OriGene Technologies, Inc.
(Rockville, MD, USA). These plasmids (50 µM) were transfected into
cells to produce negative control (vector, NC) and ZEB1-silenced
MDA-MB-231 cells using the FuGENE® HD transfection
reagent (Promega Corporation, Madison, WI, USA) according to the
manufacturer's protocols. For ZEB1 knockdown, four shRNA constructs
(cat. no. TL301174; OriGene Technologies, Inc. mixed shRNAs (15 µM
of each to obtain 60 µM) were combined and then transfected.
Following Geneticin selection, the downregulation of ZEB1 was
confirmed by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and western blot analysis.
RT-qPCR
Total RNA was isolated from cells using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
the concentration was determined using the GeneQuant II (Pharmacia
Biotech; GE Healthcare, Uppsala, Sweden) at 260 nm. The RT reaction
and cDNA synthesis were performed according to the manufacturer's
protocols (Superscript One-step RT-PCR System; Invitrogen; Thermo
Fisher Scientific, Inc.). qPCR analysis was performed on the ABI
7500 system (Applied Biosystems; Thermo Fisher Scientific, Inc.)
using the SYBR Premix Ex Taq GC kit (Takara Bio, Inc., Otsu, Japan)
at 95°C for 2 min, 94°C for 20 sec, 58°C for 20 sec and 72°C for 20
sec (40 cycles). The stem-loop primers used for PCR amplification
were synthesized by Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
All primer sequences used for analysis were as follow: ZEB1
forward, 5′-CAGGCAGATGAAGCAGGATG-3′ and reverse
5′-CAGCAGTGTCTTGTTGTTGTAG-3′; GAPDH forward,
5′-ACACCCACTCCTCCACCTTT and reverse 5′-TTACTCCTTGGAGGCCATGT. The
relative levels of ZEB1 mRNA were normalized to those of GAPDH
using the relative 2−∆∆Cq method (26).
Western blot analysis
Protein in cell lysate was extracted using RIPA
(Beyotime Institute of Biotechnology) and then the concentration
was determined by the BCA method (Beyotime Institute of
Biotechnology); it was stored at −80°C until required. A total of
60 µg protein in each sample was separated by 8% SDS-PAGE and
transferred to a nitrocellulose membrane. The nitrocellulose
membrane was then blocked in TBST containing 0.1% Tween-20 and 5%
fat-free dry milk for 2 h at 4°C, followed by incubation with the
primary antibodies, anti-vimentin (sc-6260; 1:100; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and anti-E-cadherin
(sc-21791; 1:100; Santa Cruz Biotechnology, Inc.) at 4°C overnight.
The membrane was washed 3 times with TBST prior to HRP-conjugated
secondary antibody (A0216; 1:1,000; Beyotime Institute of
Biotechnology) incubation at room temperature for 2 h, followed by
a further 3 washes with TBST. Protein detection was performed using
an enhanced chemiluminescence reagent kit (Beyotime Institute of
Biotechnology). GAPDH (AF1186; Institute of Biotechnology) was used
as the internal reference. Quantification was performed using
ImageJ version 4.0 (National Institutes of Health, Bethesda, MD,
USA).
Invasion assay
Invasion assays were performed as described
previously (23). Under aseptic
condition, Matrigel was diluted to 1 mg/ml with RPMI-1640 medium
and 100 µl/well Matrigel was added into 24-well cell culture plates
for incubation at 37°C for 30 min. Then, cells were trypsinized
(0.25%) and adjusted to 2.5×105/ml. Matrigel was used to
coat the Transwell cabin polycarbonate membrane for 1 h at 37°C.
Then, 60 µl/well of the chemotactic factor, epidermal growth factor
(10 ng/ml, Invitrogen; Thermo Fisher Scientific, Inc.) was added to
the lower Transwell chamber. Cells in serum-free culture medium
(Gibco; Thermo Fisher Scientific, Inc.) with 4 mM L-glutamine and
1% penicillin-streptomycin were used to produce a single cell
suspension at 1×105/well cells. A total of 200 µl cell
suspension was added to the wells of the upper chamber for
incubation at 37°C for 24 h. Cells that had not migrated through
the membrane were wiped away using wet cotton swabs. Following
hematoxylin staining at room temperature for 30 min and washing
with PBS, the number of cells that had migrated through the
membrane were observed and counted using an Olympus IX70 inverted
microscope (Olympus Corporation, Tokyo, Japan).
Statistical analysis
Data are presented as the mean ± standard deviation
of 3 independent experiments. SPSS version 13.0 software (SPSS,
Inc., Chicago, IL, USA) was used to evaluate the data. Analysis of
variance with a Bonferroni post hoc test were performed for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
ZEB1 shRNA inhibits the expression of
ZEB1 at the transcriptional and translational levels
In order to silence ZEB1 in MDA-MB-231 cells, 4
constructs of ZEB1 shRNA were mixed and transfected into cells. The
expression of ZEB1 at the mRNA and protein levels was then detected
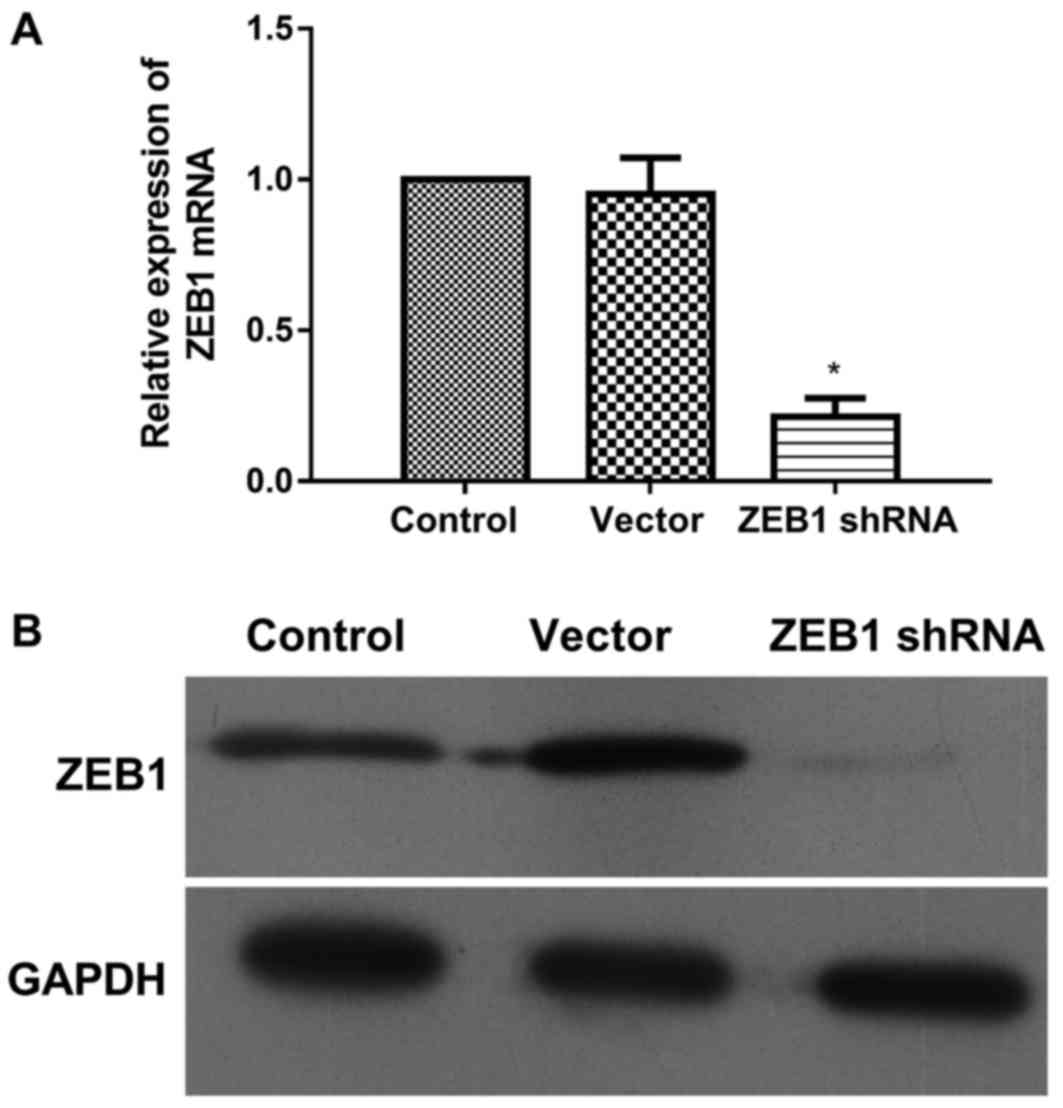
by RT-qPCR and western blotting, respectively (Fig. 1). As shown in Fig. 1A, the levels of ZEB1 mRNA in
shRNA-transfected cells was significantly downregulated to 22% of
the level presented by the control; however, the expression of ZEB1
mRNA was not significantly altered in the vector group. Similarly,
the expression of ZEB1 protein was markedly downregulated by ZEB1
shRNA, though not by the vector (Fig.
1B). Thus, the expression of ZEB1 at the transcriptional and
translational levels was downregulated by ZEB1 shRNA. These stably
transfected cells were used for the following experiments.
ZEB1 shRNA inhibits the formation of
VM
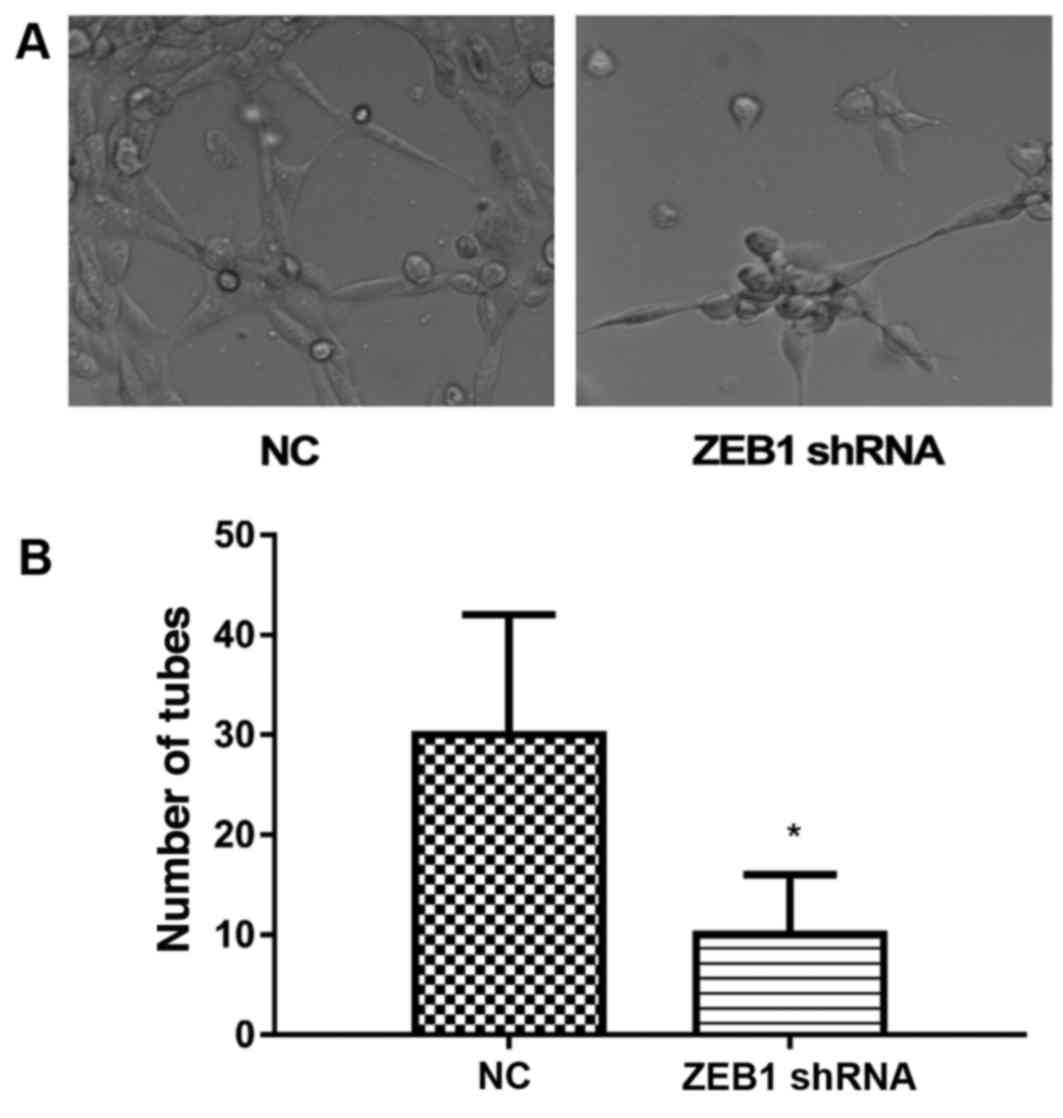
To detect the role of ZEB1 in the formation of VM,
the cells that were stably transfected with ZEB1 shRNA were
cultured on Matrigel pre-coated cell culture plates. Following 72
h, the cells transfected with vector presented marked formation of
VM (Fig. 2A). However, the
formation of VM was markedly inhibited in ZEB1 shRNA transfected
cells (Fig. 2A). For
quantification, the number of tubes observed was calculated. As
shown in Fig. 2B, the number of
tubes was significantly reduced in the ZEB1 shRNA-transfected
MDA-MB-231 cells (Fig. 2B). Thus,
the results indicated that ZEB1 shRNA inhibited the formation of
VM.
ZEB1 shRNA inhibits the EMT of
MDA-MB-231 cells
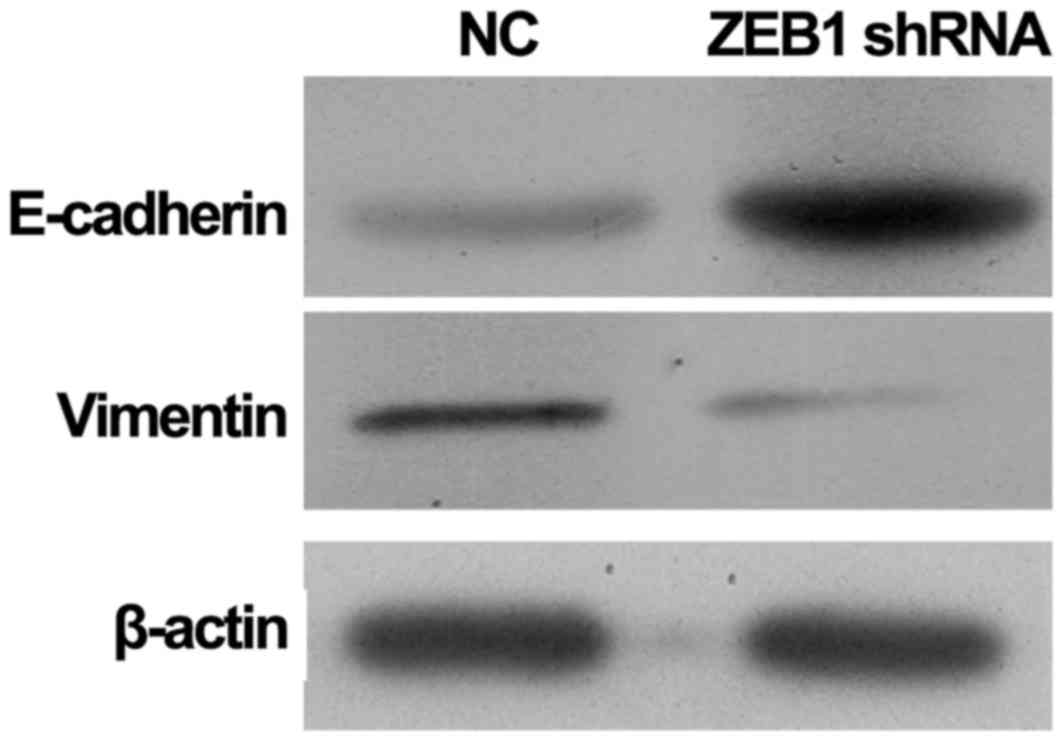
To investigate the differences in EMT in ZEB1
shRNA-transfected MDA-MB-231 cells, the present study further
detected the protein expression of E-cadherin and vimentin
(Fig. 3). The results revealed
that knockdown of ZEB1 using ZEB1 shRNA markedly inhibited the
expression of vimentin in MDA-MB-231 cells when compared with NC
cells. By contrast, the protein expression of E-cadherin in ZEB1
shRNA-transfected MDA-MB-231 cells was markedly increased when
compared with NC cells. The upregulation of E-cadherin and
downregulation of vimentin suggested that ZEB1 shRNA may inhibit
the EMT of MDA-MB-231 cells.
ZEB1 shRNA inhibits the invasion of
MDA-MB-231 cells
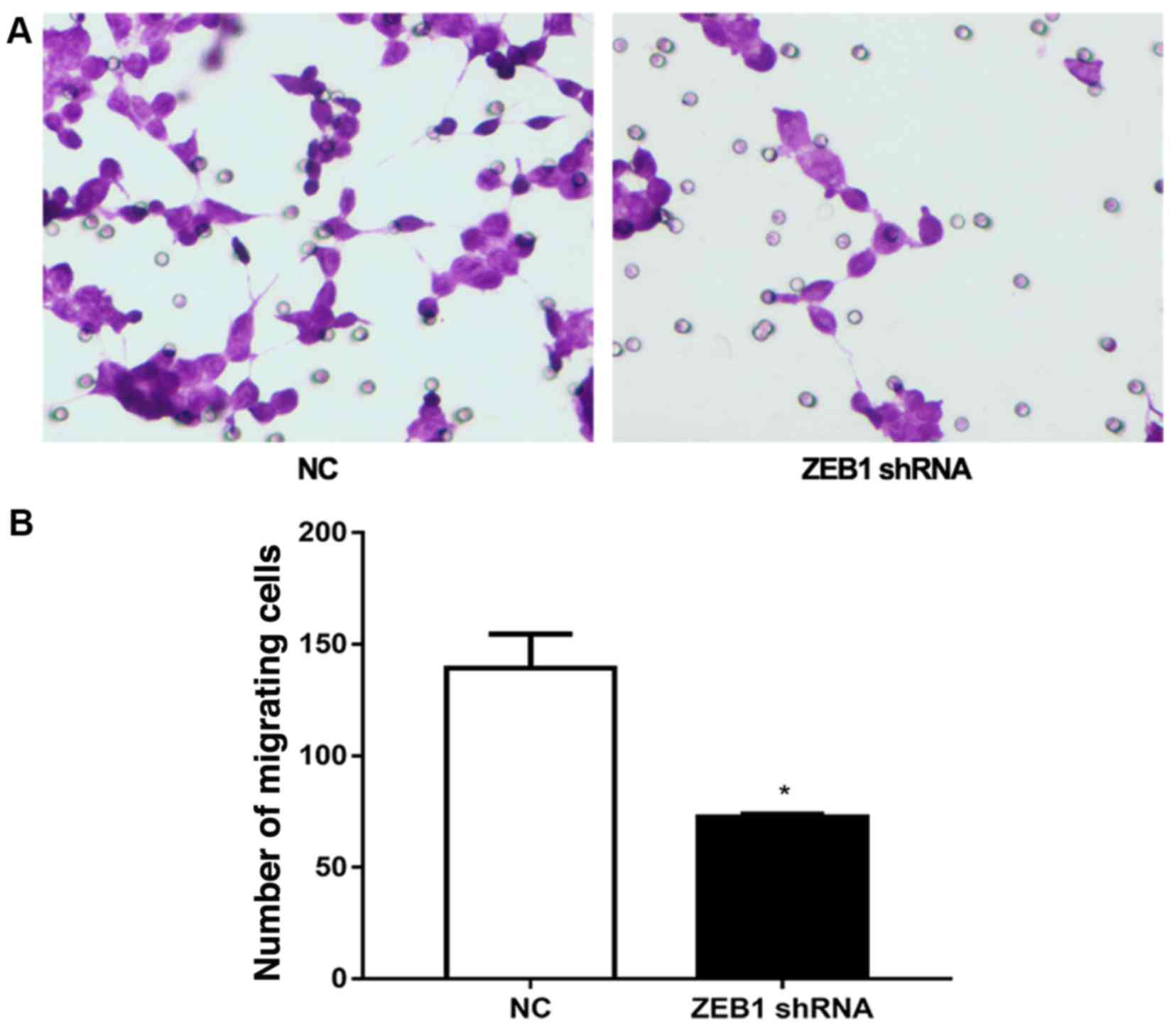
The role of ZEB1 shRNA in the metastasis of
MDA-MB-231 cells was also investigated via a Transwell assay
(Fig. 4). In cells transfected
with vector, 142±12 cells migrated. However, in cells transfected
with ZEB1 shRNA, the number of migrated cells was significantly
reduced to 75±3 cells (Fig. 4).
This result further confirmed the potential role of ZEB1 in EMT,
and also demonstrated that knockdown of ZEB1 significantly
inhibited the invasion of MDA-MB-231 cells, indicating an important
role of ZEB1 in metastasis.
ZEB1 shRNA inhibits the formation of
VM via the downregulation of flk-1
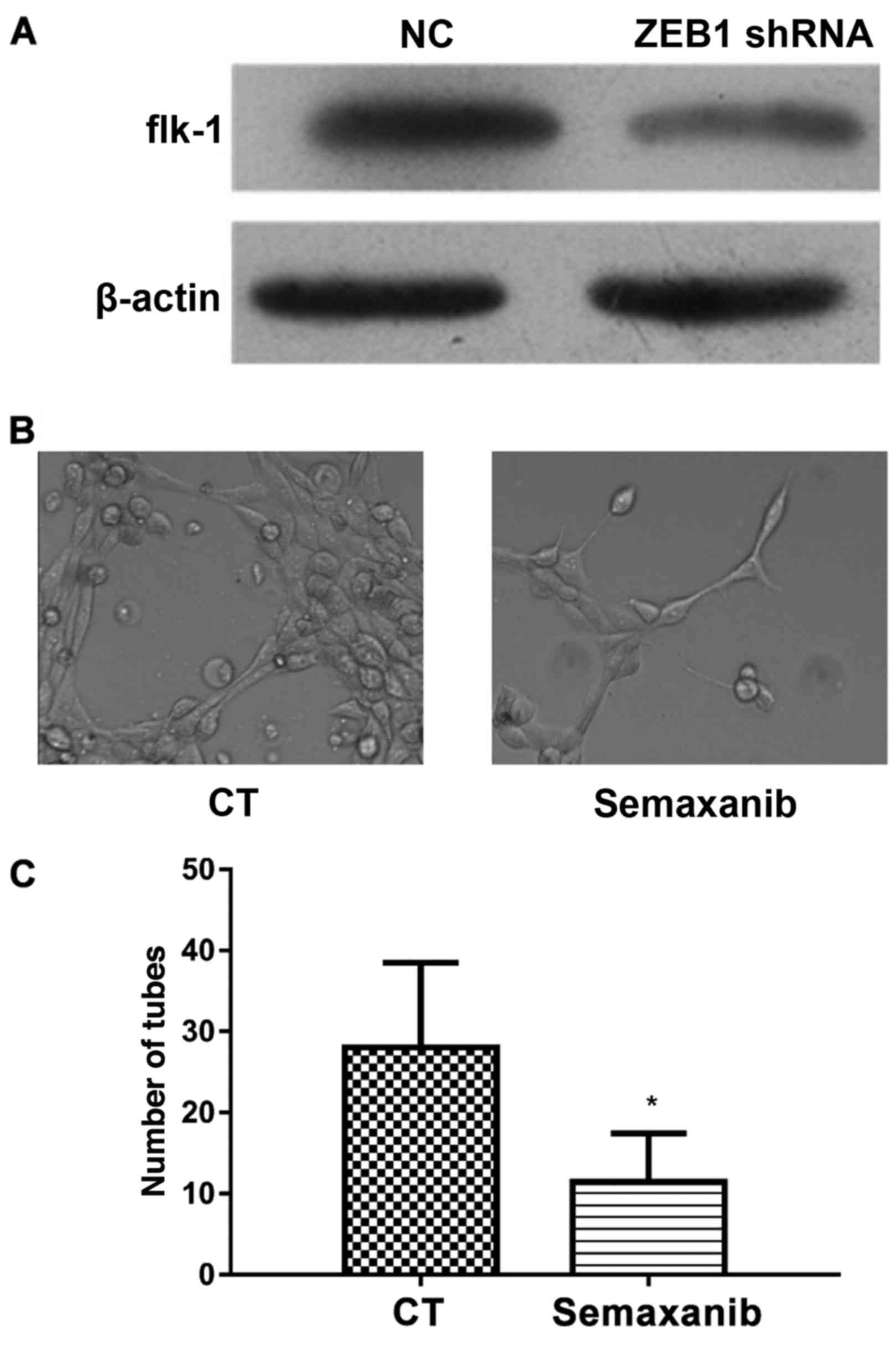
The expression of flk-1 in ZEB1 shRNA transfected
MDA-MB-231 cells and its role in the formation of VM were further
investigated (Fig. 5). The results
of western blot analysis revealed that ZEB1 shRNA markedly
inhibited the protein expression of flk-1 when compared with the
cells transfected with vector (Fig.
5A). To further inhibit flk-1 expression, MDA-MB-231 cells were
preincubated with 1 µM Semaxanib for 1 h (27). Then, the formation of VM was
observed and the results indicated that there was a marked
reduction in VM formation in cells treated with Semaxanib (Fig. 5B). As shown in Fig. 5C, the number of tubes formed was
significantly inhibited by Semaxanib pretreatment. Thus, inhibition
of flk-1 protein in MDA-MB-231 cells inhibited the formation of VM.
Taken together, these results suggested that ZEB1 shRNA may have
inhibited the formation of VM via the downregulation of flk-1.
Discussion
The present study determined whether decreasing the
expression of ZEB1 can inhibit the formation of VM in TNBC, and
also evaluated its specific function and molecular mechanism. The
results demonstrated that ZEB1 shRNA inhibited the formation of VM,
the expression of vimentin and flk-1, and cell invasion abilities;
however, it promoted the expression of E-cadherin. In addition, the
flk-1 inhibitor Semaxanib suppressed the formation of VM. Thus,
knockdown of ZEB1 inhibited the EMT and may have suppressed the
formation of VM in the human breast cancer cell line via the
downregulation of flk-1. Therefore, ZEB1 may serve important roles
in metastasis and the formation of VM in human breast cancer.
Whether VM lumen structure can be formed in
three-dimensional culture is often used to identify whether tumor
cells can form VM in vitro. Three-dimensional culture based
on Matrigel is a type of in vitro cell culture technique
(28). During the early stages of
research, studies on the molecular mechanism of VM formation
primarily focused on tumors arising from mesenchymal tissue
(29). In recent years, VM has
also been identified in tumors arising from the epithelium,
including breast and ovarian cancers; however, there are few
studies reporting the molecular mechanism of VM in epithelial tumor
(30). Low differential MDA-MB-231
cells can form a typical lumen structure in vitro (29). The results of the present study
further confirmed that the epithelial tumor cells MDA-MB-231 could
differentiate into the mesenchymal phenotype and form VM canal
structure.
The EMT process is regulated by multiple signaling
pathways and is involved in VM formation. Previous studies have
demonstrated that the transcription factor ZEB1 regulated EMT under
physiological or pathological conditions (31,32).
ZEB1 is located in the short arm of chromosome 10, and is composed
of two Zinc finger structure clusters and a homologous structure
domain. In addition, ZEB1 regulates the expression of E-cadherin
and vimentin at the transcriptional level, and enhances the
metastasis ability of cells (22,23).
In colon cancer samples with VM, Liu et al (24) revealed that ZEB1 and vimentin
expression was upregulated, and E-cadherin expression was
downregulated. Removal of ZEB1 resulted in a reduction in VM and
the recovery of the epithelial cell phenotype, which indicated that
EMT regulated by ZEB1 may serve an important role in VM formation
in colon cancer. The results of the present study demonstrated that
ZEB1 shRNA also inhibited EMT and induced the recovery of the
epithelial cell phenotype in the triple negative subtype of breast
cancer. However, the association between EMT and the formation of
VM requires further investigation.
Flk-1 (also known as VEGFR-2) is an important VEGF
receptor that is primarily expressed in endothelial cells and which
serves an important role in angiogenesis and contributes to the
formation of VM (25). The results
of the present study revealed that ZEB1 shRNA inhibited the
expression of flk-1. To further investigate the role of flk-1 in
the formation of VM, the flk-1 inhibitor Semaxanib was applied to
cells. Inhibition of flk-1 significantly inhibited the formation of
VM. Thus, knockdown of ZEB1 at least partially suppressed the
formation of VM via the downregulation of flk-1.
In addition to EMT and the roles of flk-1, the
differentiation of tumor stem-like cells may be another important
factor that should be further investigated. Cancer stem cells (CSC)
are a small number of cells that possess self-renewal abilities.
Bussolati et al (33)
injected human breast CSCs into SCID mice and revealed that some
vessels in the tumor were human-derived, indicating that the human
breast CSCs were involved angiogenesis. CD133+
spongioblastoma stem-cell-like cells also had the potential for
multi-directional differentiation, and were able to differentiate
into tumor cells and endothelial cells (34–36).
It is worth noting that previous studies have shown that ZEB1 can
maintain self-renewal abilities and has the potential for rapid
responses to differentiation cues from the action of the negative
feedback loop with the microRNA200 family (37–39).
This indicates that the transcription factor ZEB1 may participate
in the regulation of EMT, and may also be associated with
maintaining self-renewal and the potential for rapid responses to
the differentiation cues of tumor cells.
Thus, the formation of VM and the role of ZEB1 are
complex. The present study demonstrated that the knockdown of ZEB1
suppressed the formation of VM in the human breast cancer cell line
MDA-MB-231 via the downregulation of flk-1, and it also inhibited
the process of EMT. It was also revealed that ZEB1 may also promote
VM formation in TNBC by promoting tumor cells to obtain some stem
cell-like features; however, this requires further investigation.
Although MDA-MB-231 cells are a well-established cell model for the
TNBC, the group will consider other cell types in future studies.
The results of the present study further clarify the mechanism of
VM formation and provide theoretical basis for developing novel
targets for TNBC therapy.
Acknowledgements
The authors thank all members of the Department of
Breast Surgery, Qilu Hospital of Shandong University for their
suggestions and critical reading of the manuscript.
Funding
The present study was supported by a grant from the
Shandong Provincial Natural Science Foundation (Shandong, China;
grant. no. ZR2015HM068). The funders had no role in study design,
data collection and analysis, decision to publish, or preparation
of the manuscript.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
HLiu: Conception and design of the experiments,
editing the manuscript and organizing the team. WL, SS and YX:
Performance of the experiments. HLi: Analysis of the data. WL:
Wrote the paper.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cazzaniga ME, Cortesi L, Ferzi A,
Scaltriti L, Cicchiello F, Ciccarese M, Torre SD, Villa F, Giordano
M, Verusio C, et al: Metronomic chemotherapy in triple-negative
metastatic breast cancer: The future is now? Int J Breast Cancer.
2017:16830602017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chalakur-Ramireddy NKR and Pakala SB:
Combined drug therapeutic strategies for the effective treatment of
Triple Negative Breast Cancer. Biosci Rep. 38:pii: BSR20171357.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Omarini C, Guaitoli G, Pipitone S,
Moscetti L, Cortesi L, Cascinu S and Piacentini F: Neoadjuvant
treatments in triple-negative breast cancer patients: Where we are
now and where we are going. Cancer Manag Res. 10:91–103. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rabanal C, Ruiz R, Neciosup S and Gomez H:
Metronomic chemotherapy for non-metastatic triple negative breast
cancer: Selection is the key. World J Clin Oncol. 8:437–446. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reaz S, Tamkus D and Andrechek ER: Using
gene expression data to direct breast cancer therapy: evidence from
a preclinical trial. J Mol Med (Berl). 96:111–117. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sulaiman A and Wang L: Bridging the
divide: Preclinical research discrepancies between triple-negative
breast cancer cell lines and patient tumors. Oncotarget.
8:113269–113281. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horton JK, Jagsi R, Woodward WA and Ho A:
Breast cancer biology: Clinical implications for breast radiation
therapy. Int J Radiat Oncol Biol Phys. 100:23–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartsch R and Bergen E: ASCO 2017:
Highlights in breast cancer. Memo. 10:228–232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pal SK, Childs BH and Pegram M: Triple
negative breast cancer: Unmet medical needs. Breast Cancer Res
Treat. 125:627–636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yin WJ, Lu JS, Di GH, Lin YP, Zhou LH, Liu
GY, Wu J, Shen KW, Han QX, Shen ZZ and Shao ZM: Clinicopathological
features of the triple-negative tumors in Chinese breast cancer
patients. Breast Cancer Res Treat. 115:325–333. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Paez-Ribes M, Allen E, Hudock J, Takeda T,
Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D and Casanovas O:
Antiangiogenic therapy elicits malignant progression of tumors to
increased local invasion and distant metastasis. Cancer Cell.
15:220–231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason
GA, Christensen JG and Kerbel RS: Accelerated metastasis after
short-term treatment with a potent inhibitor of tumor angiogenesis.
Cancer Cell. 15:232–239. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carbone C, Moccia T, Zhu C, Paradiso G,
Budillon A, Chiao PJ, Abbruzzese JL and Melisi D: Anti-VEGF
treatment-resistant pancreatic cancers secrete proinflammatory
factors that contribute to malignant progression by inducing an EMT
cell phenotype. Clin Cancer Res. 17:5822–5832. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shirakawa K, Wakasugi H, Heike Y, Watanabe
I, Yamada S, Saito K and Konishi F: Vasculogenic mimicry and
pseudo-comedo formation in breast cancer. Int J Cancer. 99:821–828.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang JY, Sun T, Zhao XL, Zhang SW, Zhang
DF, Gu Q, Wang XH, Zhao N, Qie S and Sun BC: Functional
significance of VEGF-a in human ovarian carcinoma: Role in
vasculogenic mimicry. Cancer Biol Ther. 7:758–766. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu XM, Zhang QP, Mu YG, Zhang XH, Sai K,
Pang JC, Ng HK and Chen ZP: Clinical significance of vasculogenic
mimicry in human gliomas. J Neurooncol. 105:173–179. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun B, Qie S, Zhang S, Sun T, Zhao X, Gao
S, Ni C, Wang X, Liu Y and Zhang L: Role and mechanism of
vasculogenic mimicry in gastrointestinal stromal tumors. Hum
Pathol. 39:444–451. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun T, Zhao N, Zhao XL, Gu Q, Zhang SW,
Che N, Wang XH, Du J, Liu YX and Sun BC: Expression and functional
significance of Twist1 in hepatocellular carcinoma: Its role in
vasculogenic mimicry. Hepatology. 51:545–556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li M, Gu Y, Zhang Z, Zhang S, Zhang D,
Saleem AF, Zhao X and Sun B: Vasculogenic mimicry: A new prognostic
sign of gastric adenocarcinoma. Pathol Oncol Res. 16:259–266. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gong W, Sun B, Zhao X, Zhang D, Sun J, Liu
T, Gu Q, Dong X, Liu F, Wang Y, et al: Nodal signaling promotes
vasculogenic mimicry formation in breast cancer via the Smad2/3
pathway. Oncotarget. 7:70152–70167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zeng YE, Yao XH, Yan ZP, Liu JX and Liu
XH: Potential signaling pathway involved in
sphingosine-1-phosphate-induced epithelial-mesenchymal transition
in cancer. Oncol Lett. 12:379–382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeng Y, Yao X, Chen L, Yan Z, Liu J, Zhang
Y, Feng T, Wu J and Liu X: Sphingosine-1-phosphate induced
epithelial-mesenchymal transition of hepatocellular carcinoma via
an MMP-7/syndecan-1/TGF-β autocrine loop. Oncotarget.
7:63324–63337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Z, Sun B, Qi L, Li H, Gao J and Leng
X: Zinc finger E-box binding homeobox 1 promotes vasculogenic
mimicry in colorectal cancer through induction of
epithelial-to-mesenchymal transition. Cancer Sci. 103:813–820.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Francescone R, Scully S, Bentley B, Yan W,
Taylor SL, Oh D, Moral L and Shao R: Glioblastoma-derived tumor
cells induce vasculogenic mimicry through Flk-1 protein activation.
J Biol Chem. 287:24821–24831. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li H, Song S, Xu Y, Zhao J and Liu H:
Knockdown of ZEB1 suppresses the formation of vasculogenic mimicry
in breast cancer cell line MDA-MB-231 through downregulation of
Flk-1. Minerva Med. 108:191–193. 2017.PubMed/NCBI
|
|
28
|
Wang H, Lin H, Pan J, Mo C, Zhang F, Huang
B, Wang Z, Chen X, Zhuang J, Wang D and Qiu S: Vasculogenic mimicry
in prostate cancer: The roles of EphA2 and PI3K. J Cancer.
7:1114–1124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Timoshenko AV, Kaltner H, Andrè S, Gabius
HJ and Lala PK: Differential stimulation of VEGF-C production by
adhesion/growth-regulatory galectins and plant lectins in human
breast cancer cells. Anticancer Res. 30:4829–4833. 2010.PubMed/NCBI
|
|
30
|
Yao L, Zhang D, Zhao X, Sun B, Liu Y, Gu
Q, Zhang Y, Zhao X, Che N, Zheng Y, et al: Dickkopf-1-promoted
vasculogenic mimicry in non-small cell lung cancer is associated
with EMT and development of a cancer stem-like cell phenotype. J
Cell Mol Med. 20:1673–1685. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li S, Zhang HY, Du ZX, Li C, An MX, Zong
ZH, Liu BQ and Wang HQ: Induction of epithelial-mesenchymal
transition (EMT) by Beclin 1 knockdown via posttranscriptional
upregulation of ZEB1 in thyroid cancer cells. Oncotarget.
7:70364–70377. 2016.PubMed/NCBI
|
|
32
|
Gao HX, Yan L, Li C, Zhao LM and Liu W:
miR-200c regulates crizotinib-resistant ALK-positive lung cancer
cells by reversing epithelial-mesenchymal transition via targeting
ZEB1. Mol Med Rep. 14:4135–4143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bussolati B, Grange C, Sapino A and
Camussi G: Endothelial cell differentiation of human breast tumour
stem/progenitor cells. J Cell Mol Med. 13:309–319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chroscinski D, Sampey D and Maherali N:
Reproducibility Project; Cancer Biology: Registered report: Tumour
vascularization via endothelial differentiation of glioblastoma
stem-like cells. Elife. 4:2015.doi: 10.7554/eLife.04363. View Article : Google Scholar
|
|
35
|
Ricci-Vitiani L, Pallini R, Biffoni M,
Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G,
Larocca LM and De Maria R: Tumour vascularization via endothelial
differentiation of glioblastoma stem-like cells. Nature.
468:824–828. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fan YL, Zheng M, Tang YL and Liang XH: A
new perspective of vasculogenic mimicry: EMT and cancer stem cells
(Review). Oncol Lett. 6:1174–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brabletz S and Brabletz T: The ZEB/miR-200
feedback loop-a motor of cellular plasticity in development and
cancer? EMBO Rep. 11:670–677. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wellner U, Schubert J, Burk UC,
Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D,
zur Hausen A, et al: The EMT-activator ZEB1 promotes tumorigenicity
by repressing stemness-inhibiting microRNAs. Nat Cell Biol.
11:1487–1495. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Radisky DC: miR-200c at the nexus of
epithelial-mesenchymal transition, resistance to apoptosis, and the
breast cancer stem cell phenotype. Breast Cancer Res. 13:1102011.
View Article : Google Scholar : PubMed/NCBI
|