Introduction
Asthma is a chronic allergic lung disease and
seizures are caused by the interaction of the environmental factors
and a poor physical state. In the long run, severe irreversible
structural airway alterations with a lack of responsiveness to
treatment are frequently observed (1). Smooth muscle hypertrophy and
hyperplasia are features of airway remodeling, which significantly
contribute to the decline of lung function and frequent episodes of
asthma attacks (2). Increasing
levels of cytoskeletal proteins, inflammatory cytokines, enzymes,
receptors and adhesion molecules have been reported to be
associated with complex pathophysiology of asthma, including the
myosin light chain kinase (MLCK) (3–6).
Almost all eukaryotes produce MLCK, which is a
Ca2+/calmodulin-dependent protein kinase (CaMK) with a
catalytic core and autoregulatory segments in the C-terminus. MLCK
has a variety of different isoforms, the two major types of which
are smooth-muscle MLCK (130–150 kDa) and nonmuscle MLCK (210–220
kDa), which are emanated from the same gene (7–9). The
phosphorylation of MLC has an important role in airway smooth
muscle contraction and relaxation (10,11).
It also promotes airway inflammation and airway remodeling by
activating airway smooth muscle, fibroblasts and myoblasts, which
subsequently secrete cytokines, chemokines and extracellular matrix
(12). Previous studies have
demonstrated that MLCK regulates numerous biological functions
through up-regulation of NADPH oxidase, tumor necrosis factor
receptor 2 signaling and notch signaling (13,14).
The signaling effect of MLCK in chronic asthma has been reported by
several studies, including the regulation of the inflammatory
response and vascular permeability (15). The mechanism of MLCK in smooth
muscle cells and the immune regulation of T cells is complex,
inducing a variety of cytokines in the occurrence and development
of disease (16,17). (5-Iodonaphthalene-1-sulfonyl)
homopiperazine (ML-7), a membrane-permeable agent, is customarily
used as an MLCK inhibitor (18–20).
This inhibitor combines with the catalytic perssad of the MLCK and
then decreases the activity of the enzyme and is frequently applied
in animal and cytological experiments (21,22).
In asthma, the imbalance of the proportion of
T-helper type 1 (Th1) to Th2 cells activates the CD4+
Th2 cell immune response and the release of interleukin (IL)-13,
−25, −5, −4 and −33, prompting the transformation of B cells into
immunoglobulin (Ig)E-secreting cells (23,24).
Among these ILs, IL-25 and −33 are known as vital pro-inflammatory
mediators that induce the release of Th2-associated cytokines,
including IL-5, IL-4 and IL-13, which elevate serum IgE, as well as
airway hyperresponsiveness, remodeling and mucus hypersecretion
(25–28). However, in asthma, little is known
regarding the correlation of MLCK with Th2 cytokines.
Based on the above, the present study hypothesized
that MLCK accelerates airway remodeling through the induction of
Th2 cytokines, which may be one of the mechanisms underlying the
pathogenesis of asthma.
Materials and methods
Reagents and instruments
Anti-MLCK monoclonal antibody (cat. no. ab34829),
anti- α-SMA monoclonal antibody (mouse; cat. no. ab62736) and
anti-collagen-I monoclonal antibody (mouse; cat. no. ab48262) were
provided by Abcam (Cambridge, UK), while goat monoclonal GAPDH
antibody (cat. no. AG019-1) was from Bioworld Technology Inc. (St
Louis Park, MN, USA). Horseradish peroxidase-conjugated secondary
antibody (goat anti-rabbit cat. no. ZB-2301) were purchased from
Zhongshan Jinqiao Biotechnology Co., Ltd. (Beijing, China). ML-7
and ovalbumin (OVA) were obtained from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). IL-4 (cat. no. ZC-23216), IL-5 (cat. no.
ZC-23228), IL-13 (cat. no. ZC-23211), IL-25 (cat. no. ZC-23312) and
IL-33 (cat. no. ZC-23142) ELISA kits were from Proteintech Group,
Inc. (Chicago, IL, USA). Polyvinylidene fluoride (PVDF) membranes
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA). Enhanced chemiluminescence (ECL) reagents were obtained from
Solarbio Science & Technology Co., Ltd. (Beijing, China). The
BX51T light microscope and High-Speed Centrifuge PIC017 were
respectively provided by Olympus Corp. (Tokyo, Japan) and Heraeus
Corp. (Berlin, Germany).
Animal experiment
All animal experiments and surgical procedures were
approved by the Institutional Animal Care and Use Committee of
Shandong University (Shangdong China). A total of 45 healthy female
BABL/c mice (weight, 20–28 g; age, 6–8 weeks) were obtained from
the Animal Centre of Shandong University. The animals were bred in
a temperature- and humidity-controlled room, and given ample food
and tap water for the duration of the experimental session. Mice
were allowed a week to adapt to the environment prior. The mice
were randomly divided into three groups: The control group (PBS
treatment), the OVA group (OVA challenge+PBS treatment) and the
OVA+ML-7 group (OVA challenge+ML-7 treatment). The asthmatic models
were established by challenge with OVA. Mice in the OVA and
OVA+ML-7 groups were sensitized on days 1, 8 and 15 by
intraperitoneal injection with 100 µg OVA adsorbed to 1 mg aluminum
hydroxide. After the last sensitization, the mice were treated with
1% OVA aerosol inhalation for up to 30 min per day for 7
consecutive days. The mice in the control group were exposed to an
equivalent amount of PBS instead. In the OVA+ML-7 group, mice were
given a daily intraperitoneal injection of ML-7 (0.5 mg/kg in 0.5
ml PBS) prior to OVA inhalation challenge for the next 7 days. All
of the mice were euthanized at 24 h after the final challenge.
Bronchoalveolar lavage fluid (BALF)
analysis
After the mice were euthanized, collection of BALF
was performed immediately with isotonic sterile PBS lavage for four
times (0.5 ml each time). From each mouse, 2 ml BALF was collected
separately. The cellular influx in the BALF was examined by using a
cell counting plate. After centrifugation at 3,000 × g for 8 min at
37°C. After removing the supernatant, the precipitation cells were
evenly spread on a slide and stained with 0.5 ml Wright-Giemsa
reagent, which contains 0.5 g Wright pigment, 0.5 g giemsa pigment
and 500 ml methanol for 1 min at 37°C. Cells were identified based
on morphological features and counted in randomly selected areas of
the slide using light microscopy. The levels of IL-4, IL-5, IL-13,
IL-25 and IL-33 were measured in the supernatant of BALF using
ELISA kits.
Tissue samples
After the last challenge, the right lungs were
removed from the chest cavity and immersed in 10% neutral buffered
formalin at 25°C for 24 h. Lung samples were then embedded in
paraffin and sectioned by using a microtome (5-µm). After dewaxing
in xylene, rehydrating in graded ethanol, the sections were stained
with haematoxylin at 25°C for 3 min and eosin at 25°C for 30 sec to
assess the infiltration of inflammatory cells. All images were
assessed at a magnification of ×20.
ELISA
The left eye of the mice was removed for blood
collection. After low-temperature centrifugation at 3,000 × g at
4°C for 8 min, the serum was obtained to determine the levels of
OVA-specific (OVA-s) IgE using an ELISA kit (cat. no. YP-45821)
purchased from Zhongshan Jinqiao Biotechnology Co., Ltd., within 24
h after final challenge. The levels of IL-4, −5, −13, −25 and −33
in the supernatant of BALF were assayed with ELISA kits according
to the manufacturer's protocols.
Western blot analysis
The left lung tissue (10 mg) was minced and lysed in
ice-cold radioimmunoprecipitation buffer containing1 mM
phenylmethanesulfonyl fluoride, 1.5 M NaCl, 10% NP-40, 10 mM EDTA,
0.5 M Tris-HCl, (pH 7.4), 2.5% deoxycholic acid, and protease
inhibitors (1:100 dilution) and protease inhibitors in order to
isolate total protein, which was used to analyze the content of
α-SMA and collagen-I. A total of 100 µg protein was suspended in 5X
reducing sample buffer, followed by boiling for 3 min, and the
proteins were centrifuged at 15,000 × g for 30 min at 4°C to obtain
the supernatant for western blot analysis. The concentration of
total protein was assessed by BCA protein assay, 10 µl protein
loaded per lane for separation by 8% SDS-PAGE acrylamide gel and
subsequent transfer to a polyvinylidene difluoride membrane (Thermo
Fisher Scientific, Inc.). After a blocking at 37°C for 2 h using 5%
non-fat dried milk, and the membrane was incubated with rabbit
polyclonal α-SMA (1:1,000 dilution in TBST), collagen-I (1:500
dilution in TBST) and GAPDH antibody (1:3,000 dilution) at 4°C
overnight. After washing with Tris-buffered saline containing
Tween-20, (TBST; pH 8.0) three times, the membranes were incubated
with horseradish peroxidase-labeled goat anti-rabbit IgG as the
secondary antibody (1:6,000 dilution in TBST) at room temperature
for 2 h. The ECL method (ECL kit; Solarbio Science &
Technology, Beijing, China) was used to develop the bands, and the
gray value was determined with Image-Pro Plus 7.0 image analysis
software (Media Cybernetics, Rockville, MD, USA). The expression
levels of α-SMA and collagen-I were normalized to those of
GAPDH.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was used to analyze the transcript levels of
MLCK, α-SMA and collagen-I. The total RNA was isolated from the
lung tissues using TRIzol reagent based on the manufacturer's
protocol. Then RNA samples were treated with DNase I (Takara Bio
Inc., Otsu, Japan) for 30 min at 37°C to remove genomic DNA
contamination. Thereafter, complementary (c)DNA was generated via
RT reaction by using a PrimeScript first-strand cDNA synthesis kit
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
following the manufacturer's protocol. PCR was performed with a
Bio-Rad CFX96 Touch q-PCR system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The reaction mixture contained primers [1.0 µl
of a 10 µmol/l stock including forward and reverse (Table I)], 0.15 µl 1 µg/µl Taq DNA
polymerase, 5.0 µl 5X PCR buffer, 5.0 µl of a 1 µg/µl solution of
cDNA template and 13.85 µl 0.1% diethylpyrocarbonate-treated water.
The PCR process is as follows: Initial denaturation at 95°C for 60
sec, followed by 40 cycles of denaturation at 95°C for 5 sec,
annealing at 65°C for 30 sec, and extension at 72°C for 120 sec.
Fluorescence signals were collected at each annealing and
elongation step. The relative transcriptional levels were finally
calculated from quantification cycle values using the
2−ΔΔCq method (29).
 | Table I.Sequences of primers used for
polymerase chain reaction. |
Table I.
Sequences of primers used for
polymerase chain reaction.
| Gene | Direction | Primer | Product length
(bp) |
|---|
| MLCK | Forward |
5′-ACATCCGTCAGGAGATCAG-3 | 172 |
|
| Reverse |
5′-CACTCCGCTCTGTTAGCTC-3′ |
|
| α-SMA | Forward |
5′-CTGTCCCTCTATGCCTCTGG-3′ | 542 |
|
| Reverse |
5′-AGGGCTGTGATCTCCTTCTG-3′ |
|
| Collagen-I | Forward |
5′-TAAAGGGTCATCGTGGCTTC-3′ | 598 |
|
| Reverse |
5′-GACGGCTGAGTAGGGAACAC-3′ |
|
| GAPDH | Forward |
5′-AACTTTGGCATTGTGGAAGG-3′ | 391 |
|
| Reverse |
5′-CATCGAAGGTGGAAGAGTGG-3′ |
|
Statistical analysis
All statistical analyses were performed using SPSS
version 17.0 (SPSS, Inc., Chicago, IL, USA). Values are expressed
as the mean ± standard deviation. The levels of cytokines and the
quantity of MLCK were analyzed by one-way analysis of variance
followed by Dunnett's test. Differences between groups were
considered statistically significant at P<0.05. All experiments
were repeated three times unless otherwise stated.
Results
ML-7 reduces airway inflammation and
accumulation of inflammatory cells in a murine model of asthma
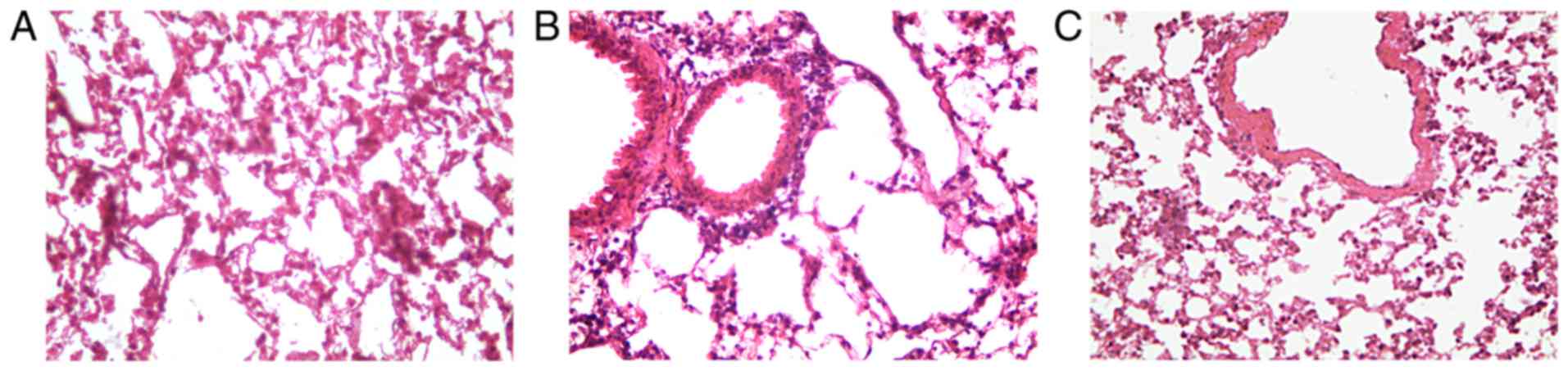
The murine model of airway inflammation was
established through repetitive OVA sensitization. Thereby, an
asthmatic phenotype similar to that observed in human asthma was
established. Histological analysis of H&E-stained lung tissue
indicated that OVA induced inflammatory cell infiltration and
pathological transformation in the lung tissues. However, treatment
of OVA-challenged mice with ML-7 significantly alleviated the
degree of tissue inflammation and infiltration. Furthermore, the
pathological changes were milder (Fig.
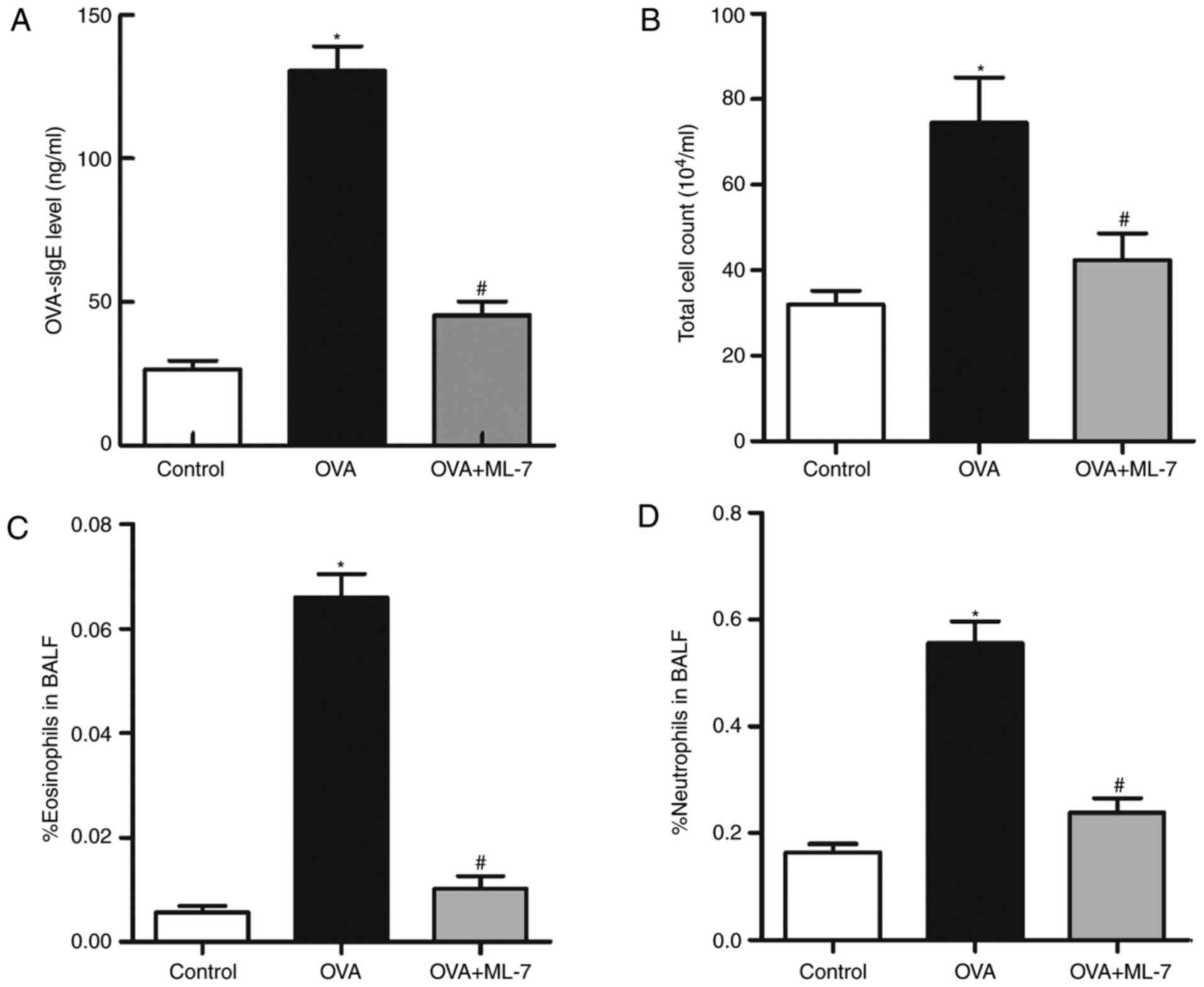
1). The serum levels of OVA-s IgE in the OVA group were
significantly higher compared with those in the control group
(131.46±10.72 vs. 25.37±4.89 ng/ml; P=0.004; Fig. 2A). Based on the histological and
serological analysis, it was determined that the model was
successfully established. The total number of inflammatory cells,
including neutrophils, eosinophils, macrophages and lymphocytes in
the BALF, are hallmarks of inflammation in the mice at the cellular
level. Particularly eosinophils are generally considered the
hallmark of the onset of asthma. Compared with the control group,
the total number of cells and eosinophils increased in the OVA
group (P<0.001 for each comparison; Fig. 2B and C). Of note, the percentage of
eosinophils in the OVA+ML-7 group was significantly decreased
compared with that in the OVA group (0.012±0.007 vs. 0.068±0.01;
P=0.0075; Fig. 2C). The total
number of cells and the percentage of neutrophils in the OVA + ML-7
group was also decreased compared with that in the OVA group
(Fig. 2B and D). This partly
reflected that ML-7 inhibited the accumulation of eosinophils in
asthma-like airway inflammation.
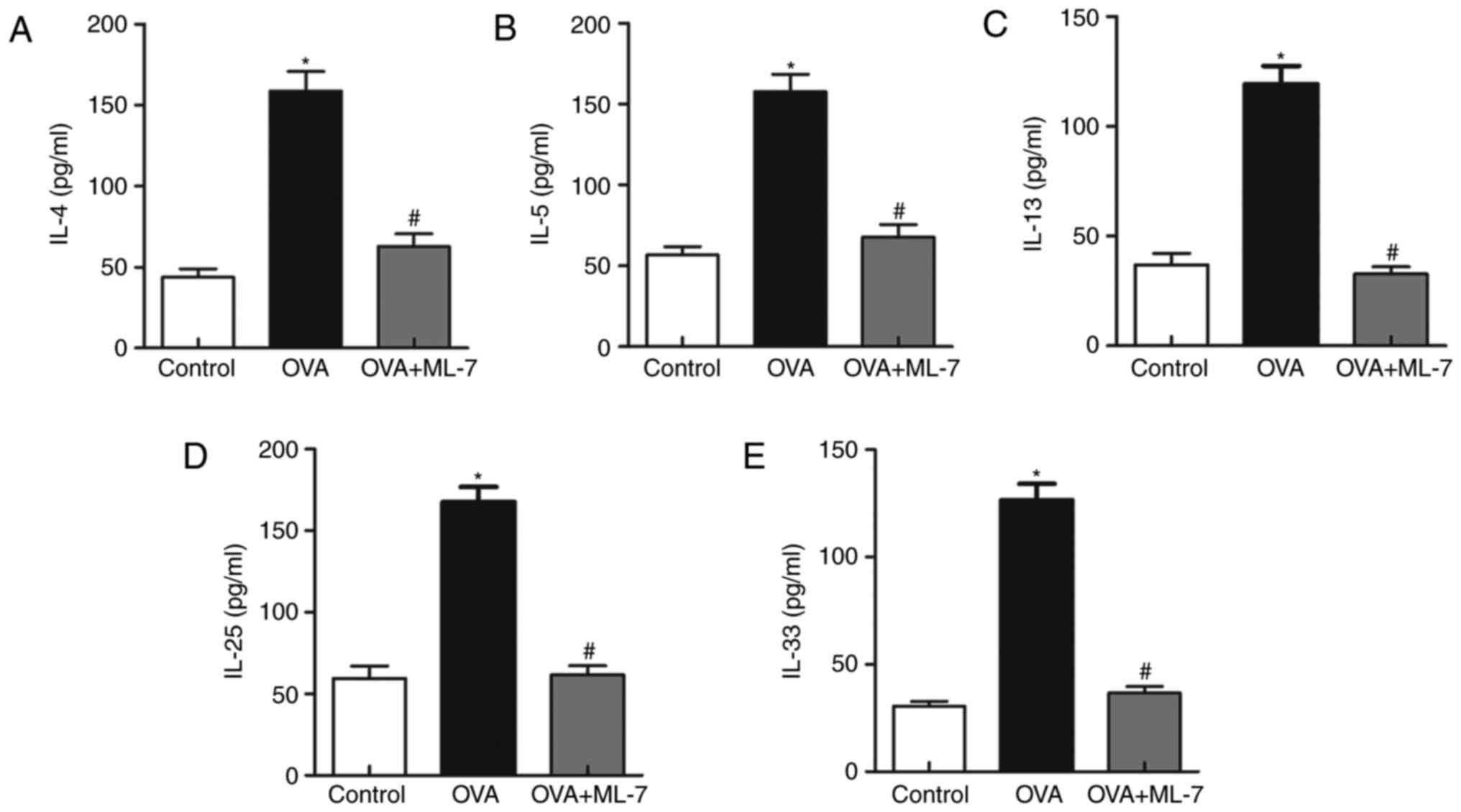
ML-7 decreases the production of
Th2-associated cytokines in the OVA-induced asthma model
MLCK has been reported to be a key mediator that
promotes the production of other cytokines. To investigate the
correlation among the mediators (IL-4, −5, −13, −25 and −33), their
levels in the BALF were assessed. The levels of Th2 cytokines in
the OVA group were significantly increased compared with those in
the control group (P<0.05 for each comparison; Fig. 3). However, treatment with ML-7
attenuated the OVA-induced increases in the cytokines (P<0.05
for each comparison; Fig. 3). This
proved that administration of ML-7 inhibited Th2-associated
inflammatory cytokine release.
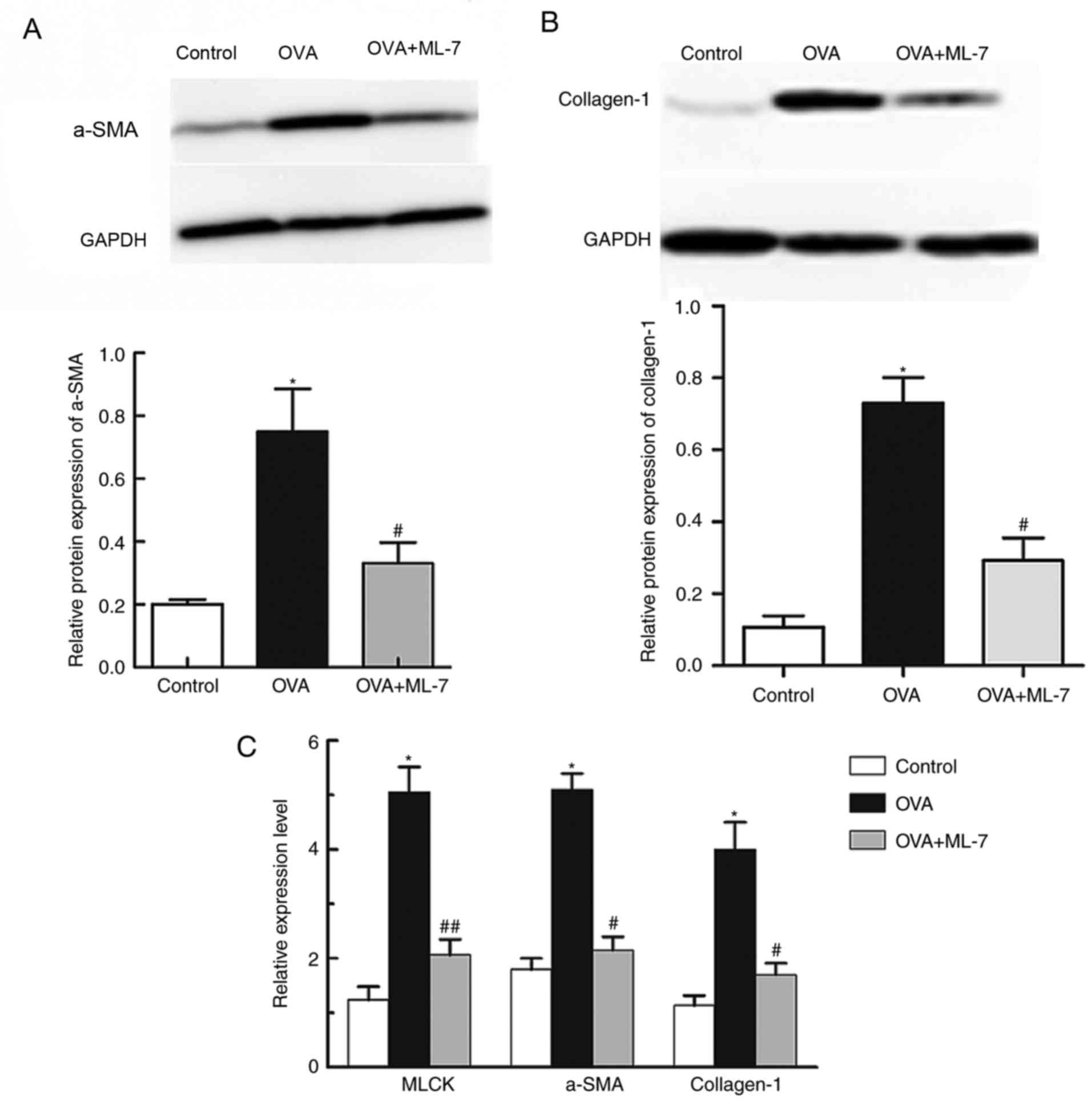
ML-7 attenuates airway remodeling in a
mouse model of asthma
In asthmatic airway remodeling, α-SMA and collagen-I
are significant pathogenic factors. The level of α-SMA and
collagen-I in the lung tissue of mice in the OVA+ML-7 group was
significantly reduced compared with that in the OVA group as
demonstrated by western blot analysis (P<0.001 for each
comparison; Fig. 4A and B). The
changes in α-SMA and collagen-I expression levels were further
confirmed by RT-qPCR analysis of lung tissue. The expression of
mRNA of α-SMA and collagen-I in asthmatic mice was significantly
higher than that in the control group (P<0.05 for each
comparison; Fig. 4). In addition,
the mRNA expression of α-SMA and collagen-I in the OVA+ML-7 group
was significantly lower than that in the OVA group (P<0.05 for
each comparison; Fig. 4). The
above results demonstrated that inhibition of MLCK markedly
inhibited airway remodeling in the OVA-induced asthma model.
ML-7 treatment completely inhibits
OVA-induced upregulation of MLCK
MLCK has a key role in the regulation of smooth
muscle contraction, and is mainly distributed in the bronchus where
it stimulates smooth muscle cells. The expression of MLCK in the
lung tissue of mice was examined using RT-qPCR. The results
revealed that the expression of MLCK mRNA in mice in the OVA group
was significantly higher than that in the control and OVA+ML-7
groups (5.05±0.72 vs. 1.21±0.37; P=0.001; Fig. 4C). Compared with that in the
control group, the expression of MLCK in the OVA+ML-7 group was not
significantly different (1.91±0.52 vs. 1.21±0.37; P=0.95; Fig. 4C), indicating that ML-7 treatment
completely inhibited OVA-induced upregulation of MLCK.
Discussion
Previous studies indicated that MLCK is involved in
a variety of T-cell immune responses in various diseases and has a
central role in asthmatic inflammation. The level of MLCK was
reported to be correlated with the severity and susceptibility to
asthma (15). The results of the
present study demonstrated that ML-7 inhibited airway inflammation
and remodeling in an OVA-induced mouse model of asthma. First, it
was revealed that the expression of MLCK was significantly higher
in OVA-challenged mice compared with that in control mice.
Furthermore, the downregulation of MLCK by ML-7 had a directly
effect on the expression and secretion of α-SMA and collagen-I,
which are indicators of airway remodeling (30).
Asthma, which is a chronic airway inflammatory
disease, occurs with a predominant Th2 immunity and the imbalance
of Th1/Th2 is the immune pathological basis of chronic asthma. MLCK
is regarded as a cytoskeletal effector, which is the key
pathophysiologic feature of asthma, including the regulation of the
inflammatory response, vascular permeability and smooth muscle
proliferation (31). In the
present study, the level of MLCK in the lungs of chronic asthma
model mice was elevated compared with that in the control group,
and the inhibition of MLCK activity by ML-7 significantly reduced
inflammatory cell infiltration. Inflammatory cytokines and
mediators are released by gathering inflammatory cells in the
airway, which may lead to mucus hypersecretion, increases in
bronchial hyperresponsiveness and airway reconstitution (32). Numerous studies have demonstrated
that the accumulation of IL-25 and −33 in asthma may stimulate the
production of IL-4, −5 and −13 via innate immune cells or other
pathways, and that anti-IL-25 or anti-IL-33 significantly reduced
the levels of IL-4, −5 and −13 (27,33).
In the present study, inhibition of MLCK activity by ML-7 also
decreased the content of IL-25 and −33. This series of links
revealed a positive correlation between MLCK and Th2 cytokines,
implying that the expression of MLCK is involved in a portion of
asthmatic immune responses, accelerating airway inflammation and
lung remodeling.
Clinically, airway remodeling causes a progressive
and irreversible loss of lung function, but the pathogenesis has
remained to be fully elucidated. The interaction of cytokines in
the airway leads to bronchoconstriction, which is thought to
gradually contribute to airway remodeling, including airway smooth
muscle hyperplasia, hypertrophy and goblet cell multiplication
(34). Among these ILs, IL-4
activates mast cells and basophils, which are involved in
triggering asthma by developing effector T cell responses,
eosinophil chemotaxis and IgE accumulation. IL-4 may modify the
differentiation of undifferentiated Th0 cells into Th2 cells, as
well as the subsequent production of cytokines and mediators
implicated in airway inflammation and obstruction (35). IL-5 is a key mediator that
participates in terminal differentiation and maturation of
eosinophils, and prolongs the survival of the cells in allergic
tissues. It may augment Ig conglutination by binding to the IL-5
receptor and promote the growth of B cells (36). IL-13 enhances mucus production,
induces goblet cell differentiation and promotes IgE synthesis
(37). IL-25 and −33 are
Th2-associated cytokines, which may accelerate airway remodeling
via numerous mechanisms. IL-25 induces airway remodeling via
complex mechanisms involving the enhancement of the expression of a
series of mediators, including connective tissue growth factor and
transforming growth factor, and subsequent increases in
extracellular matrix proteins, including fibronectin and
collagen-I, -III and -V (38).
IL-33 exacerbates airway remodeling by activating human lung
fibroblasts, which leads to upregulation of collagen-I in asthma
(39). In the present study, the
levels of Th2 cytokines were elevated in asthmatic model mice,
which was inhibited by treatment with ML-7. In addition, the
expression of α-SMA and collagen-I, also exhibited such a trend.
All of the present results suggested that ML-7, a specific
inhibitor of MLCK activity, was able to attenuate asthma-associated
airway inflammation and remodeling to a certain extent by
regulating the secretion of Th2-associated cellular
immunomodulatory factors which are vital in eosinophil
accumulation, mucus production, airway hyperresponsiveness and are
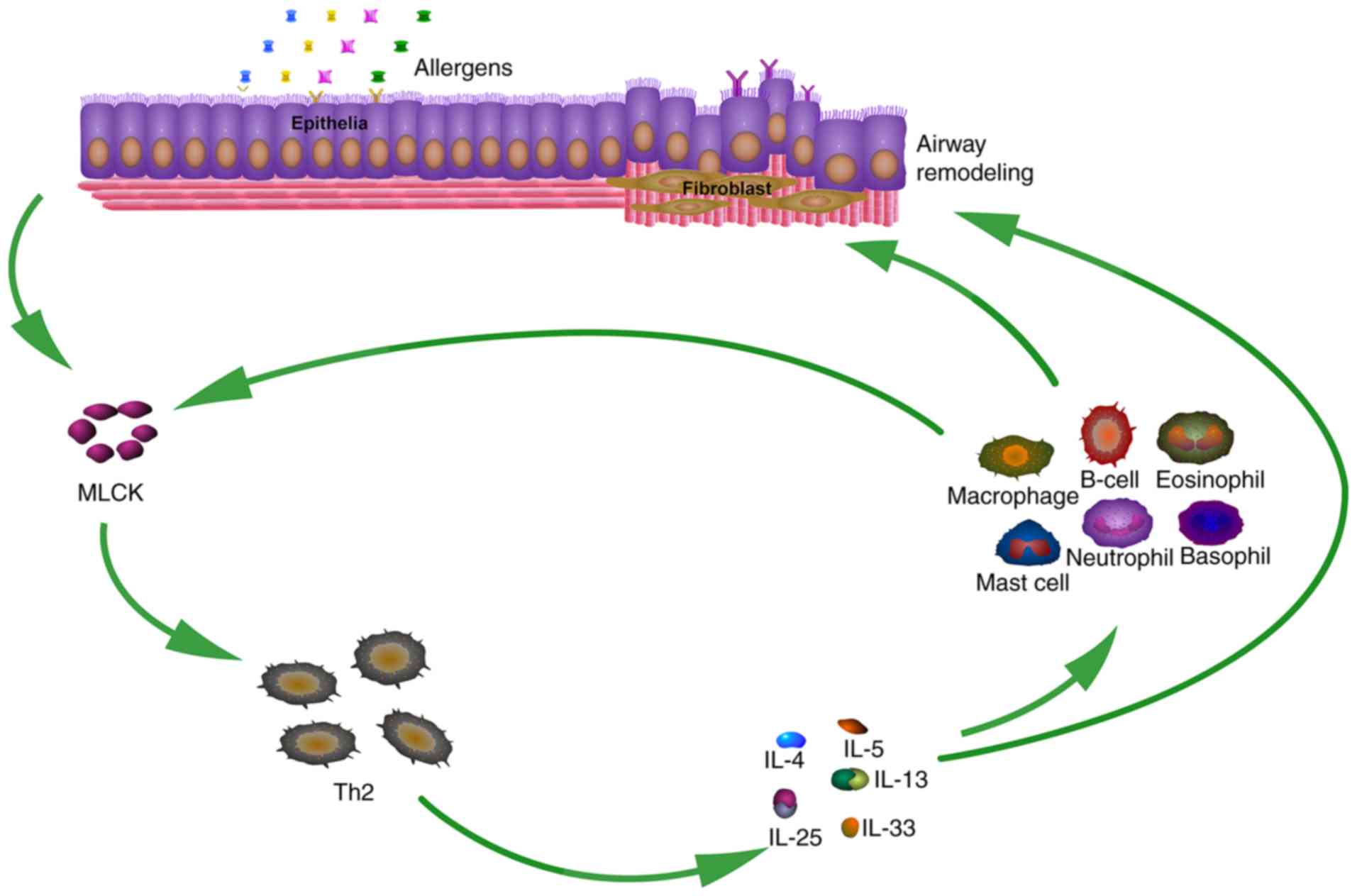
key stimulants in IgE synthesis by B cells. (Fig. 5).
Taken together, the present study revealed that MLCK
affected the development of OVA-induced airway inflammation and
remodeling in a mouse model by promoting the release of the Th2
cytokines IL-4, −5, −13, −25 and −33. Treatment with the MLCK
inhibitor ML-7 exerted protective effects against allergic airway
inflammation and remodeling in lung tissues, suggesting that it is
a potential therapeutic candidate for the treatment of asthma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural and
Science Foundation of Shandong Province, China (grant no.
ZR2014HL003).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
CH, ZZ and CZ conceived and designed the
experiments. CH, ZZ, LW and XG performed the experiments. CH, ZZ
and JL analyzed the data. LW, JL, XG and CZ contributed reagents,
materials and analysis tools. CH, ZZ and CZ wrote the paper.
Ethics approval and consent to
participate
All animal experiments and surgical procedures were
approved by the Institutional Animal Care and Use Committee of
Shandong University (Shangdong China).
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Yang ZC, Yi MJ, Shan YC, Wang C, Ran N,
Jin LY, Fu P, Feng XY, Xu L and Qu ZH: Targeted inhibition of Six1
attenuates allergic airway inflammation and remodeling in asthmatic
mice. Biomed Pharmacother. 84:1820–1825. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tabeling C, Herbert J, Hocke AC, Lamb DJ,
Wollin SL, Erb KJ, Boiarina E, Movassagh H, Scheffel J, Doehn JM,
et al: Spleen tyrosine kinase inhibition blocks airway constriction
and protects from Th2-induced airway inflammation and remodeling.
Allergy. 72:1061–1072. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jang YH, Choi JK, Jin M, Choi YA, Ryoo ZY,
Lee HS, Park PH, Kim SU, Kwon TK, Jang MH, et al: House dust mite
increases pro-Th2 cytokines, IL-25 and IL-33 via the activation of
TLR1/6 signaling. J Invest Dermatol. 137:2354–2361. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ujino M, Sugimoto N, Koizumi Y, Ro S,
Kojima Y, Asae KH, Yamashita N, Ohta K and Nagase H: Leukotriene
receptor antagonist attenuated airway inflammation and
hyperresponsiveness in a double-stranded RNA-induced asthma
exacerbation model. Allergol Int. 66S:S21–S26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khapchaev AY and Shirinsky VP: Myosin
light chain kinase MYLK1: Anatomy, interactions, functions, and
regulation. Biochemistry (Mosc). 81:1676–1697. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inam A, Shahzad M, Shabbir A, Shahid H,
Shahid K and Javeed A: Carica papaya ameliorates allergic asthma
via down regulation of IL-4, IL-5, eotaxin, TNF-α, NF-kB, and iNOS
levels. Phytomedicine. 32:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Weigand L, Foxson J, Shimoda LA
and Sylvester JT: Ca2+ signaling in hypoxic pulmonary
vasoconstriction: Effects of myosin light chain and Rho kinase
antagonists. Am J Physiol Lung Cell Mol Physiol. 293:L674–L685.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang WC, Peng YJ, Zhang GS, He WQ, Qiao
YN, Dong YY, Gao YQ, Chen C, Zhang CH, Li W, et al: Myosin light
chain kinase is necessary for tonic airway smooth muscle
contraction. J Biol Chem. 285:5522–5531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alcala DB, Haldeman BD, Brizendine RK,
Krenc AK, Baker JE, Rock RS and Cremo CR: Myosin light chain kinase
steady-state kinetics: Comparison of smooth muscle myosin II and
nonmuscle myosin IIB as substrates. Cell Biochem Funct. 34:469–474.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Connolly SC, Smith PG, Fairbank NJ, Lall
CA, Cole DJ, Mackinnon JD and Maksym GN: Chronic oscillatory strain
induces MLCK associated rapid recovery from acute stretch in airway
smooth muscle cells. J Appl Physiol (1985). 111:955–963. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Flores C, Ma SF, Maresso K, Ober C and
Garcia JG: A variant of the myosin light chain kinase gene is
associated with severe asthma in African Americans. Genet
Epidemiol. 31:296–305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao L, Grant AV, Rafaels N,
Stockton-Porter M, Watkins T, Gao P, Chi P, Muñoz M, Watson H,
Dunston G, et al: Polymorphisms in the myosin light chain kinase
gene that confer risk of severe sepsis are associated with a lower
risk of asthma. J Allergy Clin Immunol. 119:1111–1118. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Suzuki M, Nagaishi T, Yamazaki M, Onizawa
M, Watabe T, Sakamaki Y, Ichinose S, Totsuka M, Oshima S, Okamoto
R, et al: Myosin light chain kinase expression induced via tumor
necrosis factor receptor 2 signaling in the epithelial cells
regulates the development of colitis-associated carcinogenesis.
PLoS One. 9:e883692014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Basu S and Proweller A: Autoregulatory
control of smooth muscle myosin light chain kinase promoter by
notch signaling. J Biol Chem. 291:2988–2999. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou T, Wang T and Garcia JG: A nonmuscle
myosin light chain kinase-dependent gene signature in peripheral
blood mononuclear cells is linked to human asthma severity and
exacerbation status. Pulm Circ. 5:335–338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clayburgh DR, Barrett TA, Tang Y, Meddings
JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ and Turner JR:
Epithelial myosin light chain kinase-dependent barrier dysfunction
mediates T cell activation-induced diarrhea in vivo. J Clin Invest.
115:2702–2715. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang T, Moreno-Vinasco L, Ma SF, Zhou T,
Shimizu Y, Sammani S, Epshtein Y, Watterson DM, Dudek SM and Garcia
JG: Nonmuscle myosin light chain kinase regulates murine asthmatic
inflammation. Am J Respir Cell Mol Biol. 50:1129–1135. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khapchaev AY, Kazakova OA, Samsonov MV,
Sidorova MV, Bushuev VN, Vilitkevich EL, Az'muko AA, Molokoedov AS,
Bespalova ZD and Shirinsky VP: Design of peptidase-resistant
peptide inhibitors of myosin light chain kinase. J Pept Sci.
22:673–681. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Antoine TE and Shukla D: Inhibition of
myosin light chain kinase can be targeted for the development of
new therapies against herpes simplex virus type-1 infection.
Antivir Ther. 19:15–29. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng L, Geisselbrecht Y, Blanck S, Wilbuer
A, Atilla-Gokcumen GE, Filippakopoulos P, Kräling K, Celik MA,
Harms K, Maksimoska J, et al: Structurally sophisticated octahedral
metal complexes as highly selective protein kinase inhibitors. J Am
Chem Soc. 133:5976–5986. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Odani K, Kobayashi T, Ogawa Y, Yoshida S
and Seguchi H: ML-7 inhibits exocytosis of superoxide-producing
intracellular compartments in human neutrophils stimulated with
phorbol myristate acetate in a myosin light chain
kinase-independent manner. Histochem Cell Biol. 119:363–370.
2003.PubMed/NCBI
|
|
22
|
Cheng X, Wang X, Wan Y, Zhou Q, Zhu H and
Wang Y: Myosin light chain kinase inhibitor ML7 improves vascular
endothelial dysfunction via tight junction regulation in a rabbit
model of atherosclerosis. Mol Med Rep. 12:4109–4116. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chua YL, Liong KH, Huang CH, Wong HS, Zhou
Q, Ler SS, Tang Y, Low CP, Koh HY, Kuo IC, et al: Blomia
tropicalis-specific TCR transgenic Th2 cells induce inducible BALT
and severe asthma in mice by an IL-4/IL-13-dependent mechanism. J
Immunol. 197:3771–3781. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ogasawara T, Hatano M, Satake H, Ikari J,
Taniguchi T, Tsuruoka N, Watanabe-Takano H, Fujimura L, Sakamoto A,
Hirata H, et al: Development of chronic allergic responses by
dampening Bcl6-mediated suppressor activity in memory T helper 2
cells. Proc Natl Acad Sci USA. 114:pp. E741–E750. 2017; View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tashiro H, Takahashi K, Hayashi S, Kato G,
Kurata K, Kimura S and Sueoka-Aragane N: Interleukin-33 from
monocytes recruited to the lung contributes to house dust
mite-induced airway inflammation in a mouse model. PLoS One.
11:e01575712016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Glück J, Rymarczyk B, Kasprzak M and
Rogala B: Increased levels of interleukin-33 and thymic stromal
lymphopoietin in exhaled breath condensate in chronic bronchial
asthma. Int Arch Allergy Immunol. 169:51–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Salter BM, Oliveria JP, Nusca G, Smith SG,
Tworek D, Mitchell PD, Watson RM, Sehmi R and Gauvreau GM: IL-25
and IL-33 induce type 2 inflammation in basophils from subjects
with allergic asthma. Respir Res. 17:52016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mahmutovic Persson I, Akbarshahi H, Menzel
M, Brandelius A and Uller L: Increased expression of upstream
TH2-cytokines in a mouse model of viral-induced asthma
exacerbation. J Transl Med. 14:522016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo Z, Wu J, Zhao J, Liu F, Chen Y, Bi L,
Liu S and Dong L: IL-33 promotes airway remodeling and is a marker
of asthma disease severity. J Asthma. 51:863–869. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang Y, Luo X, Li X, Song X, Wei L, Li Z,
You Q, Guo Q and Lu N: Wogonin inhibits LPS-induced vascular
permeability via suppressing MLCK/MLC pathway. Vascul Pharmacol.
72:43–52. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Walsh GM: Biologics targeting IL-5, IL-4
or IL-13 for the treatment of asthma-an update. Expert Rev Clin
Immunol. 13:143–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang C, Liu Q, Chen F, Xu W, Zhang C and
Xiao W: IL-25 promotes Th2 immunity responses in asthmatic mice via
nuocytes activation. PLoS One. 11:e01623932016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chung KF: Targeting the interleukin
pathway in the treatment of asthma. Lancet. 386:1086–1096. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bagnasco D, Ferrando M, Varricchi G,
Passalacqua G and Canonica GW: A critical evaluation of anti-IL-13
and anti-IL-4 strategies in severe asthma. Int Arch Allergy
Immunol. 170:122–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Papathanassiou E, Loukides S and Bakakos
P: Severe asthma: Anti-IgE or anti-IL-5? Eur Clin Respir J.
3:318132016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jia Y, Fang X, Zhu X, Bai C, Zhu L, Jin M,
Wang X, Hu M, Tang R and Chen Z: IL-13+ type 2 innate lymphoid
cells correlate with asthma control status and treatment response.
Am J Respir Cell Mol Biol. 55:675–683. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tang W, Smith SG, Beaudin S, Dua B, Howie
K, Gauvreau G and O'Byrne PM: IL-25 and IL-25 receptor expression
on eosinophils from subjects with allergic asthma. Int Arch Allergy
Immunol. 163:5–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shan S, Li Y, Wang J, Lv Z, Yi D, Huang Q,
Corrigan CJ, Wang W, Quangeng Z and Ying S: Nasal administration of
interleukin-33 induces airways angiogenesis and expression of
multiple angiogenic factors in a murine asthma surrogate.
Immunology. 148:83–91. 2016. View Article : Google Scholar : PubMed/NCBI
|