Introduction
Transplantation is the most effective and permanent
treatment for end-stage organ failure but this option is limited
because of the acute and chronic rejection of most grafts. Chronic
rejection, which is also known as chronic allograft dysfunction, is
the main obstacle to achieving the long-term graft survival.
Chronic allograft dysfunction shows different histological
appearances in various organs. However, their common pathological
features are considered to be transplanted arteriosclerosis (TA)
(1). Various lesions of vessel
wall lead to vascular smooth muscle cells (SMC) activation.
Activated SMCs migrate from the media into the intima by
proliferating and elaborating cytokines and extracellular matrix
(ECM) proteins, eventually resulting in luminal narrowing and
vascular dysfunction (2). Matrix
metalloproteinases (MMPs) represent the family of zinc-containing
enzymes which degrade the ECM and connective tissue proteins
(3). The proteolytic effects of
MMPs play an important role in vascular remodeling, cellular
migration, and in managing of ECM proteins and adhesion molecules
(3,4). Under the physiological state, MMPs
interact with particular ECM components and their activities are
regulated at the transcription level. Endogenous tissue inhibitor
of metalloproteinases (TIMPs) can prevent excessive degradation of
ECM. An imbalance between MMPs and TIMPs could lead to the
elevation of MMPs activity that may cause pathological changes in
the vessel wall structure. The aim of our study is to reveal their
function by dynamically observing their expression and blocking
their function in allogenic transplantation groups.
Materials and methods
Animal grouping
In total, 120 Wistar and 40 Sprague Dawley (SD) rats
(male, 200–230 g) were obtained from Animal Experiment Center of
Chongqing Medical University and housed under specific
pathogen-free (SPF) conditions which were maintained at 24±2°C and
a 12-h light/dark cycle. They were randomly divided into 4 groups,
which including: i) Control group: Sham operations were performed
as control; ii) isogenic group: Orthotopic abdominal aortic
transplantation from Wistar to Wistar rats was performed; iii)
allogenic group: Orthotopic abdominal aortic transplantation from
SD to Wistar rats was performed; and v) allogenic+SB-3CT treated
group: Orthotopic abdominal aortic transplantation from SD to
Wistar rats was performed. The recipients received SB-3CT (a potent
inhibitor of MMP-2, 25 mg/kg) once daily for 28 days in the
post-transplantation period.
All animals' care and experimental protocols were
complied with the National Institutes of Health guide for the care
and use of Laboratory animals (NIH Publications no. 8023, revised
1978) and the Animal Management Rules of the Ministry of Health of
China and all experimental designs were approved by the Animal Care
and Use Committee of Second Affiliated Hospital of Chongqing
Medical University (Chongqing, China).
Orthotopic abdominal aortic
transplantation
Donor operation
The donors were anesthetized by intraperitoneal
injection of 1% pentobarbital (50 mg/kg) and then they were placed
in the supine position on the operating board with their abdomen
shaved and scrubbed by povidone-iodine. After a long midline
abdominal incision, the abdominal aorta was isolated from the level
of the left renal vein to the bifurcation via careful blunt and
sharp dissection. All aortic branches between the renal arteries
and bifurcation were ligated with the help of 9-0 suture (DE FORCE,
Shanghai Medical Instruments Co., Ltd, Shanghai, China). This
section with an average length of 1.5 to 2.0 cm can serve as the
donor material. Before separating the aorta, two sutures were tied
right near the ligations (below renal artery and above
bifurcation). Finally, the grafts were fully rinsed with heparin
sodium (200 U/ml) and then kept in lactated Ringer's solution in
4°C refrigerator.
Recipient operation
Recipients were anesthetized with isoflurane (4% for
induction and 2% for maintenance) by continuous inhalation
anesthesia. After the midline abdominal incision, the intestines
were retracted to the right side to expose the retroperitoneum and
then the intestines were covered with a square of wet sterile
gauze. The fatty tissue around the aorta and the inferior vena cava
(IVC) was gently removed using fine tissue forceps (Jinzhong,
Shanghai Medical Instruments Co., Ltd) and the infrarenal aorta was
dissected from the IVC to create enough space for two artery
clamps. Every other vessel branches were ligated during the
dissection.
Two clamps (below renal artery and above
bifurcation) were used to stop blood flow before removing the
aorta. The cut ends were rinsed with heparinized saline (200 U/ml)
and the graft was end-to-end anastomosed to the recipient through
interrupted 9-0 suture (DE FORCE). Before releasing the distal
clamp, the proximal clamp was carefully removed to avoid blood
leakage at the suture lines. The graft was immediately filled with
blood and visible pulsation could be observed. Finally, the
intestines were put back into their original place and the
abdominal incision was sutured with 4-0 nylon (Ethicon, Inc.,
Cincinnati, OH, USA). Then 1% of lidocaine was added to establish
analgesia and the rats were kept on a warming pad until they fully
recovered from the anesthesia.
Histopathological examination
The transplanted aortas were removed on the 7th,
14th, 28th and 56th day after the operation. All tissues were fixed
in 4% paraformaldehyde for 24 h immediately after surgical
resection and then embedded in paraffin. The tissue sections (5 µm)
were heated at 60°C for 1 h then dewaxed and rehydrated by
immersion in dimethylbenzene and ethanol series and hematoxylin and
eosin (H&E) staining was performed. H&E statistics were
conducted using a method described by Wiebke and colleagues
(5). Generally, digitized
histological photographs were analyzed and relative thickness (%)
of the intima was calculated by the equation R = [area
(intima)/area (media)] ×100%, for each group, a mean value of the 4
samples were calculated.
For immunohistochemistry, an antigen-retrieval
technique consisting of heating in a citrate water bath at 95°C for
20 min and cooling for 2 h, was performed after dewaxing and
rehydration to recover antigens. Endogenous peroxidase activity was
blocked by incubation in 3% hydrogen peroxide at room temperature
for 15 min. Nonspecific binding was blocked by using 5% bovine
serum albumin (BSA) at room temperature for 30 min. Finally, the
specimens were incubated overnight with antibodies against MMP-1
(cat. no. 10371-2-AP, 1:200; ProteinTech Group, Inc., Chicago, IL,
USA); TIMP-1 (cat. no. 10753-1-AP, 1:100; ProteinTech Group, Inc.);
TIMP-3 (cat. no. ab39184, 1:100; Abcam, Cambridge, UK) and the
sections were stained by DAB the next day.
For further analyses, histological sections were
clustered into fields of identical size. Subsequently, relative
staining index was calculated by counting positively stained cells
in 100 cells via Photoshop CS6 software (Adobe Systems, Inc., San
Jose, CA, USA). For each group, a mean value of the 4 samples was
calculated.
Immunohistofluorescence
Fresh tissue embedded in Tissue-Tek O.C.T. Compound
(cat. no. 4583; Sakura Finetek USA, Inc., Torrance, CA, USA) was
applied to obtain the frozen sections. After fixing in acetone for
30 min, the sections were stained with PCNA (cat. no. 13110S,
1:100; Cell Signaling Technology, Inc., Danvers, MA, USA) and added
an anti-rabbit IgG conjugated to Alexa Flour 405 (cat. no. R35551,
1:100; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Afterwards, the sections were observed with the help of a
fluorescence microscope (N-STORM 4.0; Nikon Corporation, Tokyo,
Japan) and the proliferation index was calculated by the ratio of
positively stained cells to 100 cells.
Western blotting
The protein was extracted from the tissues in a
lysis buffer with 0.2% protease inhibitor cocktail (Beyotime
Institute of Biotechnology, Haimen, China). The lysis buffer
solubilized protein was denatured in a loading buffer (Beyotime
Institute of Biotechnology) at 95°C for 5 min. The extracted 50 µg
of tissue was fractionated on a Bis-Tris gel and later was
transferred to a PVDF membrane (both from Bio-Rad Laboratories,
Inc., Hercules, CA, USA) by using semidry electroblotting. In order
to block the non-specific binding, the membranes were incubated
with 5% BSA in TBST. Then, the membranes were incubated overnight
at 4°C with antibodies against: MMP-2 (cat. no. ab37150, 1:1,000;
Abcam); β-actin (cat. no. GTX11003, 1:1,000; GeneTex, Inc., Irvine,
CA, USA); STAT3 (cat. no. ab68153, 1:2,000; Abcam); p-STAT3 (cat.
no. ab76315, 1:1,000; Abcam); IL-6 (cat. no. ab9324, 1:500; Abcam);
VCAM-1 (cat. no. ab134047, 1:2,000; Abcam); cleaved caspase-3 (cat.
no. 9664T, 1:1,000; Cell Signaling Technology, Inc.). After washing
for 5 times in TBST, the membranes were incubated with
HRP-conjugated secondary antibodies (cat. nos. AS003 and AS014;
ABclonal Biotech Co., Ltd., Woburn, MA, USA) at the dilution of
1:8,000 for 60 min at room temperature in TBST-BSA. The bands were
detected by using a chemiluminescent peroxidase substrate (ECL;
Amersham; GE Healthcare, Chicago, IL, USA) and exposed on a
ChemDoc™ MP (Bio-Rad Laboratories, Inc.).
Statistical analysis
The results were expressed as means ± SD. Two value
sets were compared by Student's t-test. For three or more values,
one-way analysis of variance followed by Bonferoni post hoc test
was used. The P<0.05 was considered statistically
significant.
Results
Relative thickness of intima
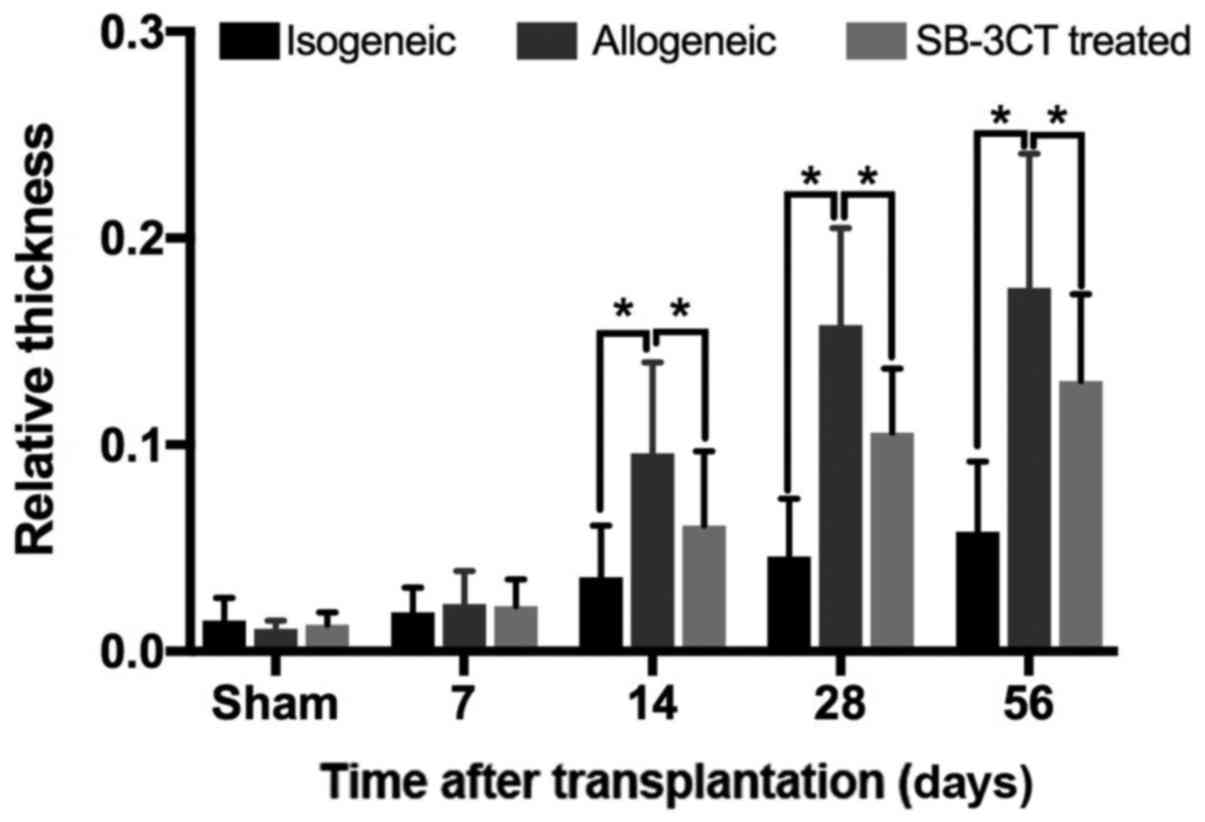
Compared to allogenic transplantation groups, the
intima of isogenic groups did not show an obvious thickening in the
early postoperative period (within 7 days after transplantation).
Analysis of specimens from allogenic groups has shown greater
thickening of the intima than in the isogenic group (P<0.05;
Fig. 1) in the late postoperative
period. At the same time, the thickness of intima in the
allogenic+SB-3CT treated group was lower compared to the allogenic
group without treatment (P<0.05; Fig. 1). As shown in Fig. 1, the mild hyperplasia in medial
layers could be observed in the isogenic group on the 14th day
after transplantation in contrast to allogenic groups where SMCs in
medial layers have shown obvious proliferation and migration into
the intima through the gap of elastic lamina.
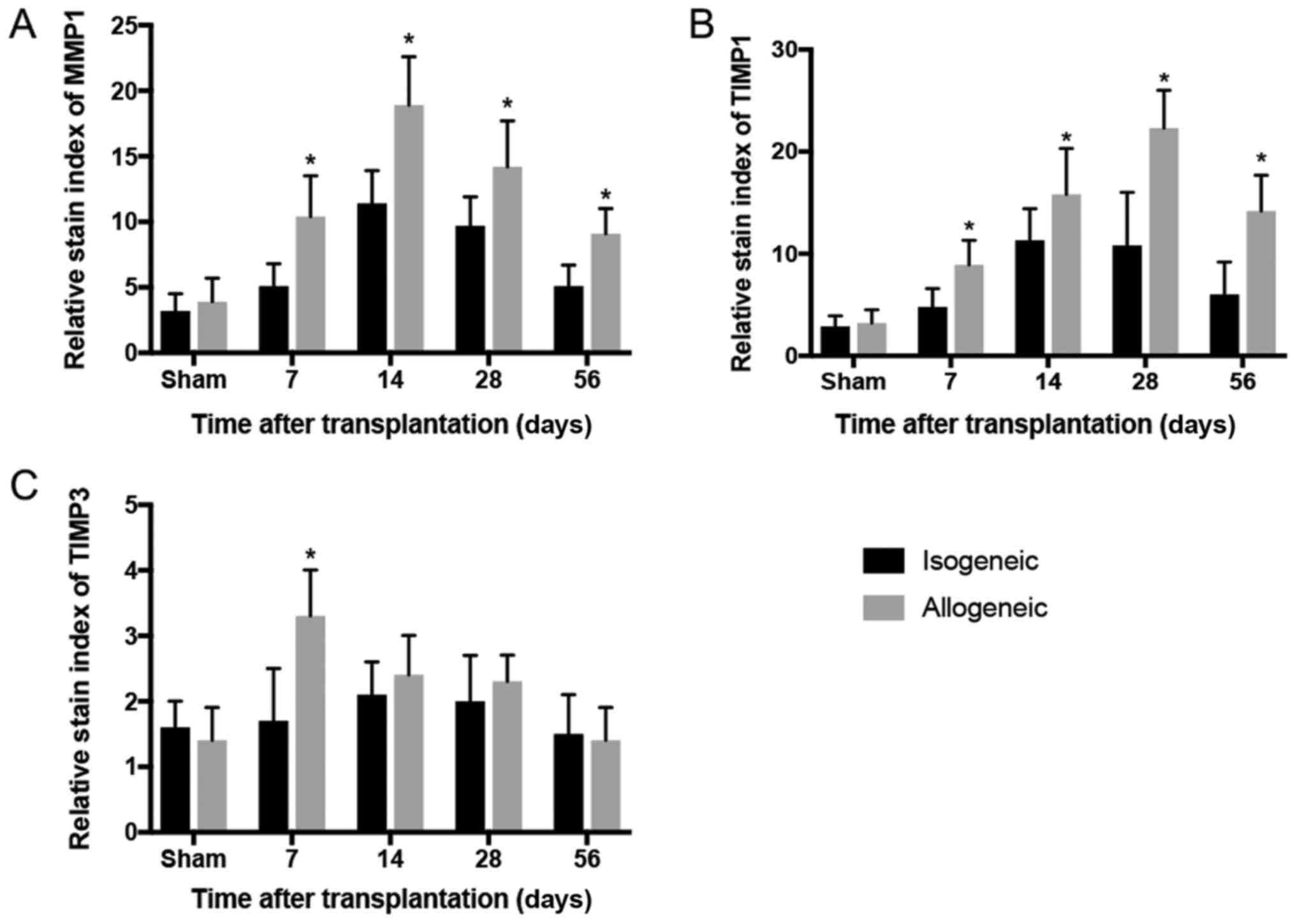
Dynamic changes of MMPs/TIMPs
The MMP-1 and TIMP-1 showed the similar trend in the
isogenic group, but after the 28th day decreased (Fig. 2). High expression of MMP-1 in
allogenic groups was noticed on the 7th day after the operation and
peaked on the 14th day (P<0.05; Fig. 2A). The high expression of TIMP-1
was demonstrated for 14 days and peaked on the 28th day followed by
a rapid drop on the 56th day (P<0.05; Fig. 2B), however, the expression of
TIMP-3 was only marginally expressed in the ECM of transplanted
grafts and statistical difference was found only on the 7th day
(P<0.05; Fig. 2C). The western
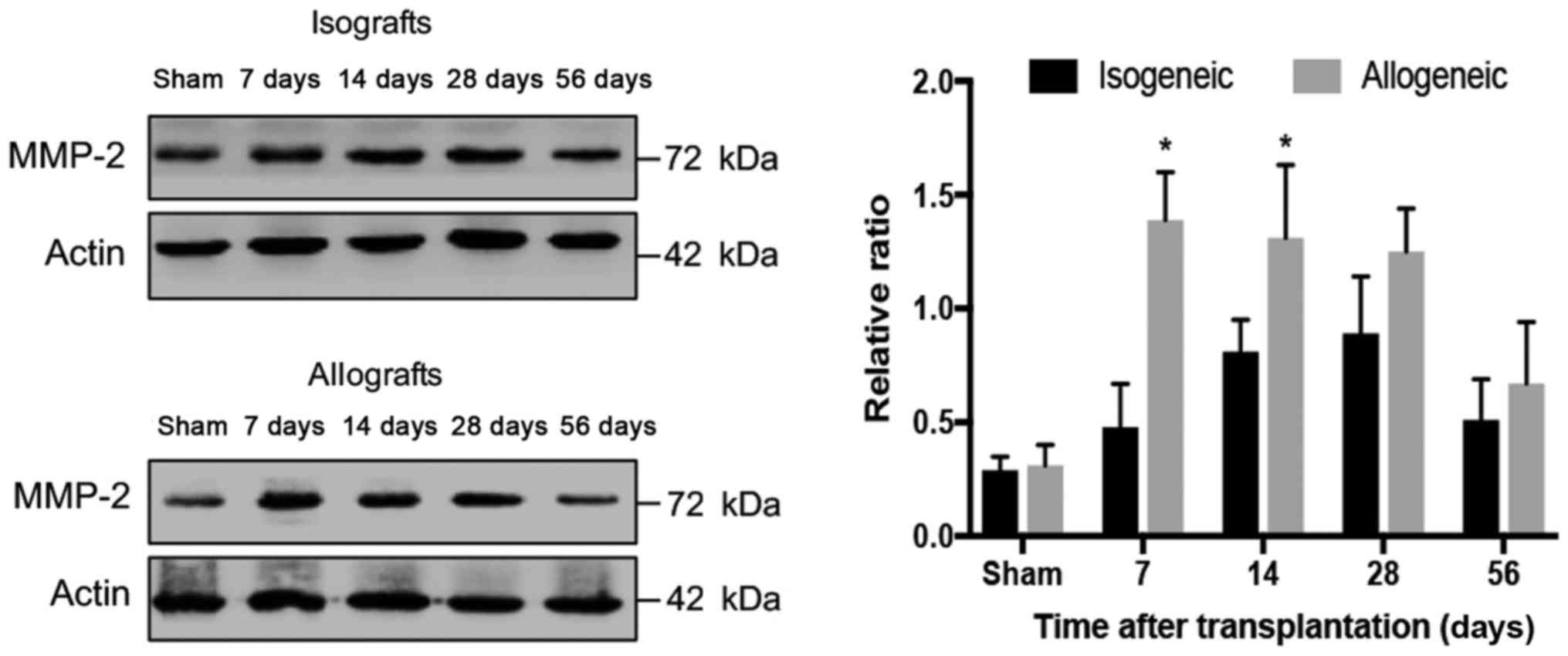
blot analysis has shown relatively low expression of MMP-2 in the
isogenic transplanted aorta and the expression of MMP-2 started to
increase only after the 14th day and peaked on the 28th day
followed by gradual reducing (P<0.05; Fig. 3). In comparison to isogenic groups,
MMP-2 was significantly upregulated on the 28th day (P<0.05;
Fig. 3).
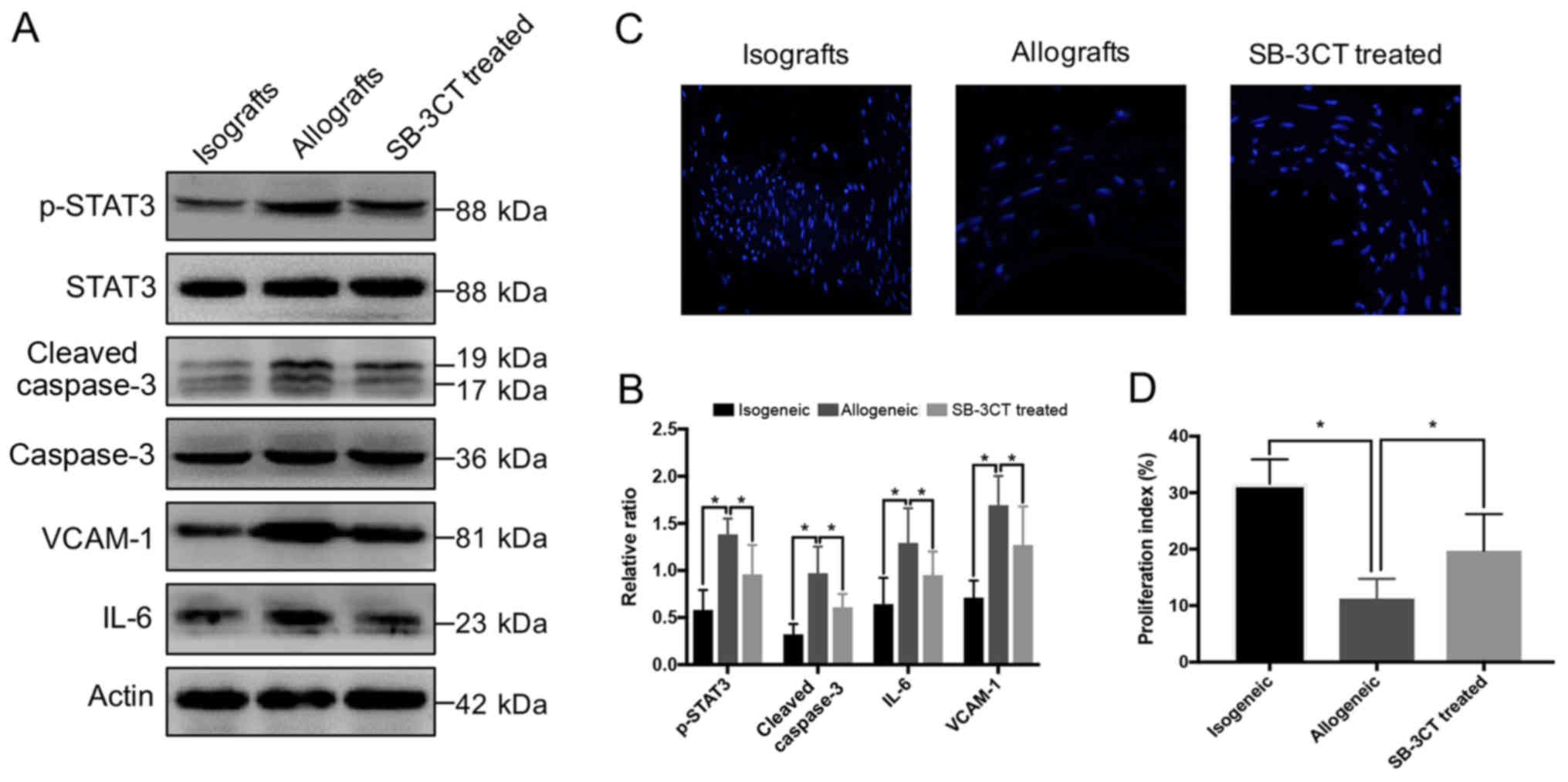
Inhibition of MMP-2 reduce aortic
inflammation and apoptosis
We further looked into the parameters related to
inflammation and apoptosis on the 7th day. Allogenic groups have
shown more severe inflammation and apoptosis compared to the
isogenic group as indicated by higher expression of p-STAT3,
VCAM-1, proinflammatory cytokine IL-6, and cleaved-caspase 3.
However, the treatment of the allogenic group by SB-3CT after
transplantation obviously reduced these damage-related parameters
(P<0.05; Fig. 4A and B).
Immunohistofluorescence has shown that in the allogenic group there
is the lowest proliferation index among the three groups while
treatment with SB-3CT protects allografts from the injury by
improving the proliferation (P<0.05; Fig. 4C and D).
Discussion
Allograft vasculopathy (AV) is a progressive and
diffuse intimal hyperplastic lesion of arteries that leads to
gradual vessel narrowing and eventually to allograft ischemia
(6). Blood vessels are regulated
constantly by neurohormonal and hemodynamic changes. Chronic
adaptation in vessels often occurs as a result of structural
changes of their construction. MMPs are important enzymes of ECM
degradation and they also regulate the biological activity of
non-matrix substrates including cytokines, chemokines, receptors,
growth factors and cell adhesion molecules (3,4,7).
Enzymatic activity is regulated at multiple levels and TIMPs
participate in controlling the local activities of MMPs in the
tissues (3).
Our study observed the expression of MMP-1, MMP-2,
TIMP-1, and TIMP-3 in both allogenic and isogenic transplantation.
Compared to the allografts, the isografts have demonstrated
relatively lower expression of MMP-1, TIMP-1, and TIMP-3.
Inhibition of MMP-2 reduced aortic inflammation and apoptosis by
increasing the proliferation in allogenic groups. Vascular cell
adhesion molecule 1 (VCAM-1) also konwn as cluster of
differentiation 106 (CD106) is a protein mediates the adhesion of
lymphocytes, monocytes, eosinophils to vascular endothelium and it
also palys an important role in in the development of
atherosclerosis (8,9). Our supression of MMP-2 by SB-3CT in
allogenic group lower the expression of VCAM-1 as well as the
proinflammatory IL-6. IL-6 transduces signals via phosphorylation
of STAT3 (pSTAT3), and STAT3 is a central regulator of inflammatory
and immune responses (9). Thus, we
might attribute the pathological changes to the imbalance of
MMPs/TIMPs expression, which leads to the disorder of degradation
of ECM and subsequently finish as arteriosclerosis of grafts.
Vascular endothelial cells (ECs) were more susceptible to
hypoxic-ischemic injuries in the early postoperative period. A
series of cytokines such as bFGF and PDGF could be released by
impaired ECs, that stimulate the expression of MMP-2 (10). Activated MMPs are able to degrade
collagen, elastin, and other extracellular molecules (11). Endothelial inflammation (such as EC
senescence, apoptosis, necrosis, and dysfunction) could be promoted
by increased MMPs activity. MMP-1 enhances ECs senescence by p53
activation (12). MMP-2 cleaves
the intercellular and cell-matrix junctions, including vascular
endothelial-cadherin and β- and γ-catenin that increase vascular
wall permeability (13,14). Some studies suggest that activated
MMPs function as growth factors of VSMCs in vitro. The
MMP-mediated VSMC proliferation in vivo contributes to
arterial intimal-medial thickening (IMT) (15,16).
In the late postoperative period, the increased
ratio of CD4+/CD8+ T cells and enormous
inflammatory cytokines can induce the gene transcription of TIMP-1
(1,17). TIMP-1 regulates the activity of
MMPs-enzymes, which are involved in the degradation of the ECM
(18). This mechanism will cause
the accumulation of collagen in the extracellular space and further
will lead to arteriosclerosis. The growing evidence from the past
decade clearly indicates that TIMPs have additional biological
activities by acting as signaling molecules (19). The cytokine-like activities of
TIMPs include the modulation of cell proliferation, apoptosis,
differentiation, and angiogenesis (20) that contribute to the formation of
atherosclerosis. Interestingly, the expression of TIMP-3 was low in
both groups. TIMPs are specific endogenous inhibitors that bind
MMPs in a 1:1 stoichiometry, however, the inhibitory properties of
TIMP-3 are different from the rest TIMPs, because TIMP-3 inhibits
ADAMTS (a disintegrin-like and metalloproteinase domain with
thrombospondin-type 1 motifs) (21). The recent biochemical data have
clarified some aspects of the relationship between ADAMTS proteins
and fibrillin microfibrils (22)
and we suspect that this might be an important reason for
arteriosclerosis.
Our present study only showed a relativly long term
(up to 56 days) of posttransplantation. In future, we would like to
perform a real long-term data (months, perhaps years) using an
in vivo model where animals receive immunosupression therapy
to study more about inflammatory cell infiltration and EC
activation to support the results of the present study.
In conclusion our study has revealed the imbalance
of MMPs/TIMPs in isogenic and allogenic orthotopic aorta
transplantation and inhibition of MMP-2 reduced the aortic
inflammation and apoptosis by increasing the proliferation in the
allogenic group. It is assumed that this imbalance leads to the
disturbance of the ECM and is related to the posttransplantation
damage.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81370577).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SDW and MHW conceived and designed the experiments.
SDW, DC and XSX performed the experiments. HB and HW analyzed the
data. MHW contributed reagents, materials and instructions for the
model establishment. SDW and MHW wrote the paper.
Ethics approval and consent to
participate
The study was approved by the Animal Care and Use
Committee of Second Affiliated Hospital of Chongqing Medical
University (Chongqing, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
von Rossum A, Laher I and Choy JC:
Immune-mediated vascular injury and dysfunction in transplant
arteriosclerosis. Front Immunol. 5:6842014.PubMed/NCBI
|
|
2
|
Rahmani M, Cruz RP, Granville DJ and
McManus BM: Allograft vasculopathy versus atherosclerosis. Circ
Res. 99:801–815. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
Structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galis ZS and Khatri JJ: Matrix
metalloproteinases in vascular remodeling and atherogenesis: The
good, the bad, and the ugly. Circ Res. 90:251–262. 2002.PubMed/NCBI
|
|
5
|
Sommer W, Knöfel AK, Izykowski N, Oldhafer
F, Avsar M, Jonigk D, Warnecke G and Haverich A: Physical exercise
reduces transplant arteriosclerosis in a mouse aorta
transplantation model. J Thorac Cardiovasc Surg. 149:330–337. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mitchell RN: Learning from rejection: What
transplantation teaches us about (other) vascular pathologies. J
Autoimmun. 45:80–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dimas G, Iliadis F and Grekas D: Matrix
metalloproteinases, atherosclerosis, proteinuria and kidney
disease: Linkage-based approaches. Hippokratia. 17:292–297.
2013.PubMed/NCBI
|
|
8
|
Kitamura H, Ohno Y, Toyoshima Y, Ohtake J,
Homma S, Kawamura H, Takahashi N and Taketomi A:
Interleukin-6/STAT3 signaling as a promising target to improve the
efficacy of cancer immunotherapy. Cancer Sci. 108:1947–1952. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calautti E, Avalle L and Poli V:
Psoriasis: A STAT3-centric view. Int J Mol Sci. 19:pii: E171.
2018.PubMed/NCBI
|
|
10
|
Solomon A, Li DQ, Lee SB and Tseng SC:
Regulation of collagenase, stromelysin, and urokinase-type
plasminogen activator in primary pterygium body fibroblasts by
inflammatory cytokines. Invest Ophthalmol Vis Sci. 41:2154–2163.
2000.PubMed/NCBI
|
|
11
|
Lakatta EG: The reality of aging viewed
from the arterial wall. Artery Res. 7:73–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Struewing IT, Durham SN, Barnett CD and
Mao CD: Enhanced endothelial cell senescence by lithium-induced
matrix metalloproteinase-1 expression. J Biol Chem.
284:17595–17606. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu WB and Huang TF: Activation of MMP-2,
cleavage of matrix proteins and adherens junctions during a snake
venom metalloproteinase-induced endothelial cell apoptosis. Exp
Cell Res. 288:143–157. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shapiro S, Khodalev O, Bitterman H,
Auslender R and Lahat N: Different activation forms of MMP-2
oppositely affect the fate of endothelial cells. Am J Physiol Cell
Physiol. 298:C942–C951. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao Q, Zhang F, Grassia G, Hu Y, Zhang Z,
Xing Q, Yin X, Maddaluno M, Drung B, Schmidt B, et al: Matrix
metalloproteinase-8 promotes vascular smooth muscle cell
proliferation and neointima formation. Arterioscler Thromb Vasc
Biol. 34:90–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Austin KM, Nguyen N, Javid G, Covic L and
Kuliopulos A: Noncanonical matrix
metalloprotease-1-protease-activated receptor-1 signaling triggers
vascular smooth muscle cell dedifferentiation and arterial
stenosis. J Biol Chem. 288:23105–23115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsieh JL, Shiau AL, Lee CH, Yang SJ, Lee
BO, Jou IM, Wu CL, Chen SH and Shen PC: CD8+ T
cell-induced expression of tissue inhibitor of metalloproteinses-1
exacerbated osteoarthritis. Int J Mol Sci. 14:19951–19970. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raffetto JD and Khalil RA: Matrix
metalloproteinases and their inhibitors in vascular remodeling and
vascular disease. Biochem Pharmacol. 75:346–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ries C: Cytokine functions of TIMP-1. Cell
Mol Life Sci. 71:659–672. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lambert E, Dassé E, Haye B and Petitfrère
E: TIMPs as multifacial proteins. Crit Rev Oncol Hematol.
49:187–198. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kashiwagi M, Tortorella M, Nagase H and
Brew K: TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4)
and aggrecanase 2 (ADAM-TS5). J Biol Chem. 276:12501–12514. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hubmacher D and Apte SS: ADAMTS proteins
as modulators of microfibril formation and function. Matrix Biol.
47:34–43. 2015. View Article : Google Scholar : PubMed/NCBI
|