Introduction
Bronchopulmonary dysplasia (BPD) is one of the most
serious complications in premature infants and remains a
substantial lifelong burden. In recent years, as significant
advances in respiratory care have been made in neonatal medicine,
the survival rates of premature infants have increased; however, it
appears that the incidence of BDP is stagnant, or even increasing
(1,2). Eunice Kennedy Shriver National
Institute of Child Health and Human Development Neonatal Network
Data reveal that the morbidity of BPD is 68% during the premature
births with a birth weight of 401–1,500 g (3). The pathogenesis of BPD is unclear and
there are no safe and effective preventative therapies (4,5).
Therefore, novel treatment strategies are required for the
prevention of BPD and it remains important to explore the
pathogenesis and clinical treatment of BPD.
Infants with severe BPD exhibit abnormal alveolar
and vascular development (6,7), and
a hyperoxia-induced model is frequently used to study BPD. In this
model, the principal pathological changes are alveolar
simplification, abnormal vascularization and varying degrees of
pulmonary fibrosis (8). Numerous
growth factors are involved in abnormal alveolar development, and
the most important is transforming growth factor-β (TGF-β).
Overexpression of TGF-β is involved in BPD via the inhibition of
alveolar development and the induction of lung fibroblast (LF)
proliferation (9). The function of
bone morphogenetic protein (BMP) 7 in BPD is, however, largely
unknown. Therefore, to investigate the effect of BMP7 on
pathological changes in the lungs of BPD is crucial. It is
important to identify improved methods of protection against, and
clinical treatment of, BPD.
BMP7 is a member of the TGF-β superfamily of growth
factors and possesses various biological functions (10–12).
BMP7 appears to inhibit the effects of TGF-β-induced fibrogenesis
as a natural antagonist (13).
BMP7 counteracts TGF-β activity during ongoing fibrogenesis in
various organs, including the liver, lungs and kidneys (14–19).
Previous research has demonstrated that BMP7 inhibits the
development of fibrosis in thioacetamide-treated rat liver and that
therapeutic application of recombinant human BMP7 or functionally
active BMP7 fragments may be advantageous for experimental fibrosis
in rats (20,21). A lack of BMP7 may lead to
extracellular matrix protein accumulation in the mesangial area,
and BMP7 serves an important function in regulating glomerular
structural homeostasis (22).
Downregulation of BMP7 expression improves renal fibrosis and
accelerates the return of renal function in experimental models of
renal disease (18,23). Experimental results have confirmed
that BMP7 may reduce asbestos-induced fibrotic alterations in the
lung (19). BMP7 mRNA was
persistently induced in didecyldimethylammonium chloride
(DDAC)-induced pulmonary damage, suggesting that BMP7 is a negative
regulator of fibrosis (24). An
additional study suggested that BMP7 opposed the TGF-β1-mediated
fibrogenic activity of pulmonary myofibroblasts in culture
(25).
However, the effect of BMP7 on LFs is insufficient
and previous studies primarily focused on lung tissue and other
organs of adult animals (19–23).
The expression of BMP7 is unclear in neonatal rats with BPD. The
primary aim of the present study was to examine the expression of
BMP7 and the association between decreased expression of BMP7 and
abnormal alveolar development. The present study additionally aimed
to demonstrate that LF proliferation is inhibited by BMP7, which is
the possible mechanism underlying abnormal alveolar
development.
Materials and methods
Animal models
A total of 24 time-dated, pregnant Wistar rats
(220–240 g) were purchased from the Center for Experimental Animals
of China Medical University (Shenyang, China). All animal
procedures were reviewed and approved by the Laboratory Animal
Ethics Committee of China Medical University. All surgeries were
performed under chloral hydrate anesthesia, and all efforts were
made to minimize animal suffering. Pups were delivered naturally at
term gestation (21 days). A total of 196 full-term newborn rats
from 20 litters were randomly marked and assigned to two groups,
and were exposed to hyperoxia (80–90% oxygen; experimental group)
or normoxia (21%; control group) beginning on the day of birth. The
inhaled oxygen concentration was measured and recorded continuously
with an analyzer equipped with a strip-chart recorder (model 572;
Servomex; Spectris plc, Egham, UK). Humidity was routinely set to
60–70%. The temperature was 25–27°C with a light/dark cycle of
10/14 h and access to food and water ad libitum. Nursing rat
dams were switched every 24 h between the hyperoxic and normoxic
chambers, to avoid oxygen toxicity and to provide equal nutrition
to each litter. Chambers were open for 30 min/day for cage
cleaning. Pups were sacrificed and lungs were harvested at the end
of 3, 7, 14 and 21 days of exposure. The left lungs were fixed in
4% paraformaldehyde for hematoxylin and eosin staining (HE), and
the right lungs were frozen at −80°C for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis.
Lung histology
Pups were anesthetized with an intraperitoneal
injection of 10% chloral hydrate (300–500 mg/kg) and sacrificed by
cervical dislocation, and their chests were opened for the
isolation of lung tissues. The left lungs were fixed in 4%
paraformaldehyde for 24 h at room temperature. Lung tissue was
dehydrated with graded alcohol, placed in xylene for 1 h and then
embedded in paraffin at 60°C. Sections of lung (4 µm) were stained
with HE at room temperature, with 10 min hematoxylin and 1 min
eosin staining. Morphological alterations were assessed using an
optical microscope (H600L; Nikon Corporation, Tokyo, Japan). From
each section, 10 random areas were examined at ×20 magnification.
The radial alveolar count (RAC) was counted with a method developed
by Emery and Mithal, as described in a previous study (26), to assess the level of alveolar
development. The RAC of each section was evaluated by two
independent pathologists who were blinded to the experimental
design.
Immunohistochemical staining
Following fixation in 4% paraformaldehyde, the lung
tissues were embedded in paraffin and sliced in 4-µm-thick
sections. The sections were dewaxed, incubated in 3%
H2O2 for 15 min to eliminate endogenous
peroxidase activity, and incubated in pancreatin (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) for 15 min at room
temperature. The sections were washed three times with PBS, blocked
with rabbit serum (OriGene Technologies, Inc., Beijing, China) and
incubated with a primary antibody targeting BMP7 (cat. no. ab56023;
1:125; Abcam, Cambridge, UK) overnight at 4°C. The tissues were
washed 3 times with PBS, and processed following the protocols
provided in the UltraSensitive serum amyloid P (goat)
immunohistochemistry kit (OriGene Technologies, Inc., Beijing,
China). Sections were developed using the peroxidase substrate
diaminobenzidine detection kit (OriGene Technologies, Inc.) and
were counterstained with hematoxylin for 30 sec at room
temperature. In control experiments, the primary antibody was
replaced with PBS. Cells with brown particles deposited in their
cytoplasm were counted as BMP7-positive cells. A total of 10
sections were selected at each time point for each group and 10
visual fields were selected from each section under a light
microscope at ×40 magnification (H600L; Nikon Corporation). The
protein expression was semi-quantitatively detected using an image
analysis system (Universal Imaging, Downingtown, PA, USA). The
staining intensity was analyzed with MetaMorph software (version
5.0; Universal Imaging). The average optical density denoted the
intensity of BMP7 protein expression.
RT-qPCR analysis
Total RNA was extracted from right lung lobes cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
and frozen at −80°C, according to the manufacturer's protocol
(Takara Bio, Inc., Otsu, Japan). A total of 1 mg RNA from each
sample was reverse-transcribed into cDNA using SuperScript III
(Invitrogen; Thermo Fisher Scientific, Inc.), according the
manufacturer's protocol. qPCR was performed on a LightCycler (7500
FAST Real-Time PCR System; Applied Biosystems; Thermo Fisher
Scientific, Inc.) using appropriate primers (synthesized by Takara
Bio, Inc.): BMP7 sense, 5′-CAGCCACCAGCAACCACT-3′ and antisense,
5′-GTCCATGCCGTCCAATCA-3′; standardized GAPDH (commodity label
DR3783). The amplification reaction was performed as follows: 40
cycles of 95°C for 10 sec, 95°C for 5 sec and 60°C for 20 sec; and
65°C for 15 sec. The relative level of mRNA expression was
calculated following normalization with GAPDH (27). Experiments were repeated six
times.
LF cell isolation and
purification
A total of 38 full-term newborn rats from 4 litters
on the day of birth were sacrificed and LFs were isolated by two
steps of trypsin digestion, as previously described (28). A tracheal cannula was placed in the
anesthetized rat for lung ventilation. Cold D-Hanks solution was
used for lung lavage via cardiopulmonary perfusion to remove blood.
The lung tissues were minced and dissociated with trypsin solution.
Following enzymatic digestion, the residual trypsin was neutralized
with the same volume of Dulbecco's modified Eagle's medium (DMEM;
Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) containing 10% fetal bovine serum (Clark Bioscience,
Richmond, VA, USA). LFs were purified by filtration through nylon
meshes and by centrifugation at 114 × g for 5 min at room
temperature. Cells were seeded in 25 cm3 culture flasks
at a density of 5×105 cells/l and incubated at 37°C in
5% CO2, with the DMEM changed each day. The cells were
trypsinized and reseeded following ~3 days of culture for further
purification. The third generation of LF cells were used for the
MTT assay and flow cytometry (FCM).
MTT assay
The effect of BMP7 on cell proliferation was
detected by MTT assay. The LF cells were seeded at a density of
10,000 cells/well in 96-well plates. Following 24 h of culture, the
cells were treated with 0 (serum-free medium), 5, 10 and 50 ng/ml
BMP7 for 12, 24 and 48 h. Subsequently, 20 µl MTT solution (5
mg/ml) was added to each well and incubated at 37°C for 4 h. The
medium was carefully aspirated and the purple formazan crystals
were solubilized with 150 µl dimethyl sulfoxide. Optical density
was measured at 490 nm using a microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA). Experiments were repeated
three times.
Cell cycle analysis
The effect of BMP7 on the cell cycle of LF cells was
analyzed by FCM. The cells were seeded at a density of
1×106 cells/ml in a 25-cm3 culture flask, and
treated with different concentrations of BMP7 (0, 5, 10 and 50
ng/ml) for 12 h, or treated with 50 ng/ml BMP-7 for different time
points (12, 24 and 48 h). Cells were harvested and fixed in 70%
ethanol at 4°C overnight. The fixed cells were centrifuged at 114 ×
g for 15 min at room temperature and washed with cold PBS three
times. Then cells were incubated with 50 µg/ml RNase A at 37°C for
30 min. Subsequently, cells were incubated with 100 µg/ml propidium
iodide (PI) in the dark at 4°C for 30 min. The DNA content of the
cells was quantified by FCM (BD CellQuest Pro; Biosciences,
Franklin Lakes, NJ, USA). Experiments were repeated six times.
Statistical analysis
SPSS software version 17.0 (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. Data are summarized as the
mean ± standard deviation. Student's t-test was used to determine
the significant differences between two groups. One-way analysis of
variance and the Bonferroni test was used to determine the
significant differences among multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Lung histology
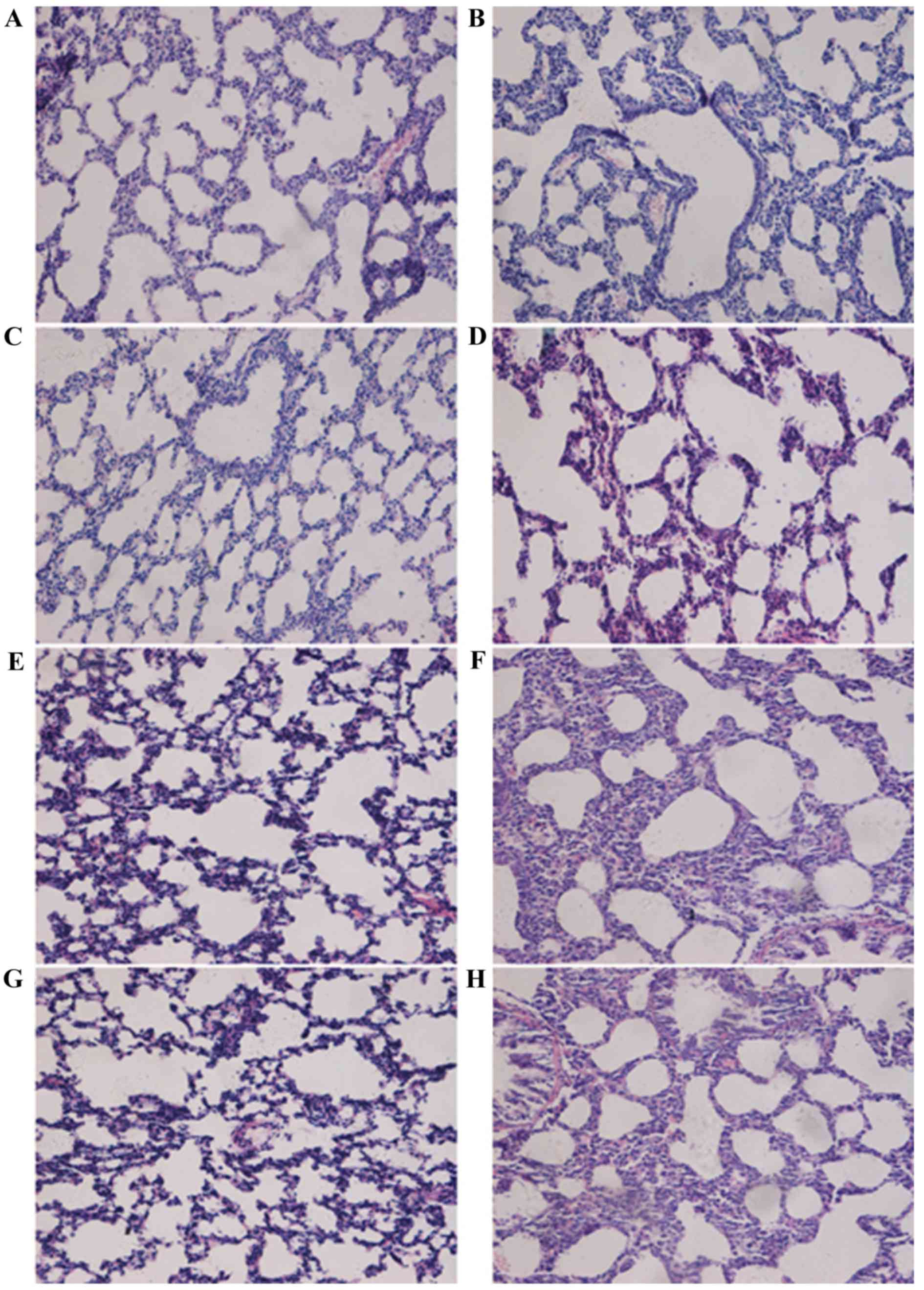
Histological analysis of lung tissues was performed
(Fig. 1). In the control group at
day 3, the lung tissues from normal full-term newborn rats
exhibited an irregular alveolus-like structure and a number of
pulmonary septa (Fig. 1A); at day
7, pulmonary septa were thinner and the number of pulmonary alveoli
increased (Fig. 1C); at day 14,
pulmonary septa became even thinner and the number of pulmonary
secondary septa and alveoli increased (Fig. 1E); at day 21, more pulmonary
secondary septa and alveoli were observed and the alveoli were
distributed more regularly and uniformly (Fig. 1G). In the hyperoxia group, at day
3, thickened pulmonary septa were observed (Fig. 1B); at day 7, there was a decreased
number of alveoli and secondary septa, and increased alveolar size
in the lung tissues (Fig. 1D). At
days 14 and 21, the alveolar space was significantly increased,
there was further evidence of pulmonary septa thickening, the
number of alveoli and secondary septa was decreased greatly, and
the normal structure of alveoli had disappeared (Fig. 1F and H). At days 7, 14 and 21, the
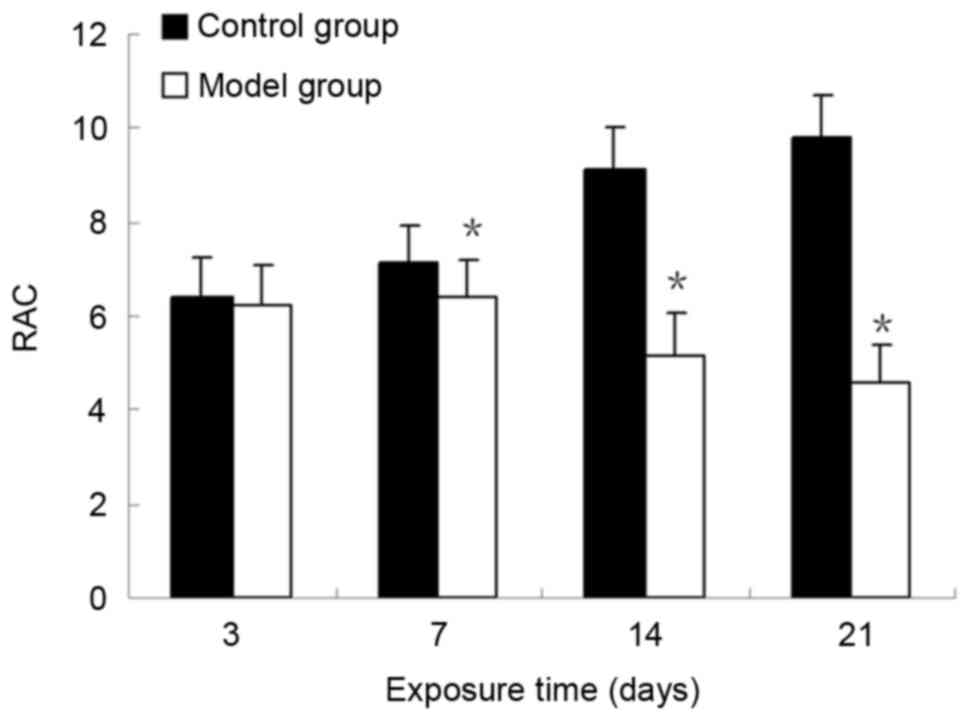
RACs of the control group were significantly increased compared
with those in the BPD model group (P<0.05; Fig. 2). These results demonstrated that
the formation of secondary septa was impaired and alveolar
development was attenuated in the model group. The RACs for
alveolar development were consistent with the results from the
histological observations.
Localization and expression of BMP7
protein in lung tissue
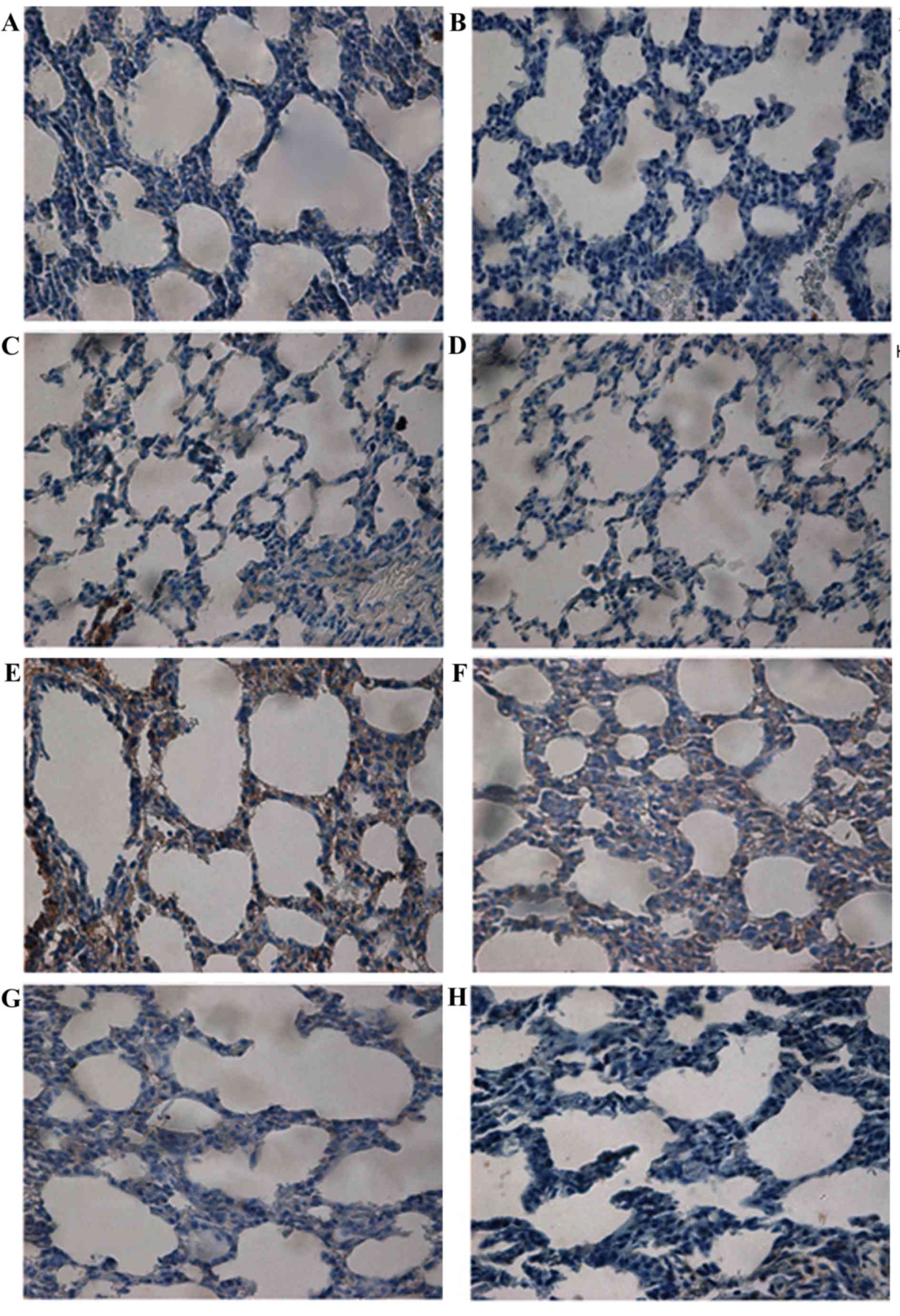
BMP7 protein expression was examined (Fig. 3). Light microscopy demonstrated
that, in the control group, there was little expression of BMP7 in
bronchial epithelial cells (Fig.
3A-D). In the model group, BMP7 distribution was significantly
increased and localized in bronchial epithelial cells in addition
to pulmonary epithelial cells and interstitial cells of the
pulmonary septa at day 3 (Fig.
3E). At day 7, cytoplasmic staining of BMP7 in pulmonary
epithelial cells and interstitial cells was decreased (Fig. 3F). The intensity of this staining
was markedly reduced in lung tissues from rats at days 14 and 21
(Fig. 3G and H, respectively).
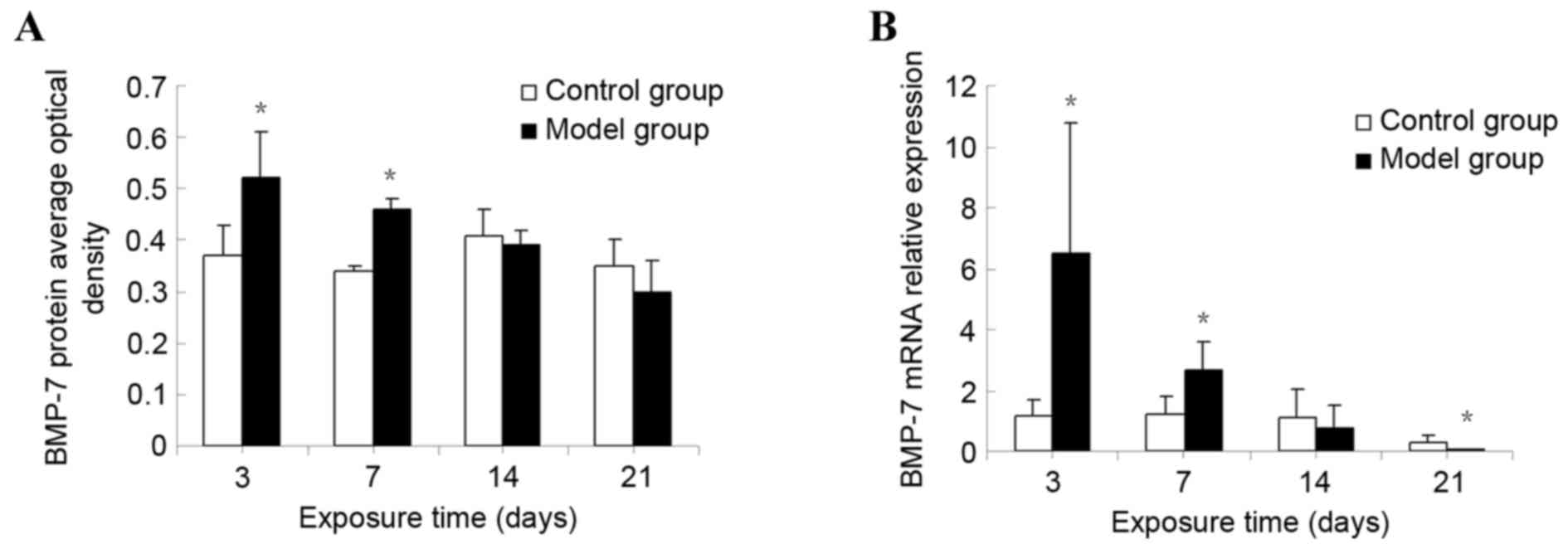
The protein expression of BMP7 in lung tissue was
detected by semi-quantitative analysis. BMP7 protein expression was
markedly increased at day 3. Significant differences between the
model group and the control group were observed (P<0.01). The
expression of BMP7 gradually decreased over time, although it
remained significantly increased compared with that of the control
group at day 7 (P<0.01). However, no statistical difference was
observed in the corresponding expression in the control group at
days 14 and 21 (P>0.05; Fig.
4A).
Expression of BMP7 mRNA in lung
tissue
The relative level of the BMP7 mRNA was markedly
increased at day 3 in the model group. Significant differences were
observed between the model and the control groups (P<0.01). The
relative BMP7 mRNA level gradually decreased, although it remained
significantly increased compared with that of the control group at
day 7 (P<0.01). No statistical difference was observed in the
corresponding expression level in the control group at day 14
(P>0.05). However, at day 21, the BMP7 mRNA level in the
hyperoxia group was significantly decreased compared with that of
the control group (P<0.01; Fig.
4B).
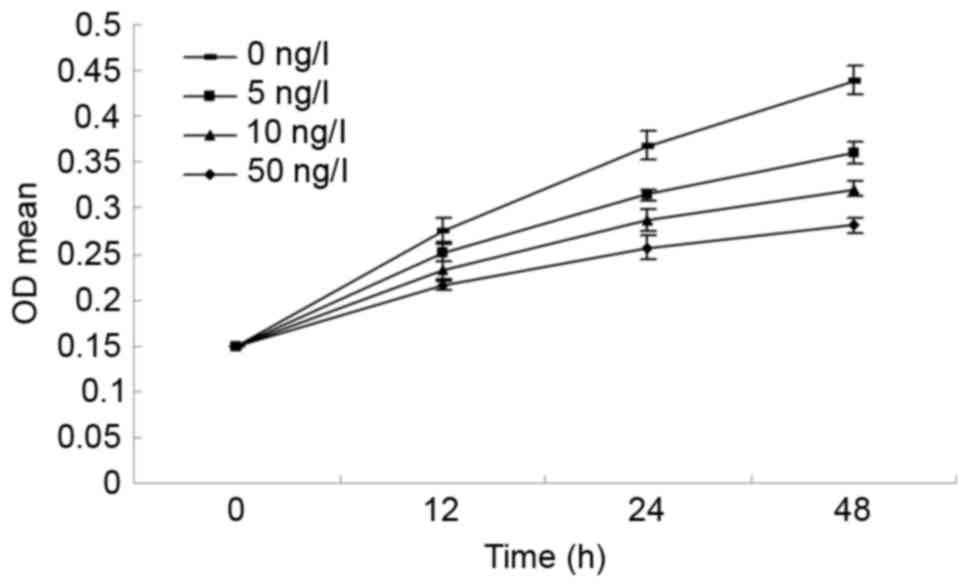
Effect of BMP7 on LF cell
proliferation of newborn rats
The cells were treated with different concentrations
of BMP7 (0, 5, 10 and 50 ng/ml) for different lengths of time (12,
24 and 48 h). As presented in Fig.
5, the MTT assay demonstrated that the proliferation of LF
cells was significantly inhibited. As the concentration of BMP7
increased, the cell proliferation rate decreased, which may suggest
that the inhibitory effect of BMP7 on LF cell proliferation is
dose-dependent. LF cells proliferated at different concentrations
of BMP7 over time; however, the cell proliferation rate decreased
as the concentration of BMP7 increased, which may suggest that the
inhibitory effect of BMP7 on LF cell proliferation is
time-dependent.
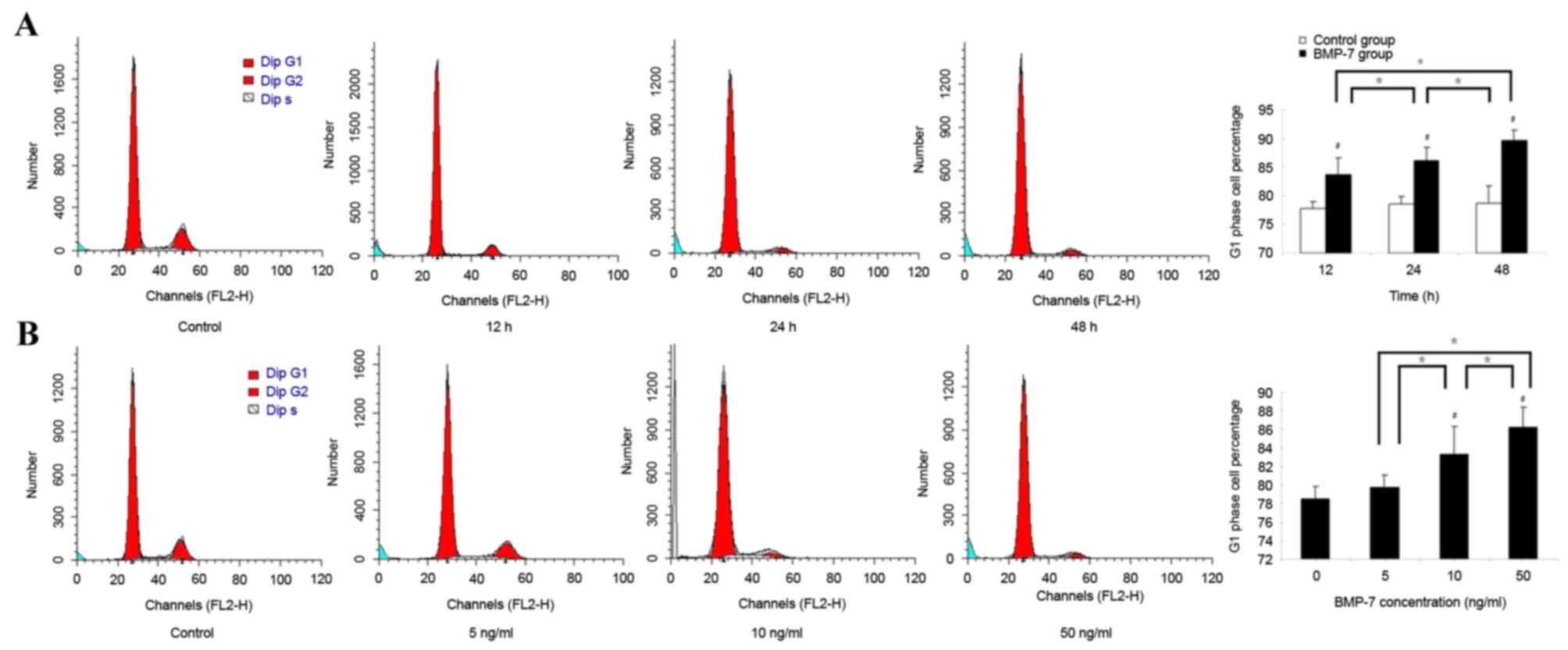
Effect of BMP7 on the cell cycle
distribution of LF cells
The cell cycle distribution of LF cells was analyzed
following treatment with 50 ng/ml BMP7 for different durations. The
percentage of LF cells in the G1 phase increased over
time. The percentage of LF cells in the G1 phase was
markedly increased following treatment with 50 ng/ml BMP7 for 12 h.
Significant differences between the BMP7 group and the control
group were observed (P<0.05). As the time of treatment with BMP7
increased, the percentage of LF cells in the G1 phase
gradually increased and remained significantly increased compared
with the control group at 24 and 48 h (P<0.05). In addition,
significant differences between the 12 and 24 h groups, and the 24
and 48 h groups, were observed (P<0.05; Fig. 6A).
Following treatment with BMP7 at different
concentrations for 24 h, the cell cycle distribution of LF cells
was also analyzed by FCM using PI staining. The percentage of LF
cells in the G1 phase increased when the concentration
of BMP7 increased. Following treatment with BMP7 at concentrations
of 10 and 50 ng/ml, the percentages of cells in the G1
phase gradually increased, and were significantly increased
compared with that of the control group (0 ng/ml; P<0.05).
Additionally, significant differences between the 5 and 10 ng/ml
groups, and between the 10 and 50 ng/ml group, were observed
(P<0.05; Fig. 6B).
Discussion
With the use of pulmonary surfactants and gentle
ventilation strategies, premature neonates with BPD more commonly
present with milder pathological alterations, demonstrating
decreased fibrosis and more lung vascular and alveolar hypoplasia
(6). However, BPD with pulmonary
fibrosis and alveolar hypoplasia pathological changes may be
observed today. Previous studies on TGF-β cytokines primarily
focused on the activity of stimulating fibroblast proliferation
(29,30). However, the mechanism of BMP7 in
neonates with BPD is little studied and the dynamic alterations in
BMP7 expression have not been studied in BPD. Therefore, the
present study was undertaken to examine the possible mechanisms
underlying the pathological alterations in neonates with BPD, and
to identify the function of BMP7 in neonatal LFs.
The present study was the first, to the best of the
authors' knowledge, to demonstrate the dynamic expression of BMP7
in neonates with BPD. It was observed that the expression of BMP7
decreased in an in vivo model, suggesting that BMP may serve
an important role in the inhibition of LF proliferation in the
early stages of abnormal alveolar development. In addition, it was
identified that LFs may be inhibited by BMP7, and that this effect
has dose-dependent and time-dependent characteristics, suggesting
that abnormal alveolar development may be postponed or prevented by
directly inhibiting LF proliferation via BMP7.
Previous studies have used a hyperoxia-induced model
to study BPD (26,31). The present study provided novel
evidence that BMP7 may be a protective cytokine, preventing
abnormal alveolar development in neonates with BPD. In this model,
the pathological alterations included pneumonedema and inflammation
at an early stage and abnormal alveolar and vascular development at
a later period; these results were consistent with a previous study
(7). It was identified that when
the expression of BMP7 decreased there were consistent alterations
in abnormal alveolar development, suggesting that BMP7 may be a
protective cytokine in the prevention of abnormal alveolar
development.
BMP7 has been demonstrated to be important during
the control of a number of important steps of embryogenesis, and
the regulation of growth, proliferation, differentiation and
apoptosis (32–34). The present study demonstrated that
the expression of BMP7 was altered in neonates with BPD. Using
immunohistochemistry, it was observed that hyperoxic exposure
markedly stimulated the expression of BMP7 protein. However, as the
hyperoxic exposure continued, the expression of BMP7 was
downregulated, as confirmed by RT-qPCR analysis. Ohnuma-Koyama
et al (24) identified that
the expression of BMP7 was continuously decreased in DDAC-induced
pulmonary fibrosis. Treatment with BMP7 may inhibit and decreased
silica-induced pulmonary fibrosis in rats (35). Restoration of the expression of
BMP7 and a BMP target gene may prevent or hinder the progression of
fibrosis in silica-induced pulmonary fibrosis (36). These previous results are
consistent with the effects of BMP-7 in experimental models of
kidney and liver fibrosis (37,38).
In addition, the present study demonstrated that
prolonged hyperoxic exposure decreased BMP7 expression. In normal
lung tissue, the expression of BMP7 was stable from beginning to
end, although the expression was decreased by hyperoxic exposure.
This alteration in the expression of BMP7 is paralleled by
prolonged hyperoxic exposure. The expression in the BPD model group
on day 14 was decreased compared with the control group, and was
significantly lower on day 21. In addition, these alterations were
more marked at the gene level. The results of the present study
demonstrated that the expression of BMP7 was inhibited by hyperoxic
exposure at the gene level in neonates with BPD, suggesting that
BMP7 possibly served an essential function in the maintenance of
the normal structure of lung tissue. Secondly, hyperoxic exposure
stimulated BMP7 expression in order to inhibit fibroblast
proliferation, preventing abnormal alveolar development; however,
with continuous hyperoxic exposure the effects of the promotive
cytokines are enhanced, and endogenous BMP7 expression is
suppressed, resulting in fibroblast proliferation and abnormal
alveolar development (39).
In order to confirm that BMP7 may be a protective
cytokine in the prevention of abnormal alveolar development, the
condition of LF proliferation was assayed in vitro by MTT
assay. The results demonstrated that the LF cell proliferation rate
decreased with the increase in BMP7 concentration. With increased
time and concentration, the LF cell proliferation rate demonstrated
a decreasing trend. These results suggested that BMP7 may inhibit
neonatal LF proliferation in vitro. A recent study
demonstrated that covalent grafting of the BMP7 peptide onto the
surface of cobalt-chrome revealed the antifibrotic activity of the
BMP7 peptide, and its capacity to reduce fibroblast adhesion and
proliferation (40). In addition,
the administration of BMP7 induced the differentiation, and
inhibited the proliferation, of podocytes (41).
An additional study demonstrated that BMP7 inhibited
cell proliferation in the subventricular zone through quantitative
inhibition of mitogenesis (42).
Miyazaki et al (43)
confirmed that BMP7 caused G1 cell cycle arrest in
androgen-insensitive prostate carcinoma cells. The effect of BMP7
on the LF cell cycle was assessed in the present study by FCM. The
experiments demonstrated that the percentage of LF cells in the
G1 phase increased as the concentration of BMP7
increased. Under the same concentration of BMP7, the percentage of
LF cells in the G1 phase was higher and increased with
time. These trends were consistent with the results of the MTT
assay, and the results suggested that BMP7 caused cell cycle arrest
in the G1 phase. BMP7 may enhance the G1/S
checkpoint activities and weaken the G2/M checkpoint
activities, causing more LF cells to remain at the G1
stage, and thus perform a function in preventing LF proliferation.
Therefore, there is evidence that the proliferation of LFs may be
inhibited by BMP7 in vivo.
A preliminary study provided certain clues that BMP7
may inhibit LF proliferation (33). During pulmonary fibrosis, BMP7
signaling decreased and TGF-β signaling increased, which suggested
that the balance between BMP7 and TGF-β signaling activities in the
lung is of importance during lung injury and repair, and is the
notable mechanism in pulmonary fibrosis (37). Whether the abnormal alveolar
development in neonatal rats with BPD is via suppression of BMP7
signaling activities, resulting in a BMP7/TGF-β signaling
imbalance, requires further study.
In conclusion, the results of the present study
demonstrated that BMP7 may be involved in the occurrence and
development of BPD. The data demonstrated that BMP7 expression
decreased in the BPD neonatal rat model, and the alterations were
inverse to the severity of the abnormal alveolar development. In
vitro experiments confirmed that BMP7 may regulate the cell
cycle of neonatal LFs and possesses the effect of inhibiting LF
proliferation. Therefore, the present study suggested that the
severity of the abnormal alveolar development may be associated
with the decreased expression of BMP7, and that BMP7 may regulate
LF proliferation, at least in part due to regulation of the LF cell
cycle, in order to resist abnormal alveolar development. However,
the specific mechanisms underlying these findings remain to be
elucidated in a future study.
Acknowledgements
The present study was supported by grants from the
Natural Science Foundation of China (grant nos. 30872781 and
81170605).
References
|
1
|
Bhandari A and Bhandari V: Pitfalls,
problems, and progress in bronchopulmonary dysplasia. Pediatrics.
123:1562–1573. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Trembath A and Laughon MM: Predictors of
bronchopulmonary dysplasia. Clin Perinatol. 39:585–601. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stoll BJ, Hansen NI, Bell EF, Shankaran S,
Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, et
al: Neonatal outcomes of extremely preterm infants from the NICHD
Neonatal Research Network. Pediatrics. 126:443–456. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Özdemir ÖM, Gözkeser E, Bir F and Yenisey
Ç: The effects of resveratrol on hyperoxia-induced lung injury in
neonatal rats. Pediatr Neonatol. 55:352–357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gien J and Kinsella JP: Pathogenesis and
treatment of bronchopulmonary dysplasia. Curr Opin Pediatr.
23:305–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jobe AH and Bancalari E: Bronchopulmonary
dysplasia. Am J Respir Crit Care Med. 163:1723–1729. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thébaud B and Abman SH: Bronchopulmonary
dysplasia: Where have all the vessels gone? Roles of angiogenic
growth factors in chronic lung disease. Am J Respir Crit Care Med.
175:978–985. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Husain AN, Siddiqui NH and Stocker JT:
Pathology of arrested acinar development in postsurfactant
bronchopulmonary dysplasia. Hum Pathol. 29:710–717. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dasgupta C, Sakurai R, Wang Y, Guo P,
Ambalavanan N, Torday JS and Rehan VK: Hyperoxia-induced neonatal
rat lung injury involves activation of TGF-{beta} and Wnt signaling
and is protected by rosiglitazone. Am J Physiol Lung Cell Mol
Physiol. 296:L1031–L1041. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boon MR, van der Horst G, van der Pluijm
G, Tamsma JT, Smit JW and Rensen PC: Bone morphogenetic protein 7:
A broad-spectrum growth factor with multiple target therapeutic
potency. Cytokine Growth Factor Rev. 22:221–229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ali IH and Brazil DP: Bone morphogenetic
proteins and their antagonists: Current and emerging clinical uses.
Br J Pharmacol. 171:3620–3632. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bragdon B, Moseychuk O, Saldanha S, King
D, Julian J and Nohe A: Bone morphogenetic proteins: A critical
review. Cell Signal. 23:609–620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Szabò H, Fiorino G, Spinelli A, Rovida S,
Repici A, Malesci AC and Danese S: Review article: Anti-fibrotic
agents for the treatment of Crohn's disease-lessons learnt from
other diseases. Aliment Pharmacol Ther. 31:189–201. 2010.PubMed/NCBI
|
|
14
|
Djamali A and Samaniego M: Fibrogenesis in
kidney transplantation: Potential targets for prevention and
therapy. Transplantation. 88:1149–1156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yanagita M: Inhibitors/antagonists of
TGF-β system in kidney fibrosis. Nephrol Dial Transplant.
27:3686–3691. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weiskirchen R, Meurer SK, Gressner OA,
Herrmann J, Borkham-Kamphorst E and Gressner AM: BMP-7 as
antagonist of organ fibrosis. Front Biosci (Landmark Ed).
14:4992–5012. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gressner OA, Rizk MS, Kovalenko E,
Weiskirchen R and Gressner AM: Changing the pathogenetic roadmap of
liver fibrosis? Where did it start; where will it go? J
Gastroenterol Hepatol. 23:1024–1035. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weiskirchen R and Meurer SK: BMP-7
counteracting TGF-beta1 activities in organ fibrosis. Front Biosci
(Landmark Ed). 18:1407–1434. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Myllärniemi M, Lindholm P, Ryynänen MJ,
Kliment CR, Salmenkivi K, Keski-Oja J, Kinnula VL, Oury TD and Koli
K: Gremlin-mediated decrease in bone morphogenetic protein
signaling promotes pulmonary fibrosis. Am J Respir Crit Care Med.
177:321–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kinoshita K, Iimuro Y, Otogawa K, Saika S,
Inagaki Y, Nakajima Y, Kawada N, Fujimoto J, Friedman SL and Ikeda
K: Adenovirus-mediated expression of BMP-7 suppresses the
development of liver fibrosis in rats. Gut. 56:706–714. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gressner OA and Gao C: Monitoring
fibrogenic progression in the liver. Clin Chim Acta. 433:111–122.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyazaki Y, Ueda H, Yokoo T, Utsunomiya Y,
Kawamura T, Matsusaka T, Ichikawa I and Hosoya T: Inhibition of
endogenous BMP in the glomerulus leads to mesangial matrix
expansion. Biochem Biophys Res Commun. 340:681–688. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morrissey J, Hruska K, Guo G, Wang S, Chen
Q and Klahr S: Bone morphogenetic protein-7 improves renal fibrosis
and accelerates the return of renal function. J Am Soc Nephrol. 13
Suppl 1:S14–S21. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ohnuma-Koyama A, Yoshida T,
Tajima-Horiuchi H, Takahashi N, Yamaguchi S, Ohtsuka R,
Takeuchi-Kashimoto Y, Kuwahara M, Takeda M, Nakashima N and Harada
T: Didecyldimethylammonium chloride induces pulmonary fibrosis in
association with TGF-β signaling in mice. Exp Toxicol Pathol.
65:1003–1009. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Izumi N, Mizuguchi S, Inagaki Y, Saika S,
Kawada N, Nakajima Y, Inoue K, Suehiro S, Friedman SL and Ikeda K:
BMP-7 opposes TGF-beta1-mediated collagen induction in mouse
pulmonary myofibroblasts through Id2. Am J Physiol Lung Cell Mol
Physiol. 290:L120–L126. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang H, Fu J, Xue X, Yao L, Qiao L, Hou A,
Jin L and Xing Y: Epithelial-mesenchymal transitions in
bronchopulmonary dysplasia of newborn rats. Pediatr Pulmonol.
49:1112–1123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kelleher MD, Naureckas ET, Solway J and
Hershenson MB: In vivo hyperoxic exposure increases cultured lung
fibroblast proliferation and c-Ha-ms expression. Am J Respir Cell
Mol Biol. 12:19–26. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakanishi H, Sugiura T, Streisand JB,
Lonning SM and Roberts JD Jr: TGF-beta-neutralizing antibodies
improve pulmonary alveologenesis and vasculogenesis in the injured
newborn lung. Am J Physiol Lung Cell Mol Physiol. 293:L151–L161.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kumarasamy A, Schmitt I, Nave AH, Reiss I,
van der Horst I, Dony E, Roberts JD Jr, de Krijger RR, Tibboel D,
Seeger W, et al: Lysyl oxidase activity is dysregulated during
impaired alveolarization of mouse and human lungs. Am J Respir Crit
Care Med. 180:1239–1252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ahn SY, Chang YS, Sung DK, Yoo HS, Sung
SI, Choi SJ and Park WS: Cell type-dependent variation in paracrine
potency determines therapeutic efficacy against neonatal hyperoxic
lung injury. Cytotherapy. 17:1025–1035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sieber C, Kopf J, Hiepen C and Knaus P:
Recent advances in BMP receptor signaling. Cytokine Growth Factor
Rev. 20:343–355. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Danesh SM, Villasenor A, Chong D, Soukup C
and Cleaver O: BMP and BMP receptor expression during murine
organogenesis. Gene Expr Patterns. 9:255–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kazama I, Mahoney Z, Miner JH, Graf D,
Economides AN and Kreidberg JA: Podocyte-derived BMP7 is critical
for nephron development. J Am Soc Nephrol. 19:2181–2191. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang G, Zhu Z, Wang Y, Gao A, Niu P and
Tian L: Bone morphogenetic protein-7 inhibits silica-induced
pulmonary fibrosis in rats. Toxicol Lett. 220:103–108. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leppäranta O, Tikkanen JM, Bespalov MM,
Koli K and Myllärniemi M: Bone morphogenetic protein-inducer
tilorone identified by high-throughput screening is antifibrotic in
vivo. Am J Respir Cell Mol Biol. 48:448–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang T, Chen SL, Lu XJ, Shen CY, Liu Y and
Chen YP: Bone morphogenetic protein 7 suppresses the progression of
hepatic fibrosis and regulates the expression of gremlin and
transforming growth factor β1. Mol Med Rep. 6:246–252.
2012.PubMed/NCBI
|
|
38
|
Wang S, de Caestecker M, Kopp J, Mitu G,
Lapage J and Hirschberg R: Renal bone morphogenetic protein-7
protects against diabetic nephropathy. J Am Soc Nephrol.
17:2504–2512. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Alejandre-Alcázar MA, Kwapiszewska G,
Reiss I, Amarie OV, Marsh LM, Sevilla-Pérez J, Wygrecka M, Eul B,
Köbrich S, Hesse M, et al: Hyperoxia modulates TGF-beta/BMP
signaling in a mouse model of bronchopulmonary dysplasia. Am J
Physiol Lung Cell Mol Physiol. 292:L537–L549. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tan HC, Poh CK, Cai Y and Wang W:
Anti-fibrosis effect of BMP-7 peptide functionalization on cobalt
chromium alloy. J Orthop Res. 31:983–990. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yamada S, Nakamura J, Asada M, Takase M,
Matsusaka T, Iguchi T, Yamada R, Tanaka M, Higashi AY, Okuda T, et
al: Twisted gastrulation, a BMP antagonist, exacerbates podocyte
injury. PLoS One. 9:e891352014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Douet V, Arikawa-Hirasawa E and Mercier F:
Fractone-heparan sulfates mediate BMP-7 inhibition of cell
proliferation in the adult subventricular zone. Neurosci Lett.
528:120–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Miyazaki H, Watabe T, Kitamura T and
Miyazono K: BMP signals inhibit proliferation and in vivo tumor
growth of androgen-insensitive prostate carcinoma cells. Oncogene.
23:9326–9335. 2004. View Article : Google Scholar : PubMed/NCBI
|