Introduction
Postmenopausal osteoporosis (OPM) is a common type
of osteoporosis in females (1). It
is a systemic, chronic bone disease that presents as microstructure
degradation of osseous tissue, decreased bone mineral density and
increased osteopsathyrosis caused by hypoovarianism and reduced
estrogen levels in the body following menopause (2). Pathological fracture is a serious
complication of the disease and has an incidence rate >70%
(3). In addition, >50% of the
female population >50 years old suffer from osteoporosis
(2). With the rapid increase of
the elderly population, the incidence rate of OPM and of fracture
is increasing annually (2).
Studies have demonstrated that the incidence rate of OPM and
fracture is increasing at a growth rate of 18% every five years
worldwide, which seriously influences the health and quality of
life of middle-aged and elderly women, and has increased the
financial and human burden on society as a whole and family members
of patients (4,5).
At present, it is universally recognized that the
root cause for OPM incidence is a disequilibrium between bone
formation and bone resorption arising from the lack of estrogen,
which leads to a disorder of bone reconstruction (6). A previous study primarily focused on
abnormally increased bone resorption mediated by osteoclasts
(7). Human bone marrow stem cells
(BMSCs) are the sourcing cell of osteoblasts in osseous tissue
(8). BMSCs serves significant role
on bone formation and bone resorption maintaining bone
reconstruction process (8). At
present, a previous study indicated that the root cause of OPM is
the abnormal differentiation of BMSCs, which leads to reduced
numbers of intraosseous osteoblasts and increased adipocytes
(9). However, the specific
regulatory mechanism of BMMSC differentiation imbalance remains to
be clarified.
MicroRNA (miRNA/miR) are highly conserved short
sequence RNAs that exist extensively in animals and plants
(10). With the exception of the Y
chromosome, the majority of other human chromosomes express miRNA
genes (11). The functional
mechanism of miRNAs is the specific inhibition or direct regulation
of the expression of target genes following degradation and
transcription through complementary binding to the 3′ untranslated
region of target mRNA (11).
According to calculations, ~30–40% of human genes are regulated by
miRNAs at the gene translation level (12). It has been demonstrated that miRNAs
serve important regulatory roles on cell proliferation,
differentiation, division, apoptosis, signal transduction and other
vital processes (11). In
addition, miRNAs have been implicated in the initiation and
development of various diseases, including cancer, cardiovascular
disease, osteoarthritis and bacterial virus infection (11). Furthermore, studies in recent years
have demonstrated that miRNAs serve important functions in
self-renewal and multidirectional differentiation processes, and
may determine the fate of stem cells (12,13).
The TGF-β receptor is a serine/threonine kinase
receptor and its signal transmission may be conducted through the
Smad signaling pathway (14,15).
TGF-β strengthens the repair capacity following bone injury
primarily through promoting enhanced cell division, as well as the
generation of osteoblasts and matrix, and type I collagen synthesis
(16). The proliferation effect of
TGF-β significantly increases the number of mesenchymal cells,
chondrocytes and osteoblasts, by expression of bone morphogenetic
protein (BMP), which may provide an increased number of target
cells for osseous tissue regeneration and rehabilitation (14).
Smad protein was discovered in a drosophila and
screwworm study originally (17).
The protein SMA in caenorhabditis elegans also has the same
effect (18). Smad protein serve a
significant role in signal transduction following Ser/Thr kinase
receptor activation (18).
Consequently, the target gene of Smad is the TGF-β receptor, which
conducts the signal of ligand and receptor function to intermediary
molecules of nucleus (18).
TGF-β/Smads regulates osteogenic differentiation in cells and
directly transduces TGF-β signals from the cytomembrane to cell
nucleus and serves an important role in differentiation (18,19).
Members of the TGF-β family primarily transfer signals via Smad
proteins (17).
Mutations in the collagen type IV α1 chain (COL4A1),
a major component of the basilar membrane, have been implicated in
various diseases including HANAC syndrome, renal disease,
porencephaly, and cataracts (20,21).
From 2005, the occurrence of the COL4A1 gene mutation and
associated hereditary disease has started to attract the attention
(22,23). The COL4A1 gene is the major
structural component of the basilar membrane (22,24).
COL4A1 is associated with bone mineral density in different parts
of the bone (25,26). Li et al (21) demonstrated that the inhibition of
miR-214-5p promotes the cell survival of MC3T3-E1 osteoblastic
cells by targeting COL4A1. In the present study, the role of the
miR-214-5p signaling pathway in adipogenic differentiation of BMSCs
was investigated.
Materials and methods
Identification of HBMSCs and
dexamethasone-induced adipogenic differentiation
The PTA-1058 HBMSC cell line was obtained from the
American Type Culture Collection (Manassas, VA, USA) and cultured
in α-minimum essential medium (α-MEM; HyClone; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (HyClone; Thermo Fisher Scientific, Inc.), penicillin
(100 IU/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and
streptomycin (100 µg/ml, Sigma-Aldrich; Merck KGaA) at 37°C in 5%
CO2. HBMSC medium supplemented with dexamethasone (10
mol/l; Sigma-Aldrich; Merck KGaA) was added into HBMSC for 2 weeks.
Oil red O staining was performed to confirm successful adipogenic
differentiation following dexamethasone treatment.
miRNA transfection
The miR-214-5p (5′-GGCCTGGCTGGACAGAGTTG-3′),
anti-miR-214-5p (5′-ACAGCAGGCACAGACAGGCAG-3′) and negative control
(5′-CCCCCCCCCCCCC-3′) used in the current study were synthesized by
Shanghai GenePharma Co., Ltd. (Shanghai, China). HBMSCs were plated
in 6-well plates (~50% confluence) and were transfected with 50 nM
miR-214-5p or anti-miR-214-5p using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. In further experiments, 50 nM
anti-miR-214-5p was transfected into cells for 4 h using
Lipofectamine® 2000, and then fresh α-MEM was
subsequently added into cells with TGF-β inhibitor (10 nM; A 77–01;
MedChemExpress China, Shanghai, China) for 48 h at 37°C.
Oil red O staining
After transfection, oil red O staining was conducted
in HBMSCs (1×105 cell/ml) at 2 weeks following
dexamethasone treatment at 37°C. Cells were washed twice with PBS
and fixed with 10% formalin for 10 min at 37°C. Subsequently, cells
were stained with filtered oil red O solution for 1 h at 37°C and
observed using a Leica Microsystem fluorescence microscope (DFC300
FX; Leica Microsystems GmbH, Wetzlar, Germany).
Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR). Total mRNA was extracted from HMBSCs using
TRIzol (Takara Biotechnology Co., Ltd., Dalian, China). A total of
2–4 µg total mRNA was synthesized to cDNA using the PrimeScript 1st
Strand cDNA Synthesis kit (Takara Bio, Inc., Otsu, Japan) at 37°C
for 1 h and 85°C for 1 min. qPCR was performed with
SYBR® Green master mix kit (cat. no. 303402; Takara Bio,
Inc., Dalian, China) and an ABI 7300 Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermal cycling
condition was set as 95°C for 10 min, followed by 40 cycles of 95°C
for 30 sec and 60°C for 30 sec. Primer sequences for qPCR are
presented in Table I. The relative
expression of miRNA and mRNA to U6 or GAPDH expression,
respectively, was measured using the comparative 2−ΔΔCq
method (27).
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward | Reverse |
|---|
| miR-214-5p |
5′-GGCCTGGCTGGACAGA-3′ |
5′-GTCACATGACAACCCAGCCT-3′ |
| ALP |
5′-TGGAGGTTCAGAAGCTCAACACCA-3′ |
5′-ATCTCGTTCTCTGAGTACCAGTC-3′ |
| Runx2 |
5′-CCGCACAACCGCACCAT-3′ |
5′-CGCTCCGGCCCACAAATCTC-3′ |
| OC |
5′-CAGCGGTGCAGAGTCCAGCAAA-3′ |
5′-GATGTGGTCAGCCAACTCGTCA-3′ |
| COL1A1 |
5′-CCTGGAAAGAATGGAGATGATG-3′ |
5′-ATCCAAACCACTGAAACCTCTG-3′ |
| PPARγ |
5′-TTATGGAGCCTAAGTTTGAGTTTGC-3′ |
5′-TTGTCTTGGATGTCCTCGATGG-3′ |
| CEBPα |
5′-GAAGTCGGTGGATAAGAACAGCA-3′ |
5′-CTCCAACACCTTCTGCTGCGT-3′ |
| Adiponectin |
5′-GTCCCTCCACCCAAGGAAACT-3′ |
5′-CTCCTGTCATTCCAGCATCTCC-3′ |
| U6 |
5′-CTCGCTTCGGCAGCACATATACT-3′ |
5′-ACGCTTCACGAATTTGCGTGTC-3′ |
| GAPDH |
5′-GGGCTGCTTTTAACTCTGGT-3′ |
5′-GCAGGTTTTTCTAGACGG-3′ |
Western blot analysis
HBMSCs were lysed using radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China) on ice for 30 min and supernatants were acquired though
centrifugation at 14,000 × g for 20 min at 4°C. Subsequently,
proteins were quantified using a bicinchoninic acid assay kit
(Beyotime Institute of Biotechnology) and 50–100 µg protein was
resolved by 8–10% SDS-PAGE and transferred to a polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). Following
blocking with 5% skim milk powder in TBS-0.1% Tween-20 (TBST) for 1
h at 37°C, membranes were incubated overnight at 4°C with the
following primary antibodies: Anti-TGF-β (cat. no. 3709; 1:2,000;
Cell Signaling Technology, Inc., Danvers, MA, USA); anti-p-Smad2
(cat. no. 8828; 1:1,000; Cell Signaling Technology, Inc.);
anti-COL4A1 (cat. no. sc-517572; 1:1,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) and anti-GAPDH (cat. no. 3683; 1:5,000; Cell
Signaling Technology, Inc.). Membranes were washed with TBST and
incubated with horseradish peroxidase-conjugated anti-rabbit or
mouse IgG secondary antibody (cat. nos. 7076 and 7074; 1:5,000;
Cell Signaling Technology, Inc.) for 1 h at room temperature. The
membranes were visualized with BeyoECL Plus (Beyotime Institute of
Biotechnology, Haimen, China) and analyzed using ImageJ 2× software
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). Experiments were
repeated three times. Data were analyzed using one-way analysis of
variance followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of miR-214-5p in
dexamethasone-induced adipogenic differentiation of HBMSCs
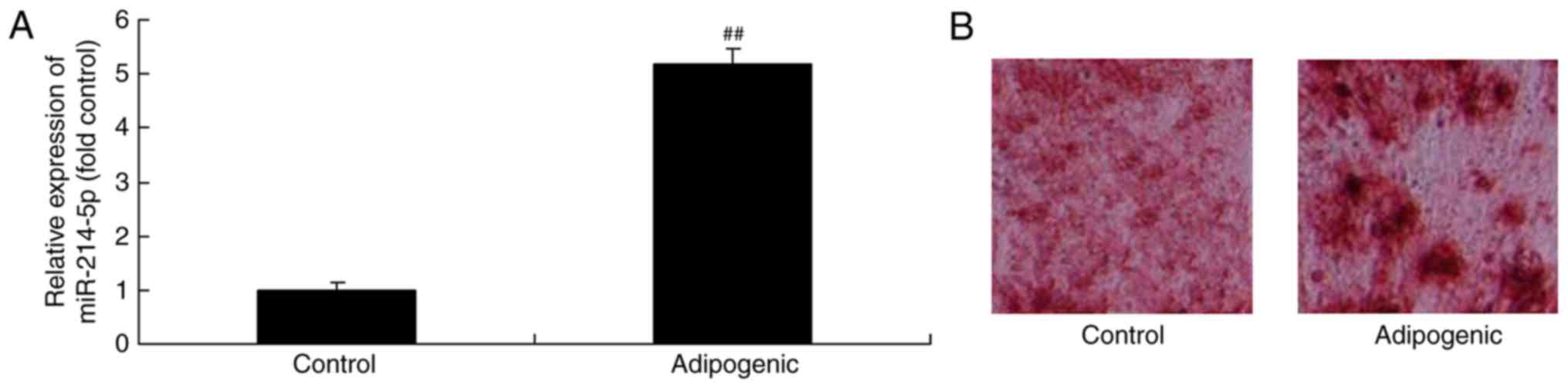
Initially, miR-214-5p expression in
dexamethasone-induced adipogenic differentiation of HBMSCs was
investigated. It was demonstrated that miR-214-5p expression in
dexamethasone-induced differentiated HBMSCs was higher compared
with the control group (Fig. 1A),
while oil red O staining demonstrated that dexamethasone treatment
led to successful adipogenic differentiation of HBMSCs (Fig. 1B).
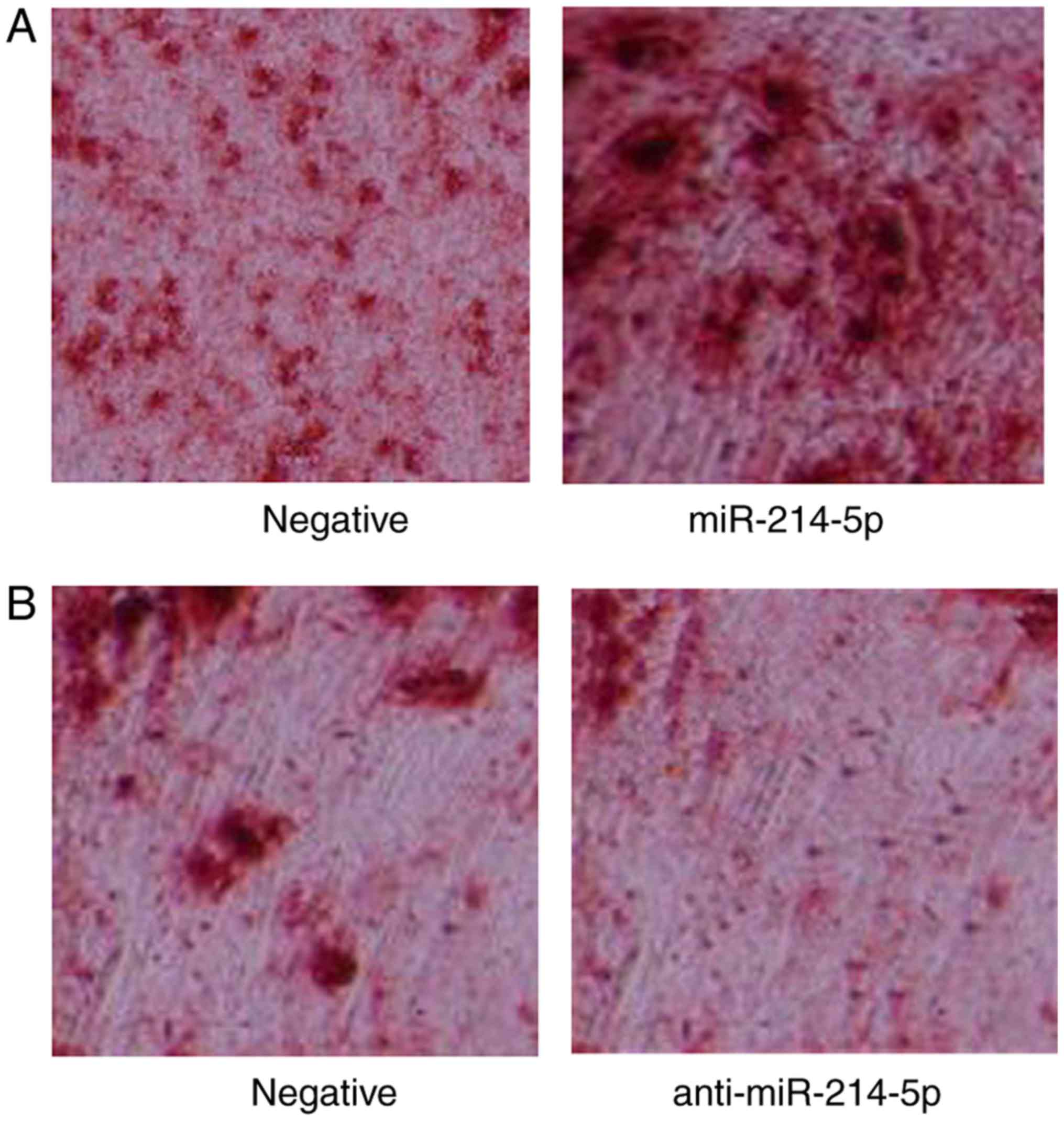
miR-214-5p promotes adipogenic
differentiation of HBMSCs
Subsequently, the effect of miR-214-5p expression on
the adipogenic differentiation of HBMSCs was investigated. Oil red
O staining was performed in miR-214-5p/anti-miR-214-5p-transfected
PTA-1058 cells at 2 weeks following dexamethasone treatment.
Notably, the results indicated that downregulation of miR-214-5p,
through transfection of anti-miR-214-5p, led to reduced oil red O
staining and thereby indicated reduced differentiation of PTA-1058
cells, compared with the control group (Fig. 2). Following overexpression of
miR-214-5p, the opposite effect was observed (Fig. 2).
Overexpression of miR-214-5p affects
alkaline phosphatase (ALP), runt-related transcription factor 2
(Runx2), OC and COL1A1 mRNA expression in HBMSCs
RT-qPCR was initially performed to confirm
successful miR-214-5p overexpression following transfection
(Fig. 3A). Subsequently, the
mechanism by which miR-214-5p affects adipogenic differentiation of
HBMSCs was investigated by measuring the mRNA expression of ALP,
Runx2, OC and COL1 in HBMSCs. RT-qPCR indicated that upregulated
expression of miR-214-5p led to downregulated expression of ALP,
Runx2, OC and COL1 mRNA in HBMSCs cells compared with the negative
control group (Fig. 3B-E).
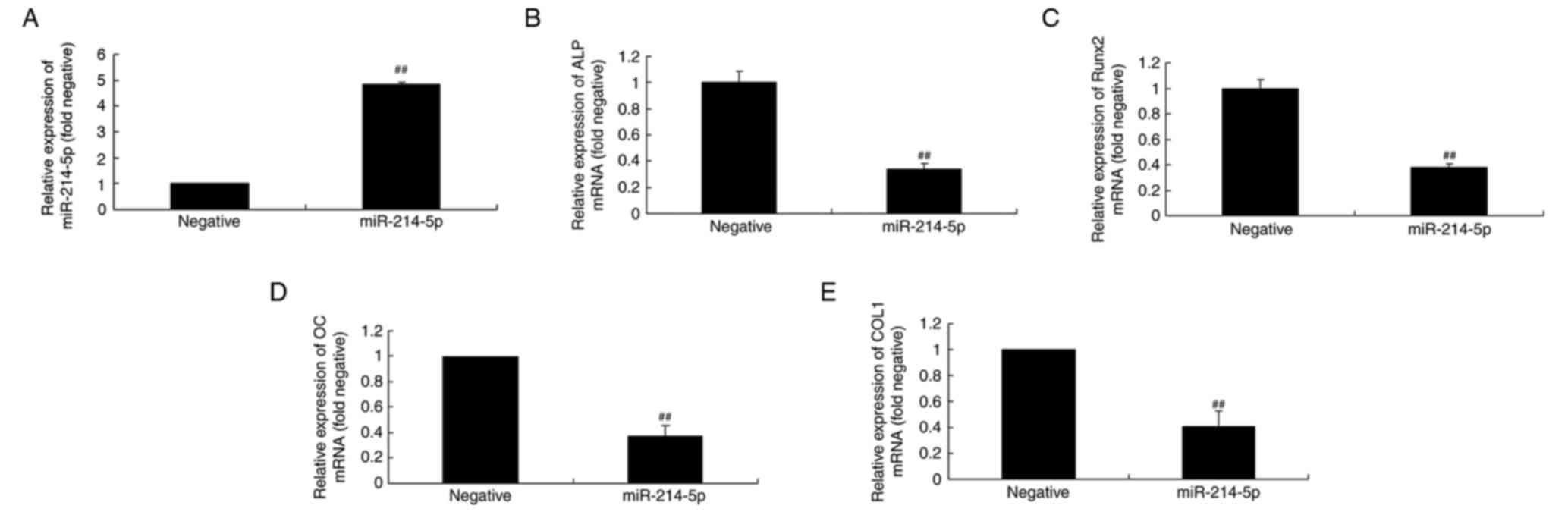 | Figure 3.Overexpression of miR-214-5p affected
ALP, Runx2, OC and COL1A1 mRNA expression. (A) RT-qPCR was
initially performed to confirm successful overexpression of
miR-214-5p following transfection. RT-qPCR was subsequently
performed to determine the effect of miR-214-5p overexpression on
(B) ALP, (C) Runx2, (D) OC and (E) COL1 mRNA expression in human
bone marrow stem cells. ##P<0.01 vs. negative control group.
miR, microRNA; ALP, alkaline phosphatase; Runx2, runt-related
transcription factor 2; OC, osteocalcin; COL1A1, collagen type IV
α1 chain; RT-qPCR, reverse transcription-quantitative polymerase
chain reaction; negative, negative control transfection group;
miR-214-5p group, overexpression of miR-214-5p group. |
Downregulation of miR-214-5p affects
ALP, Runx2, OC and COL1 mRNA expression in HBMSCs
RT-qPCR was initially performed to confirm
successful miR-214-5p downregulation following transfection of
anti-miR-214-5p (Fig. 4A).
Furthermore, as demonstrated in Fig.
4B-E, downregulation of miR-214-5p led to increased ALP, Runx2,
OC and COL1 mRNA expression in HBMSCs compared with the negative
control group.
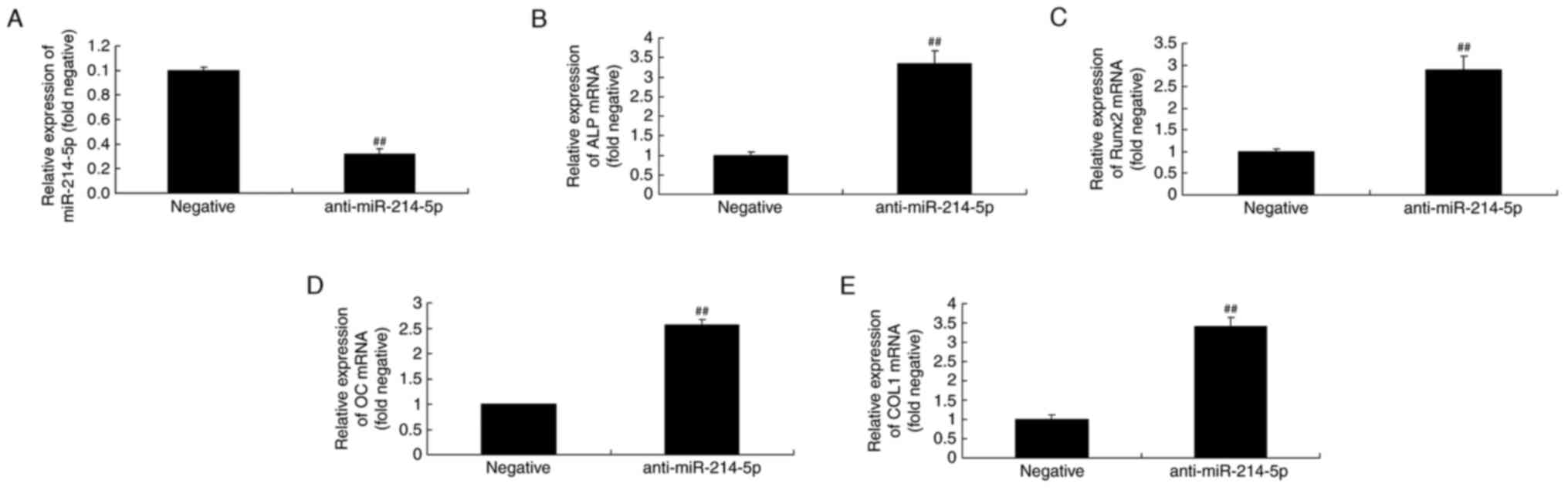 | Figure 4.Downregulation of miR-214-5p affected
ALP, Runx2, OC and COL1A1 mRNA expression. (A) RT-qPCR was
initially performed to confirm successful downregulation of
miR-214-5p following transfection. RT-qPCR was subsequently
performed to determine the effect of anti-miR-214-5p on (B) ALP,
(C) Runx2, (D) OC and (E) COL1 mRNA expression in human bone marrow
stem cells. ##P<0.01 vs. negative control group. miR, microRNA;
ALP, alkaline phosphatase; Runx2, runt-related transcription factor
2; OC, osteocalcin; COL1A1, collagen type IV α1 chain; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction;
negative, negative control transfection group; anti-miR-214-5p
group, miR-214-5p downregulation group. |
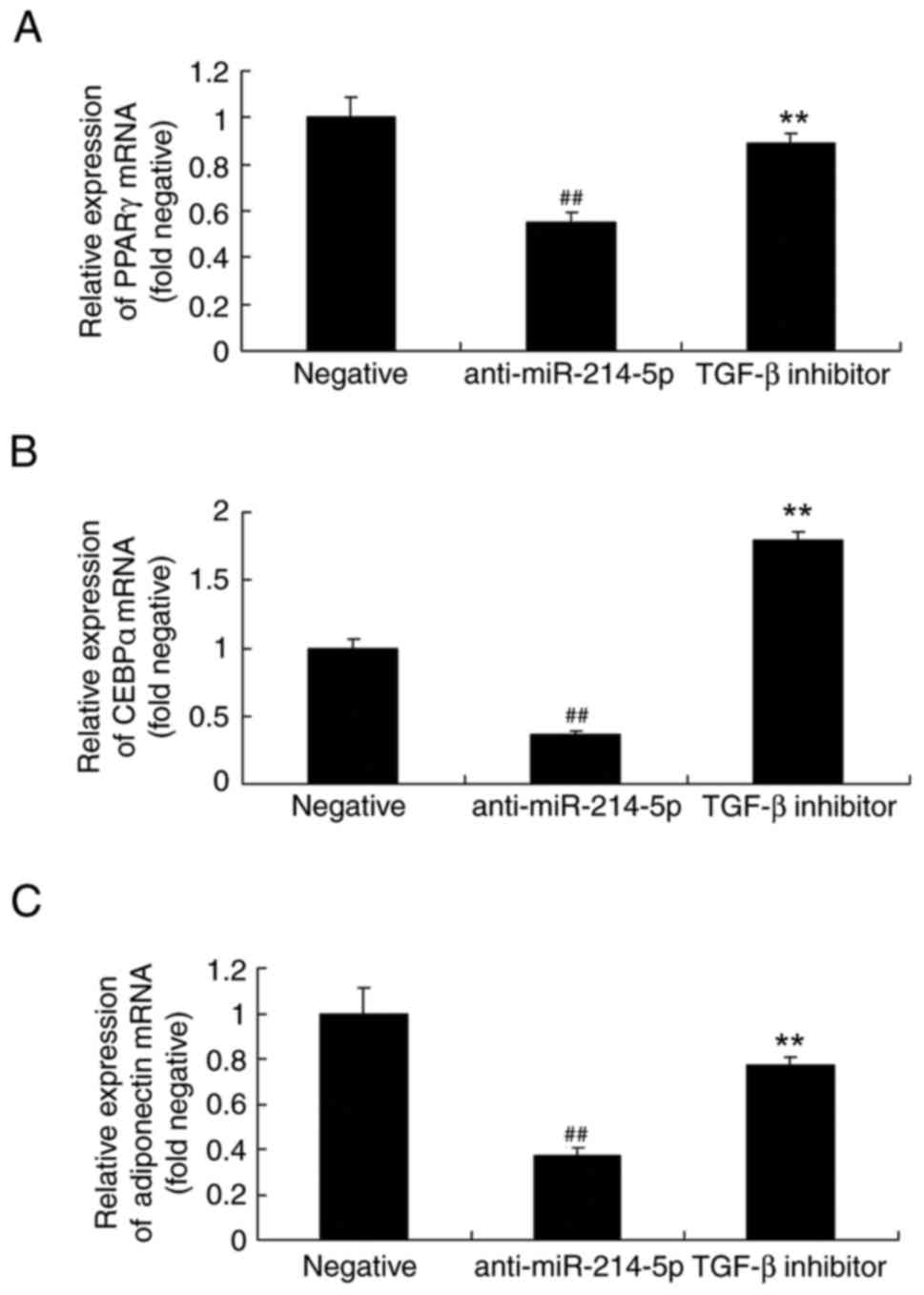
Overexpression of miR-214-5p affects
peroxisome proliferator-activated receptor γ (PPARγ),
CCAAT/enhancer-binding protein α (CEBPα) and adiponectin mRNA
expression in HBMSCs
PPARγ, CEBPα and adiponectin regulates osteogenic
differentiation of HBMSCs (28),
therefore the expression of PPARγ, CEBPα and adiponectin of HBMSCs
by miR-214-5p was investigated. The mRNA levels of PPARγ, CEBPα and
adiponectin in HBMSCs were also investigated following
overexpression of miR-214-5p. As illustrated in Fig. 5, the mRNA expression levels of
PPARγ, CEBPα and adiponectin following miR-214-5p overexpression
were higher compared with the negative control group.
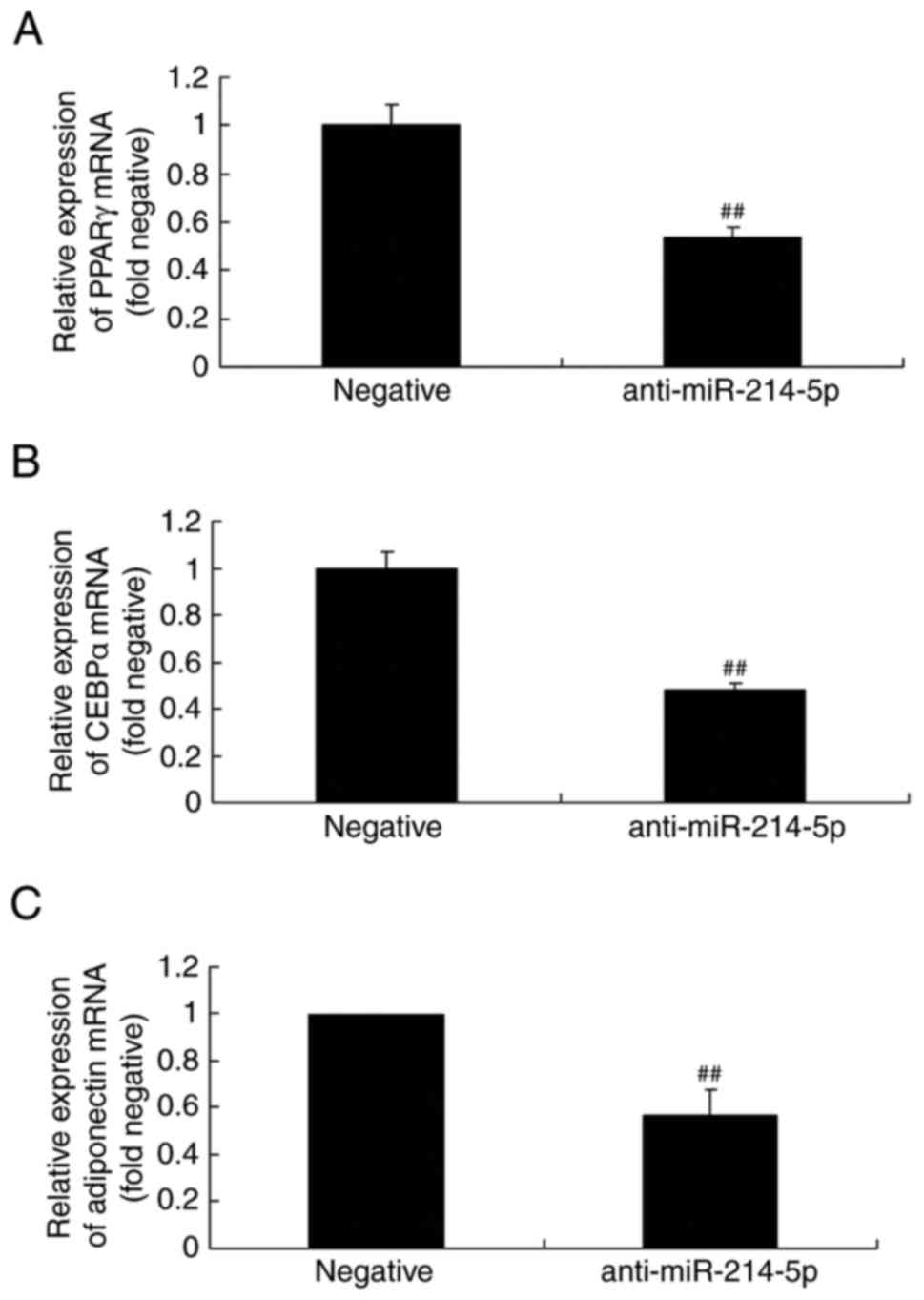
Downregulation of miR-214-5p affects
PPARγ, CEBPα and adiponectin mRNA expression in HBMSCs
By contrast, following downregulation of miR-214-5p,
the mRNA levels of PPARγ, CEBPα and adiponectin were lower compared
with the negative control group (Fig.
6).
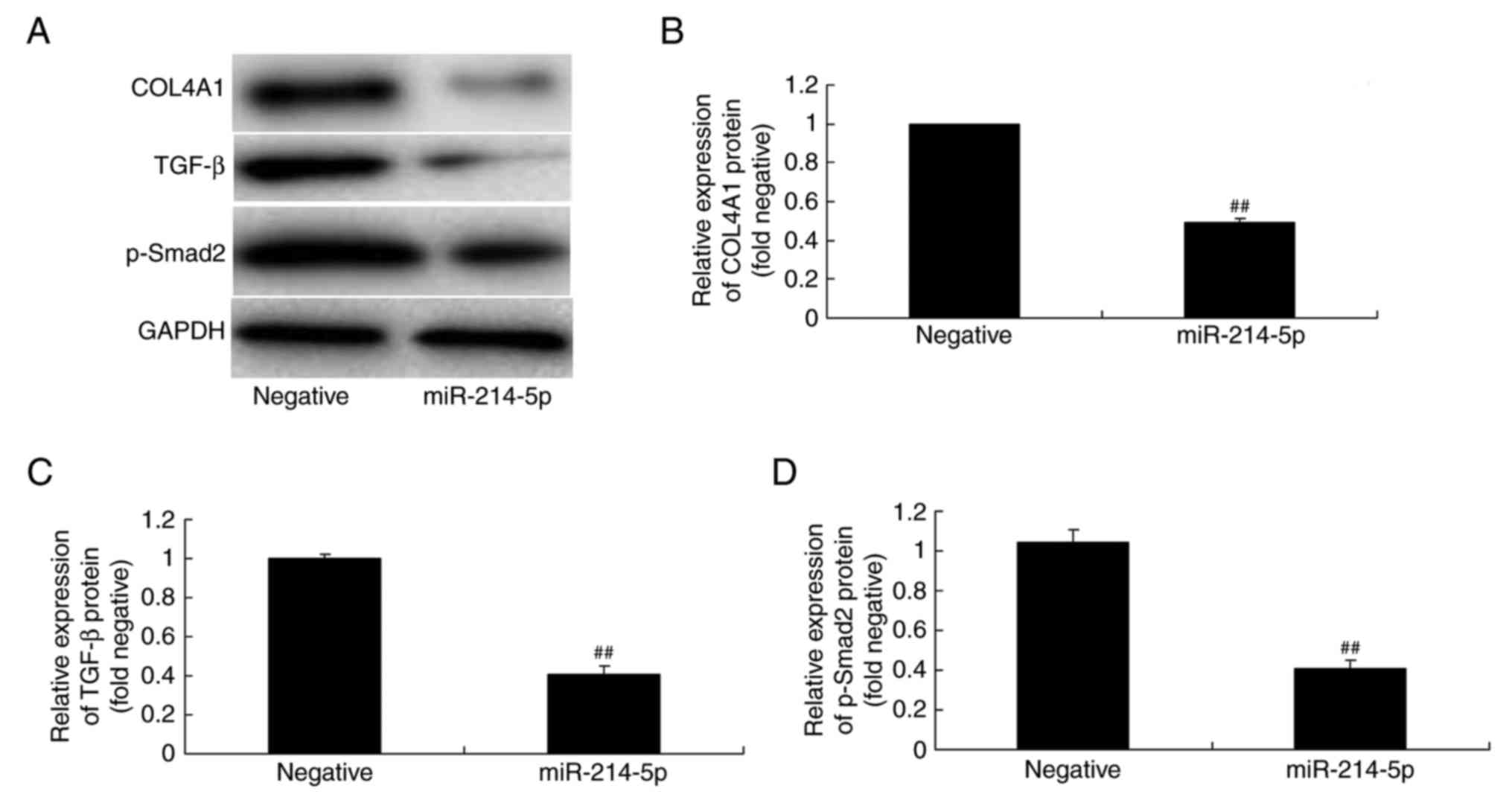
Overexpression of miR-214-5p
suppresses COL4A1, TGF-β and p-Smad2 protein expression in
HBMSCs
The association between miR-214-5p expression and
COL4A1, TGF-β and p-Smad2 protein expression in HBMSCs was
investigated by western blot analysis. The results demonstrated
that overexpression of miR-214-5p suppressed COL4A1, TGF-β and
p-Smad2 protein expression in HBMSCs, compared with the negative
control group (Fig. 7).
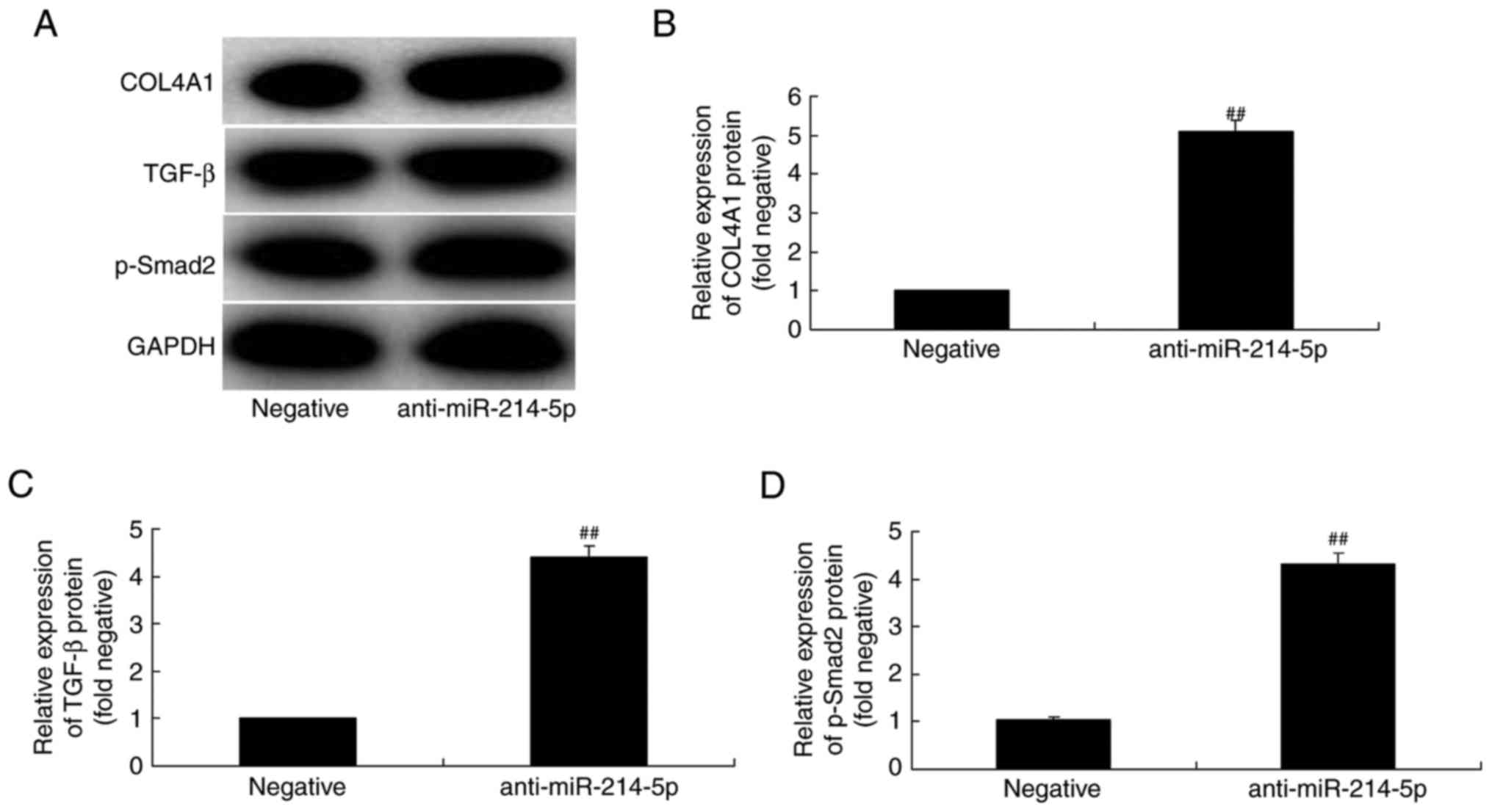
Downregulation of miR-214-5p activates
COL4A1, TGF-β and p-Smad2 protein expression in HBMSCs
By contrast, western blot analysis results following
downregulation of miR-214-5p in HBMSCs demonstrated that the
protein expression of COL4A1, TGF-β and p-Smad2 was increased,
compared with the negative control group (Fig. 8).
TGF-β inhibitor affects COL4A1, TGF-β
and p-Smad2 protein expression in HBMSCs following miR-214-5p
downregulation
The underlying mechanism of the effect of miR-214-5p
on the adipogenic differentiation of HBMSCs was further
investigated by measuring the protein expression of COL4A1, TGF-β
and p-Smad2 following miR-214-5p downregulation with or without the
addition of a TGF-β inhibitor. The results demonstrated that the
addition of the TGF-β inhibitor (10 nM for 48 h) reduced the effect
that the downregulation of miR-214-5p had on COL4A1, TGF-β and
p-Smad2 protein expression, and oil red O staining in HBMSCs
(Fig. 9A-E).
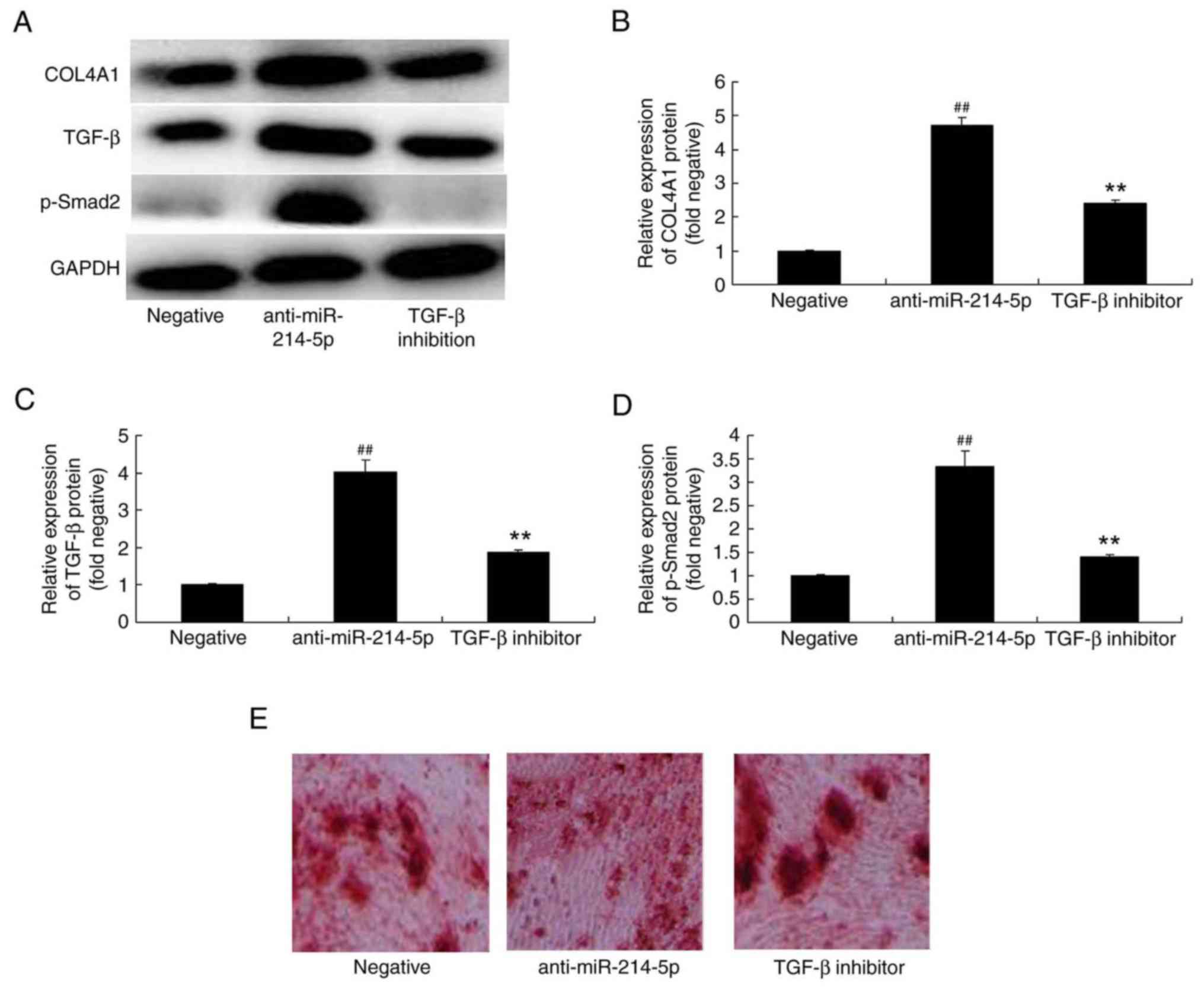 | Figure 9.TGF-β inhibitor reversed the effects
of miR-214-5p downregulation on the protein expression of COL4A1,
TGF-β and p-Smad2. (A) Western blot analysis demonstrated that
TGF-β inhibitor reversed anti-miR-214-5p-induced increases in the
protein expression of COL4A1, TGF-β and p-Smad2. Densitometric
analysis of western blotting results was performed to quantify the
protein expression of (B) COL4A1, (C) TGF-β and (D) p-Smad2
following downregulation of miR-214-5p with or without TGF-β
inhibitor treatment in human bone marrow stem cells. (E) Following
miR-214-5p downregulation with or without TGF-β inhibitor
treatment, adipogenic differentiation was assessed using oil red O
staining. Magnification, ×100. ##P<0.01 vs. negative control
group; **P<0.01 vs. anti-miR-214-5p group. TGF-β, transforming
growth factor-β; miR, microRNA; COL4A1, collagen type IV α1 chain;
p-Smad2, phosphorylated-Smad2; negative, negative control
transfection group; anti-miR-214-5p group, miR-214-5p
downregulation group; TGF-β inhibitor group, miR-214-5p
downregulation+TGF-β inhibitor group. |
TGF-β inhibitor affects ALP, Runx2, OC
and COL1 mRNA expression in HBMSCs following miR-214-5p
downregulation
The impact of a TGF-β inhibitor on the
anti-miR-214-5p-mediated effects on ALP, Runx2, OC and COL1 mRNA
expression were also investigated by RT-qPCR. The results
demonstrated that the TGF-β inhibitor affected ALP, Runx2, OC and
COL1 mRNA expression in HBMSCs following miR-214-5p downregulation
by reversing the anti-miR-214-5p-induced increase in the expression
of these genes (Fig. 10).
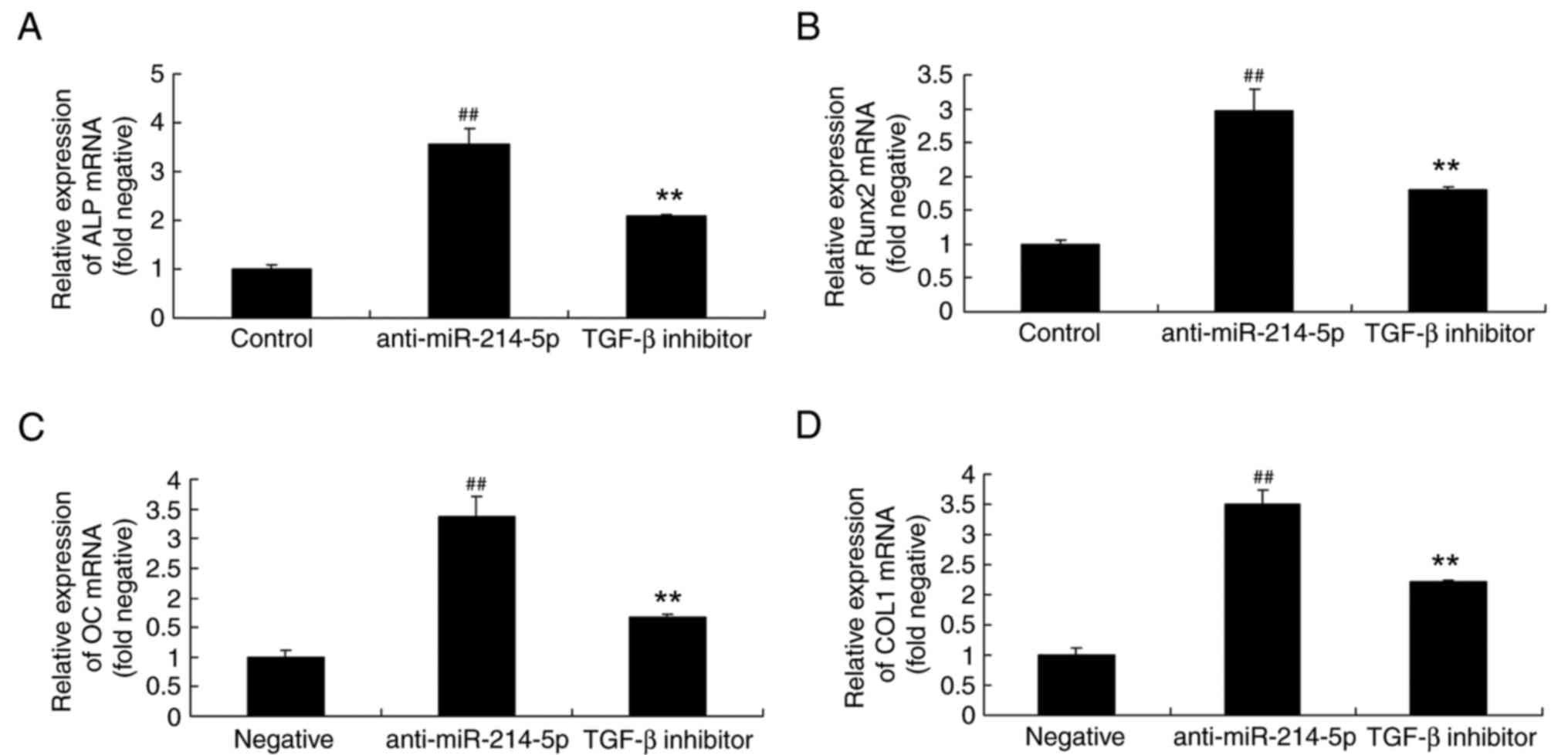 | Figure 10.TGF-β inhibitor reversed the effects
of miR-214-5p downregulation on the mRNA expression of ALP, Runx2,
OC and COL1. Reverse transcription-quantitative polymerase chain
reaction demonstrated that TGF-β inhibitor reversed the
anti-miR-214-5p-induced increases in the mRNA expression of (A)
ALP, (B) Runx2, (C) OC and (D) COL1 in human bone marrow stem
cells. ##P<0.01 vs. negative control group; **P<0.01 vs.
anti-miR-214-5p group. TGF-β, transforming growth factor-β; miR,
microRNA; ALP, alkaline phosphatase; Runx2, runt-related
transcription factor 2; OC, osteocalcin; COL1A1, collagen type IV
α1 chain; negative, negative control transfection group;
anti-miR-214-5p group, miR-214-5p downregulation group; TGF-β
inhibitor group, miR-214-5p downregulation+TGF-β inhibitor
group. |
TGF-β inhibitor affects PPARγ, CEBPα
and adiponectin mRNA expression in HBMSCs following miR-214-5p
downregulation
Similarly, following miR-214-5p downregulation in
the presence of the TGF-β inhibitor, the mRNA expression of PPARγ,
CEBPα and adiponectin in HBMSCs was increased compared with the
anti-miR-214-5p group without TGF-β inhibitor (Fig. 11).
Discussion
BMSCs are a type of stem cell deriving from
mesoblasts with self-renewal and multidirectional differentiation
potential (6). BMSC sampling is
convenient as it is easy to perform separation and cultivation
(29). BMSCs are characterized by
vulnerability to exogenous gene transfection, stable expression,
rapid in vitro proliferation and low immunogenicity
(4). Under specific in
vitro induction conditions, BMSCs are able to differentiate
into osteoblasts, reticular cells, chondroblasts, adipocytes,
neuroblasts, stromal cells and various other cell lineages. BMSCs
are the most commonly used cell line for tissue engineering in
recent years (2). Due to the
multipotent differentiation properties of BMSCs, multiple target
point injection can be performed through the arteriovenous route
conveying the BMSCs through blood circulation to the lesion, which
improved disease symptoms or signs, and promoted wound healing
(30). The present study
demonstrated that miR-214-5p expression in dexamethasone-induced
adipogenic differentiation of HBMSC group was increased compared
with control cells. Furthermore, overexpression of miR-214-5p
increased adipogenic differentiation, and suppressed ALP, Runx2, OC
and AP mRNA expression in HBMSCs. Downregulation of miR-214-5p in
HBMSCs led to opposing effects, which indicated that miR-214-5p
expression may be an important factor in adipogenic
differentiation. In the present study, only oil red O staining was
used to analyze the phenotype of BMSCs, which is a limitation as
additional methods assessing the phenotype of BMSCs are required in
future studies.
TGF-β is a type of polypeptide growth factor that is
abundantly expressed and is enriched in the osseous tissue
(15). It regulates the
proliferation and differentiation of various cell types, including
osteoblasts and osteoclasts, and affects the bone matrix synthesis
(15). At a concentration >5
ng/ml, TGF-β was demonstrated to enhance the proliferation speed of
BMSCs (15). Furthermore, TGF-β
has been reported to affect chondrocytes and inhibit the generation
of collagen type II, while in osteoblasts it induced COL1
production, thus reducing the cartilage damage period during bone
repair (15). TGF-β has also been
reported to promote matrix differentiation (31). In the present study, overexpression
of miR-214-5p suppressed TGF-β protein expression in HBMSCs.
However, only western blot analysis was used to analyze alterations
in the TGF-β protein expression following miR-214-5p
overexpression/downregulation, which is a further limitation of the
present study. Therefore, additional methods are required in order
to investigate the potential mechanisms of miR-214-5p on adipogenic
differentiation in OPM.
TGF-β and various cytokines have been previously
reported to regulate the activity of Runx2 (32), which is an essential protein for
the skeleton growth process. In vitro experiments
demonstrated that TGF-β promoted the proliferation and
differentiation of ectomesenchymal cells periosteum, promoted the
proliferation of osteogenesis (cartilage) cells and stimulated
osteogenesis-associated genes, including increases in Runx2 mRNA
expression and the synthesis of osteonectin, osteopontin and COL1
(31). Furthermore, it was
demonstrated to inhibit the generation of osteoclasts and the
activity of mature osteoclasts, thus inhibiting bone resorption and
reducing concomitant markers (27). In the present study, the results
demonstrated that downregulation of miR-214-5p induced TGF-β
protein expression in HBMSCs. Iizuka et al (33) reported that miR-214-5p may have
crucial roles in the progression of liver fibrosis through TGF-β
stimulation. So, miR-214-5p was considered to regulate TGF-β
protein expression to affect HBMSCs.
Smad proteins directly participate in signaling
transduction processes of the TGF-β superfamily (19). They function as the downstream
signal of TGF-β and transfer it to the nucleus (34). Following the detection of the Smad
genes, considerable progress and development was made concerning
the signal transduction of the TGF-β superfamily. The gene
regulatory mechanism by Smads is complex (18,34).
A single gene may be regulated by various regulatory mechanisms
that determine its expression level. Smad proteins transfer TGF-β
signals to the nucleus and precisely control the expression of
certain genes to alter cell phenotype and functions. The present
study demonstrated that overexpression of miR-214-5p suppressed
p-Smad2 protein expression in HBMSCs, which showed that miR-214-5p
inhibits the TGF-β/Smad signaling pathway to induce adipogenic
differentiation in HBMSCs.
Previous studies illustrated that COL4A1 gene
mutations were associated with cerebral hemorrhage, microaneurysm,
ocular phenotype and kidney diseases of newborns and adults
(22,35). A number of studies have
investigated the COL4A1 gene as a potential candidate gene of
osteoporosis (22,35). The COL4A1 gene can transform from
an acyl amino acid to histidine (35). The COL4A1 presented significant
correlation with the thigh bone and collar bone density (21,36).
In the present study, overexpression of miR-214-5p suppressed
COL4A1 protein expression in HBMSCs. PPARγ, CEBPα and adiponectin
regulates osteogenic differentiation of HBMSCs (28), therefore the expression of PPARγ,
CEBPα and adiponectin of HBMSCs by miR-214-5p was investigated.
Then, it was demonstrated that the mRNA expression levels of PPARγ,
CEBPα and adiponectin following miR-214-5p overexpression were
higher compared with the negative control group. So, these results
demonstrated that miR-214-5p overexpression induced PPARγ, CEBPα
and adiponectin of HBMSCs.
In conclusion, the present study demonstrated that
miR-214-5p promoted adipogenic differentiation of BMSCs by
TGF-β/Smad2/COL4A1 signaling pathway. Furthermore, it was confirmed
that miR-214-5p may also be involved in the regulation of HBMSC
adipogenic differentiation, and treatment for OPM in further
clinical.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Nature
Scientific Foundation of Guangdong Province, China (grant no.
2016A030313190), the National Nature Scientific Foundation of China
(grant nos. 81470977 and 81270835), the Science and Technology
Planning Project of Guangdong Province, China (grant nos.
2013B021800077 and 2014A020212121) and the Basic Service Charge
Young Teachers Cultivation Project of Sun Yat-sen University (grant
no. 13ykpy35).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
NN designed the experiment and wrote the manuscript.
JQ, GH and LC performed the experiment. JQ and NN analyzed the
data.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pawlowski JW, Martin BR, McCabe GP, McCabe
L, Jackson GS, Peacock M, Barnes S and Weaver CM: Impact of
equol-producing capacity and soy-isoflavone profiles of supplements
on bone calcium retention in postmenopausal women: A randomized
crossover trial. Am J Clin Nutr. 102:695–703. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pfeifer M, Kohlwey L, Begerow B and Minne
HW: Effects of two newly developed spinal orthoses on trunk muscle
strength, posture, and quality-of-life in women with postmenopausal
osteoporosis: A randomized trial. Am J Phys Med Rehabil.
90:805–815. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kessous R, Weintraub AY, Mattan Y,
Dresner-Pollak R, Brezis M, Liebergall M and Kandel L: Improving
compliance to osteoporosis workup and treatment in postmenopausal
patients after a distal radius fracture. Taiwan J Obstet Gynecol.
53:206–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Itabashi A, Yoh K, Chines AA, Miki T,
Takada M, Sato H, Gorai I, Sugimoto T, Mizunuma H, Ochi H, et al:
Bridging analysis of the efficacy and safety of bazedoxifene in
Japanese and global populations of postmenopausal women with
osteoporosis. J Bone Miner Metab. 33:61–72. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Filip R, Possemiers S, Heyerick A,
Pinheiro I, Raszewski G, Davicco MJ and Coxam V: Twelve-month
consumption of a polyphenol extract from olive (Olea europaea) in a
double blind, randomized trial increases serum total osteocalcin
levels and improves serum lipid profiles in postmenopausal women
with osteopenia. J Nutr Health Aging. 19:77–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gu JM, Wang L, Lin H, Chen DC, Tang H, Jin
XL, Xia WB, Hu YQ, Fu WZ, He JW, et al: The efficacy and safety of
weekly 35-mg risedronate dosing regimen for Chinese postmenopausal
women with osteoporosis or osteopenia: 1-year data. Acta Pharmacol
Sin. 36:841–846. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang C, Zhang GF, Han J, Liao GJ and Zou
BG: Mechanism of age-related changes of bone marrow mesenchymal
stem cells in senile osteoporosis. J Biol Regul Homeost Agents.
30:565–569. 2016.PubMed/NCBI
|
|
8
|
Li F, Zhou C, Xu L, Tao S, Zhao J and Gu
Q: Effect of stem cell therapy on bone mineral density: A
meta-analysis of preclinical studies in animal models of
osteoporosis. PLoS One. 11:e01494002016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zavrski I, Naujokat C, Niemöller K, Jakob
C, Heider U, Langelotz C, Fleissner C, Eucker J, Possinger K and
Sezer O: Proteasome inhibitors induce growth inhibition and
apoptosis in myeloma cell lines and in human bone marrow myeloma
cells irrespective of chromosome 13 deletion. J Cancer Res Clin
Oncol. 129:383–391. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heilmeier U, Hackl M, Skalicky S, Weilner
S, Schroeder F, Vierlinger K, Patsch JM, Baum T, Oberbauer E,
Lobach I, et al: Serum microRNAs are indicative of skeletal
fractures in postmenopausal women with and without type 2 diabetes
and influence osteogenic and adipogenic differentiation of
adipose-tissue derived mesenchymal stem cells in vitro. J
Bone Miner Res. 31:2173–2192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Wang Y, Yang N, Wu S, Lv Y and Xu
L: In silico analysis of the molecular mechanism of
postmenopausal osteoporosis. Mol Med Rep. 12:6584–6590. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao Z, Moore BT, Wang Y, Peng XH, Lappe
JM, Recker RR and Xiao P: MiR-422a as a potential cellular microRNA
biomarker for postmenopausal osteoporosis. PLoS One. 9:e970982014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu XD, Cai F, Liu L, Zhang Y and Yang AL:
MicroRNA-210 is involved in the regulation of postmenopausal
osteoporosis through promotion of VEGF expression and osteoblast
differentiation. Biol Chem. 396:339–347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng Q, Tang W, Sheu TJ, Du Y, Gan J, Li
H, Hong W, Zhu X, Xue S and Zhang X: Circulating TGF-β1 levels are
negatively correlated with sclerostin levels in early
postmenopausal women. Clin Chim Acta. 455:87–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun J, Zhang C, Xu L, Yang M and Yang H:
The transforming growth factor-β1 (TGF-β1) gene polymorphisms
(TGF-β1 T869C and TGF-β1 T29C) and susceptibility to postmenopausal
osteoporosis: a meta-analysis. Medicine. 94:e4612015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Utennam D, Tungtrongchitr A, Phonrat B,
Tungtrongchitr R and Preutthipan S: Association of T869C gene
polymorphism of transforming growth factor-β1 with low protein
levels and anthropometric indices in osteopenia/osteoporosis
postmenopausal Thai women. Genet Mol Res. 11:87–99. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun X, Cao Z, Zhang Q, Li M, Han L and Li
Y: Aluminum trichloride inhibits osteoblast mineralization via
TGF-β1/Smad signaling pathway. Chem Biol Interact. 244:9–15. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li B: Bone morphogenetic protein-Smad
pathway as drug targets for osteoporosis and cancer therapy. Endocr
Metab Immune Disord Drug Targets. 8:208–219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Donoso O, Pino AM, Seitz G, Osses N and
Rodriguez JP: Osteoporosis-associated alteration in the signalling
status of BMP-2 in human MSCs under adipogenic conditions. J Cell
Biochem. 116:1267–1277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsumoto T, Miyakoshi K, Fukutake M,
Ochiai D, Minegishi K and Tanaka M: Intracranial sonographic
features demonstrating in utero development of hemorrhagic brain
damage leading to schizencephaly-associated COL4A1 mutation.
J Med Ultrason. 42:445–446. 2015. View Article : Google Scholar
|
|
21
|
Li QS, Meng FY, Zhao YH, Jin CL, Tian J
and Yi XJ: Inhibition of microRNA-214-5p promotes cell survival and
extracellular matrix formation by targeting collagen type IV alpha
1 in osteoblastic MC3T3-E1 cells. Bone Joint Res. 6:464–471. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tomotaki S, Mizumoto H, Hamabata T,
Kumakura A, Shiota M, Arai H, Haginoya K and Hata D: Severe
hemolytic jaundice in a neonate with a novel COL4A1
mutation. Pediatr Neonatol. 57:522–525. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Plaisier E and Ronco P: COL4A1-Related
Disorders. In: GeneReviews®Adam MP, Ardinger HH, Pagon
RA, Wallace SE, Bean LJH, Stephens K and Amemiya A: University of
Washington, Seattle University of Washington, Seattle. GeneReviews
is a registered trademark of the University of Washington; Seattle.
All rights reserved, Seattle (WA): 1993
|
|
24
|
Wen Y, Guo X, Hao J, Xiao X, Wang W, Wu C,
Wang S, Yang T, Shen H, Chen X, et al: Integrative analysis of
genome-wide association studies and gene expression profiles
identified candidate genes for osteoporosis in Kashin-Beck disease
patients. Osteoporos Int. 27:1041–1046. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bi D, Wang H, Shang Q, Xu Y, Wang F, Chen
M, Ma C, Sun Y, Zhao X, Gao C, et al: Association of COL4A1 gene
polymorphisms with cerebral palsy in a Chinese Han population. Clin
Genet. 90:149–155. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hopwood B, Tsykin A, Findlay DM and
Fazzalari NL: Microarray gene expression profiling of
osteoarthritic bone suggests altered bone remodelling, WNT and
transforming growth factor-beta/bone morphogenic protein
signalling. Arthritis Res Ther. 9:R1002007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martin PJ, Haren N, Ghali O, Clabaut A,
Chauveau C, Hardouin P and Broux O: Adipogenic RNAs are transferred
in osteoblasts via bone marrow adipocytes-derived extracellular
vesicles (EVs). BMC Cell Biol. 16:102015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hooshmand S, Brisco JR and Arjmandi BH:
The effect of dried plum on serum levels of receptor activator of
NF-κB ligand, osteoprotegerin and sclerostin in osteopenic
postmenopausal women: A randomised controlled trial. Br J Nutr.
112:55–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Catalano A, Morabito N, Basile G,
Brancatelli S, Cucinotta D and Lasco A: Zoledronic acid acutely
increases sclerostin serum levels in women with postmenopausal
osteoporosis. J Clin Endocrinol Metab. 98:1911–1915. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saad MN, Mabrouk MS, Eldeib AM and Shaker
OG: Effect of MTHFR, TGFβ1, and TNFB polymorphisms on osteoporosis
in rheumatoid arthritis patients. Gene. 568:124–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Komatsu Y, Ibi M, Chosa N, Kyakumoto S,
Kamo M, Shibata T, Sugiyama Y and Ishisaki A: Zoledronic acid
suppresses transforming growth factor-β-induced fibrogenesis by
human gingival fibroblasts. Int J Mol Med. 38:139–147. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iizuka M, Ogawa T, Enomoto M, Motoyama H,
Yoshizato K, Ikeda K and Kawada N: Induction of microRNA-214-5p in
human and rodent liver fibrosis. Fibrogenesis Tissue Repair.
5:122012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Li A, Strait K, Zhang H, Nanes MS
and Weitzmann MN: Endogenous TNFalpha lowers maximum peak bone mass
and inhibits osteoblastic Smad activation through NF-kappaB. J Bone
Miner Res. 22:646–655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gale DP, Oygar DD, Lin F, Oygar PD, Khan
N, Connor TM, Lapsley M, Maxwell PH and Neild GH: A novel COL4A1
frameshift mutation in familial kidney disease: The importance of
the C-terminal NC1 domain of type IV collagen. Nephrol Dial
Transplant. 31:1908–1914. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Saeed H and Iqtedar M: Aberrant gene
expression profiles, during in vitro osteoblast
differentiation, of telomerase deficient mouse bone marrow stromal
stem cells (mBMSCs). J Biomed Sci. 22:112015. View Article : Google Scholar : PubMed/NCBI
|