Introduction
Fetal growth restriction (FGR) is a complex disorder
of pregnancy with varying etiology. It is characterized by the
failure of the fetus to achieve its normal growth potential and is
associated with perinatal morbidity and mortality, as well as
cardiovascular disease in adult life (1). A number of different causes have been
attributed to the development of FGR including infections, drug
abuse, as well as immunological and anatomical factors. Notably,
placental dysfunction is one of the predominant causes of FGR.
Despite extensive research into the mechanism underlying the
development of FGR, its exact etiology remains elusive. Several
hormones that are involved in pregnancy have been investigated with
regard to FGR (2). The disease is
thought to result from an abnormal placenta and thus identification
of the genes involved in abnormal placenta development may
enlighten our existing knowledge regarding the pathogenesis of
FGR.
Pregnancy-associated plasma protein A (PAPPA) is a
syncytiotrophoblast-derived metalloproteinase that cleaves the
complex formed between insulin-like growth factor (IGF) and insulin
like growth factor binding protein (IGFBP). Serum levels of PAPPA
have been examined in relation to various pathological disorders
such as stillbirth, infant death, preterm birth, and pre-eclampsia,
as well as certain chromosomal disorders and anomalies (3). It is generally accepted that plasma
levels of PAPPA are decreased in intrauterine growth restriction
(IUGR). Pregnancy-associated plasma protein A2 (PAPPA2) is a
protein that shares approximately 40% amino acid homology with
PAPPA and functions in a similar manner as an IGFBP protease. The
physiological importance of PAPPA2 is not known. However, it is
thought that PAPPA2 deficiency plays a major role in growth
retardation as documented by studies in k/o mice (4). A 25–30% lower body weight and smaller
organs were observed for the PAPPA2 k/o strain (4). In addition to PAPPA proteins,
placenta-specific-1 (PLAC-1) is a protein that has been examined as
a biomarker for genetic and gestational disorders, since its
expression is restricted to cells of the trophoblastic lineage and
is absent from adult or fetal tissues. PLAC-1 ablation is
associated with placentomegaly and IUGR (7). The exact function of this protein
remains unknown and PLAC-1 expression has been further demonstrated
in a variety of human cancers, and is likely to have a significant
role in modulating proliferation, invasion and survival of cancer
cells (6–8).
Although abnormally low PAPPA and PAPPA2 levels in
the first trimester maternal circulation are associated with
increased risk of the disease, the expression of the aforementioned
markers differs as regards the localization of the proteins in the
placenta and/or in the plasma of pregnant women. The aim of the
present study was to examine the placental expression levels of
PAPPA, PAPPA2 and PLAC-1 in pregnancies with FGR and healthy
pregnancies. Our investigation was further focused on the putative
associations of the placental expression of the aforementioned
biomarkers with the occurrence of FGR.
Materials and methods
Study group
The study comprised 16 cases of women with FGR and
16 normotensive subjects undergoing healthy pregnancy. Gestational
age (GA) was defined by the last menstrual period. GA was corrected
at 11–13 weeks of pregnancy in the cases where the gestational
dates were uncertain. Medical history and pregnancy characteristics
were recorded from maternity computerized records. An ultrasound
examination for biometry of the fetus and Doppler studies were
conducted in the third trimester of pregnancy. All the protocols
were carried out according to the International Society of
Ultrasound in Obstetrics and Gynecology (ISUOG) guidelines
(9).
During delivery of the fetus samples from the
placenta, samples were collected from both FGR and control (CT)
groups. The tissue size ranged from 4 to 6 mm3 and
tissue specimens were snap frozen in liquid N2 and
further stored at −80°C until processing. The study protocol was
approved by the Ethics Committee of the National Kapodistrian
University of Athens (Athens, Greece) and written informed consent
was obtained from all the participants.
Selection criteria
FGR was defined according to the International
Society for the study of Hypertension in Pregnancy (10). Specifically, women after 20 weeks
of gestation who presented with a fetus with reduced growth
velocity (<10th percentile) were classified as FGR subjects.
Women with pre-existing diabetes type I and II, pre-existing
hypertension and gestational diabetes mellitus were excluded from
the selection criteria. The CT group included women undergoing
pregnancies with a normal third trimester ultrasound scan.
RNA extraction and reverse
transcription
RNA was extracted with TRIzol as described
previously (11). Briefly each
tissue was cut to small sections and homogenized with 1 ml TRIzol.
The resulting mixture was vortexed with 200 µl chloroform and
centrifuged for 15 min at 18,900 g. RNA was extracted from the top
layer containing the organic phase and mixed with an equal volume
of cold isopropanol. The samples were centrifuged for 10 min at
11,200 g and the resulting RNA pellet was washed once with 70%
ethanol and resuspended in 40 µl of diethyl pyrocarbonate-treated
water. The cDNA was synthesized using a Takara cDNA synthesis kit
(Takara Bio, Inc., Otsu, Japan) following the manufacturer's
protocol. The RNA was incubated with a reaction mixture containing
reaction buffer, RNase inhibitor, reverse transcriptase and water
at 43°C for 1 h.
Quantitative polymarese chain reaction
(qPCR)
qPCR was conducted using SYBR at a final reaction
volume of 20 µl in a Mx3000 Stratagene PCR amplifier (Agilent
Technologies, Inc., Santa Clara, CA, USA). The primers were used at
a final concentration of 0.5 µM. The sequences used for
amplification of PAPPA, PAPPA2 and PLAC-1 were as follows: PAPPA,
forward: GTCATCTTTGCCTGGAAGGGAGAA at 56°C (12) and reverse:
AGGGCTGTTCAACATCAGGATGAC, PAPPA2, forward: ACTCACCCAAGAGGGCATACATGA
at 50°C (12) and reverse:
GCACTGAGCTGGCAAAGTAGATGT, PLAC-1, forward: ATTGGCTGCAGGGATGAAAG at
50°C (13) and reverse:
TGCACTCTGACCATGAACCA.
The quantification was conducted using
a standard curve for each primer set
A pool cDNA was prepared that was diluted at: 1:5,
1:25, 1:125 and 1:625. The GAPDH and TOP1 genes were
used as housekeeping genes, according to previously published
studies (14). The expression
levels were presented as normalized values of expression of each
gene.
Statistical analysis
Significant differences were determined for the
demographic parameters using the Mann-Whitney U test, while
statistical comparison of the mean levels of expression of the
PAPPA, PAPPA2 and PLAC-1 genes was conducted using independent
variables t-test. Correlation analysis was conducted using the
Pearson's correlation test. A P<0.05 was considered to indicate
a statistically significant difference.
Results
Placental levels of PAPPA, PAPPA2 and
PLAC-1
The demographic parameters of the study are shown in
Table I. The sample size consisted
of 16 pregnant women with FGR and 16 normal pregnancies. The
parameters age, height and weight of pregnant women were not
significantly different between the control and FGR pregnancies.
Significant differences were obtained for GA (P=0.024) and infant
weight (P<0.001) with the FGR group exhibiting lower mean values
of the two parameters compared to the CT group. This result was
expected given the presentation of the disease, which manifested
with lower growth characteristics of fetuses compared to normal
pregnancies. In addition to the demographic characteristics, the
placental mRNA expression levels of PAPPA, PAPPA2 and
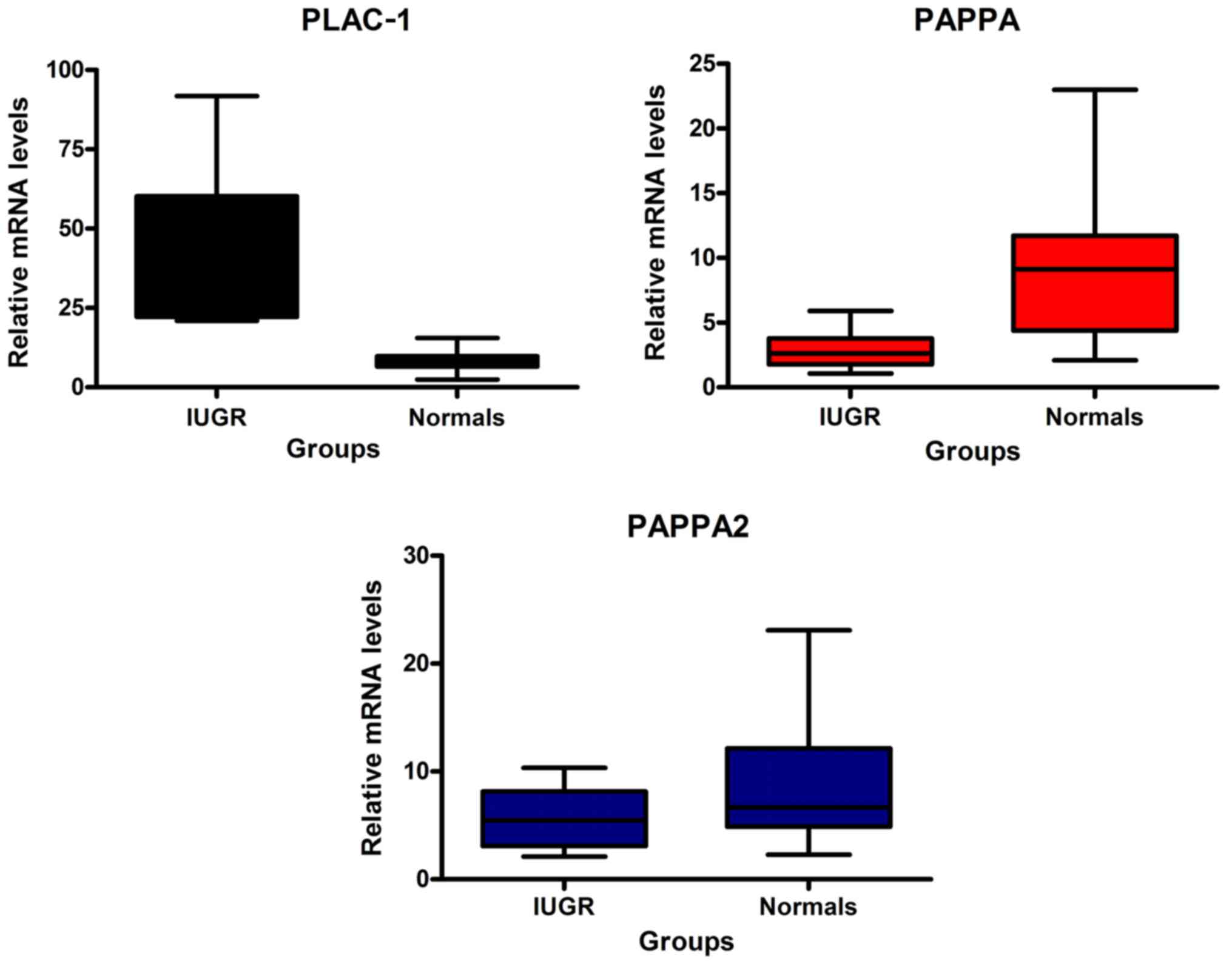
PLAC-1 were determined by qPCR. The mean expression levels
of PAPPA and PAPPA2 were lower in the IUGR group compared to those
of the CT group (Fig. 1). The
differences were statistically significant as demonstrated by the
corresponding P-values (P<0.001) (Table II). In contrast to these
observations, PLAC-1 exhibited higher values in the FGR pregnancies
compared to those of normal pregnancies (Fig. 1 and Table II), thus presenting an opposite
pattern of expression between the two groups compared to PAPPA and
PAPPA2. This difference was highly significant as determined by the
independent sample t-test (P<0.001).
 | Table I.Demographic characteristics of mother
and infants between IUGR and control group. |
Table I.
Demographic characteristics of mother
and infants between IUGR and control group.
| Variables | Groups | No. | Mean | SD | Median | Min | Max | P-value |
|---|
| Age | Control | 16 | 30.1 | 4.1 | 29.0 | 21 | 35 |
0.160 |
|
| IUGR | 16 | 33.3 | 7.6 | 33.0 | 22 | 47 |
|
|
| Total | 32 | 31.7 | 6.2 | 32.0 | 21 | 47 |
|
| Height (m) | Control | 16 | 1.6 | 0.1 | 1.6 | 1.54 | 1.76 |
0.717 |
|
| IUGR | 16 | 1.6 | 0.1 | 1.6 | 1.50 | 1.74 |
|
|
| Total | 32 | 1.6 | 0.1 | 1.6 | 1.50 | 1.76 |
|
| Weight (kg) | Control | 16 | 71.9 | 8.4 | 68.0 | 60 | 86 |
0.440 |
|
| IUGR | 16 | 69.2 | 10.2 | 68.0 | 54 | 95 |
|
|
| Total | 32 | 70.5 |
9.3 | 68.0 | 54 | 95 |
|
| Gestational age
(weeks) | Control | 16 | 40.3 |
3.1 | 41.0 | 33 | 44 |
0.024 |
|
| IUGR | 16 | 37.8 |
2.7 | 38.0 | 33 | 43 |
|
|
| Total | 32 | 39.1 |
3.1 | 38.5 | 33 | 44 |
|
| Birth weight
(kg) | Control | 16 |
3.0 |
0.5 |
3.2 | 1.64 | 3.58 | <0.001 |
|
| IUGR | 16 |
2.1 |
0.4 |
2.0 | 0.96 | 2.58 |
|
|
| Total | 32 |
2.6 |
0.7 |
2.6 | 0.96 | 3.58 |
|
 | Table II.Statistical analysis of average and
range parameters of the expression levels of PAPPA, PAPPA2
and PLAC-1 genes in the FGR and control groups. |
Table II.
Statistical analysis of average and
range parameters of the expression levels of PAPPA, PAPPA2
and PLAC-1 genes in the FGR and control groups.
| Expression
levels | Groups | No. | Mean | SD | Median | Min | Max | P-value |
|---|
| PAPPA | Control | 16 |
9.4 |
5.9 |
9.1 |
2.1 | 23.0 | <0.001 |
|
| FGR | 16 |
2.8 |
1.4 |
2.6 |
1.1 |
5.9 |
|
|
| Total | 32 |
6.1 |
5.4 |
3.9 |
1.1 | 23.0 |
|
| PAPPA2 | Control | 16 |
8.8 |
5.5 |
6.7 |
2.3 | 23.1 | <0.001 |
|
| FGR | 16 |
5.7 |
2.7 |
5.5 |
2.1 | 10.3 |
|
|
| Total | 32 |
7.2 |
4.5 |
5.9 |
2.1 | 23.1 |
|
| PLAC-1 | Control | 16 |
8.1 |
3.2 |
8.1 |
2.5 | 15.5 | <0.001 |
|
| FGR | 16 | 42.9 | 21.6 | 39.5 | 21.0 | 91.8 |
|
|
| Total | 32 | 25.5 | 23.3 | 18.3 |
2.5 | 91.8 |
|
Linear regression analysis
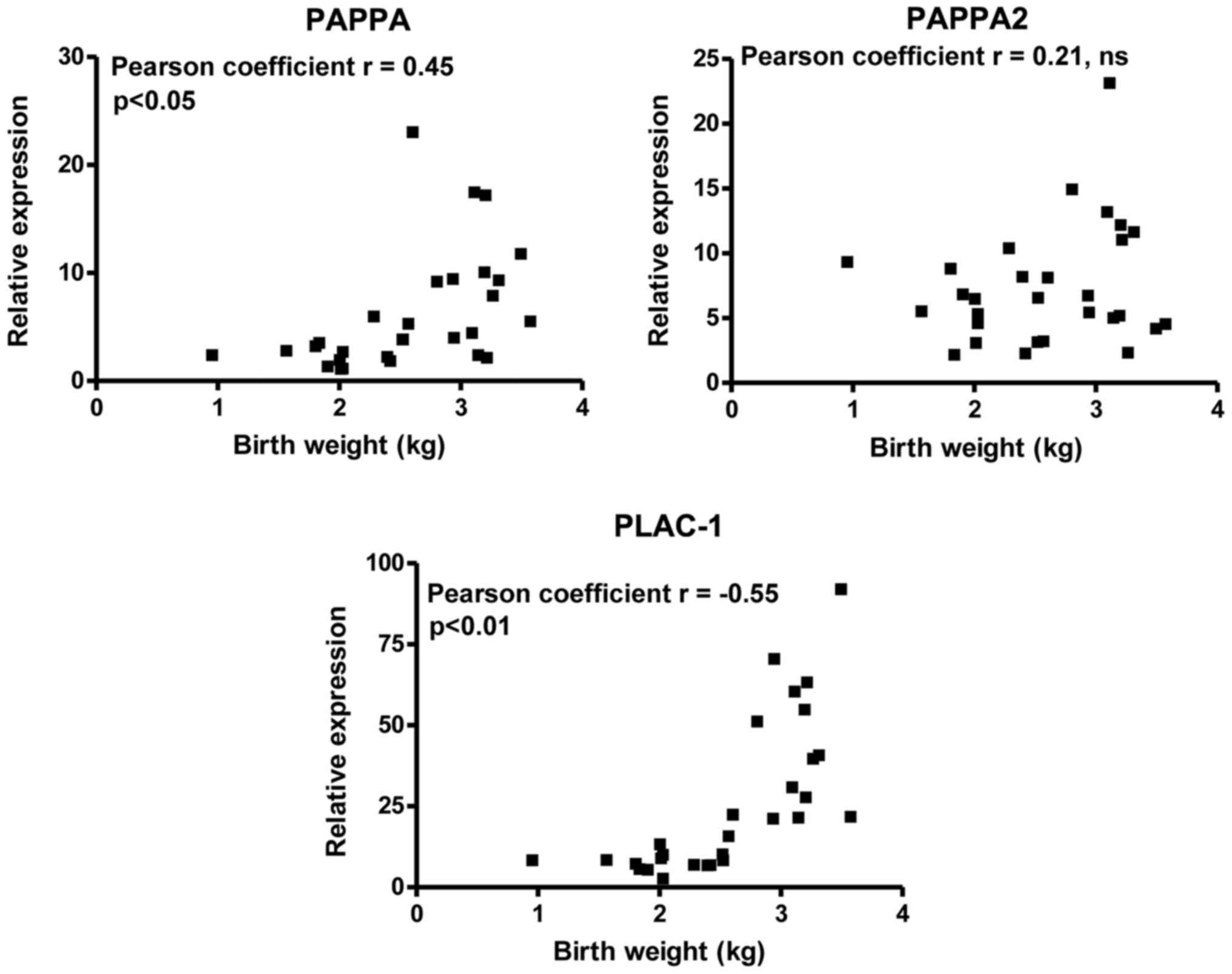
Furthermore, correlation analyses revealed positive
correlations of PLAC-1 (P< 0.05) and PAPPA (P<0.01)
expression levels with birth weight, while with regard to PAPPA2
the results were not statistically significant (Fig. 2 and Table III). No significant correlation
was noted with regard to the expression levels of these genes with
the birth weight of the FGR subjects (Table III). However, the correlation of
the birth weight of the FGR subjects with the expression levels of
PAPPA2 was stronger compared with that noted in the total
population (−0.3358 vs. 0.21, Table
III).
 | Table III.Correlation analysis of PAPPA, PAPPA2
and PLAC1 expression with birth weight in FGR pregnancies. |
Table III.
Correlation analysis of PAPPA, PAPPA2
and PLAC1 expression with birth weight in FGR pregnancies.
| Genes | P-value | r | Significance | 95% CI |
|---|
| PAPPA | 0.2 |
0.35 | No | −0.21–0.73 |
| PAPPA2 | 0.2 | −0.34 | No | −0.73–0.21 |
| PLAC-1 | 0.8 |
0.06 | No |
0.47–0.55 |
Discussion
The present study demonstrated an expression
analysis of PAPPA, PAPPA2 and PLAC-1 in pregnancies with FGR and
control pregnancies. The mean expression levels of PAPPA and PAPPA2
in placental tissues were significantly lower in FGR pregnancies
compared with healthy subjects, whereas the opposite pattern was
observed for PLAC-1. The data further demonstrated a correlation of
PAPPA and PLAC-1 expression in FGR and control subjects with birth
weight, suggesting a possible link of these biomarkers with FGR
pathology.
Previous studies have shown that PAPPA is a
biomarker that is decreased in the serum of women who present with
FGR in the first trimester (15,16).
Giudice et al have demonstrated that neonatal weights are
associated with serum PAPPA levels lower than the 25th centile
(17). The exact mechanism that
contributes to this outcome remains unclear, although PAPPA
deficiency has been shown to result in compromised fetal growth and
skeletal phenotypes in k/o mice (4). However, it is believed that serum
PAPPA levels cannot be used as a reliable biomarker for FGR
pathology, since similar biochemical measurements have been noted
for preterm delivery, stillbirth and preeclampsia (18,19).
Since PAPPA and PAPPA2 are expressed at high levels in the placenta
we hypothesized that an altered expression of these biomarkers may
influence the development of FGR. Although serum levels of PAPPA
rise and fall during the periods of FGR pregnancy we found that
placental levels of PAPPA and PAPPA2 were significantly lower in
FGR compared with control pregnancies, whereas PAPPA expression
correlated with birth weight. This finding is in agreement with the
study of Kodama et al where decreased mean levels of
placental PAPPA mRNA were reported in late onset preeclampsia
compared with healthy pregnancy (13). The possible explanation for these
observations is attributed to the levels of the substrate IGFBP-5
that are inversely related to PAPPA2 levels and regulate
cytotrophoblast invasion, a key step in placenta development
(20). With regard to PAPPA the
degradation of the similar protein IGFBP4 leads to the release of
IGF-II, which promotes placental development via trophoblast
invasion (21). Whether altered
expression of these biomarkers is the cause or the effect of FGR
remains to be determined.
The present study is in agreement with the report of
Kodama et al, with the exception of the difference in the
pathological state of the subjects examined (FGR vs. pre-eclampsia)
(13). In addition, a previous
study indicated lower PAPPA multiples of median levels in SGA
(small for GA) cases compared with control subjects (21). The same effect was noted for PAPPA2
following stratification according to maternal hypertension and
proteinuria (21). It is believed
that the changes in the expression levels of PAPPA2 and PAPPA are
associated with the expression of the substrates IGFBP-5 and IGFBP4
that regulate cytotrophoblast invasion, a key step in placenta
development (19,22).
In addition to the PAPPA biomarkers, the expression
analysis of the novel X-linked gene PLAC-1 in FGR and normal
pregnancies revealed marked differences between the two groups.
Disruption of PLAC-1 can cause hyperplasia and FGR, whereas PLAC-1
is also reported to be one of the upregulated genes in the
hyperplastic placenta generated by nuclear transfer (23). Although the association of PLAC-1
with the development of FGR during pregnancy has not been examined
to date, previous studies demonstrated elevated levels of
circulating PLAC-1 mRNA in preeclampsia that were directly related
to the disease severity (24–26).
In a similar manner, increased levels of PLAC-1 in the placenta of
FGR-women were observed in the present study, possibly suggesting a
feedback mechanism, in order to overcome the development of the
disease. Consistent with this hypothesis is the observation that
PLAC-1 participates in the maintenance of pregnancy via the
adaptation of the placenta to various physiological and
environmental stimuli (27). A key
feature of the function of PLAC-1 is the modulation of signaling
pathways that regulate the cell membrane response to the
extracellular environment, with respect to cell shape, motility and
plasticity (27).
In conclusion, the present study demonstrated the
selective overexpression of placental PLAC-1 and the reduced
expression of PAPPA and PAPPA2 in pregnancies associated with FGR
compared with healthy pregnancies. Future studies with higher
number of samples should focus on the clinical value of PLAC-1 as a
predictive biomarker for FGR, by addressing the expression of the
latter protein in the serum of women throughout the stages of
pregnancy.
Acknowledgements
We would like to thank the department of Toxicology
at the Medical School of the University of Crete for allowing us to
use the Mx3000 Stratagene PCR amplifier.
Funding
Funding was received by the Medical School of the
University of Crete.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
VPA made substantial contributions to conception and
design, or acquisition of data, or analysis and interpretation of
data and was involved in drafting the manuscript or revising it
critically for important intellectual content. AP, AV and GIP were
involved in drafting the manuscript or revising it critically for
important intellectual content. SS and NS made substantial
contributions to the acquisition of data and gave final approval of
the version to be published. DAS gave final approval of the version
to be published.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of the National Kapodistrian University of Athens
(Athens, Greece) and written informed consent was obtained from all
participants.
Consent for publication
Written informed consent was obtained from all human
subjects regarding their participation in the study.
Competing interests
D.A. Spandidos is the Editor-in-Chief for the
journal, but had no personal involvement in the reviewing process,
or any influence in terms of adjudicating on the final decision,
for this article.
Glossary
Abbreviations
Abbreviations:
|
PAPPA
|
pregnancy-associated plasma protein
A
|
|
PLAC-1
|
placenta-specific-1
|
|
PAPPA2
|
pregnancy-associated plasma protein
A2
|
|
FGR
|
fetal growth restriction
|
|
qPCR
|
quantitative polymerase chain
reaction
|
|
ISUOG
|
International Society of Ultrasound in
Obstetrics and Gynecology
|
|
GA
|
gestational age
|
References
|
1
|
Gourvas V, Dalpa E, Konstantinidou A,
Vrachnis N, Spandidos DA and Sifakis S: Angiogenic factors in
placentas from pregnancies complicated by fetal growth restriction
(Review). Mol Med Rep. 6:23–27. 2012.PubMed/NCBI
|
|
2
|
Hashimoto Y, Kawai M, Nagai S, Matsukura
T, Niwa F, Hasegawa T and Heike T: Fetal growth restriction but not
preterm birth is a risk factor for severe hypospadias. Pediatr Int.
58:573–577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patil M, Panchanadikar TM and Wagh G:
Variation of papp-a level in the first trimester of pregnancy and
its clinical outcome. J Obstet Gynaecol India. 64:116–119. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Conover CA, Boldt HB, Bale LK, Clifton KB,
Grell JA, Mader JR, Mason EJ and Powell DR: Pregnancy-associated
plasma protein-A2 (PAPP-A2): Tissue expression and biological
consequences of gene knockout in mice. Endocrinology.
152:2837–2844. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kong X, Jackman SM and Fant ME: Plac1
(placenta-specific 1) is widely expressed during fetal development
and is associated with a lethal form of hydrocephalus. Birth
Defects Res A Clin Mol Teratol. 97:571–577. 2013.PubMed/NCBI
|
|
6
|
Devor EJ, Gonzalez-Bosquet J, Warrier A,
Reyes HD, Ibik NV, Schickling BM, Newtson A, Goodheart MJ and
Leslie KK: p53 mutation status is a primary determinant of
placenta-specific protein 1 expression in serous ovarian cancers.
Int J Oncol. 50:1721–1728. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu Y, Lin X, Di X, Chen Y, Zhao H and Wang
X: Oncogenic function of Plac1 on the proliferation and metastasis
in hepatocellular carcinoma cells. Oncol Rep. 37:465–473. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koslowski M, Sahin U, Mitnacht-Kraus R,
Seitz G, Huber C and Türeci O: A placenta-specific gene ectopically
activated in many human cancers is essentially involved in
malignant cell processes. Cancer Res. 67:9528–9534. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
ACOG Committee on Practice
Bulletins-Obstetrics: ACOG practice bulletin. Diagnosis and
management of preeclampsia and eclampsia. Number 33, January 2002.
Obstet Gynecol. 99:159–167. 2002.PubMed/NCBI
|
|
10
|
Kappou D, Sifakis S, Androutsopoulos V,
Konstantinidou A, Spandidos DA and Papantoniou N: Placental mRNA
expression of angiopoietins (Ang)-1, Ang-2 and their receptor Tie-2
is altered in pregnancies complicated by preeclampsia. Placenta.
35:718–723. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Androutsopoulos VP and Tsatsakis AM:
Benzo[a]pyrene sensitizes MCF7 breast cancer cells to induction of
G1 arrest by the natural flavonoid eupatorin-5-methyl ether, via
activation of cell signaling proteins and CYP1-mediated metabolism.
Toxicol Lett. 230:304–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wagner PK, Otomo A and Christians JK:
Regulation of pregnancy-associated plasma protein A2 (PAPPA2) in a
human placental trophoblast cell line (BeWo). Reprod Biol
Endocrinol. 9:482011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kodama M, Miyoshi H, Fujito N, Samura O
and Kudo Y: Plasma mRNA concentrations of placenta-specific 1
(PLAC1) and pregnancy associated plasma protein A (PAPP-A) are
higher in early-onset than late-onset pre-eclampsia. J Obstet
Gynaecol Res. 37:313–318. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaitu'u-Lino TJ, Hastie R, Cannon P, Lee
S, Stock O, Hannan NJ, Hiscock R and Tong S: Stability of absolute
copy number of housekeeping genes in preeclamptic and normal
placentas, as measured by digital PCR. Placenta. 35:1106–1109.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gentile M, Schifano M, Lunardi S, Nanini
C, Moscuzza F, Sergiampietri C, Ciantelli M, Monacci F, Boldrini A
and Luchi C: Maternal PAPP-A levels at 11–13 weeks of gestation
predict foetal and neonatal growth. Open J Obstet Gynecol.
5:365–372. 2015. View Article : Google Scholar
|
|
16
|
Gaccioli F, Lager S, Sovio U,
Charnock-Jones S and Smith GCS: The pregnancy outcome prediction
(POP) study: Investigating the relationship between serial prenatal
ultrasonography, biomarkers, placental phenotype and adverse
pregnancy outcomes. Placenta. 59:S17–S25. 2017. View Article : Google Scholar :
|
|
17
|
Giudice I, Benintende G, Di Nicolò AM,
Mangiameli D, Carrara G, Randazzo C, Sapuppo IM and Gulisano A:
Correlation of neonatal weight with maternal serum levels of
pregnancy-associated plasma protein-A during the first trimester of
pregnancy: A retrospective study. J Perinat Med. 43:227–232. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park HJ, Shim SS and Cha DH: Combined
screening for early detection of pre-eclampsia. Int J Mol Sci.
16:17952–17974. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhong Y, Zhu F and Ding Y: Serum screening
in first trimester to predict pre-eclampsia, small for gestational
age and preterm delivery: Systematic review and meta-analysis. BMC
Pregnancy Childbirth. 15:1912015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wagner PK and Christians JK: Altered
placental expression of PAPPA2 does not affect birth weight in
mice. Reprod Biol Endocrinol. 8:902010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Qiu Q, Haider M, Bell M, Gruslin A
and Christians JK: Expression of pregnancy-associated plasma
protein A2 during pregnancy in human and mouse. J Endocrinol.
202:337–345. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kramer AW, Lamale-Smith LM and Winn VD:
Differential expression of human placental PAPP-A2 over gestation
and in preeclampsia. Placenta. 37:19–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muto M, Fujihara Y, Tobita T, Kiyozumi D
and Ikawa M: Lentiviral vector-mediated complementation restored
fetal viability but not placental hyperplasia in Plac1-deficient
mice. Biol Reprod. 94:62016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fant M, Farina A, Nagaraja R and
Schlessinger D: PLAC1 (Placenta-specific 1): A novel, X-linked gene
with roles in reproductive and cancer biology. Prenat Diagn.
30:497–502. 2010.PubMed/NCBI
|
|
25
|
Purwosunu Y, Sekizawa A, Farina A, Wibowo
N, Okazaki S, Nakamura M, Samura O, Fujito N and Okai T: Cell-free
mRNA concentrations of CRH, PLAC1, and selectin-P are increased in
the plasma of pregnant women with preeclampsia. Prenat Diagn.
27:772–777. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zanello M, Sekizawa A, Purwosunu Y, Curti
A and Farina A: Circulating mRNA for the PLAC1 gene as a second
trimester marker (14–18 weeks' gestation) in the screening for late
preeclampsia. Fetal Diagn Ther. 36:196–201. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fant ME, Fuentes J, Kong X and Jackman S:
The nexus of prematurity, birth defects, and intrauterine growth
restriction: A role for plac1-regulated pathways. Front Pediatr.
2:82014. View Article : Google Scholar : PubMed/NCBI
|