Introduction
With the rapid development of the global economy and
alterations to lifestyles and diet, the prevalence of diabetes in
the population is rapidly growing. In 2008, the prevalence of
diabetes in China was 9.7%; in 2010, it was reported that the
diabetic rate had increased to 11.7% following the introduction of
the glycated hemoglobin diagnostic criteria by the Chinese Center
For Disease Control And Prevention, based on the original diagnosis
of diabetes, with a total number of ~113 million cases (1). It is estimated that the total number
of patients with diabetes in China may be the highest globally.
Diabetic nephropathy (DN) is a microvascular disease caused by
diabetes, which is difficult to cure and may have a serious impact
on the lives of patients (2).
Research has demonstrated that DN eventually develops into
end-stage renal disease (3).
Cognitive ability refers to the ability of an
organism to perceive the surrounding environment, which is also the
basis for addressing and resolving issues, and typically includes
memory, attention, learning ability, abstract and comprehensive
analysis capabilities, information processing speed,
problem-solving ability and psychomotor speed (4). Impaired cognition is considered to be
the result of ≥1 of these aspects being impaired, and ranges from
mild cognitive impairment to severe dementia (5). Cognitive impairment is a transitional
stage between normal aging and dementia, which is a clinical
syndrome (6). In 2010, a study
demonstrated that the cognitive impairment prevalence was 20.8% in
the community population of individuals >65 years old, with the
total number of affected individuals being ~23.86 million in China
(7).
Oxidative stress is an important factor that leads
to nerve cell damage. Abnormally deposited Aβ may induce oxidative
stress, and lead to the loss of synaptic function and hindered
neuronal metabolism, which serves a key role in the pathogenesis of
cognitive impairment (8).
Following injection of amyloid-β (Aβ) into the temporal lobe cortex
of rats, it was reported that the oxidative stress level was
increased in brain tissue, neurons were injured and the number of
activated astrocytes was significantly increased (9). Additional experimental results
revealed that there is a positive association between cognitive
impairment and oxidative stress, for example, Aβ deposition in the
brain may lead to the generation of oxidative stress, and oxidative
stress also promotes Aβ deposition in the brain, reducing its
clearance rate and thereby speeding up the progression of cognitive
impairment (10).
Previous studies concerning the pathogenesis of DN
have demonstrated that multiple factors, including abnormal gene
regulation, inflammation, oxidative stress, an abnormal endoplasmic
reticulum system, accumulation of advanced glycation end-products,
polyol and activation of protease C, are involved in the occurrence
and development of diabetic nephropathy (11,12).
These processes are all inextricably associated with the local
microinflammation of the kidney (12).
Mitogen-activated protein kinases (MAPKs) are
widespread in vertebrates. The MAPK signaling pathway is an
important eukaryotic signal transduction network and serves a key
role in the gene expression regulation process (13). MAPK contains three classic
subfamilies, including extracellular regulated kinase (ERK) 1/2,
p38 MAPK and c-Jun N-terminal kinase (JNK) (13). Certain studies have revealed that
the activities of p38 MAPK and JNK are significantly increased in
kidney tissues with DN, indicating that the MAPK signaling pathway
is closely associated with DN (14,15).
Esculin is a derivative of coumarin, which is also
an active ingredient of ash bark, and has antibacterial,
anti-inflammatory, anti-allergy and skin protective effects
(16,17). In addition, esculin was reported to
treat increased capillary fragility and prevent peripheral vascular
diseases (18). Esculin exhibits
various activities, including anti-inflammatory, antibacterial,
anticoagulation and analgesic effects, and has a significant
diuretic effect in mice (18). In
addition, esculin inhibits aldose reductase in the rat eye lens,
serves as a growth inhibitor of Bacillus subtilis (19). The present study aimed to determine
whether esculin may ameliorate cognitive impairment in experimental
DN and to determine the potential underlying mechanisms.
Materials and methods
Animals, DN model and study
design
Experiments were performed in accordance with the
Guide for the Care and Use of Laboratory Animals of the First
Affiliated Hospital of Harbin Medical University (Harbin, China),
and were approved by the ethics committee of the First Affiliated
Hospital of Harbin Medical University. Male C57BL/6J 6-week-old
mice (20–22 g, n=38) were obtained from the Animal Laboratory of
Harbin Medical University and housed in cages under controlled
environmental conditions (23–24°C, 55–60% humidity, 0.038%
CO2 and a 12 h light/dark cycle) and allowed freely
access to food and water. Following acclimatization for one week,
the mice were randomly divided into five groups: Control (n=6), DN
model (n=8), 5 mg/kg esculin (n=8), 10 mg/kg esculin (n=8) and 20
mg/kg esculin (n=8). Mice from the DN model group, and 5, 10 and 20
mg/kg esculin groups, were injected intravenously with a single low
dose of streptozotocin (30 mg/kg; STZ; Sigma-Aldrich, Merck KGaA,
Darmstadt, Germany). In control group, mice were injected
intravenously with normal saline. At 2 weeks after STZ injection,
mice from the DN model group, and 5, 10 and 20 mg/kg esculin
groups, were injected intravenously with normal saline, or 5, 10 or
20 mg/kg esculin for two days for 2 weeks. Following this, mice
were subjected to functional analysis tests, such as the Morris
water maze test and assessment of biochemical and urine
parameters.
Morris water maze (MWM) test
Briefly, the MWM test for mice consisted of a
stainless-steel circular tank (diameter, 90 cm; height, 50 cm). It
was painted white on the inside and a circular platform of 9 cm in
diameter was submerged 0.5–1 cm below the surface of water
(28–32°C). The tank was divided into four quadrants and the end of
each line marked four cardinal points: North, south, east and west.
The maze was placed in water at 37°C, and mice placed in it were
observed. The experiment began by lowering the animals into the
pool and releasing them into the water at water-level (not dropped)
so that they were facing and close to the side wall for 60 sec. The
latency and swimming distance were recorded. Subsequently, mice
were placed on the platform for 20 sec, and next training was
performed following 60 sec of rest. This experiment was repeated
six times and each day was recorded. On day 5 of the training, the
platform was removed, and the number of crossings of the platform
location within 120 sec (crossing number) was recorded.
Assessment of biochemical parameters
and urine parameters
Serum samples were collected after centrifugation at
1,000 × g for 10 min at 4°C to determine glucose levels (cat. no.
F006) using ELISA KITS according to the manufacturer's protocol of
each kit (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China). Urine was collected to measure urinary albumin excretion
(cat. no. C035-2) and serum creatinine (C012-1) using ELISA KITS
according to the manufacturer's protocol of each kit (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China).
Determination of tumor necrosis factor
(TNF)-α, interleukin (IL)-6, superoxide dismutase (SOD),
malondialdehyde (MDA), monocyte chemoattractant protein (MCP)-1 and
intracellular adhesion molecule (ICAM)-1 levels
A total of 50 mg right kidney tissue of mice was
precisely weighed and normal saline (1:9 w/v) was added to kidney
tissue samples. The kidney was homogenized and centrifuged at 6,000
× g for 10 min at 4°C. The levels of TNF-α (cat. no. H052), IL-6
(cat. no. H007), SOD (cat. no. A001-1-1), MDA (cat. no. A003-1),
MCP-1 (cat. no. H115) and ICAM-1 (cat. no. H065) were measured with
ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China), according to the manufacturer's protocol.
Western blot analysis
A total of 50 mg right kidney tissue of mice was
precisely weighed and homogenized using PRO-PREP Protein Extraction
solution (Intron Biotechnology, Inc., Seongnam, Korea) at 4°C for
30 min. Protein concentrations were measured with BCA assay kit
(Bio-Rad Laboratories Inc., Hercules, CA, USA). Each protein sample
(60–80 µg) was loaded onto a 8–10% SDS-PAGE gel and transferred
onto a polyvinylidene difluoride membrane (Immobilon; EMD
Millipore, Billerica, MA, USA). The membranes were blocked with 5%
skimmed milk in PBS containing 0.1% Tween-20 solution for 1 h at
room temperature. The membrane was probed with antibodies against
activator protein (AP)-1 (9165, 1:2,000; Cell Signaling Technology,
Inc., Danvers, MA, USA), phosphorylated (p)-p38 MAPK (4511,
1:1,000; Cell Signaling Technology, Inc.), p-JNK (9255, 1:2,000;
Cell Signaling Technology, Inc.), p-ERK1/2 (4370, 1:2,000; Cell
Signaling Technology Inc.) and anti-GAPDH (5174, 1:5,000; Cell
Signaling Technology Inc.) overnight at 4°C. The membrane was
subsequently incubated with goat anti-rabbit IgG-HRP (cat. no.
sc-2030, 1:5,000; Santa Cruz Biotechnology Inc., Dallas, TX, USA) s
for 1 h at room temperature, followed by exposure to Enhanced
Chemiluminescence substrate solution kit (Santa Cruz Biotechnology
Inc.) and quantified using ImageJ-ProPlus 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
All results are presented as the mean ± standard
error of the mean using SPSS 19.0 (SPSS, Inc., Chicago, IL, USA).
Data were analyzed using one-way or repeated measure analysis of
variance followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Esculin ameliorates cognitive
impairment in experimental DN mice
A DN mouse model was generated by treatment with
STZ, and the effect of esculin on cognitive impairment was
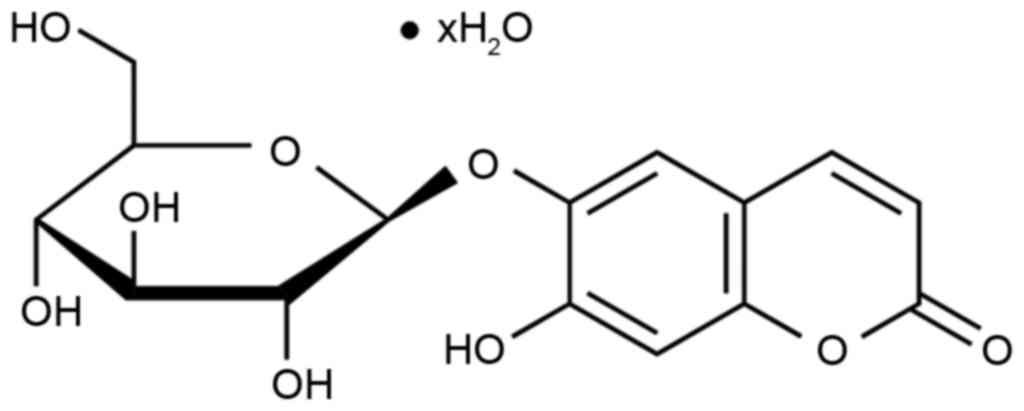
investigated. The chemical structure of esculin is depicted in
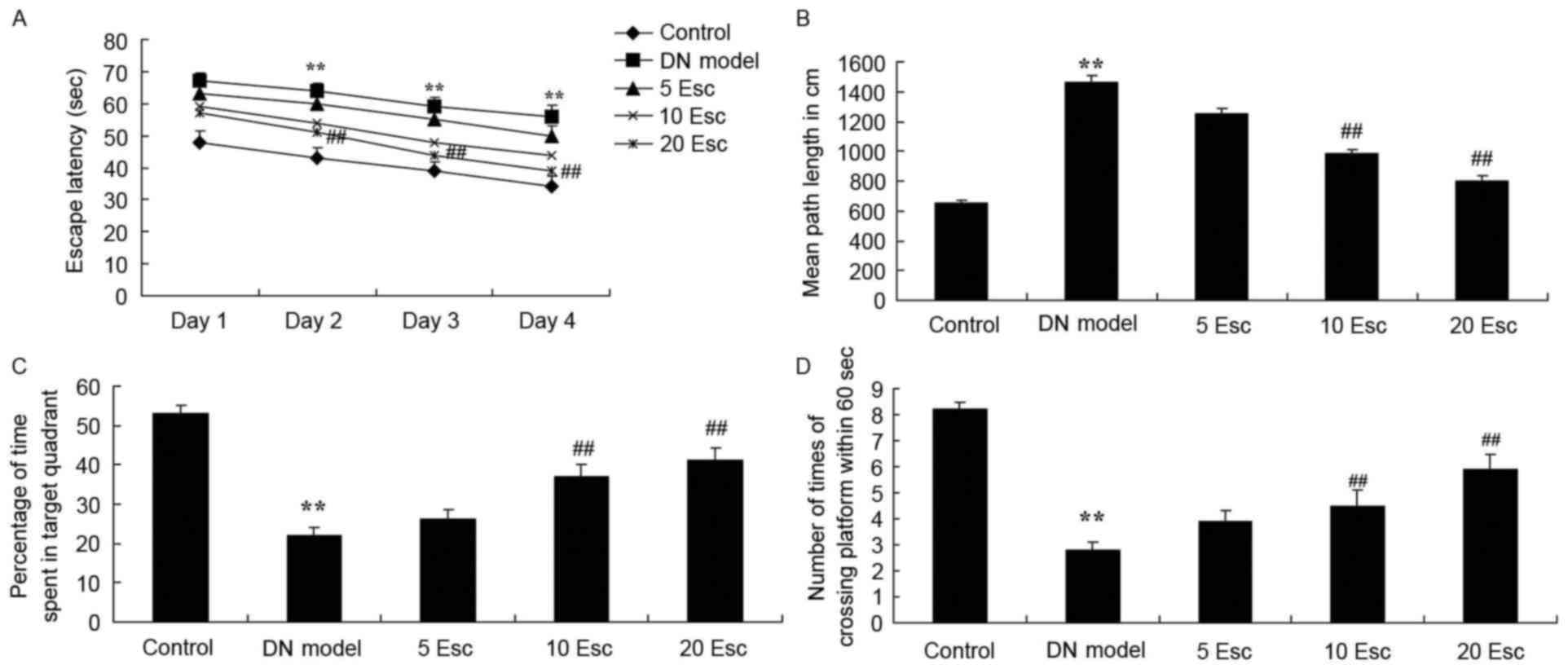
Fig. 1. As demonstrated in
Fig. 2A and B, the time of escape
latency and mean path length in DN model mice were enhanced
compared with the control group. Esculin treatment at 10 and 20
mg/kg significantly inhibited these indices in DN mice, compared
with the DN model group (Fig. 2A and
B). Furthermore, the percentage of time spent in the target
quadrant and the number of times crossing the platform in the DN
model group were reduced compared with the control group (Fig. 2C and D). Esculin (10 and 20 mg/kg)
significantly increased the percentage of time spent in the target
quadrant and the number of times crossing the platform in DN mice,
compared with the DM model group (Fig.
2C and D).
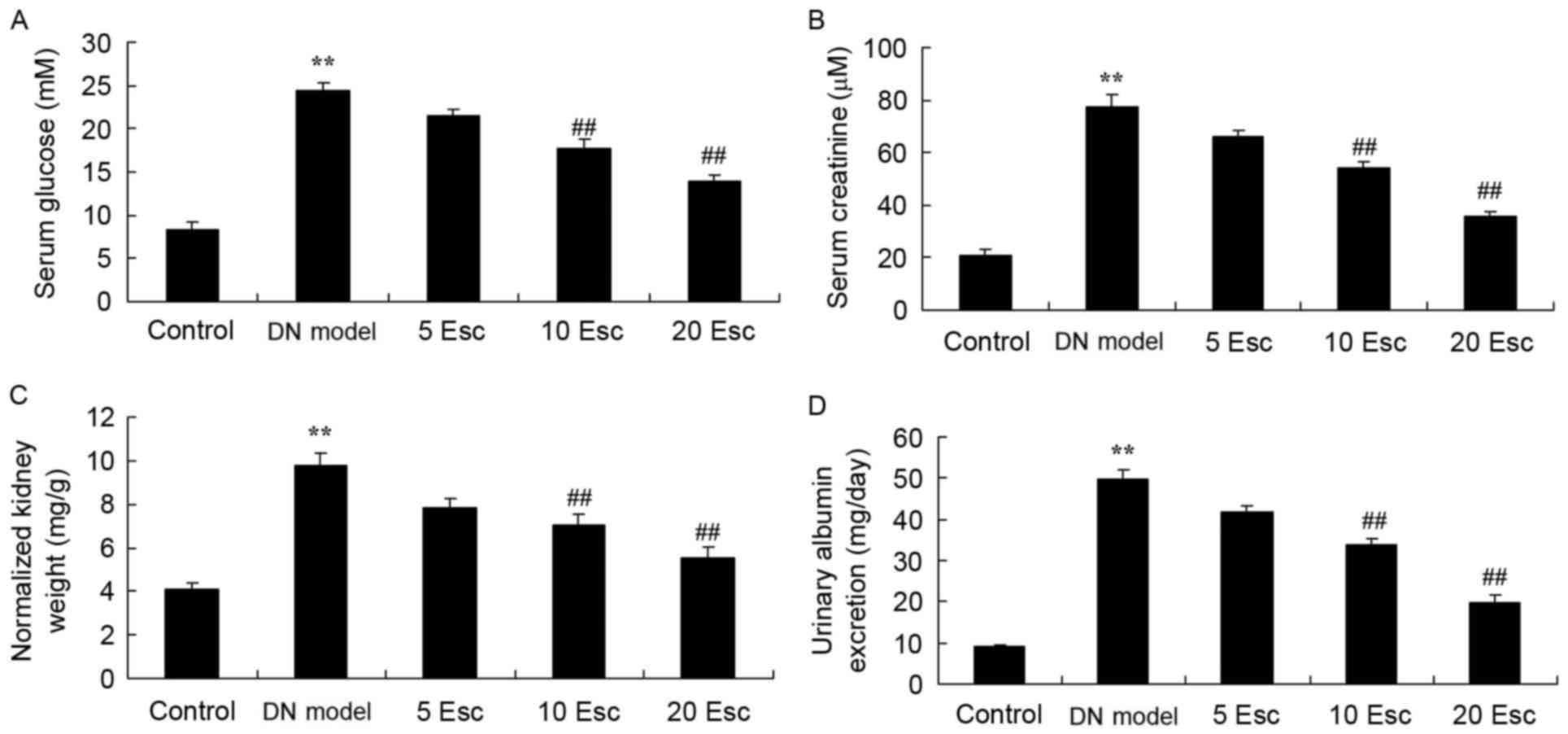
Esculin ameliorates renal function in
experimental diabetic nephropathy mice
The effect of esculin on the renal function of DN
mice following treatment with STZ was determined. In the DM model
group, serum glucose, serum creatinine, normalized kidney weight
and urinary albumin excretion were enhanced, compared with control
animals (Fig. 3). Treatment with
esculin at 10 and 20 mg/kg significantly inhibited serum glucose,
serum creatinine, normalized kidney weight and urinary albumin
excretion in DN mice, compared with the DN model group (Fig. 3).
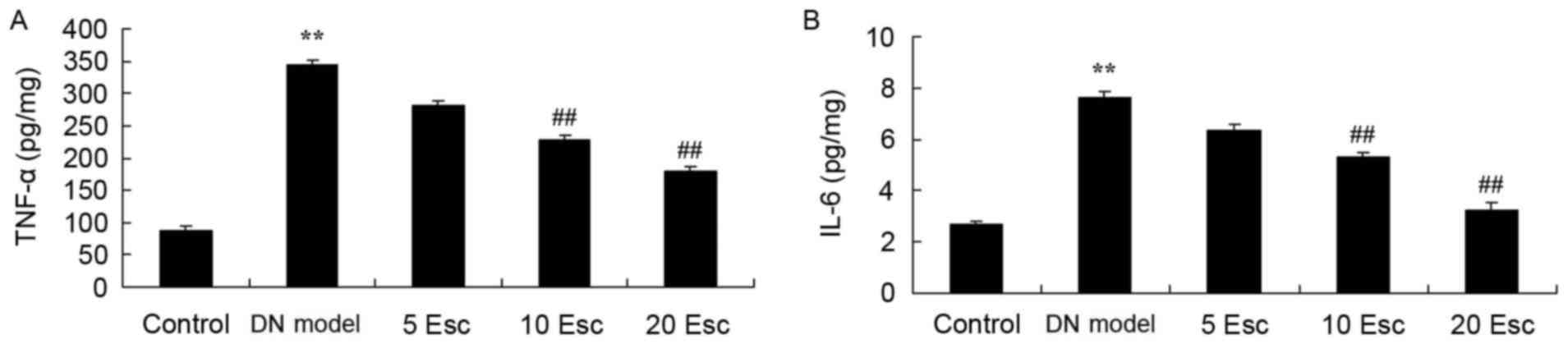
Esculin ameliorates inflammatory
reaction in experimental DN mice
The anti-inflammatory effect of esculin in
experimental DN mice was investigated, and TNF-α and IL-6 levels
were measured using ELISA kits. As demonstrated in Fig. 4, TNF-α and IL-6 levels in the DN
model group were enhanced compared with control mice. However, the
levels of TNF-α and IL-6 in DN mice were significantly attenuated
following treatment with esculin at 10 and 20 mg/kg, compared with
the DN model group (Fig. 4).
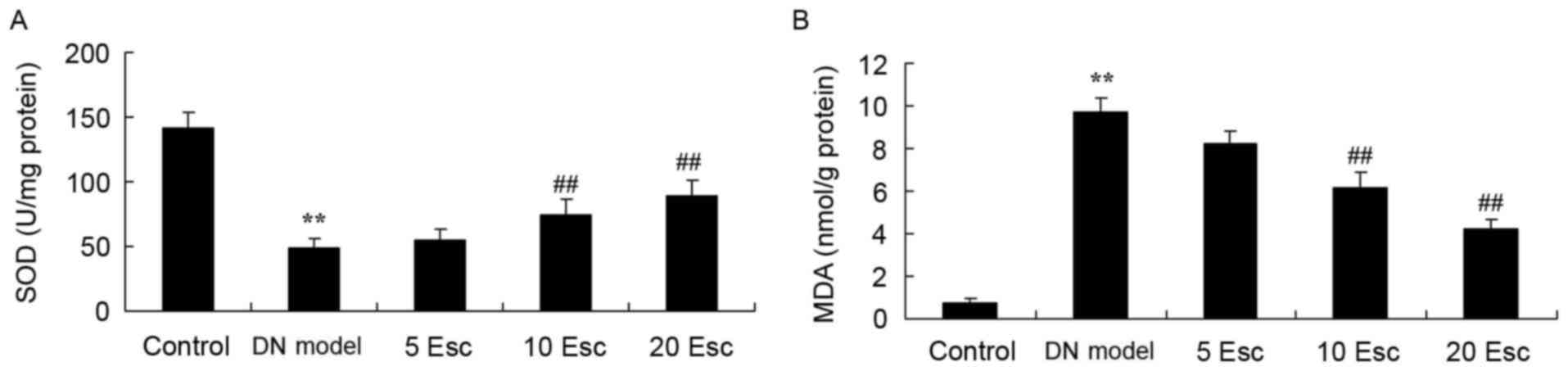
Esculin ameliorates oxidative stress
in experimental DN mice
To confirm the effect of esculin, oxidative stress
in experimental DN mice was determined. In the kidney samples, SOD
activity was significantly decreased and MDA levels were
significantly increased in the DM model compared with control
animals (Fig. 5). The inhibition
of SOD activity and increased MDA levels in DN mice were
significantly reversed by treatment with esculin at 10 and 20
mg/kg, compared with the DN model group (Fig. 5).
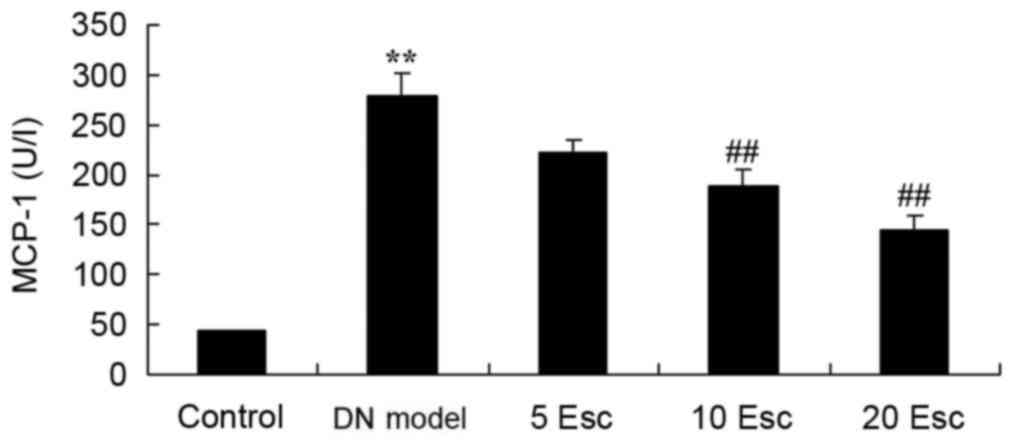
Esculin ameliorates MCP-1 activity in
experimental DN mice
MCP-1 activity was measured using an ELISA kit. As
demonstrated in Fig. 6, there was
a significant increase in MCP-1 activity in the DN model group
compared with control animals. The activation of MCP-1 activity in
DM mice was significantly inhibited by treatment with esculin at 10
and 20 mg/kg, compared with the DN model group (Fig. 6).
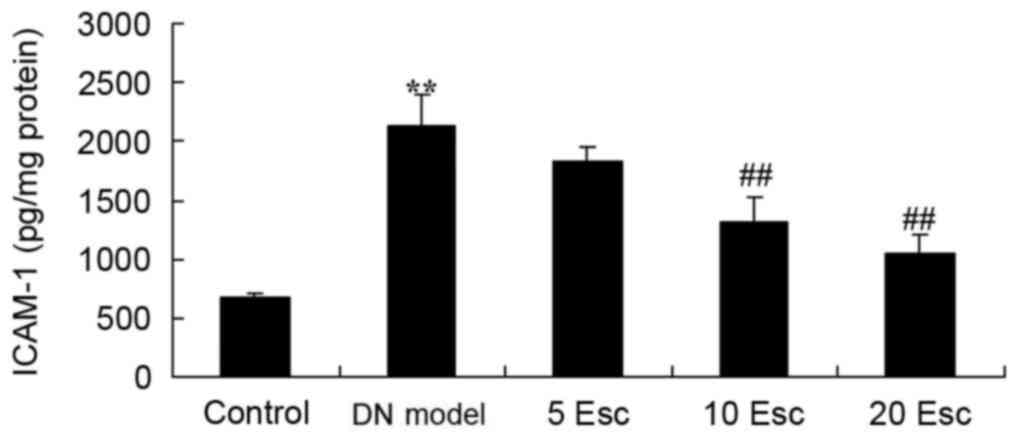
Esculin ameliorates ICAM-1 levels in
experimental DN mice
ICAM-1 levels in experimental DN mice was
determined. There was a significant increase in ICAM-1 levels in
the DN model group compared with the control group (Fig. 7). Treatment with esculin at 10 and
20 mg/kg significantly inhibited ICAM-1 levels in DN mice, compared
with the DN model group (Fig.
7).
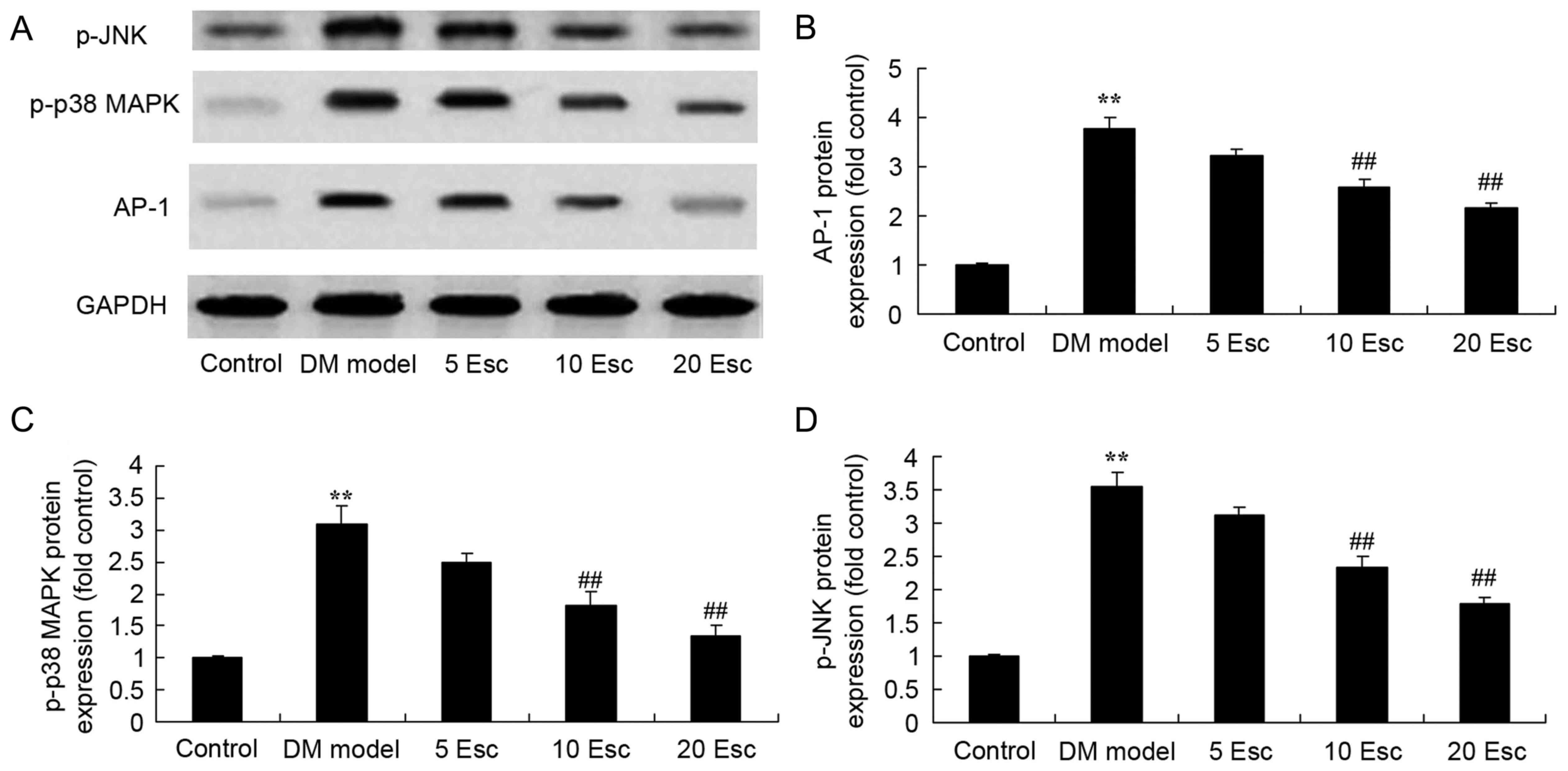
Esculin ameliorates AP-1, p-p38 MAPK
and p-JNK protein expression in experimental DN mice
To investigate the effect of esculin on AP-1, p-p38
MAPK and p-JNK protein expression in experimental DN mice, AP-1
protein expression was detected using western blot analysis. As
demonstrated in Fig. 8, increased
AP-1, p-p38 MAPK and p-JNKprotein expression in the DN model group
was observed compared with the control group. Treatment with
esculin at 10 and 20 mg/kg significantly suppressed AP-1, p-p38
MAPK and p-JNK protein expression in DN mice, compared with the DN
model group (Fig. 8).
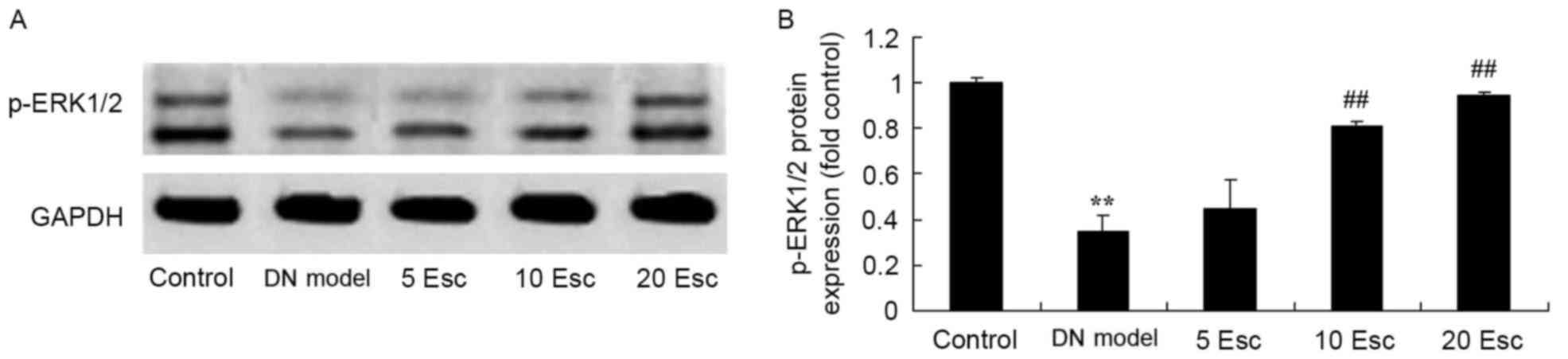
Esculin induces p-ERK 1/2 protein
expression in experimental DN mice
To further investigate the effect of esculin, p-ERK
1/2 protein expression was measured by western blot analysis. p-ERK
1/2 protein expression in the DN model group was significantly
suppressed compared with control group (Fig. 9). Esculin at 10 and 20 mg/kg
significantly enhanced p-ERK 1/2 protein expression in DN mice,
compared with the DM model group (Fig.
9).
Discussion
With the acceleration of social aging, the incidence
of DN and cognitive impairment is increasing (20). The type 2 diabetes prevalence rate
among the elderly population is >10%, which is increasing
annually in China. Diabetes and dementia are two major diseases
that affect the health of the elderly (20). Therefore, methods for the early
prevention of dementia are urgently required (21). Diabetes may cause cognitive
impairment and, with worsening progression of the disease, it may
develop into dementia. Early clinical cognitive impairment is
frequently neglected, and when it is identified, it is usually in
the acute phase of cognitive impairment or dementia (22). The current treatments available are
not satisfactory, and this ultimately has a large impact on the
patients themselves, family and society (23). Therefore, cognitive function
estimation in elderly diabetes patients has attracted increased
attention from clinicians (4). In
the present study, it was demonstrated, perhaps for the first time,
that esculin ameliorated cognitive impairment and improved renal
function in experimental DN mice.
In animals, reactive oxygen species (ROS) induced
oxidative stress to leading to loss of cytotoxicity in different
tissues, including the brain, and may have certain benefits for
cell homeostasis (24). However,
as a primary factor for the occurrence and deterioration of
numerous diseases, including aging and degenerative diseases of the
nervous system, oxidative stress injury is aggravated due to
excessive and/or continuously increased levels of ROS (25). When ROS levels become excessive,
mitochondria are also damaged. If mitochondrial function becomes
abnormal, increased ROS may be generated and cannot be quickly
cleared, which leads to the accumulation of ROS in the body, thus
resulting in lipid peroxidation, protein and DNA oxidative damage,
destruction of the function and structure of biological membranes,
protein denaturation, abnormally altered function and structure of
biofilm, and DNA strand breaks and base modifications to cause
mutations or cell death (26,27).
Additionally, Zheng et al (16) reported that esculin exhibited a
protective effect in adjuvant-induced arthritic rats via
attenuation of proinflammatory cytokines and oxidative stress.
Oxidative stress may increase the generation of
cytokines by various mechanisms. Oxygenated derivatives, as
secondary messengers, activate the transcription factors nuclear
factor-κB (NF-κB) and AP-1, thus leading to the transcription of
cytokines, growth factors and protein-coding genes in the
extracellular matrix (25). NF-κB
serves an important role in the activation of glomerular mesangial
cells and renal dysfunction (28).
AP-1 mediates high glucose to induce the generation of transforming
growth factor (TGF)-β in mesangial cells. When AP-1 has genetic
mutations in the TGF-β binding sites, the high glucose-mediated
TGF-β level will not be increased (28). Peroxynitrite may enhance diabetic
inflammation as it may reduce the bioavailability of nitric oxide
and accelerate the migration of macrophages, causing the release of
inflammatory and profibrotic cytokines, further stimulating the
generation of ROS (28).
Therefore, the generation of cytokines induced by oxidative stress
is likely to further increase the level of oxidative stress, thus
forming a vicious cycle. Interactions between oxidative stress and
different inflammatory cytokines have been identified (25). The present study demonstrated that
esculin ameliorates AP-1 protein expression in experimental DN
mice.
Various types of inflammatory cells, including
leucocytes, granulocytes, monocytes and macrophages, are involved
in the progression of DN (11).
Research concerning the reduction of renal macrophage accumulation
using an immunosuppression strategy demonstrated that inflammation
enhances the development of DN (29). In the present study, esculin
ameliorated the inflammatory reaction in experimental DN mice. Niu
et al (17) indicated that
esculin exhibited potent anti-inflammatory activities in
lipopolysaccharide-stimulated mice via regulation of TNF-α and IL-6
production, and the MAPK signaling pathway.
The association between oxidative stress and MCP-1
is not clear, and further research is required (30). It has been reported that, in animal
experimental studies, increased levels of oxidative stress may lead
to increased macrophage accumulation, and improve the expression
levels of ICAM-1 and MCP-1 in type 1 diabetic kidneys (31). The oxidative stress levels in rat
mesangial cells and the levels of MCP-1 were increased (30). MCP-1 levels in the plasma of
patients with type 1 diabetes was associated with oxidative stress
in the plasma but treatment with vitamin E reduced these level
(30). In conclusion, these
studies indicated that oxidative stress may increase the levels of
MCP-1 expression (32). In the
present study, Esculin ameliorated MCP-1 and ICAM-1 levels in
experimental DN mice. Wang et al (19) revealed that esculin improved
dyslipidemia, inflammation responses and renal damage in
STZ-induced diabetic rats via ICAM-1.
Certain studies have demonstrated that the
activation of the MAPK signaling pathway may serve a role in the
acceleration of the extracellular maxtrix deposition process
(33). A previous study
demonstrated that the activity of MAPK was significantly increased
in DN in in vitro cultured mesangial cells and also in renal
tubular epithelial cells, which indicated that the MAPK pathway may
serve an important role in the development of DN (34). Smad is the classic pathway involved
in renal fibrosis; however, it has been demonstrated that collagen
generated in human mesangial cells may activate the MAPK/Smad
pathway (35). Previous results
demonstrated that the generation of TGF-β was decreased following
specific blocking of the MAPK pathway, which indicated that the
generation of fibrotic cytokines and collagen may occur via the
MAPK pathway (35,36). The present study demonstrated that
esculin significantly suppressed p-p38 MAPK and p-JNK protein
expression, and increased p-ERK1/2 protein expression, in
STZ-induced diabetic rats. Therefore, MAPK signaling may be
involved in the effect of esculin on diabetes. Additionally, Niu
et al (17) indicated that
esculin exhibited potent anti-inflammatory effects in
LPS-stimulated mice through regulation of TNF-α and IL-6
production, and the MAPK pathway.
In conclusion, the present study demonstrated that
esculin ameliorated cognitive impairment in experimental DN, and
exhibited anti-oxidative stress and anti-inflammatory effects,
potentially via the MAPK signaling pathway. These results may
provide initial experimental evidence to support the treatment of
cognitive impairment in DN mice with esculin.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zilişteanu DS, Atasie T and Voiculescu M:
Efficacy of long-term low-dose sulodexide in diabetic and
non-diabetic nephropathies. Rom J Intern Med. 53:161–169.
2015.PubMed/NCBI
|
|
2
|
Imamura S, Hirai K and Hirai A: The
glucagon-like peptide-1 receptor agonist, liraglutide, attenuates
the progression of overt diabetic nephropathy in type 2 diabetic
patients. Tohoku J Exp Med. 231:57–61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schutte E, Lambers Heerspink HJ, Lutgers
HL, Bakker SJ, Vart P, Wolffenbuttel BH, Umanath K, Lewis JB, de
Zeeuw D and Gansevoort RT: Serum bicarbonate and kidney disease
progression and cardiovascular outcome in patients with diabetic
nephropathy: A Post Hoc analysis of the RENAAL (Reduction of end
points in non-insulin-dependent diabetes with the angiotensin II
antagonist losartan) study and IDNT (Irbesartan diabetic
nephropathy trial). Am J Kidney Dis. 66:450–458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ryan CM, Williams TM, Finegold DN and
Orchard TJ: Cognitive dysfunction in adults with type 1
(insulin-dependent) diabetes mellitus of long duration: Effects of
recurrent hypoglycaemia and other chronic complications.
Diabetologia. 36:329–334. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gorska-Ciebiada M, Saryusz-Wolska M,
Borkowska A, Ciebiada M and Loba J: C-reactive protein, advanced
glycation end products, and their receptor in type 2 diabetic,
elderly patients with mild cognitive impairment. Front Aging
Neurosci. 7:2092015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ba-Tin L, Strike P and Tabet N: Diabetic
peripheral microvascular complications: Relationship to cognitive
function. Cardiovasc Psychiatry Neurol. 2011:7234342011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frier BM: Cognitive functioning in type 1
diabetes: The diabetes control and complications trial (DCCT)
revisited. Diabetologia. 54:233–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Price TO, Farr SA, Niehoff ML, Ercal N,
Morley JE and Shah GN: Protective effect of topiramate on
hyperglycemia-induced cerebral oxidative stress, pericyte loss and
learning behavior in diabetic mice. Int Libr Diabetes Metab.
1:6–12. 2015.PubMed/NCBI
|
|
9
|
Wang X, Song X, Takata T, Miichi Y, Yokono
K and Sakurai T: Amyloid-beta neurotoxicity restricts glucose
window for neuronal survival in rat hippocampal slice cultures. Exp
Gerontol. 45:904–908. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang ZJ, Zou W, Yuan J, Zhang P, Tian Y,
Xiao ZF, Li MH, Wei HJ and Tang XQ: Antidepressant-like and
anxiolytic-like effects of hydrogen sulfide in
streptozotocin-induced diabetic rats through inhibition of
hippocampal oxidative stress. Behav Pharmacol. 26:427–435. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou X, Zhang F, Hu X, Chen J, Wen X, Sun
Y, Liu Y, Tang R, Zheng K and Song Y: Inhibition of inflammation by
astaxanthin alleviates cognition deficits in diabetic mice. Physiol
Behav. 151:412–420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wong TY and McIntosh R: Systemic
associations of retinal microvascular signs: A review of recent
population-based studies. Ophthalmic Physiol Opt. 25:195–204. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohashi N, Urushihara M, Satou R and Kobori
H: Glomerular angiotensinogen is induced in mesangial cells in
diabetic rats via reactive oxygen species-ERK/JNK pathways.
Hypertens Res. 33:1174–1181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang T, Chen SS, Chen R, Yu DM and Yu P:
Reduced beta 2 glycoprotein I improve diabetic nephropathy via
inhibiting TGF-β1-p38 MAPK pathway. Int J Clin Exp Med.
8:6852–6865. 2015.PubMed/NCBI
|
|
15
|
Lim AK, Nikolic-Paterson DJ, Ma FY, Ozols
E, Thomas MC, Flavell RA, Davis RJ and Tesch GH: Role of MKK3-p38
MAPK signalling in the development of type 2 diabetes and renal
injury in obese db/db mice. Diabetologia. 52:347–358. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng L, Yang L, Wang Z, Chen C and Su Y:
Protective effect of Esculin in adjuvant-induced arthritic (AIA)
rats via attenuating pro-inflammatory cytokines and oxidative
stress. Cell Mol Biol (Noisy-le-grand). 61:1–5. 2015.PubMed/NCBI
|
|
17
|
Niu X, Wang Y, Li W, Zhang H, Wang X, Mu
Q, He Z and Yao H: Esculin exhibited anti-inflammatory activities
in vivo and regulated TNF-α and IL-6 production in LPS-stimulated
mouse peritoneal macrophages in vitro through MAPK pathway. Int
Immunopharmacol. 29:779–786. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Z, Zhang L and Chen G: Carbon
nanotube/poly(ethylene-co-vinyl acetate) composite electrode for
capillary electrophoretic determination of esculin and esculetin in
Cortex Fraxini. Electrophoresis. 30:3419–3426. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang YH, Liu YH, He GR, Lv Y and Du GH:
Esculin improves dyslipidemia, inflammation and renal damage in
streptozotocin-induced diabetic rats. BMC Complement Altern Med.
15:4022015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakamura T, Sato E, Amaha M, Kawagoe Y,
Maeda S and Yamagishi S: Addition of aliskiren to angiotensin II
receptor blockers ameliorates renal tubular injury and reduces
intima media thickness of carotid artery in patients with diabetic
nephropathy. Int J Cardiol. 155:294–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kimura S, Inoguchi T, Yokomizo H, Maeda Y,
Sonoda N and Takayanagi R: Randomized comparison of pitavastatin
and pravastatin treatment on the reduction of urinary albumin in
patients with type 2 diabetic nephropathy. Diabetes Obes Metab.
14:666–669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martynyuk L, Martynyuk L, Ruzhitska O and
Martynyuk O: Effect of the herbal combination Canephron N on
diabetic nephropathy in patients with diabetes mellitus: Results of
a comparative cohort study. J Altern Complement Med. 20:472–478.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bakris GL, Agarwal R, Chan JC, Cooper ME,
Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack
C, et al: Effect of Finerenone on Albuminuria in patients with
diabetic nephropathy: A randomized clinical trial. JAMA.
314:884–894. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim BH, Lee ES, Choi R, Nawaboot J, Lee
MY, Lee EY, Kim HS and Chung CH: Protective effects of curcumin on
renal oxidative stress and lipid metabolism in a rat model of type
2 diabetic nephropathy. Yonsei Med J. 57:664–673. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Persson P, Friederich-Persson M, Fasching
A, Hansell P, Inagi R and Palm F: Adenosine A2 a receptor
stimulation prevents proteinuria in diabetic rats by promoting an
anti-inflammatory phenotype without affecting oxidative stress.
Acta Physiol (Oxf). 214:311–318. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang M, Feng L, Gu J, Ma L, Qin D, Wu C
and Jia X: The attenuation of Moutan Cortex on oxidative stress for
renal injury in AGEs-induced mesangial cell dysfunction and
streptozotocin-induced diabetic nephropathy rats. Oxid Med Cell
Longev. 2014:4638152014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pérez-Gallardo RV, Noriega-Cisneros R,
Esquivel-Gutiérrez E, Calderón-Cortés E, Cortés-Rojo C,
Manzo-Avalos S, Campos-García J, Salgado-Garciglia R, Montoya-Pérez
R, Boldogh I and Saavedra-Molina A: Effects of diabetes on
oxidative and nitrosative stress in kidney mitochondria from aged
rats. J Bioenerg Biomembr. 46:511–518. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nam JS, Cho MH, Lee GT, Park JS, Ahn CW,
Cha BS, Lim SK, Kim KR, Ha HJ and Lee HC: The activation of
NF-kappaB and AP-1 in peripheral blood mononuclear cells isolated
from patients with diabetic nephropathy. Diabetes Res Clin Pract.
81:25–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gorska-Ciebiada M, Saryusz-Wolska M,
Borkowska A, Ciebiada M and Loba J: Serum soluble adhesion
molecules and markers of systemic inflammation in elderly diabetic
patients with mild cognitive impairment and depressive symptoms.
Biomed Res Int. 2015:8261802015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang X, Wang Y and Gao G: High glucose
induces rat mesangial cells proliferation and MCP-1 expression via
ROS-mediated activation of NF-κB pathway, which is inhibited by
eleutheroside E. J Recept Signal Transduct Res. 36:152–157. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ellina O, Chatzigeorgiou A, Kouyanou S,
Lymberi M, Mylona-Karagianni C, Tsouvalas E and Kamper EF:
Extracellular matrix-associated (GAGs, CTGF), angiogenic (VEGF) and
inflammatory factors (MCP-1, CD40, IFN-γ) in type 1 diabetes
mellitus nephropathy. Clin Chem Lab Med. 50:167–174. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zafra-Stone S, Yasmin T, Bagchi M,
Chatterjee A, Vinson JA and Bagchi D: Berry anthocyanins as novel
antioxidants in human health and disease prevention. Mol Nutr Food
Res. 51:675–683. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu H, Shi Y, Deng X, Su Y, Du C, Wei J,
Ren Y, Wu M, Hou Y and Duan H: Inhibition of c-Src/p38 MAPK pathway
ameliorates renal tubular epithelial cells apoptosis in db/db mice.
Mol Cell Endocrinol. 417:27–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rane MJ, Song Y, Jin S, Barati MT, Wu R,
Kausar H, Tan Y, Wang Y, Zhou G, Klein JB, et al: Interplay between
Akt and p38 MAPK pathways in the regulation of renal tubular cell
apoptosis associated with diabetic nephropathy. Am J Physiol Renal
Physiol. 298:F49–F61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng X, Gao W, Dang Y, Liu X, Li Y, Peng
X and Ye X: Both ERK/MAPK and TGF-Beta/Smad signaling pathways play
a role in the kidney fibrosis of diabetic mice accelerated by blood
glucose fluctuation. J Diabetes Res. 2013:4637402013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lakshmanan AP, Thandavarayan RA, Watanabe
K, Sari FR, Meilei H, Giridharan VV, Sukumaran V, Soetikno V,
Arumugam S, Suzuki K and Kodama M: Modulation of AT-1R/MAPK cascade
by an olmesartan treatment attenuates diabetic nephropathy in
streptozotocin-induced diabetic mice. Mol Cell Endocrinol.
348:104–111. 2012. View Article : Google Scholar : PubMed/NCBI
|