Introduction
Airway inflammation has been regarded as the most
important pathological characteristic of asthma patients (1). Infiltration of various inflammatory
cells into the airway submucosa is also the basis of airway
hyper-responsiveness (AHR) and airway remodeling (2). It has been verified that the
development of chronic airway inflammation in asthma is due to
inappropriate airway immune responses to specific pathogens or
allergens from the outside environment (3). A disrupted immune response of airway
epithelial cells induces the occurrence and development of
complicated airway inflammation in asthma (4,5). As
they are situated between the host and the outside environment,
airway epithelial cells are the physical defense line against
microorganisms, gases and allergens. In recent years, the
structural and functional homeostasis of airway epithelial cells in
asthma pathogenesis has attracted increasing attention (5,6).
Airway epithelial cells rapidly identify and respond to microbes,
tissue damage or cellular stress via expression of pattern
recognition receptors (PRRs). Epithelial PRR activation then leads
to the release of cytokines, chemokines and antimicrobial peptides,
which further attract and activate innate and adaptive immune cells
(7,8). Notably, an increasing number of
studies have confirmed that multiple pro-inflammatory mediators
(including cytokines and chemokines) serve a major role in the
recruitment and invasion of airway inflammatory cells (7,9,10).
Chemokines are the most direct and immediate cell
factors that selectively induce the migration of specific
inflammatory cells by binding with different receptors (11). They are a group of low molecular
weight (mostly 8–10 KDa) polypeptides that are designated for their
targeted cell chemotaxis. There are four conserved cysteines in the
protein structure of chemokine molecules. Chemokines are divided
into the following four families (CC, CXC, C and CX3C) based on
where other amino acids are inserted between the first two
cysteines near the N-terminus (12). By interacting with corresponding
receptors, chemokines strongly contribute to the chemotaxis of
neutrophils, eosinophils, basophils, monocytes, mast cells,
dendritic cells (DCs), natural killer (NK) cells, T lymphocytes and
B lymphocytes. There is not a simple one-to-one correspondence
between chemokines and chemokine receptors. One chemokine receptor
can be activated by different chemokines and one chemokine can bind
to different chemokine receptors (13). The CC family contains ~28 types of
chemokines with strong chemotaxis effects on almost all
inflammatory cells (except neutrophils). The CXC chemokine family
contains more than 15 types of chemokines, which possess potent
effects on the recruitment of neutrophils and monocytes. Besides
these four traditional cytokine families, there is a growing number
of cytokines showing strong chemotactic effects which are also
classified as chemokines (14,15).
Studies have shown that a variety of cells are involved in
expression and secretion of chemokines, including macrophages,
monocytes, eosinophils, basophils, neutrophils, mast cells, DCs and
lymphocytes (10,11,13).
The participation of a variety of effector cells leads to the
cascade of events triggering the activation of diverse immune
responses. It has been identified that airway epithelial cells act
as the key orchestrator to airway inflammation in asthma (16). Airway epithelial cells selectively
produce a number of different chemokines that can induce various
types of inflammatory cells which would release a variety of
inflammatory factors. Such inflammatory factors cause the immediate
phase reaction of airway inflammation mediated by immunoglobin
(IgE) and induce chronic persistence airway inflammation with
eosinophils and T helper cell (Th)2 lymphocytes (9,17).
The present review focused on the expression and biological
characteristics of chemokines in airway epithelial cells and the
role of these epithelial chemokines in the pathogenesis of airway
inflammation in asthma (Table
I).
 | Table I.Airway epithelium chemokine
expression associated with asthma (8,9,13). |
Table I.
Airway epithelium chemokine
expression associated with asthma (8,9,13).
| System name | Generic name | Full name | Receptor | Cell types
affected |
|---|
| CC family |
|
|
|
|
|
CCL2 | MCP-1 | Monocyte
chemotactic protein-1 | CCR2, 10 | Monocyte, T
lymphocyte, basophil, NK cell |
|
CCL3 | MIP-1α | Macrophage
inflammatory protein-1α | CCR1, 3, 5 | Macrophage,
eosinophil, T lymphocyte, DCs, neutrophil, NK cell |
|
CCL4 | MIP-1β | Macrophage
inflammatory protein-1β | CCR5,8 | Lymphocyte,
monocyte |
|
CCL5 | RANTES | Regulated upon
activation normal T-cell expressed and secreted | CCR1, 3, 5 | Eosinophil,
monocyte, memory T cell, CD4+T cell, basophil |
|
CCL11 | Eotaxin-1 | Eotaxin-1 | CCR3, 5 | Eosinophil |
|
CCL13 | MCP-4 | Monocyte
chemotactic protein-4 | CCR2, 3 | T lymphocyte,
eosinophil, basophil, monocyte |
|
CCL17 | TARC | Thymus activation
regulated chemokine | CCR4 | Th2 cell |
|
CCL20 | MIP-3α | Macrophage
inflammatory protein-3α | CCR6 | iDCs, T lymphocyte,
B lymphocyte |
|
CCL22 | MDC | Macrophage-derived
chemokine | CCR4 | Th2 cell, DCs, NK
cells |
| CXC family |
|
|
|
|
|
CXCL1 | GRO-α | Growth-regulated
oncogene-α | CXCR1, 2 | Neutrophil |
|
CXCL5 | ENA-78 | Epithelial-derived
neutrophil-activating peptide 78 | CXCR2 | Neutrophil |
|
CXCL8 | IL-8 | Interleukin-8 | CXCR1, 2 | Neutrophil,
eosinophil, T lymphocyte, NK cell |
|
CXCL10 | IP-10 |
Interferon-inducible protein-10 | CXCR3, 4 | Eosinophil,
neutrophil, monocyte, T lymphocyte |
| Non CC or CXC
family |
|
|
|
|
|
TSLP | No | Thymic stromal
lymphopoietin | TSLPR | DCs, Th0 cell, Th2
cell |
|
IL-33 | No | Interleukin-33 | IL-33R, ST2 | DCs, Th2 cell,
ILC2s |
Expression properties of epithelial
chemokines
For airway epithelial cells, the expression of
chemokines is regulated at several levels. Different expression
properties of chemokines from diverse research models all indicate
the importance of chemokines during the pathogenesis of airway
inflammation in asthma (18).
Expression properties of CC family
chemokines in airway epithelium
The early immune response is mediated mainly by
inflammatory cytokines interleukin (IL)-1 and tumor necrosis
factor-α (TNF-α). It has been reported that IL-1β and TNF-α are
present in the epithelial environment within hours of infection
(19,20). IL-1β and TNF-α then induce the
expression of chemokines in epithelial cells (11). It has previously been demonstrated
that TNF-α stimulates the expression of C-C motif chemokine ligands
CCL2, CCL4, CCL5, CCL11 and CCL20. Furthermore, airway epithelial
cells release CCL5 and CCL20 upon stimulation by IL-1β (18). IL-1β can also induce the expression
of CCL3 and CCL4 in airway epithelial cells by activating nuclear
factor (NF)-κB. In addition, NF-κB also activates transcription of
the gene encoding CCL2 as its promoter contains an NF-κB binding
site (21,22).
Several other inflammatory cytokines can induce the
expression of certain chemokines. Previous studies have revealed
that TNF-α in combination with IL-4 or IL-13 upregulates the
expression and secretion of CCL17 in epithelial cells (23,24).
It has also been indicated that CCL11 and CCL20 are produced
following IL-4 or IL-13 stimulation (25). Furthermore, it has been
demonstrated that clusterin induces the production of CCL20 by
regulating the oxidative stress environment in airway epithelial
cells from mice studies (26).
Previous studies have demonstrated that microRNA-34a,
15-lipoxygenase and histamine are important regulators for CCL22 in
airway epithelial cells (27–29).
In addition, airway epithelial cells produce
chemokines in response to immunological environmental factors
including microbial and viral stimuli (11). Viral or bacterial infection induces
the secretion of high levels of CCL2, CCL5 and CCL20 by airway
epithelial cells (30).
Furthermore, infection by viruses increases CCL5 expression in
different types of cells. For example, respiratory syncytial virus
infection results in enhanced expression of CCL5 in human nasal
mucosa and gland epithelial cells, and infection by influenza virus
induces CCL5 expression in human bronchial tissues and nasal polyp
epithelial cells (31). In
addition, Der p allergens from house dust mites induce CCL17
expression in bronchial epithelial cells which appears to be
mediated by a disintegrin and metalloproteinase-dependent
phosphorylation of epidermal growth factor receptor and subsequent
activation of mitogen-activated protein kinase (MAPK) and NF-κB
(32).
Expression properties of CXC family
chemokines in airway epithelium
It has been demonstrated that IL-17 promotes the
expression of CXCL1 and CXCL5 in bronchial epithelial cells and
that IL-17 potentially promotes CXCL1 expression mainly through the
extracellular regulated protein kinases (ERK) or MAPK pathway
(33). At the same time, different
cytokines stimulate CXCL8 secretion under the control of different
signaling pathways, including NF-κB, ERK, c-Jun N-terminal kinase
(JNK) and MAPK pathways (34).
Additionally, Janus kinase (JAK) and the synergistic effect of
TNF-α and interferon-γ can induce CXCL10 expression in airway
epithelial cells (35). On this
basis, these associated signaling molecules have also been used as
potential targets of anti-inflammatory treatment. It has been
identified that the inhibition of JAK pathway in the airway
epithelium may provide an alternative anti-inflammatory approach to
glucocorticosteroid-resistant diseases in vitro (36). It has also been observed that the
long-acting β2 agonists downregulate poly I:C-induced CXCL10
expression in bronchial epithelial cells via the β2
adrenoreceptor-cyclic adenosine monophosphate and JNK pathways
in vitro (37).
Expression properties of other
chemokines in airway epithelium
Various cytokines including thymic stromal
lymphopoietin (TSLP) and IL-33 secreted by airway epithelial cells
have previously been identified to possess very strong recruitment
effects on inflammatory cells. They are also classified as
chemokines not belonging to the traditional four classic chemokine
families (38,39).
Previous studies have revealed that TNF-α and IL-1β
can induce the expression of IL-33 (40). Park et al (41) verified that TNF-α stimulates IL-33
expression in primary nasal epithelial cells and A549 cells via the
NF-κB, ERK and MAPK pathways in vitro. Furthermore, IL-33
has been reported to induce the production of TSLP in bronchial
epithelial cells following activation by antigens (42). Respiratory syncytial virus
infection can also rapidly transfer stress signals through the JNK
and MAPK pathways via direct stimulation of epithelial cells to
upregulate expression of TSLP (43).
Functional properties of chemokines and
associations between chemokines and asthma
Effect of chemokines on DCs and T
cells
Recruitment of Th2 cells and the subsequent
production of Th2-type cytokines form the main characteristics of
asthma airway inflammation (44).
DCs are sentinels of the adaptive immune system as they can induce
the differentiation of Th2 cells. The key role of DCs in asthma
pathogenesis has been a subject of attention for more than 15 years
(1).
In the pathogenesis of asthma, the secretion of
CCL20, IL-33 and TSLP is substantially increased following stress
by allergens and other pathogenic substances. C-C chemokine
receptor CCR6, IL-33R and TSLPR (corresponding receptors for CCL20,
IL-33 and TSLP respectively) are expressed on immature DCs (iDCs).
By binding with these receptors, CCL20, IL-33 and TSLP activate
iDCs and promote their maturation (26,42).
DCs then migrate to the T cell zone in mediastinal lymph nodes,
where they activate T cells via antigen presentation and
co-stimulation. Furthermore, TSLP upregulates the expression of
surface co-stimulatory molecules, including CD40, CD80 and CD86 on
DCs. Under the influence of secreted cytokines and
membrane-expressed molecules including OX40 L, Jagged1, IL-6 and
leukotrienes C4, activated inflammatory DCs interact with naive
CD4+T cells and induce the differentiation of Th2, Th17
and follicular helper T cells (45,46).
CCR3 and CCR4 surface receptors of Th2 cells directly bind to
chemokines secreted by epithelial cells. CCR4 interacts with
CCL17/CCL22, and CCR3 interacts with CCL13 (27). Animal studies have indicated that
targeted blocking of CCR4/CCR3 receptors can significantly reduce
the eosinophil ratio in the bronchoalveolar lavage fluid of
asthmatic model mice (47).
Following prompting by the actions of these chemokines, Th2 cells
infiltrate into the site of inflammation where they secrete
cytokines including IL-4, IL-5, IL-13 and TNF-α (Fig. 1). These cytokines in turn cause
enhanced airway mucus secretion and airway epithelial structure
destruction, further provoking airway inflammation and AHR
(48,49). IL-4 induces B cell activation and
the secretion of IgE; IL-13 causes goblet cell metaplasia, AHR and
increases the expression of adhesion molecules on vascular
endothelial cells (44,50). Furthermore, IL-4 and IL-13 can also
promote the secretion of CCL17 by airway epithelial cells, and
IL-13 can promote the secretion of CCL22 (23). These chemokines, which result from
cascade amplification, further induce the aggregation of Th2 cell
infiltration. The specific enhanced expression of TSLP in airway
epithelial cells results in initial CD4+T lymphocyte
proliferation and Th2 cell differentiation (38). Conversely, targeting TSLP with
short hairpin RNA or antibodies, alleviates airway inflammation and
decreases epithelial CCL17 in a murine model of asthma (51,52).
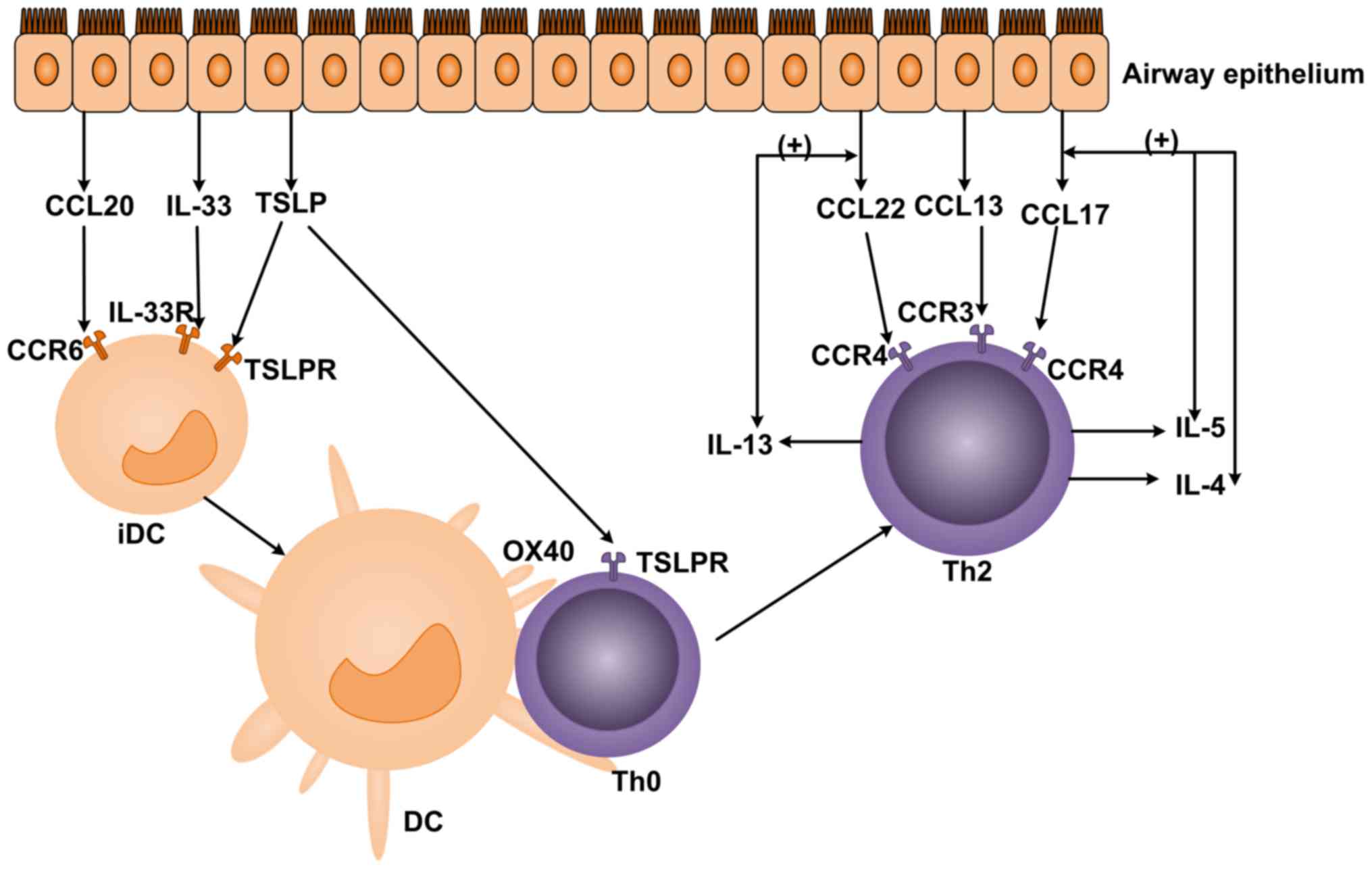 | Figure 1.The effect of chemokines on DCs and T
cells. CCL20, IL-33 and TSLP secreted by airway epithelial cells
combine with CCR6, IL-33R and TSLPR on iDCs to serve a role in
chemotaxis. CCL13, CCL17 and CCL22 combine with CCR3 and CCR4 on
Th2 cells to induce chemotaxis. DCs, dendritic cells; CCL, C-C
motif chemokine ligand; IL, interleukin; TSLP, thymic stromal
lymphopoietin; CCR, C-C chemokine receptor; IL-33R, IL-33 receptor;
TSLPR, TSLP receptor. |
Effect of chemokines on eosinophils,
neutrophils and innate lymphoid cells (ILC)2
In addition to activated DCs and Th2 cells,
eosinophils also act as key effector cells in the airway
inflammation of asthma. The invasion of eosinophils in the airway
is closely associated with the severity of asthma (53). Eosinophils initially form in the
bone marrow and differentiate from progenitor cells. By rolling
adhesion and exudation, eosinophils interact with endothelial
cells. Eosinophils are activated to release granule-associated
proteins, which cause airway epithelial injury, smooth muscle
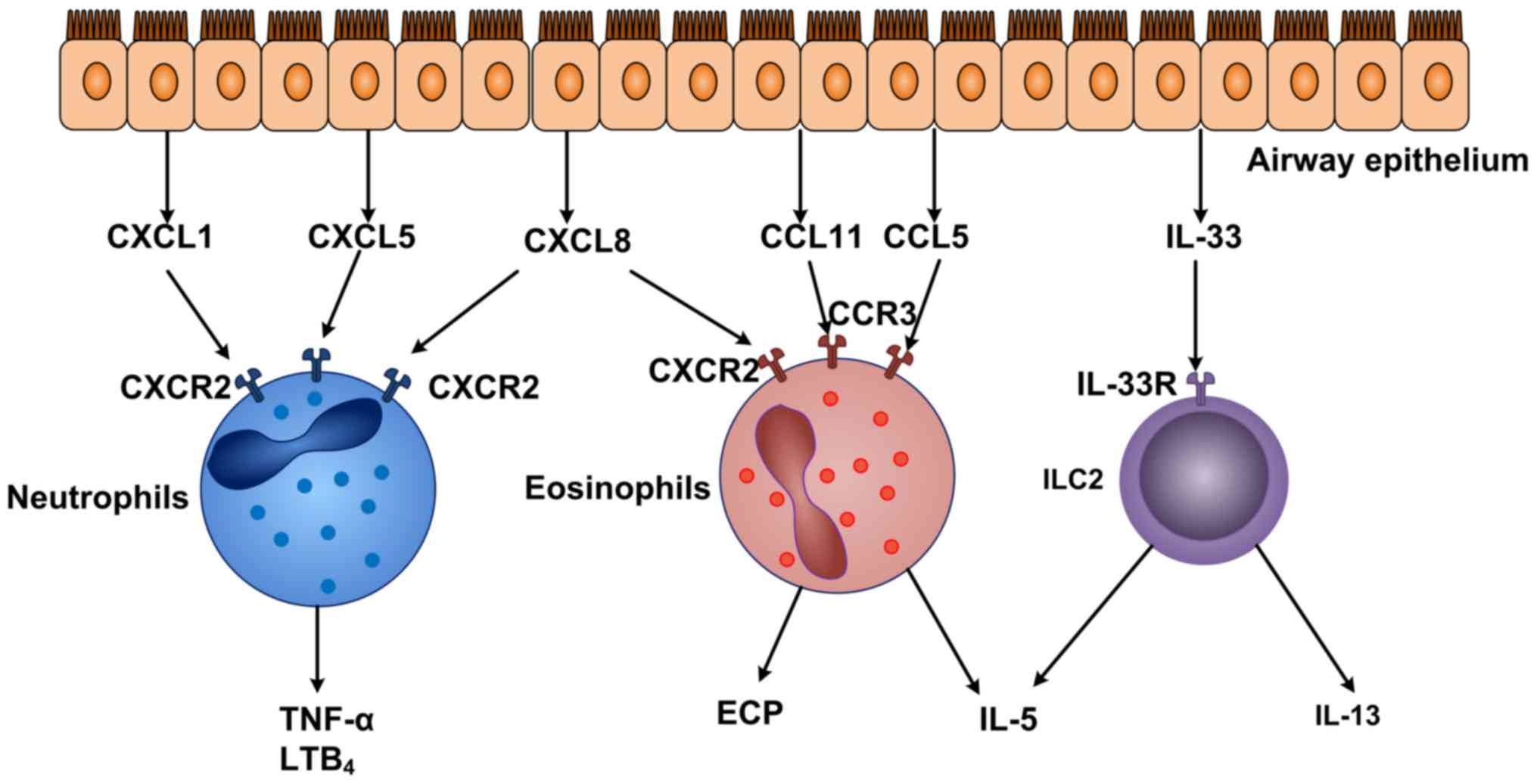
contraction, inflammatory cell infiltration and AHR (54). In this process, CCL5 and CCL11
function as potent eosinophil chemoattractants by binding to CCR3
(Fig. 2). CCL11 also induces
eosinophil chemotaxis via the activation of ERK and MAPK pathways
(55). CXCL8 and CXCL10 can also
bind to CXCR2 and CXCR3, respectively, on eosinophils to induce the
recruitment of eosinophils. Following the recruitment to the airway
inflammation area, eosinophils release various inflammatory
mediators and toxic proteins (including eosinophil cationic
protein, myelin basic protein, palmitic acid and IL-5 which
further aggravate airway inflammation. Eosinophils also produce
protein particles to cause tissue damage (56) and respiratory burst (57,58).
Although asthma is classically associated with
eosinophilia and Th2 cytokines, certain asthma patients exhibit a
neutrophil-predominant phenotype without evident Th2 cytokines.
CXCL8 and CXCL10 possess a strong chemotactic effect on neutrophils
(Fig. 2). Takaku et al
(58) identified that CXCL10 and
CXCL8 are elevated in asthma phenotypes with increasing eosinophils
and neutrophils in airways. In addition, CXCL8, CXCL1 and CXCL5 can
bind to CXCR2 (a specific surface receptor on neutrophils), which
can activate neutrophils and attract them to inflammation sites.
These chemokines also promote expression of adhesion molecules,
(including CD11a, -b, -c and CD18) and cause cell deformation,
eosinophil degranulation and respiratory burst (59–61).
In addition, TNF-α, leukotriene B4 and other inflammatory mediators
produced by activated neutrophils further exacerbate airway
inflammation, leading to airway submucosal edema and goblet cell
metaplasia (62).
An increasing number of studies have demonstrated
that ILC2 s contribute to the initiation and maintenance of the
adaptive Th2 immune response (63,64).
By binding to IL-33R on ILC2 cells, IL-33 promotes production of
IL-5 and IL-13 by ILC2 (Fig. 2).
IL-13 secreted by ILC2 cells can bind to IL-13R on macrophages,
which further induce the activation of macrophages (65). In addition, early eosinophilia in
the lung is driven by IL-5 that also supports the development of
eosinophils in the bone marrow. Consequently, ILC2 cells contribute
to Th2 cell-mediated lung inflammation in the pathogenesis of
asthma (66).
Effect of chemokines on monocytes
Monocytes express specific high-affinity receptors
for CCL2, CXCL10, CCR2 and CXCR3 (9). Macrophage recruitment occurs via a
chemotactic gradient of monocyte selective chemokines. Following
activation and recruitment, monocytes release superoxide anions and
lysozymes (67). At the same time,
surface-specific adhesion molecules CD11c and CD11b are expressed
on monocytes, which are involved in the regulation of airway
inflammation. In addition, the increased expression of integrin β2
and α4 is accompanied by upregulated IL-1 and IL-6 (68). Interactions between mucosal
epithelial cells and macrophages are pivotal to allergic lung
inflammation. Increased expression of CCL2 has been reported in
asthmatic airway epithelial cells, blocking the CCL2-CCR2 axis and
attenuating the asthma phenotype in other animal models of asthma
(69).
Conclusion
The airway immune response is mediated by airway
epithelial cells through the secretion of chemokines. First,
chemokines selectively induce various inflammatory cells to
accumulate directly at the site of inflammation. Chemokines further
induce stromal and inflammatory cells and produce more chemokines,
resulting in a cascade effect that results in more severe tissue
damage indirectly. The present review provides novel considerations
for asthma airway inflammation research from a chemokine
perspective, and a fresh approach to the clinical therapy of
asthma.
Acknowledgements
Not applicable.
Funding
This review was funded by grants from the National
Natural Science Foundation of China (grant nos. 81270065, 81370116,
81570026, 81670002 and 3167188), the Hunan Natural Science
Foundation (grant nos. 2013JJ4030, 2015JJ3170, 2015JJ2147 and
2017JJ2402), the National Basic Research Program of China (973
Program; grant no. 2012CB518104), the Open Foundation of Hunan
College Innovation Program (grant nos. 16K097 and 14K109) and the
Youth Support Program of China Science Communication and the
Fundamental Research Funds for the Central Universities of Central
South University [grant no. 45 (2015)].
Availability of data and materials
Not applicable.
Authors' contributions
ChL and XQ conceived and designed this review. XZ,
YX, XQ and HL collected the relevant papers. CL, MT and JJ obtained
and analyzed the relevant data from the references. ChL wrote the
review.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ishmael FT: The inflammatory response in
the pathogenesis of asthma. J Am Osteopath Assoc. 111 11 Suppl
7:S11–S17. 2011.PubMed/NCBI
|
|
2
|
KleinJan A: Airway inflammation in asthma:
Key players beyond the Th2 pathway. Curr Opin Pulm Med. 22:46–52.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Papi A, Brightling C, Pedersen SE and
Reddel HK: Asthma. Lancet. 391:783–800. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang D, Kang R, Xiao W, Wang H, Calderwood
SK and Xiao X: The anti-inflammatory effects of heat shock protein
72 involve inhibition of high-mobility-group box 1 release and
proinflammatory function in macrophages. J Immunol. 179:1236–1244.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hallstrand TS, Hackett TL, Altemeier WA,
Matute-Bello G, Hansbro PM and Knight DA: Airway epithelial
regulation of pulmonary immune homeostasis and inflammation. Clin
Immunol. 151:1–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Holgate ST: The sentinel role of the
airway epithelium in asthma pathogenesis. Immunol Rev. 242:205–219.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mitchell PD and O'Byrne PM:
Epithelial-derived cytokines in asthma. Chest. 151:1338–1344. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao W, Li L, Wang Y, Zhang S, Adcock IM,
Barnes PJ, Huang M and Yao X: Bronchial epithelial cells: The key
effector cells in the pathogenesis of chronic obstructive pulmonary
disease? Respirology. 20:722–729. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Erle DJ and Sheppard D: The cell biology
of asthma. J Cell Biol. 205:621–631. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mitchell PD and O'Byrne PM: Biologics and
the lung: TSLP and other epithelial cell-derived cytokines in
asthma. Pharmacol Ther. 169:104–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smit JJ and Lukacs NW: A closer look at
chemokines and their role in asthmatic responses. Eur J Pharmacol.
533:277–288. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Castan L, Magnan A and Bouchaud G:
Chemokine receptors in allergic diseases. Allergy. 72:682–690.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guerreiro R, Santos-Costa Q and
Azevedo-Pereira JM: The chemokines and their receptors:
Characteristics and physiological functions. Acta Medica
Portuguesa. 24 Suppl 4:S967–S976. 2011.
|
|
14
|
Fall N, Bove KE, Stringer K, Lovell DJ,
Brunner HI, Weiss J, Higgins GC, Bowyer SL, Graham TB, Thornton S
and Grom AA: Association between lack of angiogenic response in
muscle tissue and high expression of angiostatic ELR-negative CXC
chemokines in patients with juvenile dermatomyositis: possible link
to vasculopathy. Arthritis Rheum. 52:3175–3180. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Osei-Kumah A, Wark PA, Smith R and Clifton
VL: Asthma during pregnancy alters immune cell profile and airway
epithelial chemokine release. Inflamm Res. 59:349–358. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iosifidis T, Garratt LW, Coombe DR, Knight
DA, Stick SM and Kicic A: Airway epithelial repair in health and
disease: Orchestrator or simply a player? Respirology. 21:438–448.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fuke S, Betsuyaku T, Nasuhara Y, Morikawa
T, Katoh H and Nishimura M: Chemokines in bronchiolar epithelium in
the development of chronic obstructive pulmonary disease. Am J
Respir Cell Mol Biol. 31:405–412. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Post S, Rozeveld D, Jonker MR, Bischoff R,
van Oosterhout AJ and Heijink IH: ADAM10 mediates the house dust
mite-induced release of chemokine ligand CCL20 by airway
epithelium. Allergy. 70:1545–1552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Julkunen I, Melen K, Nyqvist M, Pirhonen
J, Sareneva T and Matikainen S: Inflammatory responses in influenza
A virus infection. Vaccine. 19 Suppl 1:S32–S37. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van de Veerdonk FL, Netea MG, Dinarello CA
and Joosten LA: Inflammasome activation and IL-1beta and IL-18
processing during infection. Trends Immunol. 32:110–116. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Z, Bryan JL, DeLassus E, Chang LW,
Liao W and Sandell LJ: CCAAT/enhancer-binding protein beta and
NF-κB mediate high level expression of chemokine genes CCL3 and
CCL4 by human chondrocytes in response to IL-1β. J Biol Chem.
285:33092–33103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu WT, Li MQ, Liu W, Jin LP, Li DJ and Zhu
XY: IL-33 enhances proliferation and invasiveness of decidual
stromal cells by up-regulation of CCL2/CCR2 via NF-κB and ERK1/2
signaling. Mol Hum Reprod. 20:358–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Faffe DS, Whitehead T, Moore PE, Baraldo
S, Flynt L, Bourgeois K, Panettieri RA and Shore SA: IL-13 and IL-4
promote TARC release in human airway smooth muscle cells: Role of
IL-4 receptor genotype. Am J Physiol Lung Cell Mol Physiol.
285:L907–L914. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hijnen D, De Bruin-Weller M, Oosting B,
Lebre C, De Jong E, Bruijnzeel-Koomen C and Knol E: Serum thymus
and activation-regulated chemokine (TARC) and cutaneous T
cell-attracting chemokine (CTACK) levels in allergic diseases: TARC
and CTACK are disease-specific markers for atopic dermatitis. J
Allergy Clin Immunol. 113:334–340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou X, Hu H, Balzar S, Trudeau JB and
Wenzel SE: MAPK regulation of IL-4/IL-13 receptors contributes to
the synergistic increase in CCL11/eotaxin-1 in response to TGF-β1
and IL-13 in human airway fibroblasts. J Immunol. 188:6046–6054.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hong GH, Kwon HS, Moon KA, Park SY, Park
S, Lee KY, Ha EH, Kim TB, Moon HB, Lee HK and Cho YS: Clusterin
modulates allergic airway inflammation by attenuating
CCL20-mediated dendritic cell recruitment. J Immunol.
196:2021–2030. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He M, Song G, Yu Y, Jin Q and Bian Z:
LPS-miR-34a-CCL22 axis contributes to regulatory T cell recruitment
in periapical lesions. Biochem Biophys Res Commun. 460:733–740.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kimura S, Tanimoto A, Wang KY, Shimajiri
S, Guo X, Tasaki T, Yamada S and Sasaguri Y: Expression of
macrophage-derived chemokine (CCL22) in atherosclerosis and
regulation by histamine via the H2 receptor. Pathol Int.
62:675–683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abrial C, Grassin-Delyle S, Salvator H,
Brollo M, Naline E and Devillier P: 15-Lipoxygenases regulate the
production of chemokines in human lung macrophages. Br J Pharmacol.
172:4319–4330. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schneider D, Hong JY, Bowman ER, Chung Y,
Nagarkar DR, McHenry CL, Goldsmith AM, Bentley JK, Lewis TC and
Hershenson MB: Macrophage/epithelial cell CCL2 contributes to
rhinovirus-induced hyperresponsiveness and inflammation in a mouse
model of allergic airways disease. Am J Physiol Lung Cell Mol
Physiol. 304:L162–L169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Renois F, Jacques J, Talmud D, Deslée G,
Lévêque N and Andréoletti L: Respiratory echovirus 30 and
coxsackievirus B5 can induce production of RANTES, MCP-1 and IL-8
by human bronchial epithelial cells. Virus Res. 152:41–49. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Heijink IH, Marcel Kies P, van Oosterhout
AJ, Postma DS, Kauffman HF and Vellenga E: Der p, IL-4 and TGF-beta
cooperatively induce EGFR-dependent TARC expression in airway
epithelium. Am J Respir Cell Mol Biol. 36:351–359. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Herjan T, Yao P, Qian W, Li X, Liu C,
Bulek K, Sun D, Yang WP, Zhu J, He A, et al: HuR is required for
IL-17-induced Act1-mediated CXCL1 and CXCL5 mRNA stabilization. J
Immunol. 191:640–649. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Katanov C, Lerrer S, Liubomirski Y,
Leider-Trejo L, Meshel T, Bar J, Feniger-Barish R, Kamer I,
Soria-Artzi G, Kahani H, et al: Regulation of the inflammatory
profile of stromal cells in human breast cancer: Prominent roles
for TNF-α and the NF-κB pathway. Stem Cell Res Therapy. 6:872015.
View Article : Google Scholar
|
|
35
|
Song Y, Lin Q, Zheng J, Zhu X and Yang S:
PPAR-γ agonist inhibits the expressions of chemokines induced by
IFN-γ and TNF-α in renal tubular epithelial cells. Xi Bao Yu Fen Zi
Mian Yi Xue Za Zhi. 30:673–676. 2014.(In Chinese). PubMed/NCBI
|
|
36
|
Fenwick PS, Macedo P, Kilty IC, Barnes PJ
and Donnelly LE: Effect of JAK Inhibitors on Release of CXCL9,
CXCL10 and CXCL11 from human airway epithelial cells. PLoS One.
10:e01287572015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chien JW, Chu YT, Yang SN, Kuo CH, Wang
WL, Kuo PL, Jong YJ and Hung CH: Long-acting beta 2 agonists
suppress IP-10 expression in human bronchial epithelial cells. J
Investig Med. 60:1048–1053. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takahashi N, Sugaya M, Suga H, Oka T,
Kawaguchi M, Miyagaki T, Fujita H and Sato S: Thymic stromal
chemokine TSLP acts through Th2 cytokine production to induce
cutaneous T-cell lymphoma. Cancer Res. 76:6241–6252. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Prefontaine D, Nadigel J, Chouiali F,
Audusseau S, Semlali A, Chakir J, Martin JG and Hamid Q: Increased
IL-33 expression by epithelial cells in bronchial asthma. J Allergy
Clin Immunol. 125:752–754. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sei H, Oshima T, Shan J, Wu L, Yamasaki T,
Okugawa T, Kondo T, Tomita T, Fukui H, Watari J and Miwa H:
Esophageal epithelial-derived IL-33 Is upregulated in patients with
heartburn. PLoS One. 11:e01542342016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Park IH, Park JH, Shin JM and Lee HM:
Tumor necrosis factor-α regulates interleukin-33 expression through
extracellular signal-regulated kinase, p38 and nuclear factor-κB
pathways in airway epithelial cells. Int Forum Allergy Rhinol.
6:973–980. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nygaard U, Hvid M, Johansen C, Buchner M,
Fölster-Holst R, Deleuran M and Vestergaard C: TSLP, IL-31, IL-33
and sST2 are new biomarkers in endophenotypic profiling of adult
and childhood atopic dermatitis. J Eur Acad Dermatol Venereol.
30:1930–1938. 2016.PubMed/NCBI
|
|
43
|
Golebski K, van Tongeren J, van Egmond D,
de Groot EJ, Fokkens WJ and van Drunen CM: Specific Induction of
TSLP by the Viral RNA Analogue Poly (I:C) in primary epithelial
cells derived from nasal polyps. PLoS One. 11:e01528082016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Amin K: The Role of the T lymphocytes and
remodeling in asthma. Inflammation. 39:1475–1482. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Froidure A, Vandenplas O, D'Alpaos V,
Evrard G and Pilette C: Persistence of asthma following allergen
avoidance is associated with proTh2 myeloid dendritic cell
activation. Thorax. 70:967–973. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lin CL, Hsiao G, Wang CC and Lee YL:
Corrigendum to ‘Imperatorin exerts antiallergic effects in
Th2-mediated allergic asthma via induction of IL-10-producing
regulatory T cells by modulating the function of dendritic cells’
[Pharmacol. Res. (2016) 111–121]. Pharmacological Res. 124:1572017.
View Article : Google Scholar
|
|
47
|
Purandare AV, Wan H, Somerville JE, Burke
C, Vaccaro W, Yang X, McIntyre KW and Poss MA: Core exploration in
optimization of chemokine receptor CCR4 antagonists. Bioorg Med
Chem Lett. 17:679–682. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Penaloza-MacMaster P, Kamphorst AO,
Wieland A, Araki K, Iyer SS, West EE, O'Mara L, Yang S, Konieczny
BT, Sharpe AH, et al: Interplay between regulatory T cells and PD-1
in modulating T cell exhaustion and viral control during chronic
LCMV infection. J Exp Med. 211:1905–1918. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liao J, Liang G, Xie S, Zhao H, Zuo X, Li
F, Chen J, Zhao M, Chan TM and Lu Q: CD40L demethylation in CD4(+)
T cells from women with rheumatoid arthritis. Clin Immunol.
145:13–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Alasandagutti ML, Ansari MS, Sagurthi SR,
Valluri V and Gaddam S: Role of IL-13 genetic variants in
signalling of asthma. Inflammation. 40:566–577. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen YL and Chiang BL: Targeting TSLP With
shRNA alleviates airway inflammation and decreases epithelial CCL17
in a murine model of asthma. Mol Ther Nucleic Acids. 5:e3162016.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu J, Dong F, Wang RA, Wang J, Zhao J,
Yang M, Gong W, Cui R and Dong L: Central role of cellular
senescence in TSLP-induced airway remodeling in asthma. PLoS One.
8:e777952013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Walsh CJ, Zaihra T, Benedetti A, Fugère C,
Olivenstein R, Lemière C, Hamid Q and Martin JG: Exacerbation risk
in severe asthma is stratified by inflammatory phenotype using
longitudinal measures of sputum eosinophils. Clin Exp Allergy.
46:1291–1302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rose CE Jr, Lannigan JA, Kim P, Lee JJ, Fu
SM and Sung SS: Murine lung eosinophil activation and chemokine
production in allergic airway inflammation. Cell Mol Immunol.
7:361–374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Asosingh K, Vasanji A, Tipton A, Queisser
K, Wanner N, Janocha A, Grandon D, Anand-Apte B, Rothenberg ME,
Dweik R and Erzurum SC: Eotaxin-rich proangiogenic hematopoietic
progenitor cells and CCR3+ endothelium in the atopic asthmatic
response. J Immunol. 196:2377–2387. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
George L and Brightling CE: Eosinophilic
airway inflammation: Role in asthma and chronic obstructive
pulmonary disease. Ther Adv Chronic Dis. 7:34–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kikuchi I, Kikuchi S, Kobayashi T,
Hagiwara K, Sakamoto Y, Kanazawa M and Nagata M: Eosinophil
trans-basement membrane migration induced by interleukin-8 and
neutrophils. Am J Respir Cell Mol Biol. 34:760–765. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Takaku Y, Nakagome K, Kobayashi T,
Hagiwara K, Kanazawa M and Nagata M: IFN-γ-inducible protein of 10
kDa upregulates the effector functions of eosinophils through beta2
integrin and CXCR3. Respir Res. 12:1382011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Henkels KM, Frondorf K, Gonzalez-Mejia ME,
Doseff AL and Gomez-Cambronero J: IL-8-induced neutrophil
chemotaxis is mediated by Janus kinase 3 (JAK3). FEBS Lett.
585:159–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sawant KV, Xu R, Cox R, Hawkins H, Sbrana
E, Kolli D, Garofalo RP and Rajarathnam K: Chemokine CXCL1-mediated
neutrophil trafficking in the lung: Role of CXCR2 activation. J
Innate Immu. 7:647–658. 2015. View Article : Google Scholar
|
|
61
|
Disteldorf EM, Krebs CF, Paust HJ, Turner
JE, Nouailles G, Tittel A, Meyer-Schwesinger C, Stege G, Brix S,
Velden J, et al: CXCL5 drives neutrophil recruitment in
TH17-mediated GN. J Am Soc Nephrol. 26:55–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mosca T, Menezes MC, Silva AV, Stirbulov R
and Forte WC: Chemotactic and phagocytic activity of blood
neutrophils in allergic asthma. Immunol Invest. 44:509–520. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Drake LY, Iijima K and Kita H: Group 2
innate lymphoid cells and CD4+ T cells cooperate to mediate type 2
immune response in mice. Allergy. 69:1300–1307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Halim TY, Steer CA, Matha L, Gold MJ,
Martinez-Gonzalez I, McNagny KM, McKenzie AN and Takei F: Group 2
innate lymphoid cells are critical for the initiation of adaptive T
helper 2 cell-mediated allergic lung inflammation. Immunity.
40:425–435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhu J: T helper 2 (Th2) cell
differentiation, type 2 innate lymphoid cell (ILC2) development and
regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine.
75:14–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Molofsky AB, Van Gool F, Liang HE, Van
Dyken SJ, Nussbaum JC, Lee J, Bluestone JA and Locksley RM:
Interleukin-33 and interferon-gamma counter-regulate group 2 innate
lymphoid cell activation during immune perturbation. Immunity.
43:161–174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang J, Vodovotz Y, Fan L, Li Y, Liu Z,
Namas R, Barclay D, Zamora R, Billiar TR, Wilson MA, et al:
Injury-induced MRP8/MRP14 stimulates IP-10/CXCL10 in
monocytes/macrophages. FASEB J. 29:250–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Carta S, Tassi S, Delfino L, Omenetti A,
Raffa S, Torrisi MR, Martini A, Gattorno M and Rubartelli A:
Deficient production of IL-1 receptor antagonist and IL-6 coupled
to oxidative stress in cryopyrin-associated periodic syndrome
monocytes. Ann Rheum Dis. 71:1577–1581. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mellado M, Martin de Ana A, Gomez L,
Martinez C and Rodriguez-Frade JM: Chemokine receptor 2 blockade
prevents asthma in a cynomolgus monkey model. J Pharmacol Exp Ther.
324:769–775. 2008. View Article : Google Scholar : PubMed/NCBI
|