Introduction
Chondrosarcoma is the second most commonly occurring
primary bone malignancy, which affects the pelvis, long bones and
the spine, in addition to the larynx, head and neck, and it
eventually metastasizes (1–3).
Currently, chondrosarcoma remains largely incurable due to poor
prognosis and a high rate of recurrence (4). Thus, the development of innovative
therapies for this disease is required.
Pyrroloquinoline quinone (PQQ) is a nutrient widely
distributed in nature and serves as a noncovalently bound redox
cofactor in a series of bacterial quinoprotein dehydrogenases
(5). PQQ scavenges reactive oxygen
species (ROS) in bacteria (6).
Previous studies have demonstrated that PQQ protected isolated
liver mitochondria from damage following oxidative stress (7) and scavenged superoxide radicals
(8,9). A previous study revealed that PQQ
could induce apoptosis in human promonocytic leukemia U937 and
lymphoma EL-4 cells, in addition to Jurkat cell programmed death
(7). The underlying mechanism may
be relevant to the increase in intracellular ROS and the depletion
of glutathione (GSH) (10). In
addition, PQQ may induce tumor cell (A549, Neuro-2A and HCC-LM3)
apoptosis by decreasing the expression of B-cell lymphoma 2
(11).
Oxidative stress arises from an imbalanced redox
status between the production of ROS and the biological system able
to remove them. ROS, including superoxide
(O2−), hydroxyl radical (•OH) and
H2O2, are constantly generated in aerobic
organisms (12). ROS can cause
fatal lesions in cells under oxidative stress, leading to a number
of diseases including cancer (13). High levels of ROS are detrimental
and induce cell apoptosis or necrosis (14,15).
Recently, ‘oxidation therapy’ has been developed by inducing
cytotoxic oxidative stress for cancer treatment. A number of
antitumor agents, including vinblastine, cisplatin, doxorubicin,
camptothecin and several others have exhibited antitumor activity
via the ROS-dependent activation of apoptotic cell death,
suggesting the potential use of ROS as an antitumor agent (16). However, the mechanisms underlying
the role of PQQ in regulating chondrosarcoma cells have not been
fully elucidated.
The present study examined the role of PQQ in
chondrosarcoma cells, and identified that PQQ could increase cell
apoptosis and the level of ROS. PQQ reduced the ability of
scavenging oxygen free radicals by inhibiting the activation of
superoxide dismutase (SOD)1 and SOD2, and the formation of GSH,
causing an increased level of ROS. Additionally, an animal model
was established in vivo, which identified that PQQ inhibited
the proliferation of transplanted tumor cells, increased cell
apoptosis through ROS and increased DNA damage. Thus, PQQ may be a
desirable drug for cancer treatment in the future.
Materials and methods
Cell culture and reagents
The chondrosarcoma SW1353 cells, osteosarcoma Saos-2
cells and 293 cells were purchased from American Type Culture
Collection (Manassas, VA, USA). Human XJH B lymphocytes were
purchased from the Institute of Biochemistry and Cell Biology, the
Chinese Academy of Sciences (Shanghai, China). All cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and
1% penicillin/streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). All cells were maintained at 37°C with 5% CO2
in an incubator with a constant humidity. PQQ was purchased from
Sigma-Aldrich (Merck KGaA).
Cell cytotoxicity death analysis
Cell cytotoxic death was assessed using a
CytoTox-Glo™ Cytotoxicity assay (Promega Corporation,
Madison, WA, USA) according to the manufacturer's protocols.
Briefly, all of the cells were seeded in at a density of
1×104 cells per well in 3 ml culture medium and
incubated at 37°C in 5% CO2 for 6 h. All of cells were
incubated in culture medium with indicated concentration of PQQ (0,
40, 80, 120 and 200 µM) for 24 h or 120 µM PQQ on SW1353 cells at
different time points (6, 12, 24, 36 and 48 h). A total of 50 µl
CytoTox-Glo™ Reagent was first added and incubated at
room temperature for 15 min, luminescence was measured to determine
dead cell luminescence. Then lysis reagent was added and
luminescence was measured to determine total cell luminescence
after 15 min of incubation at room temperature. The luminescent
signal was adjusted to reflect the ‘live cell’ contribution by
subtracting the initial dead cell signal.
Western blot analysis
The SW1353 cells were lysed with cell lysis buffer
10X (cat. no. 9803; Cell Signaling Technology, Inc., Danvers, MA,
USA), which contained protease inhibitors (Sigma-Aldrich; Merck
KGaA). Then cells were centrifuged at 12,000 × g for 5 min at 4°C.
The supernatant was collected and a bicinchoninic acid Protein
Assay kit (Sigma-Aldrich; Merck KGaA) was used to measure the
protein concentration. The protein samples (20 µg/lane) were
separated by 10% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). Membranes
were blocked with Tris-buffered saline (TBS) and 0.1% Tween-20
(TBS/T) containing 5% bovine serum albumin (Sangon Biotech Co.,
Ltd., Shanghai, China) at 37°C for 2 h, and incubated overnight at
4°C with primary antibodies (Abcam, Cambridge, MA, USA; SOD1 and
SOD2; 1:1,000 in TBS/T; SOD1; cat. no. ab13498; SOD2, cat. no.
ab13534). The membrane was washed 3 times with TBS/T and then
incubated with a horseradish peroxidase (HRP)-labeled secondary
antibody (Cell Signaling Technology, Inc.; 1:2,000 in TBS/T; cat
no. 7074) for 2 h at room temperature. The protein bands were
detected by chemiluminescence (GE Healthcare, Piscataway, NJ, USA).
Bands were quantified by densitometry using Image Lab 5.0 software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) and β-actin (Cell
Signaling Technology, Inc.; 1:2,000; cat no. 4970) was used as an
internal control.
Cell apoptosis analysis
SW1353 and Saos-2 cells were seeded into 6-well
plates at a density of 2×105 cells/well with the
complete medium and grown for 24 h. Following incubation, cells
were exposed to 120 µM PQQ or 200 µl PBS (control group) at 37°C in
a 5% CO2 humidified atmosphere. Following treatment for
48 h, cells were processed with trypsin EDTA (0.25%) solution and
centrifuged at 1,000 × g for 5 min at 4°C. Following washing twice
with PBS, 10 µl Annexin V-fluorescein isothiocyanate and 5 µl
propidium iodide (BD Biosciences, Franklin Lakes, NJ, USA) were
added and incubated for 15 min in the dark at room temperature.
Subsequently, apoptotic cells were detected by flow cytometry using
a FACSC alibur system with Cell Quest software version 5.1 (BD
Biosciences).
Intracellular ROS measurement
2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA)
was used as a probe for cellular ROS in the present study. SW1353
cells (2×105/well) in a 6-well plate were incubated with
PQQ (120 µM) for 24 h. Following incubation in serum-free medium
containing 20 µM DCFH-DA for 20 min at 37°C, cells in each well
were washed three times with PBS, digested by pancreatic enzymes at
37°C for 3 min and immediately subjected to ROS measurement by flow
cytometry analysis using Cytomics FC 500 MCL (Beckman Coulter,
Inc., Brea, CA, USA). The wavelength was 488 nm for excitation and
525 nm for emission.
Hydroxyl radical (•OH)
measurement
The deoxyribose degradation method was used to
detect the level of •OH as described by Baliga (17). Briefly, 2-deoxy-d-ribose at 3 mM
was added to cells just prior to the addition of PQQ. After
incubation, 0.5 ml of medium was collected and mixed with 0.5 ml of
1% (w/v) 2-thiobarbituric acid in 50 mM NaOH and 0.5 ml of 2.8%
(w/v) trichloroacetic acid. The mixture was then heated at 100°C
for 15 min, cooled, and extracted with n-butanol. The supernatant
was measured for absorbance at 532 nm.
GSH production
A GSH Assay kit (Beyotime Institute of
Biotechnology, Shanghai, China) was used to assess the content of
GSH according to the manufacturer's protocols (18).
Tumor xenograft experiments
Experimental procedures were conducted in conformity
with institutional guidelines (The Second Affiliated Hospital of
Zhejiang University School of Medicine, Zhejiang, China) for the
care and use of laboratory animals, and ethical approval was
obtained from The Second Affiliated Hospital of Zhejiang University
School of Medicine. Procedures also conformed to the National
Institutes of Health Guide for Care and Use of Laboratory Animals
(19). A mouse model of
chondrosarcoma was established using SW1353 cells to generate
subcutaneous xenografts. A total of 12 Male BALB/c nude mice (6
week-old, 18–22 g) were obtained from the Shanghai Laboratory
Animal Center, Chinese Academy Sciences (Shanghai, China), and
housed in a controlled 12-h light/dark cycle environment at 20–25°C
with free access to food and water. The mice were randomly divided
into 2 groups (n=6/group). Briefly, ~1×106 cells/mouse
were injected subcutaneously once into the lateral flanks of BALB/c
nude mice and 1 tumor per mouse was grown over 10–14 days until
they almost reached 100–150 mm3. The mice were then
intraperitoneally injected with 50 mg/kg PQQ or 200 µl PBS once
daily for 10 days. Following 10 days, the mice were sacrificed and
the tumor xenografts were harvested. The tumor volume was measured
and calculated as follows: Volume=(width2 × length)/2.
The tumor tissues were stored in −80°C.
Immunohistochemistry
Sections (4-µm) of tumor tissues which were cut
using a LEICA RM2235 (Leica Microsystems GmbH, Wetzlar, Germany)
and then were fixed in a 10% formalin solution (Sigma-Aldrich;
Merck KGaA) for 24 h at room temperature, embedded in paraffin wax
(Sigma-Aldrich; Merck KGaA). Then they were deparaffinized with
xylene for 10 min twice, 100% ethyl alcohol (Sangon Biotech Co.,
Ltd., Shanghai, China) for 5 min twice, 95% ethyl alcohol for 5
min, 85% ethyl alcohol for 5 min, 75% ethyl alcohol for 5 min, PBS
washed three times. Next, the endogenous peroxidase activity was
quenched using 3% H2O2 and nonspecific
binding was blocked with 5% FBS at room temperature for 20 min.
Following incubation with primary antibody proliferating cell
nuclear antigen (PCNA) and index γ-H2A histone member X (γ-H2AX;
Abcam, Cambridge, MA, USA; PCNA cat. no. ab18197, γ-H2AX; cat. no.
ab2893; all diluted 1:1,000 in TBS/T) overnight at 4°C, the
sections were further incubated with the corresponding
HRP-conjugated secondary antibody (Cell Signaling Technology, Inc.;
1:2,000 in TBS/T; cat. no. 7074) for 1 h at room temperature. The
target protein expression was visualized using
3,3′-diaminobenzidine and hematoxylin counterstaining was also
performed at room temperature for 3 min. The negative control was
established using PBS rather than the primary antibody. The
sections were observed under light microscopy (Olympus Corporation,
Tokyo, Japan). The positive rates were measured using Image-Pro
Plus v 6.0 software (Media Cybernetics, Inc., Rockville, MD,
USA).
In situ quantification of apoptotic
cells
Apoptotic cells were detected using terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
staining and an in situ cell death detection kit (Roche
Diagnostics, Basel, Switzerland) according to the manufacturer's
protocols. Stained sections were visualized under a fluorescence
microscope. TUNEL-positive cells with brown staining and all cells
with nuclear hematoxylin counterstaining for 30 sec at room
temperature were counted within 5 randomly selected fields under
high-power magnification (DM-2,500; Leica Microsystems GmbH). The
index of apoptosis was expressed as the ratio of positively stained
apoptotic cells to the total number of cells counted, ×100%.
Statistical analysis
Data were expressed as the mean ± standard deviation
(n=3) and analysis was performed using Prism 5 software (Graph Pad
Software, Inc., La Jolla, CA, USA). UCSF DOCK 6.5 Software
(University of California, San Francisco, CA, USA) was used to
analyze the molecular docking. Statistical differences between two
groups were examined with the Student's t-test and multiple groups
were compared using one-way analysis of variance followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
PQQ enhances the apoptotic rate in
chondrosarcoma SW1353 cells
To determine whether PQQ has a role in causing
chondrosarcoma cell death, a cell cytotoxicity assay was performed
to measure cell viability. SW1353, Saos-2, 293 and XJH B cells were
treated with different concentrations of PQQ (0, 40, 80, 120 and
200 µM) for 24 h. The results demonstrated that chondrosarcoma
SW1353 cells and osteosarcoma Saos-2 cells had a greater percentage
of apoptosis than normal human 293 and XJH B cells, which increased
in a PQQ concentration-dependent manner (Fig. 1A). It was identified that 120 µM
PQQ treatment significantly increased the cell death rate in a
time-dependent manner as measured by a cell cytotoxicity analysis
(Fig. 1B). Flow cytometry was used
to measure the apoptotic rates of chondrosarcoma SW1353 cells. It
was identified that treatment with PQQ markedly increased the
number of apoptotic SW1353 and Saos-2 cells when compared with the
PBS treatment groups (Fig. 1C and
D). These results indicated that cell death was increased in a
dose- and time-dependent manner, while the effect on normal cells
was relatively small.
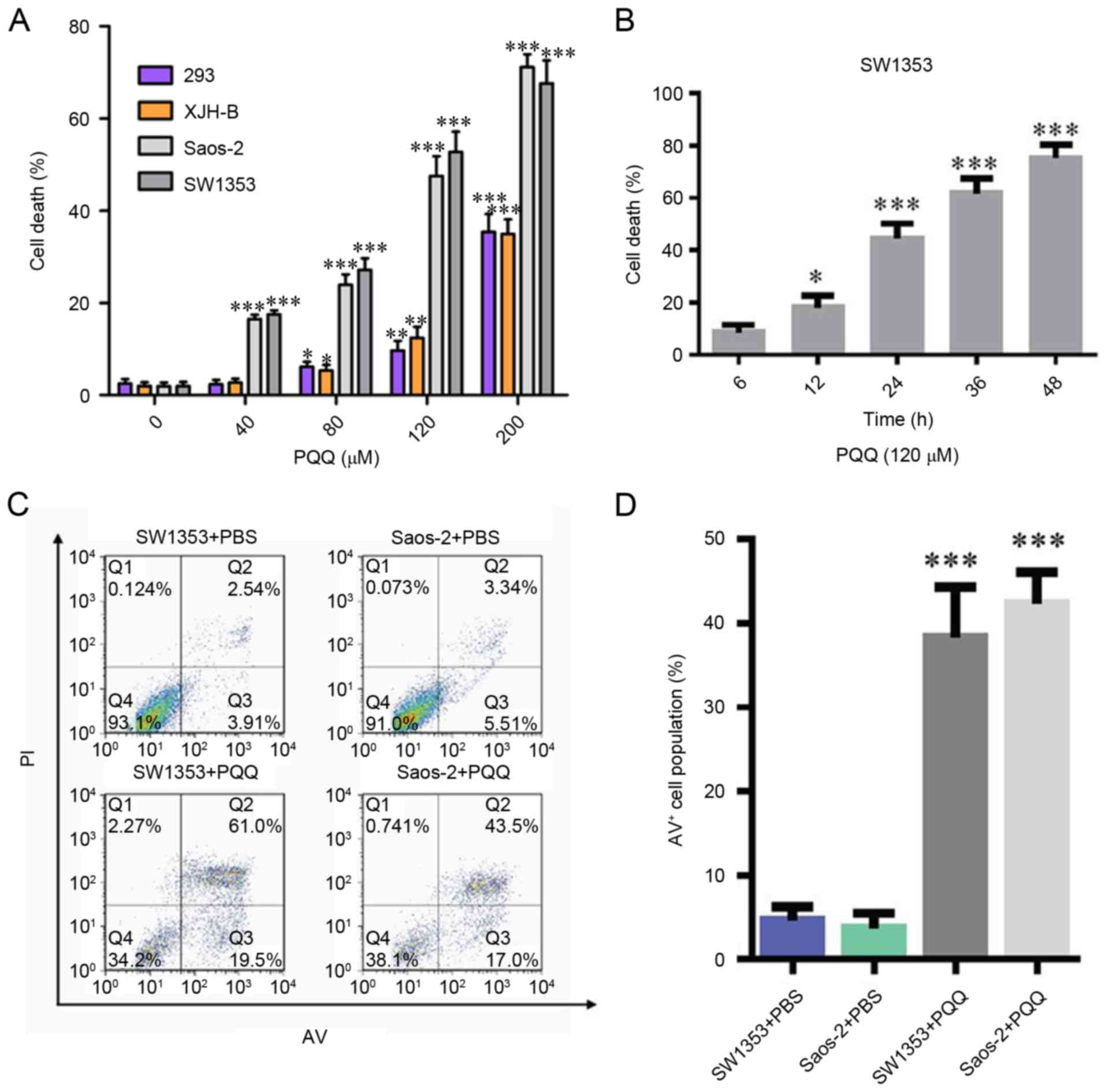 | Figure 1.(A) Different PQQ sensitivity in
SW1353, Saos-2, 293 and XJH B cells. All cells were incubated with
PQQ (0, 40, 80, 120 or 200 µM) for 48 h. Cell viability was
measured using cell cytotoxicity assay. *P<0.05, **P<0.01,
***P<0.001 vs. PQQ (0 µM). (B) Effects of PQQ (120 µM) on SW1353
cells at different time points (6, 12, 24, 36 and 48 h). *P<0.05
and ***P<0.001 vs. 6 h. (C) The number of apoptotic cells was
determined by flow cytometry in SW1353 and Saos-2 cells following
treatment with PQQ for 24 h. (D) The rate of apoptosis represented
as a histogram. ***P<0.001 vs. PBS treatment. PQQ,
pyrroloquinoline quinone; PI, propidium iodide; AV, Annexin
V-fluorescein isothiocyanate. |
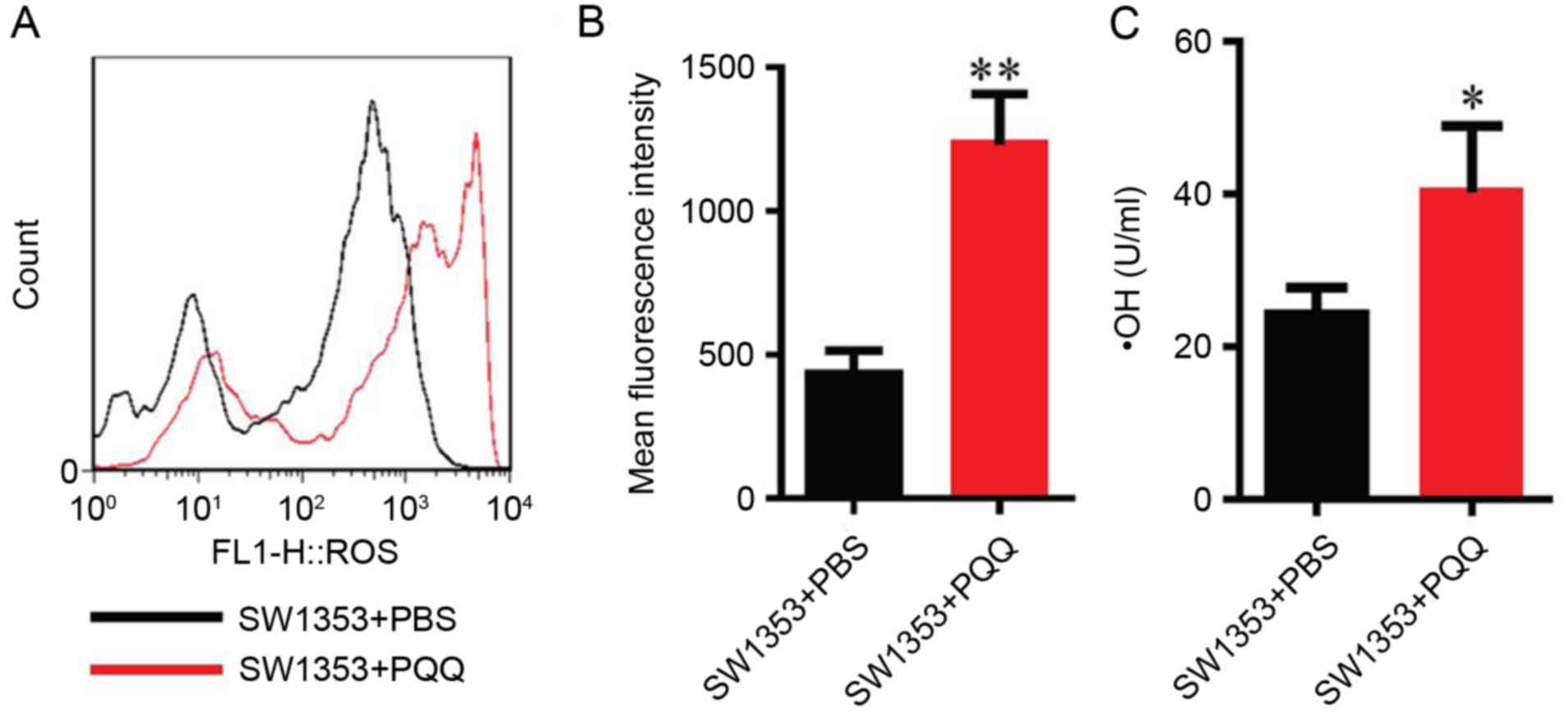
PQQ enhances the level of ROS
To determine whether PQQ-induced cell apoptosis was
associated with oxidative stress levels in chondrosarcoma SW1353
cells, DCFH-DA staining and flow cytometry were used to detect the
changes in the levels of ROS and hydroxyl radicals in SW1353 cells
treated with 120 µM PQQ for 24 h. It was identified that the level
of ROS and hydroxyl radicals increased when compared with the
control PBS group (P<0.05 and P<0.01; Fig. 2). These results demonstrated that
PQQ treatment increased oxidative stress levels in chondrosarcoma
SW1353 cells.
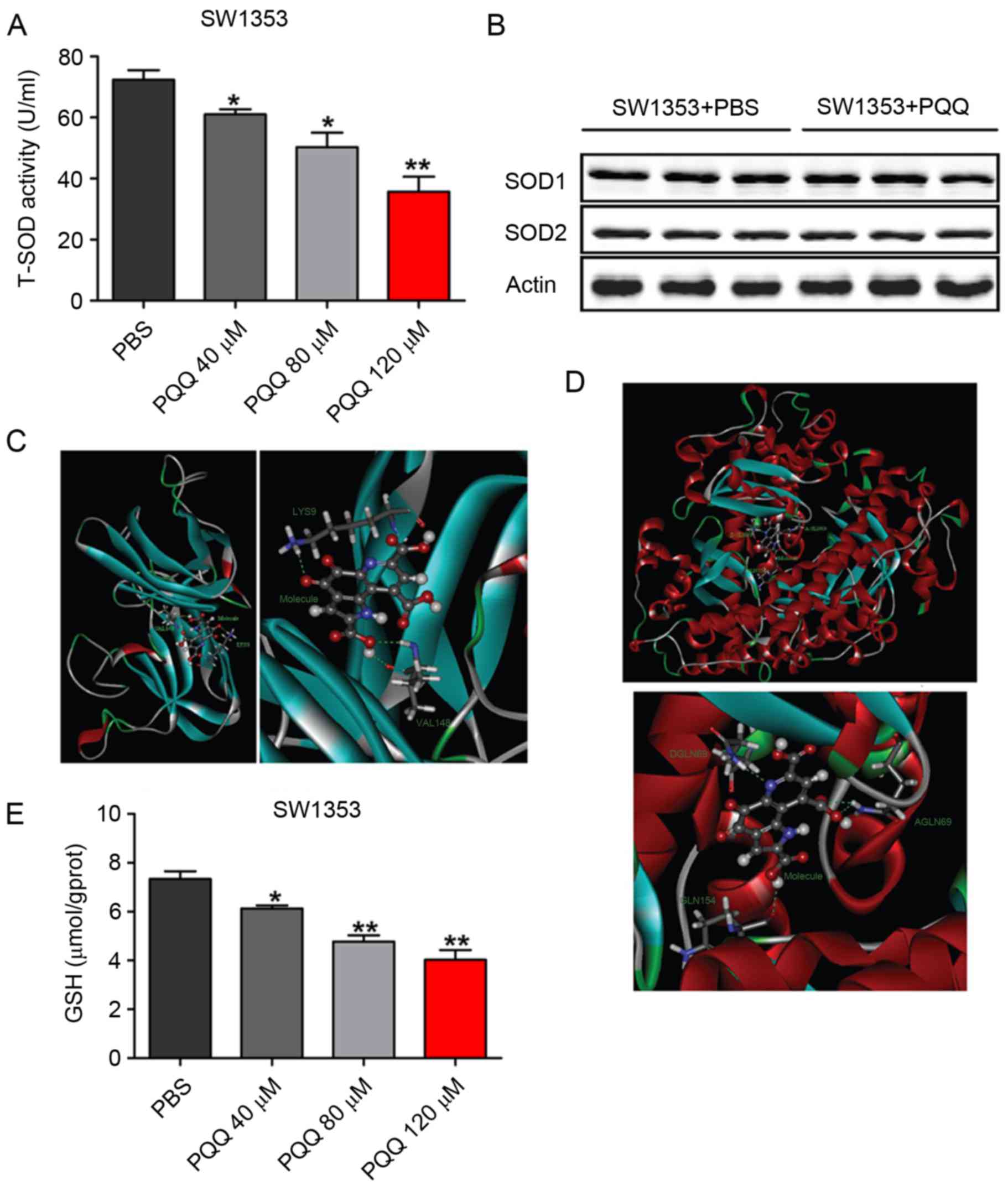
PQQ inhibits the activity of SOD1 and
SOD2, and reduces GSH production
To investigate the molecular mechanism of
PQQ-induced oxidative stress in chondrosarcoma SW1353 cells,
western blotting was performed to detect the total SOD activity and
to examine the expression of SOD1 and SOD2 protein. The results
indicated that, when compared with control, the total SOD activity
was reduced when cells were treated with different concentration of
PQQ (0, 40, 80 and 120 µM) PQQ for 24 h (Fig. 3A); however, no significant
difference was observed in SOD1 and SOD2 protein expression
(Fig. 3B). Molecular docking
software UCSF DOCK 6.5 (University of California) was used to test
molecular docking simulations of PQQ with SOD1 and SOD2 protein,
respectively. It was observed that PQQ formed 3 hydrogen bonds with
the amino acids that surround the SOD1 activity center (Fig. 3C; Table I). Similarly, PQQ formed 4 hydrogen
bonds with the amino acids that surround the SOD1 activity center
(Fig. 3D; Table II). The content of GSH was also
detected, and it was identified that GSH content was significantly
decreased following treatment with different concentrations of PQQ
(0, 40, 80 and 120 µM) for 24 h (Fig.
3E). These results indicated that PQQ can decrease the level of
ROS by inhibiting the activities of SOD1 and SOD2, and the
production of GSH, reducing the ability of scavenging oxygen free
radicals in chondrosarcoma SW1353 cells.
 | Table I.Pyrroloquinoline quinone combined with
superoxide dismutase 1 hydrogen bond. |
Table I.
Pyrroloquinoline quinone combined with
superoxide dismutase 1 hydrogen bond.
| X-H…Y |
d(X-H)(A) |
d(H…Y)(A) |
d(X…Y)(A) | ∠XHY(°) |
|---|
|
Molecule:O13…H22:
LYS9 | 1.04 | 1.90 | 2.69 | 130 |
|
Molecule:O12…HN:
VAL148 | 1.00 | 2.06 | 2.79 | 128 |
|
Molecule:H27…O: VAL148 | 0.95 | 1.97 | 2.76 | 139 |
 | Table II.Pyrroloquinoline quinone combined
with superoxide dismutase 2 hydrogen bond. |
Table II.
Pyrroloquinoline quinone combined
with superoxide dismutase 2 hydrogen bond.
| X-H…Y |
d(X-H)(A) |
d(H…Y)(A) |
d(X…Y)(A) | ∠XHY(°) |
|---|
|
Molecule:O20…HE21:GLN69 | 1.00 | 2.02 | 2.77 | 129 |
|
Molecule:O21…HE21:GLN69 | 1.00 | 2.28 | 3.06 | 134 |
|
Molecule:N16…HE22:GLN69:D | 1.00 | 2.11 | 3.10 | 170 |
|
Molecule:H27…O:GLN154 | 0.95 | 2.13 | 2.81 | 127 |
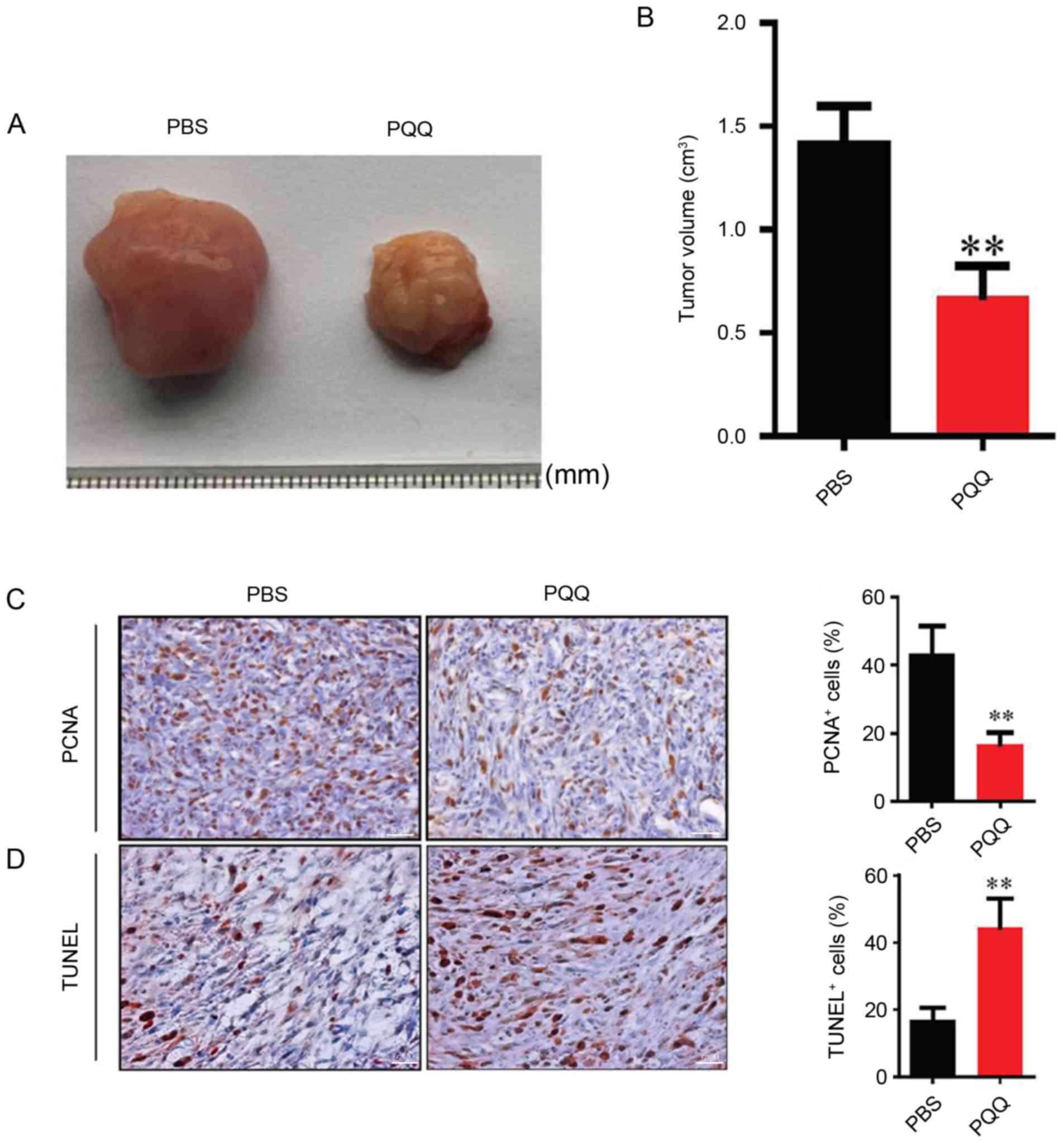
PQQ increases the inhibitory effect on
tumorigenesis in vivo
To clarify the effect of PQQ in vivo, SW1353
cells were xenografted into BALB/c nude mice. When compared with
the PBS control, the tumor volume of the PQQ treated group was
significantly smaller (Fig. 4A and
B). Immunohistochemistry analysis demonstrated that the
percentage of PCNA positive cells was decreased and in TUNEL
analysis the number of apoptotic cells increased when compared with
the PBS control (Fig. 4C and D).
These findings indicated that PQQ may inhibit the proliferation and
promote the apoptosis of tumor cells.
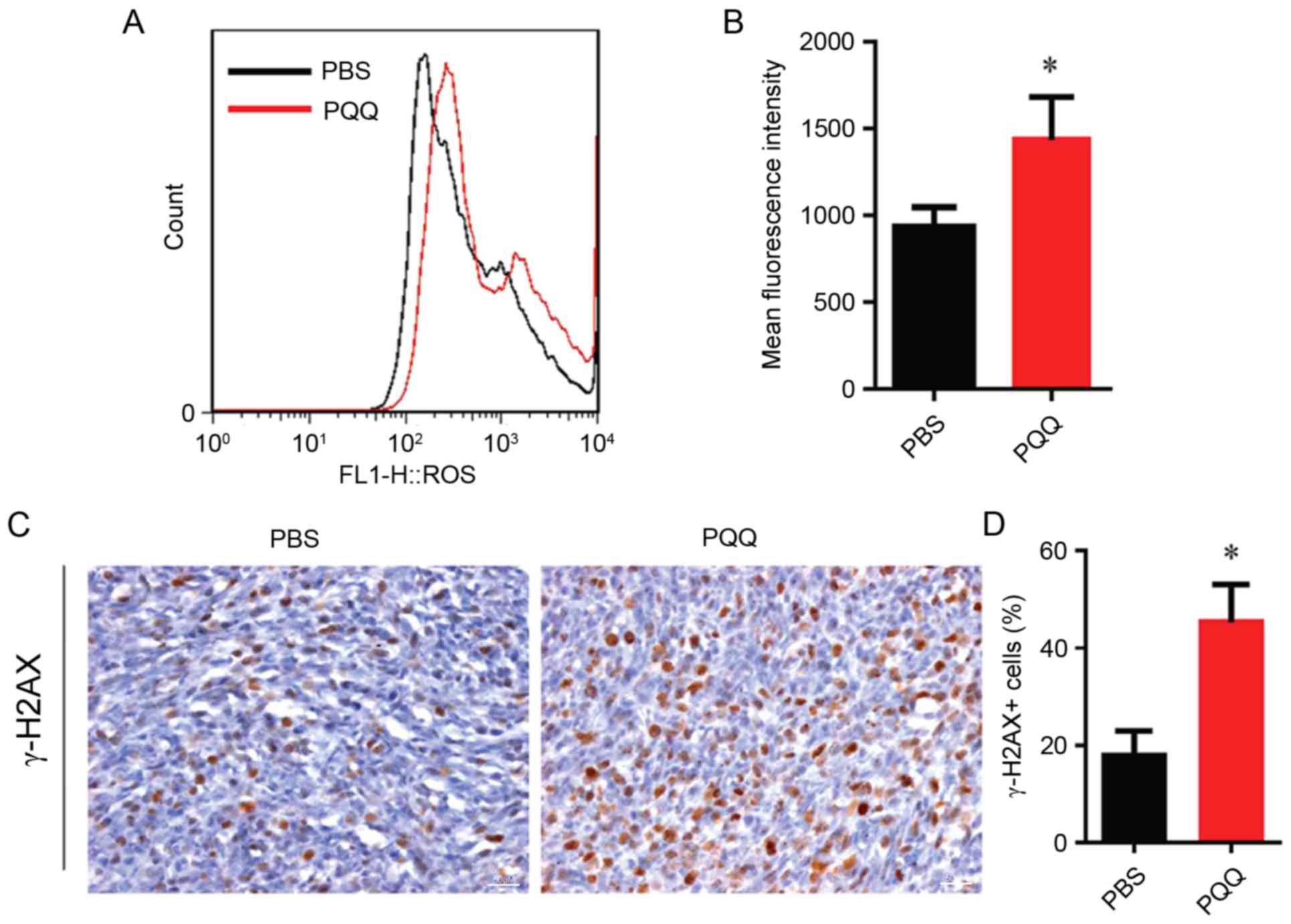
PQQ increases the level of ROS and DNA
damage in tumor xenograft cells
To clarify whether PQQ decreased the proliferation
of tumor xenograft cells and if increased apoptosis was associated
with ROS, the ROS and DNA damage associated index γ-H2AX was
detected in the tumors. The results demonstrated that the levels of
ROS in the PQQ treated group were significantly higher compared
with the PBS control group (Fig. 5A
and B), and the percentage of γ-H2AX was significantly
increased (Fig. 5C and D). These
results suggested that PQQ increased intracellular ROS and caused
DNA damage in transplanted cells.
Discussion
Chondrosarcoma is a malignant tumor of mesenchymal
origin that is generally locally aggressive and tends to produce
early systemic metastases. However, chondrosarcoma does not respond
to chemotherapy or radiation. Therefore, novel therapies are
required. PQQ was first identified in bacteria and is also likely
to be important in mammals (20–22).
Previous studies on PQQ have been focused on its activities as an
antioxidant and redox modulator (7), a cardio- and neuro-protectant
(23,24), and its radio-protective effects on
the hemopoietic system (25). PQQ
has a potent antitumor effect and possesses a significant cytotoxic
effect on human lung adenocarcinoma, hepatocarcinoma, melanoma
cells and brain cancer; however, it exhibits little effect on
normal cells, which suggests that PQQ may be an effective
therapeutic drug in the future (7,11,26).
However, the molecular mechanism of PQQ underlying its effect on
chondrosarcoma remains to be elucidated.
The present study examined cell death following
treatment with or without different concentrations of PQQ in cancer
and normal cells. It was identified that cell death increased in a
dose- and time-dependent manner following treatment with PQQ, while
the effect on normal cells was relatively small. Cell death occurs
in a variety of ways, including apoptosis, necrosis and cell
autophagy (27,28); however, the most frequently studied
and most common type is apoptosis. In the present study it was
hypothesized whether the effect of PQQ on chondrosarcoma cells was
achieved by promoting the rate of apoptosis in chondrosarcoma
cells. In the present study, the apoptotic level following PQQ
treatment in SW1353 and Saos-2 cells was investigated by flow
cytometry. The results demonstrated a large level of apoptosis
occurred following PQQ treatment, and the proportion of cell
apoptosis was comparable to that of the cytotoxic cell death rate,
indicating that PQQ may mainly induce apoptosis to kill
chondrosarcoma cells. Previous studies have reported that PQQ may
cause apoptosis by inhibiting the synthesis of GSH and producing
H2O2 via autoxidation to increase the level
of oxidative stress in tumor cells (7,11).
It is well known that ROS induces cell death, including apoptosis.
In addition, the enhancement of ROS production has long been
associated with the apoptotic response induced by several
anticancer agents (29–31). The status of intracellular redox is
regulated by antioxidant enzymes (including SOD, catalase and GSH
peroxidase) and non-enzymatic antioxidants (such as GSH and vitamin
C) (11). The levels of ROS and
hydroxyl radicals in the PQQ treated and the PBS control groups
were compared using flow cytometry and deoxyribose degradation
method. The results suggested that PQQ can cause a significant
increase in oxidative stress levels in the SW1353 chondrosarcoma
cell line.
It was demonstrated that treatment with PQQ had
inhibitory effects on tumorigenesis and decreased tumor size when
compared with the control in vivo, which is similar to the
results of the in vitro studies. However, it is unclear
whether the mechanism of PQQ inhibition in the xenograft tumors is
consistent with the in vitro results. These findings
indicated that the levels of ROS in the PQQ treated group were
significantly higher than those in the PBS control group, and the
percentage of γ-H2AX positive cells in the DNA injury-associated
indexes was significantly increased. The results suggested that PQQ
may inhibit the proliferation of tumor xenograft cells by promoting
oxidative stress and increasing the percentage of γ-H2AX. In
conclusion, the mechanism underlying PQQ-induced apoptosis may be
associated with increasing the levels of ROS, suggesting that PQQ
may be a potential drug for chondrosarcoma therapies.
Glossary
Abbreviations
Abbreviations:
|
PQQ
|
pyrroloquinoline quinone
|
|
ROS
|
reactive oxygen species
|
|
O2−
|
superoxide
|
|
OH
|
hydroxyl radical
|
|
TBS/T
|
Tris-buffered saline/0.1% Tween-20
|
References
|
1
|
Bauer HC, Brosjö O, Kreicbergs A and
Lindholm J: Low risk of recurrence of enchondroma and low-grade
chondrosarcoma in extremities. 80 patients followed for 2–25 years.
Acta Orthop Scand. 66:283–288. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eriksson AI, Schiller A and Mankin HJ: The
management of chondrosarcoma of bone. Clin Orthop Relat Res. 44–66.
1980.PubMed/NCBI
|
|
3
|
Bovée JV, Cleton-Jansen AM, Taminiau AH
and Hogendoorn PC: Emerging pathways in the development of
chondrosarcoma of bone and implications for targeted treatment.
Lancet Oncol. 6:599–607. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bao X, Ren T, Huang Y, Wang S, Zhang F,
Liu K, Zheng B and Guo W: Induction of the mesenchymal to
epithelial transition by demethylation-activated microRNA-125b is
involved in the anti-migration/invasion effects of arsenic trioxide
on human chondrosarcoma. J Exp Clin Cancer Res. 35:1292016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McIntire WS: Newly discovered redox
cofactors: Possible nutritional, medical, and pharmacological
relevance to higher animals. Annu Rev Nutr. 18:145–177. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Misra HS, Khairnar NP, Barik A, Indira
Priyadarsini K, Mohan H and Apte SK: Pyrroloquinoline-quinone: A
reactive oxygen species scavenger in bacteria. FEBS Lett.
578:26–30. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He K, Nukada H, Urakami T and Murphy MP:
Antioxidant and pro-oxidant properties of pyrroloquinoline quinone
(PQQ): Implications for its function in biological systems. Biochem
Pharmacol. 65:67–74. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smidt CR, Steinberg FM and Rucker RB:
Physiologic importance of pyrroloquinoline quinone. Proc Soc Exp
Biol Med. 197:pp. 19–26. 1991; View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bishop A, Gallop PM and Karnovsky ML:
Pyrroloquinoline quinone: A novel vitamin? Nutr Rev. 56:287–293.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shankar BS, Pandey R, Amin P, Misra HS and
Sainis KB: Role of glutathione in augmenting the anticancer
activity of pyrroloquinoline quinone (PQQ). Redox Rep. 15:146–154.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Min Z, Wang L, Jin J, Wang X, Zhu B, Chen
H and Cheng Y: Pyrroloquinoline quinone induces cancer cell
apoptosis via mitochondrial-dependent pathway and down-regulating
cellular Bcl-2 protein expression. J Cancer. 5:609–624. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pizzimenti S, Toaldo C, Pettazzoni P,
Dianzani MU and Barrera G: Thetwo-faced effects of reactive oxygen
species and the lipid peroxidation product4-hydroxynonenal in the
hallmarks of cancer. Cancers (Basel). 2:338–363. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tripathi DN, Chowdhury R, Trudel LJ, Tee
AR, Slack RS, Walker CL and Wogan GN: Reactive nitrogen species
regulate autophagy through ATM-AMPK-TSC2 mediated suppression of
mTORC1. Proc Natl Acad Sci USA. 110:pp. E2950–E2957. 2013;
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Finkel T and Holbrook NJ: Oxidants,
oxidative stress and the biology of ageing. Nature. 408:239–247.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Martindale JL and Holbrook NJ: Cellular
response to oxidative stress: Signalling for suicide and survival.
J Cell Physiol. 192:1–15. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fang J, Nakamura H and Iyer AK: Regulation
of replicative potential of cells. J Drug Target. 15:475–486. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baliga R, Zhang Z, Baliga M, Ueda N and
Shah SV: In vitro and in vivo evidence suggesting a role for iron
in cisplatin-induced nephrotoxicity. Kidney Int. 53:394–401. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie X, Zhao Y, Ma CY, Xu XM, Zhang YQ,
Wang CG, Jin J, Shen X, Gao JL, Li N, et al: Dimethyl fumarate
induces necroptosis in colon cancer cells through GSH depletion/ROS
increase/MAPKs activation pathway. Br J Pharmacol. 172:3929–3943.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X, Bao Y, Dong Z, Chen Q, Guo H,
Ziang C and Shao J: WP1130 attenuates cisplatin resistance by
decreasing P53 expression in non-small cell lung carcinomas.
Oncotarget. 8:49033–49043. 2017.PubMed/NCBI
|
|
20
|
Salisbury SA, Forrest HS, Cruse WB and
Kennard O: A novel coenzyme from bacterial primary alcohol
dehydrogenases. Nature. 280:843–844. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Killgore J, Smidt C, Duich L,
Romero-Chapman N, Tinker D, Reiser K, Melko M, Hyde D and Rucker
RB: Nutritional importance of pyrroloquinoline quinone. Science.
245:850–852. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Steinberg FM, Gershwin ME and Rucker RB:
Dietary pyrroloquinoline quinone: Growth and immune response in
BALB/c mice. J Nutr. 124:744–753. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tao R, Karliner JS, Simonis U, Zheng J,
Zhang J, Honbo N and Alano CC: Pyrroloquinoline quinone preserves
mitochondrial function and prevents oxidative injury in adult rat
cardiac myocytes. Biochem Biophys Res Commun. 363:257–262. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hara H, Hiramatsu H and Adachi T:
Pyrroloquinoline quinone is a potentneuroprotective nutrient
against 6-hydroxydopamine-induced neurotoxicity. Neurochem Res.
32:489–495. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiong XH, Zhao Y, Ge X, Yuan SJ, Wang JH,
Zhi JJ, Yang YX, Du BH, Guo WJ, Wang SS, et al: Production and
radioprotective effects of pyrroloquinoline quinone. Int J Mol Sci.
12:8913–8923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sato K and Toriyama M: Effect of
pyrroloquinoline quinone (PQQ) on melanogenic protein expression in
murine B16 melanoma. J Dermatol Sci. 53:140–145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fadeel B and Orrenius S: Apoptosis: A
basic biological phenomenon with wide-ranging implications in human
disease. J Intern Med. 258:479–517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Clarke PG: Developmental cell death:
Morphological diversity and multiple mechanisms. Anat Embryol
(Berl). 181:195–213. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tibodeau JD, Benson LM, Isham CR, Owen WG
and Bible KC: The Anticanceragent chaetocin is a competitive
substrate and inhibitor ofthioredoxin reductase. Antioxid Redox
Signal. 11:1097–1106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Madan E, Prasad S, Roy P, George J and
Shukla Y: Regulation of apoptosis by resveratrol through JAK/STAT
and mitochondria mediated pathway in human epidermoid carcinoma
A431 cells. Biochem Biophys Res Commun. 377:1232–1237. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Z, Huang SL, Li MM, Huang ZS, Lee KS
and Gu LQ: Inhibition of thioredoxin reductase by mansonone F
analogues: Implications for anticancer activity. Chem Biol
Interact. 177:48–57. 2009. View Article : Google Scholar : PubMed/NCBI
|