Introduction
Adiponectin (APN; additionally termed Acrp30, AdipoQ
and GBP28) is an adipocytokine that is secreted by adipocytes. APN
has received attention due to its insulin-sensitizing effects and
possible therapeutic use for metabolic disorders (1,2). APN
exerts antidiabetic actions by modulating glucose, fatty acid
metabolism and insulin sensitivity (3–7).
Apart from its antiatherosclerotic and insulin-sensitizing effects,
APN additionally exhibits anti-inflammatory and antiapoptotic roles
(8). Research has indicated that
APN suppresses the phagocytic activity and inflammatory cytokine
production of macrophages stimulated with lipopolysaccharide
(9). APN additionally reduces
cerebral and myocardial ischemia/reperfusion (I/R) injury,
decreases proinflammatory cytokine production and reduces cellular
apoptosis, and infarction of the brain and heart, via its
anti-inflammatory, antioxidative stress and antiapoptotic effects
(4,10). It has been suggested that decreased
levels of serum APN are associated with an increased risk of
cardiovascular disease (CVD) development and obesity-associated
types of cancer, including colon, breast, endometrial and prostate
cancer (7,11–16).
These results indicate that APN may possess a protective role
against I/R injury that is not specific to a particular organ.
Lung I/R injury (LIRI), a primary graft dysfunction,
leads to substantial morbidity and mortality following lung
transplantation (LTx). Ischemia is unavoidable during LTx, and the
subsequent effect of reperfusion results in marked lung
inflammation, oxidative stress and cellular damage (1,2,17). A
study has identified that there were 1.5 million cases of mortality
caused by diabetes in 2012, an increase from 1 million in 2000
(18). Diabetes has reached
epidemic proportions in the general adult population of China
(19). People with diabetes
mellitus and prediabetes who are awaiting LTx remain at risk of
mortality following LTx; donors with a history of diabetes mellitus
are associated with an increased risk of mortality in the recipient
following LTx (20,21).
There is increasing evidence that the lung is a
target organ for diabetic microangiopathy in patients with either
type 1 or type 2 diabetes mellitus (22,23).
Previous studies have indicated the physiological and structural
abnormalities of diabetic lungs. Diabetic hyperglycemia damages the
respiratory system due to pulmonary interstitial injury caused by
microangiopathy, and may contribute to autonomic neuropathy
(24). Decreased lung function has
been associated with diabetes in cross-sectional and longitudinal
studies (25,26). It has additionally been reported
that restrictive, although not obstructive, ventilatory dysfunction
is associated with the development of prediabetes and precedes the
development of type 2 diabetes mellitus (27).
A previous study reported that APN attenuated
lipopolysaccharide-induced acute lung injury. Therefore, APN may
have protective effects in LIRI within normal rats; the present
study aimed to investigate whether APN serves a protective role
LIRI in rats with diabetes mellitus.
Materials and methods
Animals
All procedures in the present study were approved by
the Institutional Animal Care and Use Committee of Harbin Medical
University. The animals used in the present study were supplied by
the Animal Center of the Second Affiliated Hospital of Harbin
Medical University (Harbin, China; no. SYXK, 2013–002). The
experiments involved 36 male Wistar rats aged 8–9 weeks (200–250
g). The rats were fed with a high-fat diet or standard laboratory
chow and were provided with water ad libitum. All
experimental animals were housed in the same feeding environment on
a 12-h light/dark cycle at a temperature of 24±2°C with a humidity
of 60±10% prior to the establishment of the model.
Animal preparation
A total of 18 male rats were fed a high-fat diet
(15% lard, 5% sesame oil, 20% sucrose, 2.5% cholesterol and 57.5%
normal chow) for 4 weeks, followed by administration of
streptozotocin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany; 35
mg/kg, dissolved to 0.1 M, pH 4.5) by intraperitoneal injection.
The rats with a fasting plasma glucose ≥11.1 mmol/l (measured in
blood from the tail) were considered diabetic and, therefore, as a
model of type 2 diabetes mellitus (3). The control rats were fed a standard
diet and injected with an equivalent volume of citrate buffer.
Following anesthesia via intraperitoneal injection of 30 mg/kg
sodium pentobarbital, each rat was intubated with a tracheal
cannula. The rats were ventilated with 40% O2 at 45–55
strokes/min and a tidal volume of 8–10 ml/kg body weight. A
24-gauge catheter was inserted into the right femoral vein for drug
and liquid administration. The right femoral artery was cannulated
with a 24-gauge catheter connected to a fluid-filled pressure
transducer to continuously monitor the blood pressure. The animals
were positioned in the right lateral decubitus position and their
chests were opened. The left lung hilum was clamped 5 min
post-administration of heparin (50 IU/animal) with a non-clash
microclip at the end of expiration. Subsequently, the lung was
subjected to 90 min of ischemia and 4 h of reperfusion.
Pentobarbital sodium was infused to maintain stable anesthesia, and
rocuronium bromide was used to maintain muscle relaxation
throughout the present study. Following reperfusion, blood samples
were collected from the femoral artery. Subsequently, the animals
were sacrificed with an overdose of pentobarbital sodium (100
mg/kg, intravenous).
Experimental protocols
All rats were randomly assigned to the normal or
diabetic groups. There were three subgroups in each group; the
normal groups comprised: i) Sham group (NS), in which the left lung
hilum was not clamped; ii) an I/R group (NIR group), in which the
lung was subjected to 90 min of ischemia and 4 h of reperfusion;
and iii) an I/R + APN globular domain (gAPN) (NIRA group), in which
10 µg gAPN was injected 10 min prior to reperfusion (5). The diabetic groups comprised: i) Sham
group (DS), in which the left lung hilum was not clamped; ii) an
I/R group (DIR), in which the lung was subjected to 90 min of
ischemia and 4 h of reperfusion and iii) an I/R + gAPN group
(DIRA), in which 10 µg gAPN was injected 10 min prior to
reperfusion.
Blood gas analysis
Arterial blood gas was measured prior to ischemia as
a baseline, at 90 min following ischemia, and at 60 and 120 min
following reperfusion. These time points were recorded as
T0-T3. At the end of the experiment, blood
from the femoral artery was collected and measured using a
conventional analyzer (Rapid Lab 348; Bayer AG, Leverkusen,
Germany).
Measurement of inflammatory cytokine
levels in the bronchoalveolar lavage fluid (BALF), wet-weight to
dry-weight ratio (W/D) ratio and myeloperoxidase (MPO) activity in
the lung
The left lung was lavaged three times with 3 ml cold
sterile saline. BALF samples were centrifuged (206 × g for 10 min,
4°C) and were stored at −80°C (28). Interleukin (IL)-6 and tumor
necrosis factor-α (TNF-α) expression levels in the BALF were
measured with ELISA kits (cat. nos. PR6000B and PRTA00,
respectively; R&D Systems, Inc., Minneapolis, MN, USA). The
upper lobe of the lung graft was desiccated at 80°C for 72 h to
measure the W/D ratio. A portion of the lung graft tissue was
frozen, homogenized and processed for MPO detection with a
colorimetric assay kit (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China) the change in spectrophotometric absorbance at 460
nm and was expressed as the optical density unit per gram of
tissue.
Malondialdehyde (MDA) levels,
superoxide dismutase (SOD) activity and nitric oxide (NO)
production in the lung
In the lung homogenate supernatants from the left
lung, MDA levels and SOD activity were determined using MDA and SOD
assay kits (cat. no. A003-1 and cat. no. A001-1-1, respectively;
Nanjing Jiancheng Bioengineering Institute, Nanjing, China). NO
levels were measured with a commercially available kit (cat. no.
A012-1 Nanjing Jiancheng Bioengineering Institute). These
experiments were performed according to the manufacturers'
protocols.
Histological evaluation and
scoring
The middle of the left lung was immediately fixed in
10% formalin at 4°C for 24 h. After 24 h, tissues were dehydrated,
embedded in paraffin, sectioned at 6 µm and stained with
hematoxylin and eosin for 30 min at room temperature. All images
were acquired with a Nikon Eclipse 80i microscope (Nikon
Corporation, Tokyo, Japan). Evaluation was based on the following
criteria: i) Neutrophil infiltration; ii) airway epithelial cell
damage; iii) interstitial edema; iv) hyaline membrane formation;
and v) hemorrhage. Each section had five scores corresponding with
the following five criteria as determined by degree of
deterioration: Normal=0; minimal alteration=1; mild alteration=2;
moderate alteration=3; and severe alteration=4. The lung injury
score (LIS) for each criterion was recorded (29).
Terminal deoxynucleotidyl transferase
dUTP nick end-labeling (TUNEL) staining for apoptosis
Cellular apoptosis was examined using TUNEL assays
(Nanjing Jiancheng Bioengineering Institute). The lung tissues were
placed in 10% formalin at room temperature overnight for paraffin
embedding and then were sectioned at 5 µm and were processed for
TUNEL. Then they were dehydrated, incubated with 0.9% NaCl for 5
min, then rinsed with PBS for 5 min, fixed in 4% paraformaldehyde
at room temperature for 15 min, then rinsed twice with PBS for 5
min each time. The sections were mixed with biotinylated
nucleotides and terminal deoxynucleotidyl transferase, covered with
coverslips and incubated at 37°C for 60 min. Following another PBS
wash, lung tissue was blocked with 0.3% hydrogen peroxide, and
incubated horseradish peroxidase streptavidin at room temperature
for 30 min, then washed 3 times with PBS and stained with
hematoxylin at room temperature for 3 min. The number of positive
cells per section was counted in five random fields from every
specimen with a Nikon Eclipse 80i microscope (magnification, ×40;
Nikon Corporation). and evaluated using the apoptotic index (AI).
The AI is a measure of the number of positive cells per 100 cells
counted in five different fields from the same section.
Immunohistochemistry
The lung tissues were placed in 10% formalin at room
temperature overnight for paraffin embedding and were processed for
immunohistochemical staining. Paraffin embedded specimens were
sectioned at 5 µm, deparaffinized and hydrated in PBS. Then, the
sections were incubated in 3% H2O2 for 10 min
and rinsed with PBS. A primary antibody against cleaved caspase-3
(cat. no. 9661; 1:200; Cell Signaling Technology, Inc., Danvers,
MA, USA) was applied, followed by washing and incubation in a
biotinylated secondary antibody (Vector Labs, Burlingame, CA, USA)
for 30 min. A Nikon Eclipse 80i microscope (Nikon Corporation) was
used to obtain the images.
Western blot analysis
Tissues were lysed with radioimmunoprecipitation
assay lysis buffer containing a protease inhibitor cocktail (Roche
Diagnostics, Basel, Switzerland). Equal amounts of proteins were
measured by BCA (Beyotime Institute of Biotechnology, Shanghai,
China). Cell lysates with each protein (10–20 µg/well) were
separated by 10–12% SDS-PAGE and transferred to nitrocellulose
membranes (Pall Life Sciences, Port Washington, NY, USA). Following
blocking with 5% non-fat milk for 1.5–2 h at room temperature, the
membranes were probed with primary antibodies against β-actin (cat.
no. sc-47778; 1:2,000; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), 5′adenosine monophosphate-activated protein kinase (AMPK;
cat. no. 5831; 1:500), p-AMPK (cat. no. 2535; 1:200) endothelial
nitric oxide synthase (eNOS; cat. no. 32027; 1:1,000) and inducible
nitric oxide synthase (iNOS; cat. no. 13120; 1:500; all Cell
Signaling Technology, Inc.) at 4°C overnight. Following washing and
incubating with rabbit (cat. no. 7074; 1:10,000) or mouse (cat. no.
7076; 1:10,000; both Cell Signaling Technology, Inc.) secondary
antibodies at room temperature for 1 h in the dark, the blots were
visualized using an enhanced chemiluminescence reagent (GE
Healthcare Bio-Sciences, Pittsburgh, PA, USA), Intensities of blots
were determined by densitometric analysis using ImageJ version 1.61
software (National Institutes of Health, Bethesda, MD, USA) and
normalized to β-actin.
Statistical analyses
Statistical analyses were performed using SAS
software (version 9.4; SAS Institute Inc., Cary, NC, USA). One-way
or two-way analysis of variance (ANOVA) followed by the
Student-Newman-Keuls or Dunnett's tests were used for the
statistical comparisons between multiple groups. The non-parametric
method with the Kruskal-Wallis test was used for the analysis of
LIS data. P<0.05 was considered to indicate a statistically
significant with 3–6 independent experiments. Data are presented as
the mean ± standard deviation.
Results
Pulmonary oxygenation function,
histological examination and lung edema
The histological structure of the alveoli was normal
in the lungs of the NS group, while the lung tissues from the I/R
group were markedly damaged, with intra-alveolar edema, hemorrhage
and interstitial thickening. The basal membranes and alveolar walls
were thickened in the rats with diabetes mellitus. These
histological alterations were more notable in the DIR group and
resulted in an increased cumulative LIS and W/D ratio compared with
the NIR group (Fig. 1).
Additionally, the partial pressure of arterial oxygen
(PaO2)/fraction of inspired oxygen (FiO2) was
decreased at T1, T2 and T3 in the
DIR group compared with the NIR group (Table I). Conversely, these alterations
were ameliorated in the DIRA and NIRA groups compared with the DIR
and NIR groups, respectively (Fig.
1). However, the LIS and W/D ratios were increased, and the
PaO2/FiO2 was decreased at T2 and
T3 in the DIRA group compared with the NIRA group
(Table I).
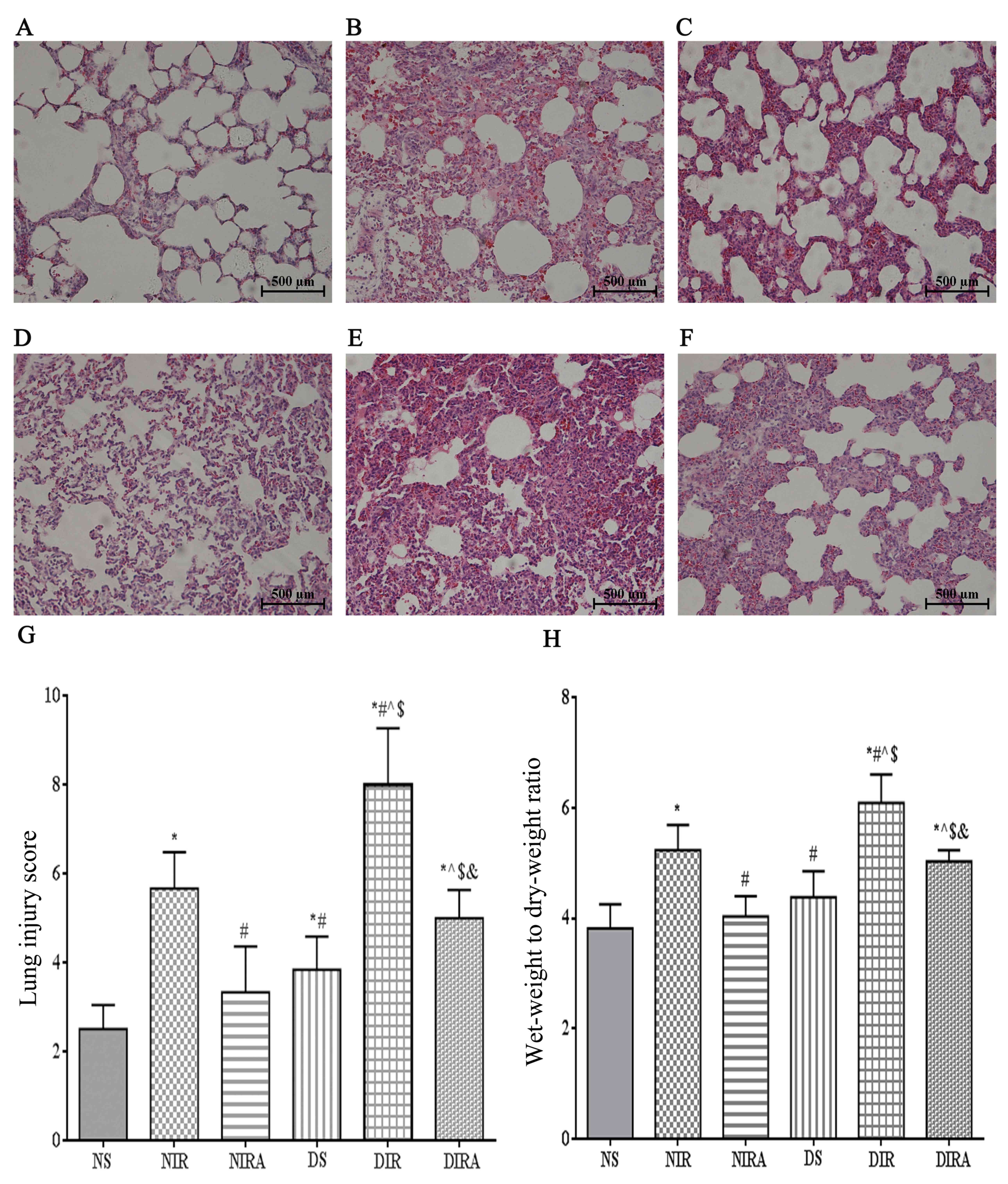 | Figure 1.Diabetes mellitus increases
susceptibility to lung I/R injury. Adiponectin improved the
IR-induced pathological alterations in lung tissue (hematoxylin and
eosin staining, lung injury score, and wet-weight to dry-weight
ratio). (A) NS, (B) NIR, (C) NIRA, (D) DS, (E) DIR and (F) DIRA
groups. Scale bar=500 µM. (G) Lung injury score and (H) wet-weight
to dry-weight ratio were determined. The results are expressed as
the mean ± standard deviation. *P<0.05 vs. NS group,
#P<0.05 vs. NIR group, ^P<0.05 vs. NIRA
group, $P<0.05 vs. DS group and
&P<0.05 vs. DIR group. DIR, diabetic I/R group,
in which the lung was subjected to 90 min of ischemia and 4 h of
reperfusion; DIRA, diabetic I/R + gAPN group, in which 10 µg gAPN
was injected 10 min prior reperfusion; DS, diabetic sham group, in
which the left lung hilum was not clamped; NIR, I/R, in which the
lung was subjected to 90 min of ischemia and 4 h of reperfusion;
NIRA, I/R + gAPN, in which 10 µg gAPN was injected 10 min prior to
reperfusion; NS, Sham group, in which the left lung hilum was not
clamped. I/R, ischemia/reperfusion; gAPN, adiponectin globular
domain. |
 | Table I.Effects of gAPN on pulmonary
oxygenation. |
Table I.
Effects of gAPN on pulmonary
oxygenation.
| Group | T0 | T1 | T2 | T3 |
|---|
| NS |
349.7500±34.70339 |
276.1667±18.21378 |
321.2083±33.45647 |
347.6250±39.13430 |
| NIR |
340.2500±69.56634 |
202.0417±17.91188a |
242.2500±37.09144a |
234.1250±8.93833a |
| NIRA |
341.6667±43.32974 |
188.7083±28.02294a |
298.6667±48.61443b |
357.2500±57.97111b |
| DS |
379.5000±38.02828 |
241.8833±21.36227a–c |
298.6667±48.61443a,b |
291.9583±54.47715b |
| DIR |
331.3333±67.44992 |
161.8917±39.01699a,b,d |
192.2500±22.51777a–d |
168.7917±22.67235a–d |
| DIRA |
396.9167±100.8507 |
159.7083±37.90957a,d,e |
236.3333±28.56075a,c,d,e |
217.2500±42.53528a,c,d,e |
Inflammatory cytokines and MPO
activity
The expression levels of IL-6 and TNF-α were higher
in the DS and NIR groups compared with the NS group, and the levels
of IL-6 and TNF-α were higher in the DIR group compared with the
NIR group. MPO activity exhibited the same trend as that of IL-6
expression levels. Treatment with gAPN prior to reperfusion reduced
MPO activity, and IL-6 and TNF-α expression levels in the NIRA and
DIRA groups compared with the NIR and DIR groups. Additionally, MPO
activity, and IL-6 and TNF-α expression levels were higher in the
DIRA group compared with the NIRA group (Fig. 2).
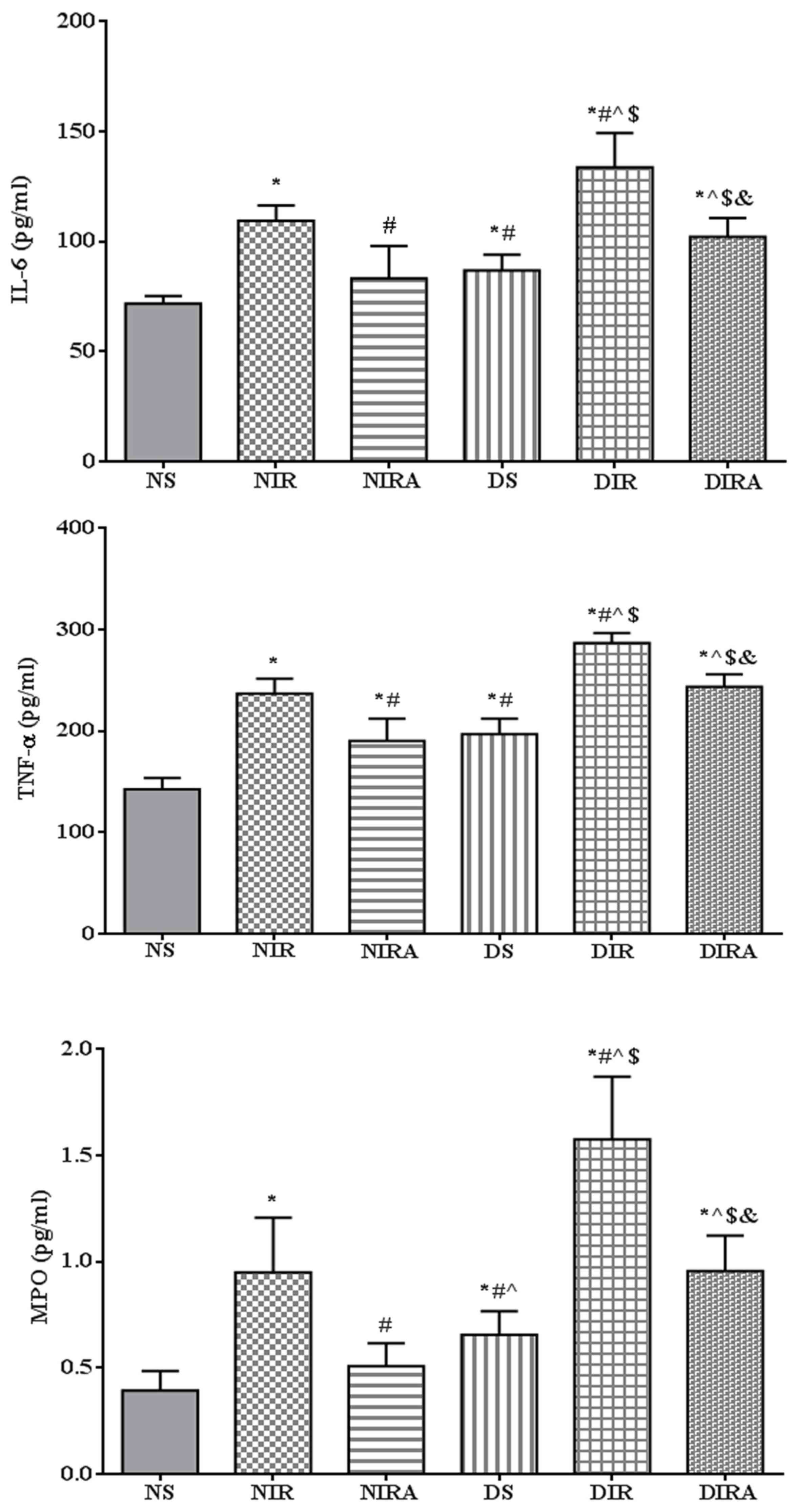 | Figure 2.APN reduces the levels of IL-6 and
TNF-α, in addition to MPO activity, following ischemia/reperfusion.
The results are expressed as the mean ± standard deviation.
*P<0.05 vs. NS group, #P<0.05 vs. NIR group,
^P<0.05 vs. NIRA group, $P<0.05 vs. DS
group and &P<0.05 vs. DIR group. NIR, I/R, in
which the lung was subjected to 90 min of ischemia and 4 h of
reperfusion; NIRA, I/R + gAPN, in which 10 µg gAPN was injected 10
min prior to reperfusion; NS, Sham group, in which the left lung
hilum was not clamped; DIR, diabetic I/R group, in which the lung
was subjected to 90 min of ischemia and 4 h of reperfusion; DIRA,
diabetic I/R + gAPN group, in which 10 µg gAPN was injected 10 min
prior reperfusion; DS, diabetic sham group, in which the left lung
hilum was not clamped; MPO, myeloperoxidase; TNF-α, tumor necrosis
factor-α; gAPN, adiponectin globular domain; IL-6, interleukin-6;
I/R, ischemia/reperfusion. |
Oxidative stress
SOD activity was decreased, and MDA and NO
production levels were increased in the DS group compared with the
NS group. In the NIR and DIR groups, SOD activity was significantly
decreased, and the MDA and NO production levels were significantly
increased compared with the NS and DS groups. In the NIRA and DIRA
groups, SOD activity was increased and MDA and NO production were
decreased compared with the NIR and DIR group, respectively. SOD
activity was lower, and the MDA and NO production levels were
higher, in the DIRA group compared with the NIRA group (Fig. 3).
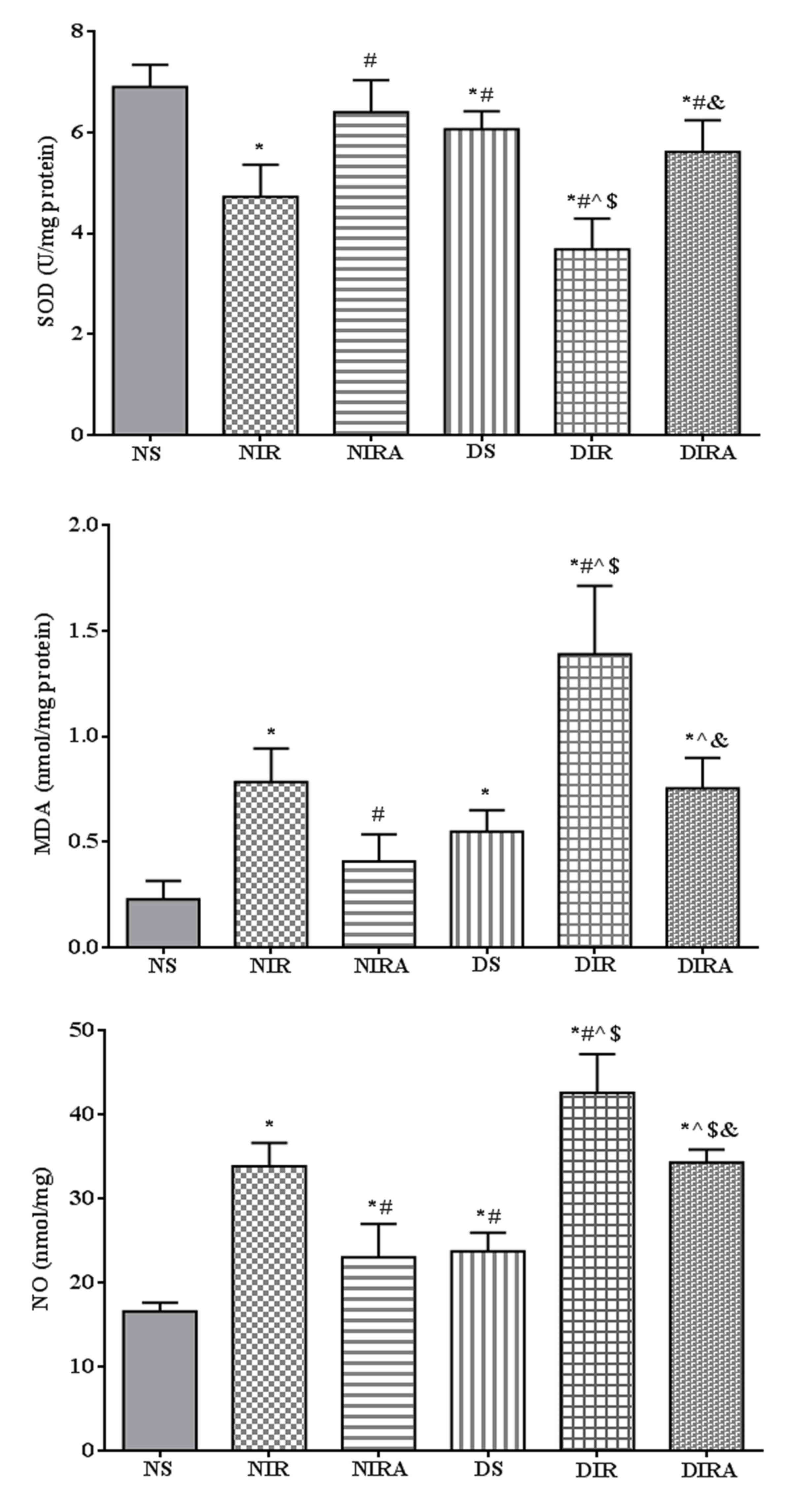 | Figure 3.APN attenuates oxidative stress
induced by I/R. The results are expressed as mean ± standard
deviation. *P<0.05 vs. NS group, #P<0.05 vs. NIR
group, ^P<0.05 vs. NIRA group, $P<0.05
vs. DS group and &P<0.05 vs. DIR group. DIR,
diabetic I/R group, in which the lung was subjected to 90 min of
ischemia and 4 h of reperfusion; DIRA, diabetic I/R + gAPN group,
in which 10 µg gAPN was injected 10 min prior reperfusion; DS,
diabetic sham group, in which the left lung hilum was not clamped;
NIR, I/R, in which the lung was subjected to 90 min of ischemia and
4 h of reperfusion; NIRA, I/R + gAPN, in which 10 µg gAPN was
injected 10 min prior to reperfusion; NS, Sham group, in which the
left lung hilum was not clamped. I/R, ischemia/reperfusion; gAPN,
adiponectin globular domain; SOD, superoxide dismutase; MDA,
malondialdehyde; NO, nitric oxide. |
Apoptosis
The percentages of TUNEL-positive cells were
increased in the DS and NIR groups compared with the NS group, and
were further increased in the DIR group compared with the NIR
group. Following treatment with gAPN prior to reperfusion, the
percentage of TUNEL-positive cells was decreased in the DIRA and
NIRA groups compared with the DIR and NIR groups (Fig. 4). The percentage of
caspase-3-positive cells exhibited a pattern that was similar to
that of the TUNEL-positive cells mentioned above (Fig. 5).
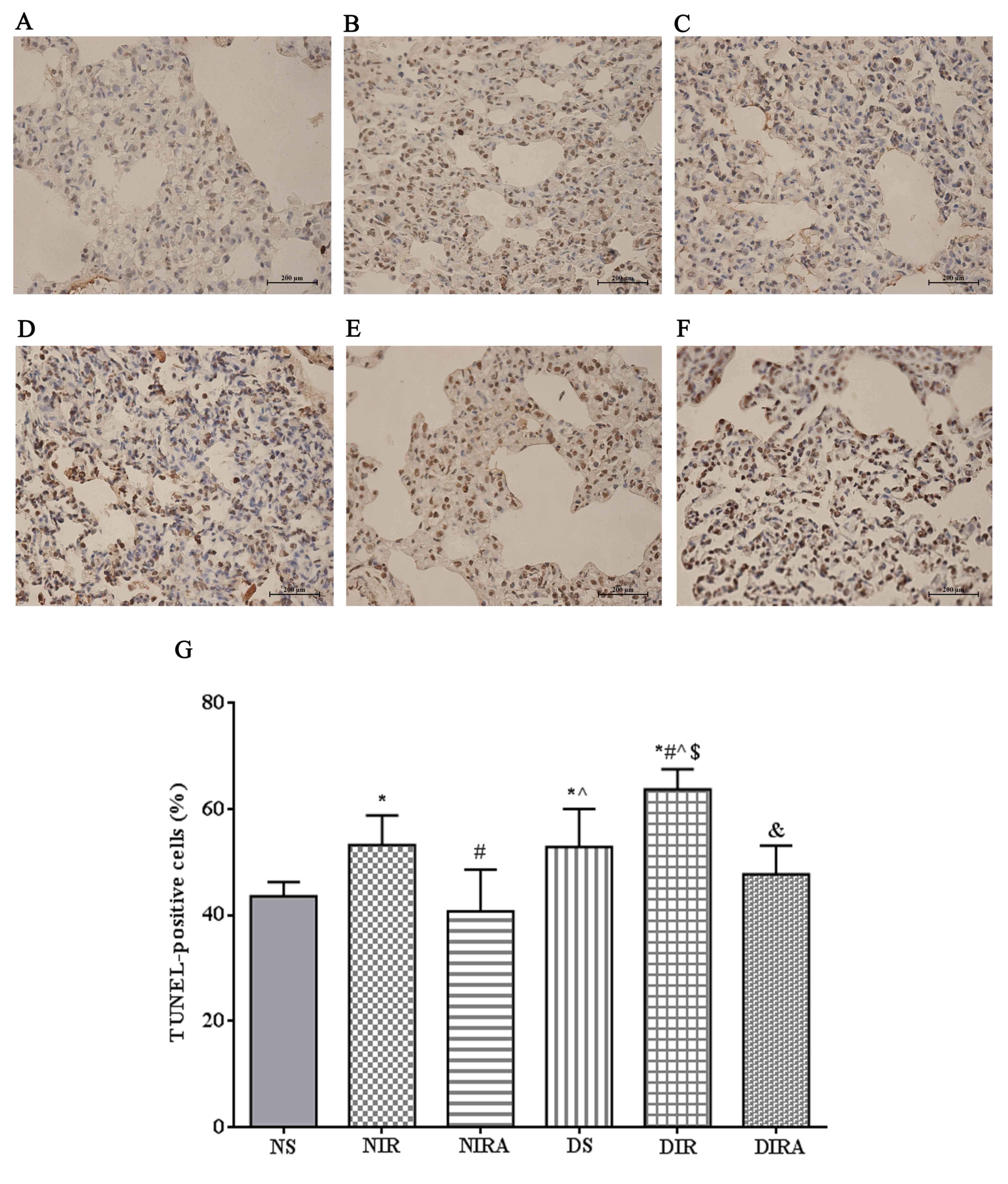 | Figure 4.APN inhibits I/R-induced apoptosis,
as demonstrated via TUNEL staining. TUNEL-positive cells observed
under a light microscope were identified by brown-stained nuclei.
(A) NS, (B) NIR, (C) NIRA, (D) DS, (E) DIR and (F) DIRA groups.
Scale bar=200 µM. (G) Percentages of TUNEL-positive cells. The
results were expressed as the mean ± standard deviation. *P<0.05
vs. NS group, #P<0.05 vs. NIR group,
^P<0.05 vs. NIRA group, $P<0.05 vs. DS
group and &P<0.05 vs. DIR group. DIR, diabetic
I/R group, in which the lung was subjected to 90 min of ischemia
and 4 h of reperfusion; DIRA, diabetic I/R + gAPN group, in which
10 µg gAPN was injected 10 min prior reperfusion; DS, diabetic sham
group, in which the left lung hilum was not clamped; NIR, I/R, in
which the lung was subjected to 90 min of ischemia and 4 h of
reperfusion; NIRA, I/R + gAPN, in which 10 µg gAPN was injected 10
min prior to reperfusion; NS, Sham group, in which the left lung
hilum was not clamped; TUNEL, terminal deoxynucleotidyl transferase
dUTP nick end-labeling; I/R, ischemia/reperfusion; gAPN,
adiponectin globular domain. |
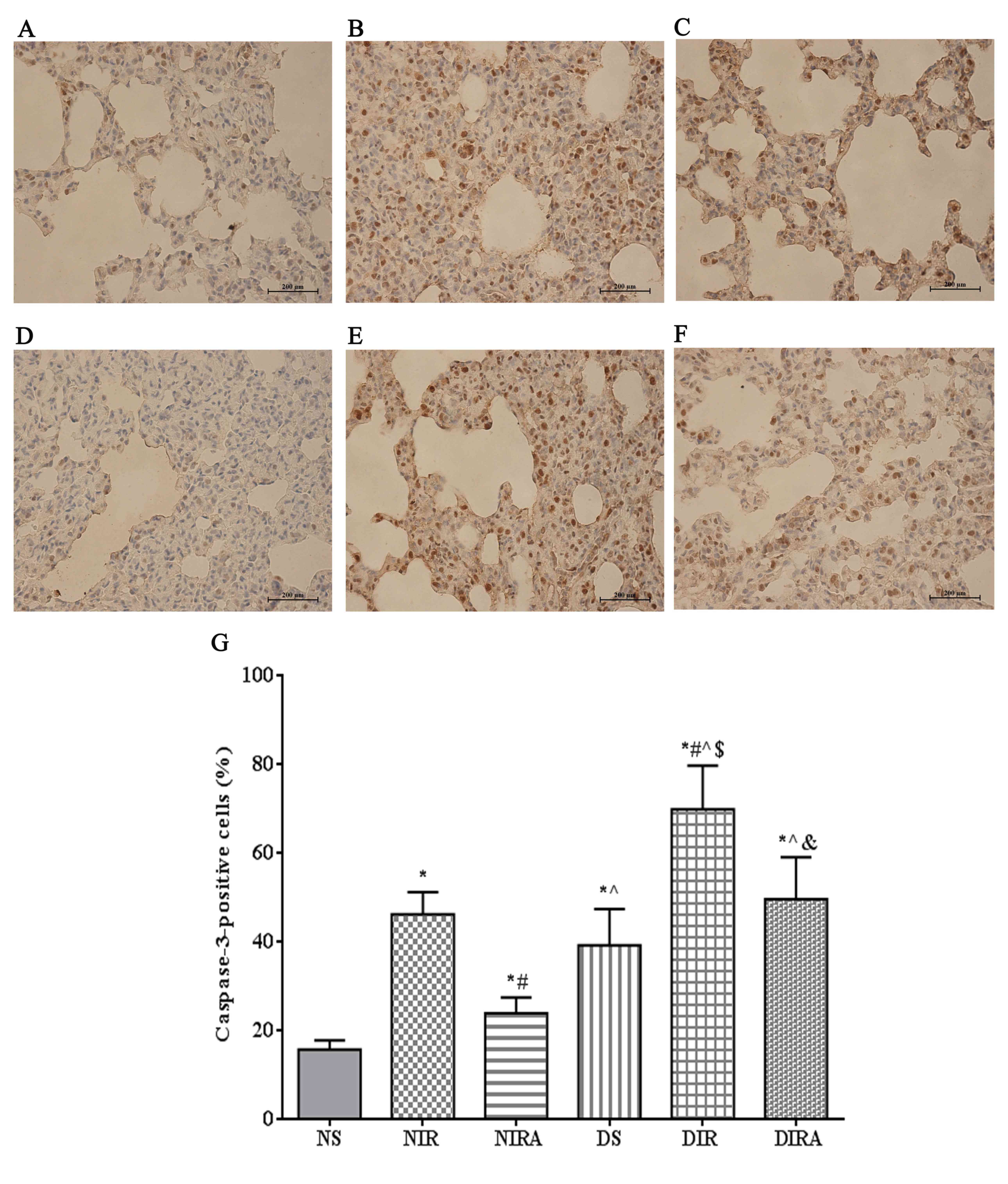 | Figure 5.APN inhibits I/R-induced caspase-3
expression. Caspase-3-positive cells observed under a light
microscope were identified by brown-stained nuclei. (A) NS, (B)
NIR, (C) NIRA, (D) DS, (E) DIR and (F) DIRA groups. Scale bar=200
µM. (G) Percentages of caspase-3-positive cells. The results are
expressed as the mean ± standard deviation. *P<0.05 vs. NS
group, #P<0.05 vs. NIR group, ^P<0.05
vs. NIRA group, $P<0.05 vs. DS group and
&P<0.05 vs. DIR group. DIR, diabetic I/R group,
in which the lung was subjected to 90 min of ischemia and 4 h of
reperfusion; DIRA, diabetic I/R + gAPN group, in which 10 µg gAPN
was injected 10 min prior reperfusion; DS, diabetic sham group, in
which the left lung hilum was not clamped; NIR, I/R, in which the
lung was subjected to 90 min of ischemia and 4 h of reperfusion;
NIRA, I/R + gAPN, in which 10 µg gAPN was injected 10 min prior to
reperfusion; NS, Sham group, in which the left lung hilum was not
clamped. I/R, ischemia/reperfusion; gAPN, adiponectin globular
domain. |
p-AMPK, eNOS and iNOS protein
expression
The results of the present study demonstrated that
p-AMPK/AMPK levels were increased in the DIR and NIR groups
compared with the NS and DS groups, and were decreased in the DS
group compared with the NS group (Fig.
6). Treatment with gAPN increased p-AMPK/AMPK levels in the
NIRA and DIRA groups compared with the NIR and DIR groups.
Additionally, p-AMPK/AMPK levels were decreased in the DIRA group
compared with the NIRA group. The eNOS levels exhibited a pattern
that was similar to that of p-AMPK/AMPK, whereas iNOS levels
exhibited a trend that was the inverse of that of p-AMPK/AMPK
(Fig. 6).
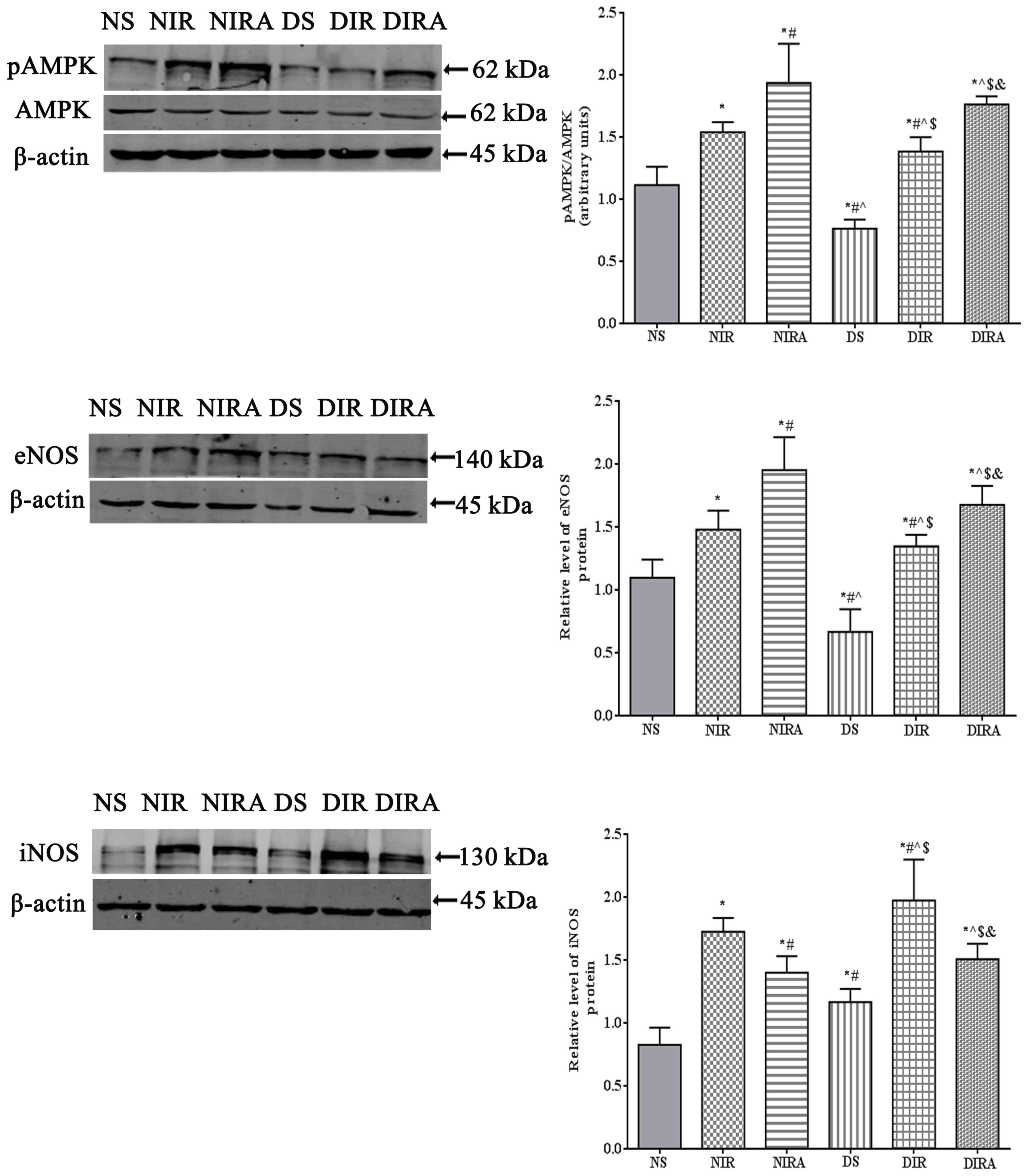 | Figure 6.APN activates p-AMPK and eNOS
expression and attenuates iNOS expression. The results are
expressed as mean ± standard deviation. *P<0.05 vs. NS group,
#P<0.05 vs. NIR group, ^P<0.05 vs. NIRA
group, $P<0.05 vs. DS group and
&P<0.05 vs. DIR group. DIR, diabetic I/R group,
in which the lung was subjected to 90 min of ischemia and 4 h of
reperfusion; DIRA, diabetic I/R + gAPN group, in which 10 µg gAPN
was injected 10 min prior reperfusion; DS, diabetic sham group, in
which the left lung hilum was not clamped; NIR, I/R, in which the
lung was subjected to 90 min of ischemia and 4 h of reperfusion;
NIRA, I/R + gAPN, in which 10 µg gAPN was injected 10 min prior to
reperfusion; NS, Sham group, in which the left lung hilum was not
clamped. p-AMPK, phosphorylated 5′ adenosine
monophosphate-activated protein kinase; I/R, ischemia/reperfusion;
gAPN, adiponectin globular domain; eNOS, endothelial nitric oxide
synthase; iNOS, inducible nitric oxide synthase. |
Discussion
The present study reported three primary findings:
i) Type 2 diabetes mellitus exacerbates LIRI in a manner that is
characterized by a high level of inflammatory cytokines, and a
large increase in oxidative stress and cellular apoptosis; ii)
conditioning via pretreatment with gAPN alleviates LIRI in
vivo by reducing the inflammatory response, lung edema, LIS,
oxidative stress and apoptosis, and by improving pulmonary
oxygenation in normal rats and DM rats; and iii) treatment with
gAPN increases the activation of AMPK in rats, regardless of
treatment. To the best of our knowledge, the present study was the
first to provide direct evidence that the administration of gAPN as
a bolus 10 min prior to reperfusion reverses the adverse effects of
type 2 diabetes on LIRI.
APN monomers possess an amino-terminal collagen-like
domain and a carboxy-terminal globular domain that generates
trimers, hexamers and high-molecular-weight multimers (30). The three multimeric forms have been
detected in the circulation, associated with numerous serum
proteins previously characterized in humans (31). The APN globular head has
additionally been detected in the trimeric form in human and mouse
plasma, albeit at low concentrations (32,33).
APN is one of a number of proteins secreted by adipose cells that
may couple the regulation of insulin sensitivity with energy
metabolism and serve to associate obesity with insulin resistance
(34). It has been reported that
gAPN has increased biological activity (>20-fold) compared with
the full-length form (33,35). gAPN is more potent in the
stimulation of fatty acid oxidation in skeletal muscles compared
with full-length APN and has more rapid action than full-length APN
following a single in vivo dose (32). In addition, Tao et al
(4) reported that, although the
levels of gAPN may be markedly low in the circulation, gAPN may be
the final active ligand of APN at its target cells.
It has been demonstrated that APN decreases
proinflammatory cytokine levels and reduces apoptosis and
oxidative/nitrative stress (4,5,10).
In the present study, the LIRI of normal rats was mitigated
following administration of APN. Compared with the NIRA group, the
W/D ratio, LIS, IL-6 expression levels, TNF-α expression levels and
MPO activity were increased; oxidative stress and apoptosis were
more severe in the NIR group. Therefore, APN had a protective
effect on cerebral and myocardial I/R injury (10,36),
in addition to LIRI. Low levels of APN may increase the risk of
type 2 diabetes mellitus and CVD development; however, the
association between circulating APN concentrations and CVD risk is
moderate compared with the strong association with type 2 diabetes
mellitus risk (6). In the present
study, diabetes mellitus in rats induced more severe lung injury
and aggravated LIRI compared with normal rats in the same state.
Compared with the NIR group, the W/D ratio, LIS, IL-6 expression
levels, TNF-α expression levels and MPO activity were higher;
oxidative stress and apoptosis were more severe in the DIR group.
These changes were ameliorated in the DIRA group compared with the
DIR group. Therefore, the administration of exogenous APN mitigated
LIRI in rats with diabetes mellitus. Additionally, compared with
the NIRA group, the W/D ratio, LIS, IL-6 expression levels, TNF-α
expression levels and MPO activity were higher; oxidative stress
and apoptosis were more severe within the DIRA group. Therefore,
APN had a protective effect on LIRI, and this protective effect was
inhibited in rats with diabetes mellitus.
AMPK is a highly conserved heterotrimeric kinase
that functions as a metabolic switch, thereby coordinating the
cellular enzymes involved in carbohydrate and fat metabolism to
enable adenosine 5′-triphosphate conservation and synthesis
(37). The metabolic effects of
APN are similar to those elicited by the activation of AMPK in the
liver and muscles (7,11,12),
leading to the hypothesis that APN may act via the stimulation of
this enzyme. AMPK signaling in endothelial cells is required for
the proangiogenic effects of APN (12,38),
antiapoptotic effects (39),
stimulation of NO production (40), reduction of myocardial infarct size
and myocardial apoptosis in a mouse model of heart I/R (36), and attenuation of acute lung injury
(41,42). The majority of the metabolic
regulatory function of APN occurs via the AMPK signaling axis
(35), whereas APN-mediated
inhibition of the inflammatory response and elicitation of
vasodilatation/vasculoprotection occurs primarily via the AMPK/eNOS
axis (38–40). Previous research revealed that AMPK
activation reduces TLR4-induced neutrophil activation and
diminishes the severity of neutrophil-driven proinflammatory
processes, including acute lung injury (43). AMPK is involved in modulating acute
inflammatory reactions and AMPK activation inhibited cytokine
production (44). AMPK may
additionally inhibit the formation of reactive oxygen species via
NADPH oxidase and stimulate NO production by eNOS (45,46).
Consistent with these studies, the present study demonstrated that
APN activated AMPK expression, protected against LIRI by countering
inflammation, inhibited oxidative stress and inhibited apoptosis in
rats. In type 2 diabetes mellitus, tissue responses to insulin are
significantly reduced, and numerous studies have demonstrated that
APN shares numerous biological functions with insulin (47). Therefore, rats with diabetes
mellitus may exhibit downregulated phosphorylation of AMPK. In the
present study, p-AMPK/AMPK was decreased in diabetes mellitus rats
compared with normal rats under the same conditions.
APN directly stimulates NO production via the
phosphorylation of eNOS by AMPK (48). In the present study, treatment with
gAPN decreased NO production; however, eNOS expression was
increased after treatment with gAPN. In contrast to eNOS
expression, iNOS expression was enhanced following I/R and
treatment with gAPN inhibited iNOS expression. A previous study
suggested that APN may increase NO production by eNOS under
physiological conditions; whereas, under pathological conditions,
where iNOS expression is stimulated, APN inhibits NO overproduction
by inhibiting iNOS expression, thus protecting tissues (5,48).
These observations are consistent with those of the present study,
in which treatment with gAPN decreased NO production and iNOS
expression in the NIRA and DIRA groups compared with the NIR and
DIR groups, respectively.
Overall, the present study suggested that APN may
serve an important protective role in diabetes mellitus-exacerbated
LIRI in rats. Further studies are required to clarify the
underlying mechanisms through which APN may protect against
diabetes mellitus-exacerbated LIRI in rats.
There were a number of limitations to the present
study; as the present study was terminated 4 h subsequent to
reperfusion, extended observations of the recipients of APN are
required, and the therapeutic effects of pretreatment with APN on
lung function requires further investigation. In the present study,
normal rats and diabetes mellitus rats were administered the same
dose of APN. The protective effects of APN were eliminated in
diabetes mellitus rats; an increased dose of APN may exhibit more
protective effects within diabetes mellitus rats. An APN knockout
mouse model is required to investigate the underlying mechanisms of
action of APN.
The results of the present study demonstrated that
gAPN may exert potent protective functions against LIRI within rats
with type 2 diabetes mellitus and the anti-inflammatory,
antioxidative stress and antiapoptotic properties of APN may
contribute considerably to its beneficial effects. AMPK is a key
transcription factor in this process. These molecules may be
considered as potential novel therapeutic targets following
LTx.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DL and XC designed the present study. DL performed
the experiments, collected and analyzed data. LS, JW and CM
provided experimental support. DL wrote the manuscript and JW, LS
and XC proofread the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The protocol for the present study was approved by
Institutional Committee on Animal Care and Use of Harbin Medical
University (Harbin, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fernandez LG, Sharma AK, LaPar DJ, Kron IL
and Laubach VE: Adenosine A1 receptor activation attenuates lung
ischemia-reperfusion injury. J Thorac Cardiovasc Surg.
145:1654–1659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
den Hengst WA, Gielis JF, Lin JY, Van
Schil PE, De Windt LJ and Moens AL: Lung ischemia-reperfusion
injury: A molecular and clinical view on a complex
pathophysiological process. Am J Physiol Heart Circ Physiol.
299:H1283–H1299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen GM, Hu N, Liu L, Xie SS, Wang P, Li
J, Xie L, Wang GJ and Liu XD: Pharmacokinetics of verapamil in
diabetic rats induced by combination of high-fat diet and
streptozotocin injection. Xenobiotica. 41:494–500. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tao L, Gao E, Jiao X, Yuan Y, Li S,
Christopher TA, Lopez BL, Koch W, Chan L, Goldstein BJ and Ma XL:
Adiponectin cardioprotection after myocardial ischemia/reperfusion
involves the reduction of oxidative/nitrative stress. Circulation.
115:1408–1416. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yi W, Sun Y, Gao E, Wei X, Lau WB, Zheng
Q, Wang Y, Yuan Y, Wang X, Tao L, et al: Reduced cardioprotective
action of adiponectin in high-fat diet-induced type II diabetic
mice and its underlying mechanisms. Antioxid Redox Signal.
15:1779–1788. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sattar N, Wannamethee G, Sarwar N,
Tchernova J, Cherry L, Wallace AM, Danesh J and Whincup PH:
Adiponectin and coronary heart disease: A prospective study and
meta-analysis. Circulation. 114:623–629. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kahn BB, Alquier T, Carling D and Hardie
DG: AMP-activated protein kinase: Ancient energy gauge provides
clues to modern understanding of metabolism. Cell Metab. 1:15–25.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Whitehead JP, Richards AA, Hickman IJ,
Macdonald GA and Prins JB: Adiponectin-a key adipokine in the
metabolic syndrome. Diabetes Obes Metab. 8:264–280. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thakur V, Pritchard MT, McMullen MR and
Nagy LE: Adiponectin normalizes LPS-stimulated TNF-alpha production
by rat Kupffer cells after chronic ethanol feeding. Am J Physiol
Gastrointest Liver Physiol. 290:G998–G1007. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen B, Liao WQ, Xu N, Xu H, Wen JY, Yu
CA, Liu XY, Li CL, Zhao SM and Campbell W: Adiponectin protects
against cerebral ischemia-reperfusion injury through
anti-inflammatory action. Brain Res. 1273:129–137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kola B, Boscaro M, Rutter GA, Grossman AB
and Korbonits M: Expanding role of AMPK in endocrinology. Trends
Endocrinol Metab. 17:205–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Viollet B, Foretz M, Guigas B, Horman S,
Dentin R, Bertrand L, Hue L and Andreelli F: Activation of
AMP-activated protein kinase in the liver: A new strategy for the
management of metabolic hepatic disorders. J Physiol. 574:41–53.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ouchi N, Kobayashi H, Kihara S, Kumada M,
Sato K, Inoue T, Funahashi T and Walsh K: Adiponectin stimulates
angiogenesis by promoting cross-talk between AMP-activated protein
kinase and Akt signaling in endothelial cells. J Biol Chem.
279:1304–1309. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ouchi N, Kihara S, Arita Y, Maeda K,
Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T,
et al: Novel modulator for endothelial adhesion molecules:
Adipocyte-derived plasma protein adiponectin. Circulation.
100:2473–2476. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumada M, Kihara S, Sumitsuji S, Kawamoto
T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka
H, et al: Association of hypoadiponectinemia with coronary artery
disease in men. Arterioscler Thromb Vasc Biol. 23:85–89. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bouhali T, Brisson D, St-Pierre J,
Tremblay G, Perron P, Laprise C, Vohl MC, Vissers MN, Hutten BA,
Després JP, et al: Low plasma adiponectin exacerbates the risk of
premature coronary artery disease in familial hypercholesterolemia.
Atherosclerosis. 196:262–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Laubach VE and Kron IL: Pulmonary
inflammation after lung transplantation. Surgery. 146:1–4. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng H, Wu J, Jin Z and Yan LJ: Potential
biochemical mechanisms of lung injury in diabetes. Aging Dis.
8:7–16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J,
Shan Z, Liu J, Tian H, Ji Q, et al: Prevalence of diabetes among
men and women in China. N Engl J Med. 362:1090–1101. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hackman KL, Snell GI and Bach LA: An
unexpectedly high prevalence of undiagnosed diabetes in patients
awaiting lung transplantation. J Heart Lung Transplant. 32:86–91.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ambur V, Taghavi S, Jayarajan S, Kadakia
S, Zhao H, Gomez-Abraham J and Toyoda Y: The impact of lungs from
diabetic donors on lung transplant recipients†. Eur J Cardiothorac
Surg. 51:285–290. 2017.PubMed/NCBI
|
|
22
|
Kuitert LM: The lung in diabetes-yet
another target organ? Chron Respir Dis. 5:67–68. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pitocco D, Fuso L, Conte EG, Zaccardi F,
Condoluci C, Scavone G, Incalzi RA and Ghirlanda G: The diabetic
lung-a new target organ? Rev Diabet Stud. 9:23–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Williams JG, Morris AI, Hayter RC and
Ogilvie CM: Respiratory responses of diabetics to hypoxia,
hypercapnia, and exercise. Thorax. 39:529–534. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dennis RJ, Maldonado D, Rojas MX, Aschner
P, Rondón M, Charry L and Casas A: Inadequate glucose control in
type 2 diabetes is associated with impaired lung function and
systemic inflammation: A cross-sectional study. BMC Pulm Med.
10:382010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Engström G, Hedblad B, Nilsson P, Wollmer
P, Berglund G and Janzon L: Lung function, insulin resistance and
incidence of cardiovascular disease: A longitudinal cohort study. J
Intern Med. 253:574–581. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim CH, Kim HK, Kim EH, Bae SJ, Jung YJ,
Choi J and Park JY: Association of restrictive ventilatory
dysfunction with the development of prediabetes and type 2 diabetes
in Koreans. Acta Diabetol. 52:357–363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chiang CH, Hsu K, Yan HC, Harn HJ and
Chang DM: PGE1, dexamethasone, U-74389G, or Bt2-cAMP as an additive
to promote protection by UW solution in I/R injury. J Appl Physiol
(1985). 83:583–590. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pirat A, Zeyneloglu P, Aldemir D, Yücel M,
Ozen O, Candan S and Arslan G: Pretreatment with simvastatin
reduces lung injury related to intestinal ischemia-reperfusion in
rats. Anesth Analg. 102:225–232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kadowaki T, Yamauchi T, Kubota N, Hara K,
Ueki K and Tobe K: Adiponectin and adiponectin receptors in insulin
resistance, diabetes, and the metabolic syndrome. J Clin Invest.
116:784–792. 2006. View
Article : Google Scholar
|
|
31
|
Wang Y, Xu LY, Lam KS, Lu G, Cooper GJ and
Xu A: Proteomic characterization of human serum proteins associated
with the fat-derived hormone adiponectin. Proteomics. 6:3862–3870.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fruebis J, Tsao TS, Javorschi S,
Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE and Lodish HF:
Proteolytic cleavage product of 30-kDa adipocyte complement-related
protein increases fatty acid oxidation in muscle and causes weight
loss in mice. Proc Natl Acad Sci USA. 98:pp. 2005–2010. 2001;
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamauchi T, Kamon J, Waki H, Terauchi Y,
Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N,
et al: The fat-derived hormone adiponectin reverses insulin
resistance associated with both lipoatrophy and obesity. Nat Med.
7:941–946. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iwashima Y, Katsuya T, Ishikawa K, Ouchi
N, Ohishi M, Sugimoto K, Fu Y, Motone M, Yamamoto K, Matsuo A, et
al: Hypoadiponectinemia is an independent risk factor for
hypertension. Hypertension. 43:1318–1323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamauchi T, Kamon J, Minokoshi Y, Ito Y,
Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al:
Adiponectin stimulates glucose utilization and fatty-acid oxidation
by activating AMP-activated protein kinase. Nat Med. 8:1288–1295.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shibata R, Sato K, Pimentel DR, Takemura
Y, Kihara S, Ohashi K, Funahashi T, Ouchi N and Walsh K:
Adiponectin protects against myocardial ischemia-reperfusion injury
through AMPK- and COX-2-dependent mechanisms. Nat Med.
11:1096–1103. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hardie DG, Carling D and Carlson M: The
AMP-activated/SNF1 protein kinase subfamily: Metabolic sensors of
the eukaryotic cell? Annu Rev Biochem. 67:821–855. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shibata R, Ouchi N, Kihara S, Sato K,
Funahashi T and Walsh K: Adiponectin stimulates angiogenesis in
response to tissue ischemia through stimulation of amp-activated
protein kinase signaling. J Biol Chem. 279:28670–28674. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kobayashi H, Ouchi N, Kihara S, Walsh K,
Kumada M, Abe Y, Funahashi T and Matsuzawa Y: Selective suppression
of endothelial cell apoptosis by the high molecular weight form of
adiponectin. Circ Res. 94:e27–e31. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen H, Montagnani M, Funahashi T,
Shimomura I and Quon MJ: Adiponectin stimulates production of
nitric oxide in vascular endothelial cells. J Biol Chem.
278:45021–45026. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Konter JM, Parker JL, Baez E, Li SZ,
Ranscht B, Denzel M, Little FF, Nakamura K, Ouchi N, Fine A, et al:
Adiponectin attenuates lipopolysaccharide-induced acute lung injury
through suppression of endothelial cell activation. J Immunol.
188:854–863. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He Y, Zou L, Zhou Y, Hu H, Yao R, Jiang Y,
Lau WB, Yuan T, Huang W, Zeng Z and Cao Y: Adiponectin ameliorates
the apoptotic effects of paraquat on alveolar type cells via
improvements in mitochondrial function. Mol Med Rep. 14:746–752.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao X, Zmijewski JW, Lorne E, Liu G, Park
YJ, Tsuruta Y and Abraham E: Activation of AMPK attenuates
neutrophil proinflammatory activity and decreases the severity of
acute lung injury. Am J Physiol Lung Cell Mol Physiol.
295:L497–L504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Becker J, Delayre-Orthez C, Frossard N and
Pons F: Regulation of inflammation by PPARs: A future approach to
treat lung inflammatory diseases? Fundam Clin Pharmacol.
20:429–447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Alba G, El Bekay R, Alvarez-Maqueda M,
Chacón P, Vega A, Monteseirín J, Santa María C, Pintado E, Bedoya
FJ, Bartrons R and Sobrino F: Stimulators of AMP-activated protein
kinase inhibit the respiratory burst in human neutrophils. FEBS
Lett. 573:219–225. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Song P and Zou MH: Regulation of NAD(P)H
oxidases by AMPK in cardiovascular systems. Free Radic Biol Med.
52:1607–1619. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Berg AH, Combs TP, Du X, Brownlee M and
Scherer PE: The adipocyte-secreted protein Acrp30 enhances hepatic
insulin action. Nat Med. 7:947–953. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Huang PH, Sata M, Nishimatsu H, Sumi M,
Hirata Y and Nagai R: Pioglitazone ameliorates endothelial
dysfunction and restores ischemia-induced angiogenesis in diabetic
mice. Biomed Pharmacother. 62:46–52. 2008. View Article : Google Scholar : PubMed/NCBI
|




















