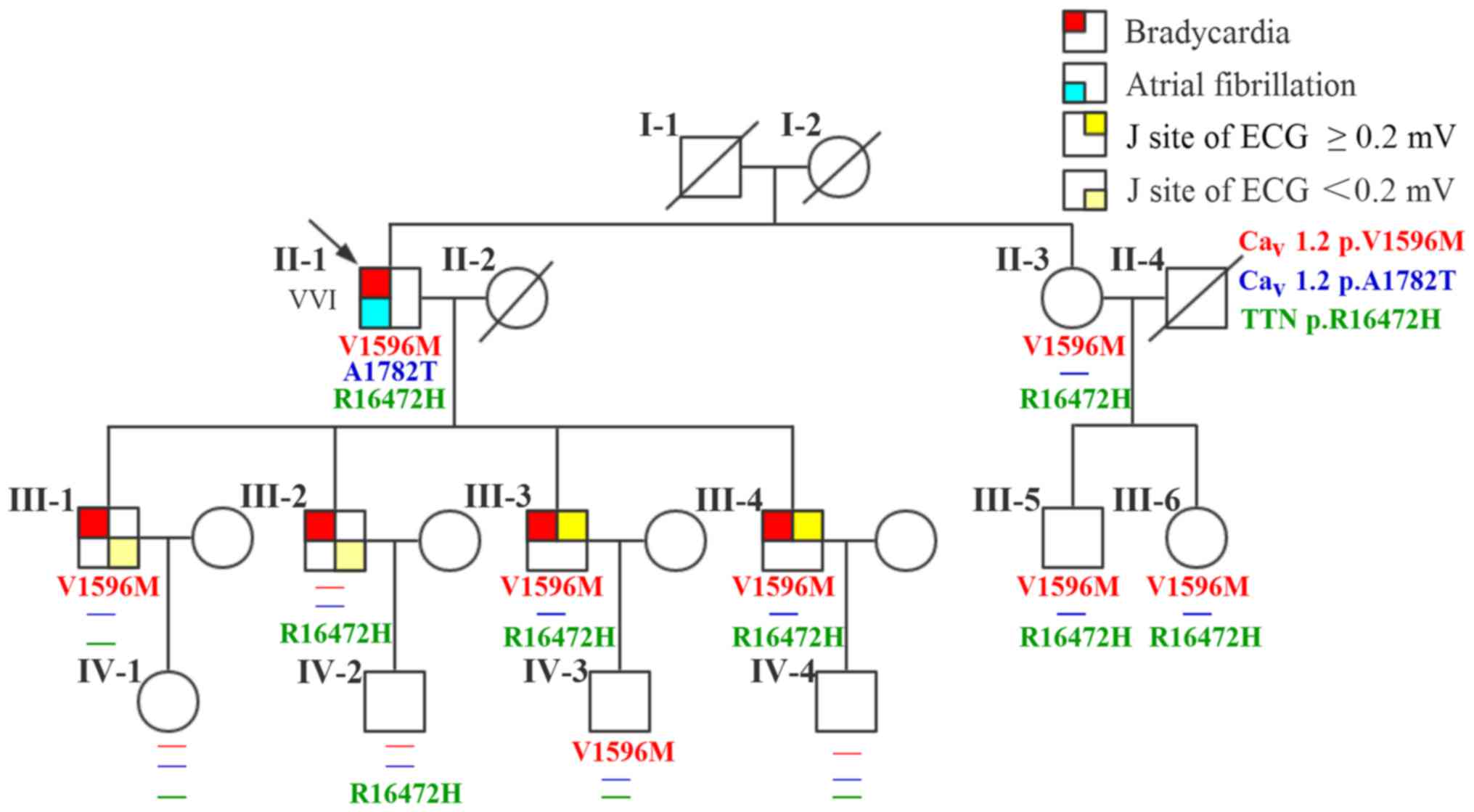
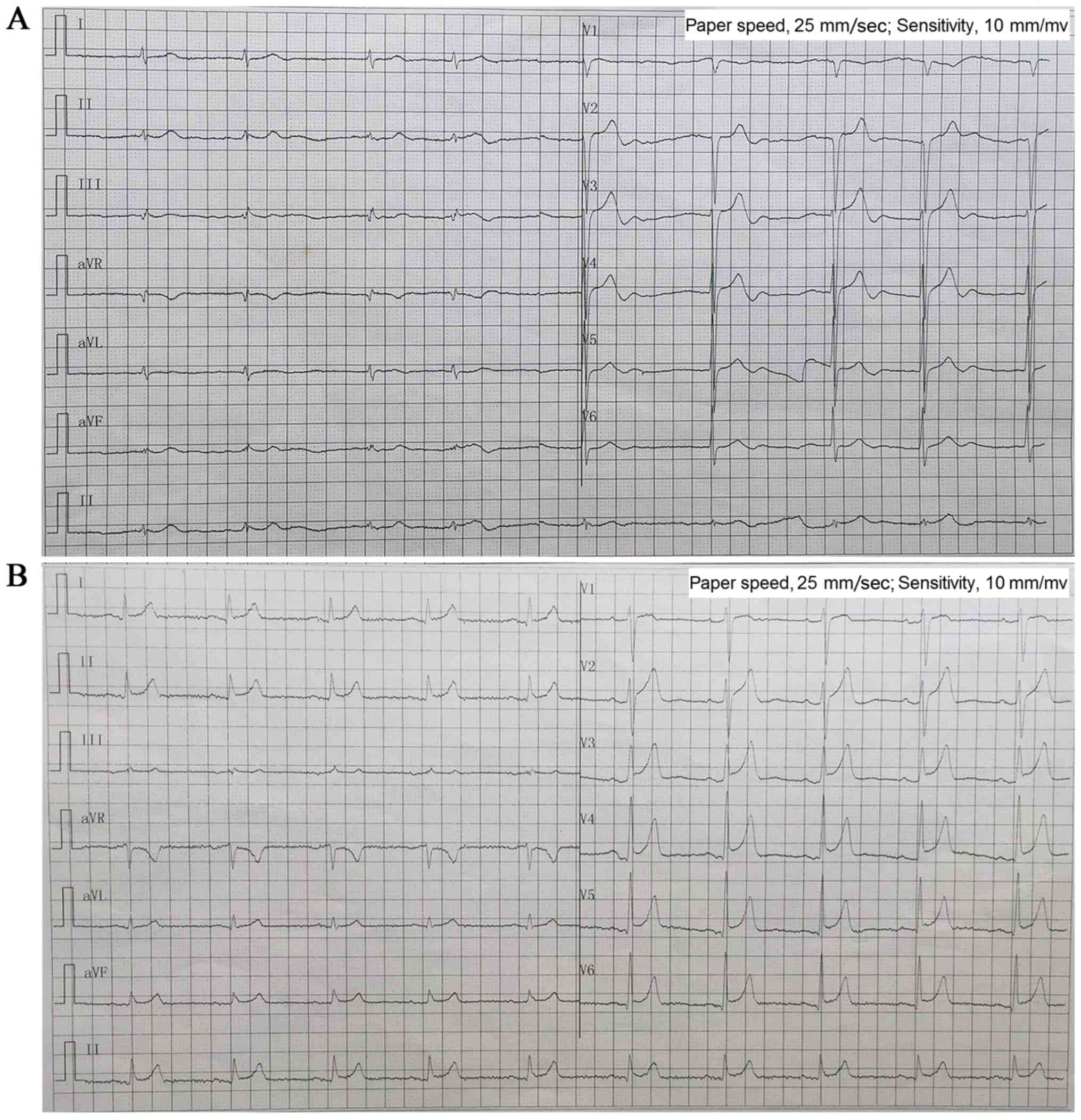
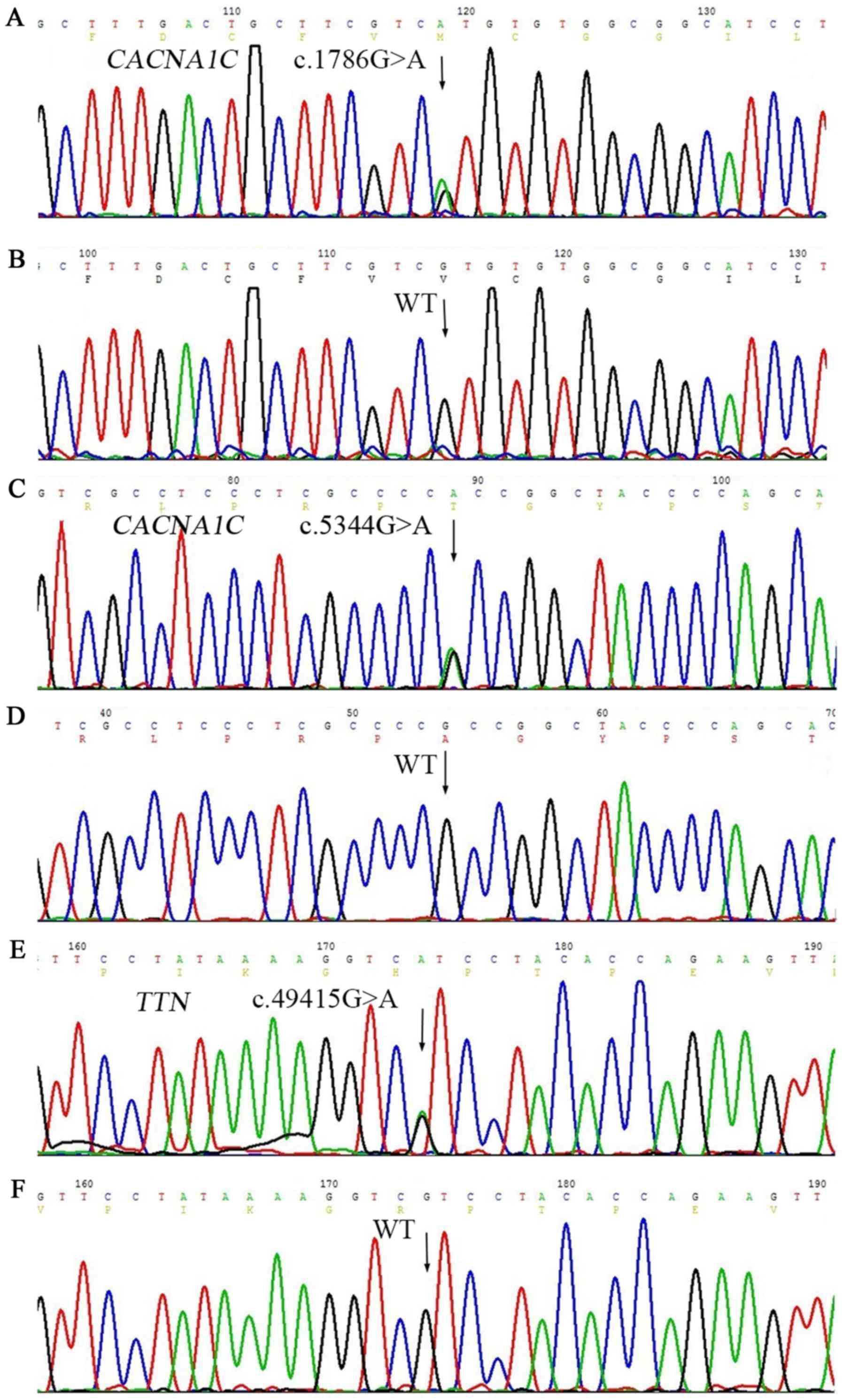
|
1
|
Sanders P, Lau DH and Kalman JK: Sinus
node abnormalitiesCardiac Electrophysiology: From Cell to Bedside.
Zipes D and Jalife J: 6th. Elsevier Saunders; Philadelphia: pp.
691–696. 2014, View Article : Google Scholar
|
|
2
|
Semelka M, Gera J and Usman S: Sick sinus
syndrome: A review. Am Fam Physician. 87:691–696. 2013.PubMed/NCBI
|
|
3
|
Mond HG and Proclemer A: The 11th world
survey of cardiac pacing and implantable
cardioverter-defibrillators: Calendar year 2009-a World Society of
Arrhythmia's project. Pacing Clin Electrophysiol. 34:1013–1027.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayashi M, Shimizu W and Albert CM: The
spectrum of epidemiology underlying sudden cardiac death. Circ Res.
116:1887–1906. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Monfredi O and Boyett MR: Sick sinus
syndrome and atrial fibrillation in older persons-A view from the
sinoatrial nodal myocyte. J Mol Cell Cardiol. 83:88–100. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Platzer J, Engel J, Schrott-Fischer A,
Stephan K, Bova S, Chen H, Zheng H and Striessnig J: Congenital
deafness and sinoatrial node dysfunction in mice lacking class D
L-type Ca2+ channels. Cell. 102:89–97. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baig SM, Koschak A, Lieb A, Gebhart M,
Dafinger C, Nürnberg G, Ali A, Ahmad I, Sinnegger-Brauns MJ, Brandt
N, et al: Loss of Ca(v)1.3 (CACNA1D) function in a human
channelopathy with bradycardia and congenital deafness. Nat
Neurosci. 14:77–84. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosso R, Kogan E, Belhassen B, Rozovski U,
Scheinman MM, Zeltser D, Halkin A, Steinvil A, Heller K, Glikson M,
et al: J-point elevation in survivors of primary ventricular
fibrillation and matched control subjects: Incidence and clinical
significance. J Am Coll Cardiol. 52:1231–1238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Derval N, Simpson CS, Birnie DH, Healey
JS, Chauhan V, Champagne J, Gardner M, Sanatani S, Yee R, Skanes
AC, et al: Prevalence and characteristics of early repolarization
in the CASPER registry: Cardiac arrest survivors with preserved
ejection fraction registry. J Am Coll Cardiol. 58:722–728. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mahida S, Derval N, Sacher F, Berte B,
Yamashita S, Hooks DA, Denis A, Lim H, Amraoui S, Aljefairi N, et
al: History and clinical significance of early repolarization
syndrome. Heart Rhythm. 12:242–249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burashnikov E, Pfeiffer R,
Barajas-Martinez H, Delpón E, Hu D, Desai M, Borggrefe M,
Häissaguerre M, Kanter R, Pollevick GD, et al: Mutations in the
cardiac L-type calcium channel associated with inherited J-wave
syndromes and sudden cardiac death. Heart Rhythm. 7:1872–1882.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haïssaguerre M, Chatel S, Sacher F,
Weerasooriya R, Probst V, Loussouarn G, Horlitz M, Liersch R,
Schulze-Bahr E, Wilde A, et al: Ventricular fibrillation with
prominent early repolarization associated with a rare variant of
KCNJ8/KATP channel. J Cardiovasc Electrophysiol. 20:93–98. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo Q, Ren L, Chen X, Hou C, Chu J, Pu J
and Zhang S: A novel mutation in the SCN5A gene contributes to
arrhythmogenic characteristics of early repolarization syndrome.
Int J Mol Med. 37:727–733. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haïssaguerre M, Derval N, Sacher F, Jesel
L, Deisenhofer I, de Roy L, Pasquié JL, Nogami A, Babuty D,
Yli-Mayry S, et al: Sudden cardiac arrest associated with early
repolarization. N Engl J Med. 358:2016–2023. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gussak I and Antzelevith C: Early
repolarization syndrome: Clinical characteristics and possible
cellular and ionic mechanisms. J Electrocardiol. 33:299–309. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He J, Wu J, Jiao Y, Wagner-Johnston N,
Ambinder RF, Diaz LA Jr, Kinzler KW, Vogelstein B and Papadopoulos
N: IgH gene rearrangements as plasma biomarkers in Non-Hodgkin's
lymphoma patients. Oncotarget. 2:178–185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu J, Matthaei H, Maitra A, Dal Molin M,
Wood LD, Eshleman JR, Goggins M, Canto MI, Schulick RD, Edil BH, et
al: Recurrent GNAS mutations define an unexpected pathway for
pancreatic cyst development. Sci Transl Med. 3:92ra662011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu YB, Gan JH, Luo JW, Zheng XY, Wei SC
and Hu D: Splicing mutation of a gene within the Duchenne muscular
dystrophy family. Genet Mol Res. 15:Jul 14–2016.doi:
10.4238/gmr.15028258. View Article : Google Scholar
|
|
19
|
Li R, Li Y, Kristiansen K and Wang J:
SOAP: Short oligonucleotide alignment program. Bioinformatics.
24:713–714. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mizusawa Y: Recent advances in genetic
testing and counseling for inherited arrhythmias. J Arrhythm.
32:389–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Verheijck EE, van Ginneken AC, Wilders R
and Bouman LN: Contribution of L-type Ca2+ current to
electrical activity in sinoatrial nodal myocytes of rabbits. Am J
Physiol. 276:H1064–H1077. 1999.PubMed/NCBI
|
|
22
|
Crotti L, Celano G, Dagradi F and Schwartz
PJ: Congenital long QT syndrome. Orphanet J Rare Dis. 3:182008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Splawski I, Timothy KW, Sharpe LM, Decher
N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM,
Condouris K, et al: Ca(V)1.2 calcium channel dysfunction causes a
multisystem disorder including arrhythmia and autism. Cell.
119:19–31. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Antzelevitch C, Pollevick GD, Cordeiro JM,
Casis O, Sanguinetti MC, Aizawa Y, Guerchicoff A, Pfeiffer R, Oliva
A, Wollnik B, et al: Loss-of-function mutations in the cardiac
calcium channel underlie a new clinical entity characterized by
ST-segment elevation, short QT intervals, and sudden cardiac death.
Circulation. 115:442–449. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jones SA, Boyett MR and Lancaster MK:
Declining into failure: The age-dependent loss of the L-type
calcium channel within the sinoatrial node. Circulation.
115:1183–1190. 2007.PubMed/NCBI
|
|
26
|
Kapplinger JD, Tester DJ, Alders M, Benito
B, Berthet M, Brugada J, Brugada P, Fressart V, Guerchicoff A,
Harris-Kerr C, et al: An international compendium of mutations in
the SCN5A-encoded cardiac sodium channel in patients referred for
Brugada syndrome genetic testing. Heart Rhythm. 7:33–46. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Walsh R, Peters NS, Cook SA and Ware JS:
Paralogue annotation identifies novel pathogenic variants in
patients with Brugada syndrome and catecholaminergic polymorphic
ventricular tachycardia. J Med Genet. 51:35–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gerull B, Gramlich M, Atherton J, McNabb
M, Trombitás K, Sasse-Klaassen S, Seidman JG, Seidman C, Granzier
H, Labeit S, et al: Mutations of TTN, encoding the giant muscle
filament titin, cause familial dilated cardiomyopathy. Nat Genet.
30:201–204. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Satoh M, Takahashi M, Sakamoto T, Hiroe M,
Marumo F and Kimura A: Structural analysis of the titin gene in
hypertrophic cardiomyopathy: Identification of a novel disease
gene. Biochem Biophys Res Commun. 262:411–417. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carmignac V, Salih MA, Quijano-Roy S,
Marchand S, Al Rayess MM, Mukhtar MM, Urtizberea JA, Labeit S,
Guicheney P, Leturcq F, et al: C-terminal titin deletions cause a
novel early-onset myopathy with fatal cardiomyopathy. Ann Neurol.
61:340–351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nicolao P, Xiang F, Gunnarsson LG,
Giometto B, Edström L, Anvret M and Zhang Z: Autosomal dominant
myopathy with proximal weakness and early respiratory muscle
involvement maps to chromosome 2q. Am J Hum Genet. 64:788–792.
1999. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Herman DS, Lam L, Taylor MR, Wang L,
Teekakirikul P, Christodoulou D, Conner L, DePalma SR, McDonough B,
Sparks E, et al: Truncations of titin causing dilated
cardiomyopathy. N Engl J Med. 366:619–628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brun F, Barnes CV, Sinagra G, Slavov D,
Barbati G, Zhu X, Graw SL, Spezzacatene A, Pinamonti B, Merlo M, et
al: Titin and desmosomal genes in the natural history of
arrhythmogenic right ventricular cardiomyopathy. J Med Genet.
51:669–676. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mohler PJ, Splawski I, Napolitano C,
Bottelli G, Sharpe L, Timothy K, Priori SG, Keating MT and Bennett
V: A cardiac arrhythmia syndrome caused by loss of ankyrin-B
function. Proc Natl Acad Sci USA. 101:pp. 9137–9142. 2004;
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nof E, Luria D, Brass D, Marek D, Lahat H,
Reznik-Wolf H, Pras E, Dascal N, Eldar M and Glikson M: Point
mutation in the HCN4 cardiac ion channel pore affecting synthesis,
trafficking, and functional expression is associated with familial
asymptomatic sinus bradycardia. Circulation. 116:463–470. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Benson DW, Wang DW, Dyment M, Knilans TK,
Fish FA, Strieper MJ, Rhodes TH and George AL Jr: Congenital sick
sinus syndrome caused by recessive mutations in the cardiac sodium
channel gene (SCN5A). J Clin Invest. 112:1019–1028. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Giudicessi JR and Ackerman MJ:
Determinants of incomplete penetrance and variable expressivity in
heritable cardiac arrhythmia syndromes. Transl Res. 161:1–14. 2013.
View Article : Google Scholar : PubMed/NCBI
|