Introduction
Diabetic kidney disease (DKD) is the leading cause
of kidney failure in patients with diabetes. Renal interstitial
fibrosis may occur during the prophase of DKD and lead to the
deterioration of renal function, independently of the glomerular
lesions (1–3). However, the underlying mechanism of
renal interstitial fibrosis in DKD remains to be fully elucidated.
DKD has long been considered a non-inflammatory disease. However,
certain studies previously reported that inflammation serves an
important role in the pathogenesis of DKD and various types of
inflammatory cells were involved in the occurrence of DKD (4,5). It
has also been reported that mast cells, derived from cluster of
differentiation (CD) 34-positive multipotent bone marrow progenitor
cells, may be one type of inflammatory cell that participate in the
process of renal interstitial fibrosis in DKD (6), however, the specific mechanism
remains unclear.
Tranilast [N-(3′,4′-dimethoxycinnamic)-anthranilic
acid] is a cell membrane stabilizer that has been widely used in
the treatment of inflammatory diseases due to its role in
inhibiting the release of histamine and other chemical mediators
(7). Previous studies have
demonstrated that tranilast attenuates renal interstitial fibrosis
in the rat model of obstructive nephropathy (8), suppresses the progression of
peritoneal fibrosis in rats with chronic renal failure and
decreases fibrosis in myocardial infarction (9,10).
It has also been reported that tranilast inhibits the release of
transforming growth factor (TGF)-β1, decreases the accumulation of
extracellular matrix and suppresses oxidative stress (11,12).
However, the role and mechanisms underlying the effects of
tranilast on renal interstitial fibrosis in DKD are unclear. In the
present study, the association between mast cell infiltration and
renal interstitial fibrosis of DKD was analyzed, and the role and
mechanisms by which tranilast inhibits renal interstitial fibrosis
were investigated.
Materials and methods
Rat model of DKD and experimental
grouping
A total of 30 healthy male Sprague-Dawley rats
(6-weeks old; 181.65±5.15 g) were purchased from the Animal
Department at Xiangya Medical School, Central South University
(Changsha, China). All rats had free access to food and water with
a 12-h dark/light cycle in a climate-controlled room (temperature,
18–25°C; humidity, 65–80%; CO2, 0.03%). Following
feeding under adaptation conditions for 3 days, rats were randomly
divided into normal control (n=6) and DKD model (n=24) groups. The
normal control animals were fed with normal food and received once
daily intraperitoneal injection of citrate buffer [0.1 mol/l, (pH
4.5)] at a dose of 10 ml/kg, while the rat model of DKD was
performed as described previously (13,14).
The rats were fed with a high-sugar and high-fat diet for 8 weeks
followed by once daily intraperitoneal injection of streptozotocin
(30 mg/kg; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) dissolved
in citrate buffer. Blood and urine glucose was detected 72 h
following streptozotocin injection. The diabetes model was
considered to be successful when fasting blood glucose was >13.8
mmol/l, random blood glucose was >16.7 mmol/l, and urine
glucose, detected by Benedict's test, was ++++. The rat model of
DKD was considered to be successfully established if urine protein,
determined by the Lowry procedure, was >30 mg for 24 h following
the establishment of the diabetes model. During the experiment,
blood glucose was measured every 3 days using a tail vein sample. A
certain amount of long-acting insulins (0.4–3.2 units) was injected
into the rats of which blood glucose was higher than 26.0 mmol/l,
but random blood glucose was maintained above 16.7 mmol/l.
DKD model rats were subsequently randomly divided
into the following three groups (n=8 for each group): DKD model
group; low-dose tranilast group (200 mg/kg/day) and high-dose
tranilast group (400 mg/kg/day) (11). The DKD model rats were only fed
1.5% sodium carboxymethyl cellulose solution, while the rats
treated with tranilast had tranilast administrated twice daily,
which was dissolved in 1.5% sodium carboxymethyl cellulose
solution. By the 8th week following tranilast treatment, the 24 h
urine samples of all rats were collected to determine the albumin
concentrations, and rats were intraperitoneally anesthetized with
ketamine/xylazine (80 mg/kg+10 mg/kg) and sacrificed. Blood samples
for measuring creatinine were obtained from the abdominal aorta
prior to the kidneys being harvested and weighed. All animals used
in the present study were appropriately processed following
protocols approved in advance by the Animal Care and Use Committee
at Central South University. All procedures performed in
experiments with animals were in accordance with the ethical
standards of the Department of Nephrology, Second Xiangya Hospital
at which the present study was conducted.
Biochemical analysis
In the present study, the levels of blood glucose
were estimated using an Accu-Chek glucometer (Roche Diagnostics,
Basel, Switzerland). To assess the urinary albumin excretion rate
(UAER), the albumin concentrations for the urine samples were
detected using the Urine micro-albumin assay kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). The serum for detecting
creatinine were collected by centrifugation of whole blood (0.5 ml
each of samples) at 3,000 × g for 15 min following blood
agglutination at room temperature, and serum creatinine (Scr) was
detected using a BioAssay Systems Creatinine assay kit (BioAssay
Systems, Hayward, CA, USA).
Masson's trichrome staining for
detecting renal tubulointerstitial fibrosis
The 4% paraformaldehyde-fixed (at room temperature
for 24 h), paraffin-embedded tissue sections (3-µm) were dewaxed
routinely in xylene for 10 min and then in graded alcohol (100, 95,
80 and 70%), 5 min each, and washed in dH2O. Hematoxylin
was used to stain nuclei for 3 min at room temperature. Following
flushing with water for 30–60 sec, sections were stained in Ponceau
acid fuchsin solution (ponceau 0.7 g, acid fuchsin 0.3 g, distilled
water 99 ml and glacial acetic acid l ml) for 5–10 min, rinsed with
tap water for 1 min, stained in 1% phosphomolybdic acid solution
for 1 min and transferred into 2% aniline blue liquid to dye for 3
min. Following washing with water, sections were dehydrated in
graded ethanol and made transparent in xylene for 1 min, and
subsequently enclosed with neutral gum. All the procedures were
done at room temperature. Collagen fibers were stained blue. Slides
were observed under a light microscope (Olympus CX41; Olympus
Corporation, Tokyo, Japan). Images were taken of 10 non-overlapping
interstitial areas in each section. A high-definition color medical
analysis system with image analysis software (HMIAS-2000; Qianping
Image Technology Co., Ltd., Wuhan, China) was used for automatic
measurement and analysis, and the percentage of tubulointerstitial
area in the total area visualized in each section was
calculated.
Toluidine blue staining
Modified toluidine blue staining was performed to
detect mast cells (15,16). Paraffin sections (3-µm) were
deparaffinized and rehydrated according to the above procedures,
and immersed in toluidine blue for 3 min and differentiated with
0.5% glacial acetic acid for 10–20 sec until mast cell nuclei were
visible at room temperature. Tissue sections were cleared with
xylene and sealed with neutral gum, and subsequently photographed
under a light microscope. A total of three images at high
magnification (×200) were randomly selected for calculating the
number of mast cells.
Immunohistochemical staining
(IHC)
Paraffin sections (3-µm) were dewaxed and hydrated
with the above method, and immersed in 0.01 mol/l citrate buffer
(pH 6.0) at 90°C for 5 min for microwave antigen retrieval.
Sections were then incubated with 3% H2O2 for
10 min at room temperature to eliminate endogenous peroxidase
activity prior to blocking in 10% goat serum (Beyotime Institute of
Biotechnology, Haimen, China) for 30 min at 37°C. Sections were
subsequently incubated with anti-complement C3a receptor 1 (C3aR;
1:200; cat. no. sc-20138) and anti-proto-oncogene c-kit (c-kit;
1:400; cat. no. sc-5535) polyclonal antibodies (Santa Cruz
Biotechnology Inc., Dallas, TX, USA), anti-fibronectin (FN; 1:400;
cat. no. BA1772) and anti-stem cell factor (SCF; 1:400; cat. no.
A01254) polyclonal antibodies (Boster Biological Technology Co.,
Ltd., Wuhan, China), and anti-collagen I polyclonal antibody
(Col-I; 1:400; cat. no. T40227R; Meridian Life Science, Inc.,
Memphis, TN, USA) overnight at 4°C. Following rinsing, the sections
were incubated with horseradish peroxidase-labeled goat anti-rabbit
immunoglobulin (Ig)G secondary antibodies for 1 h at 37°C (1:500;
cat. no. ab6721; Abcam, Cambridge, UK), stained with
3,3′-diaminobenzidine for 30–60 sec and counterstained with
hematoxylin for 3 min at room temperature. Negative controls for
specific labeling were performed in parallel by replacing the
primary antibody with a normal rabbit serum (cat. no. A7016;
Beyotime Institute of Biotechnology). The slides were examined and
photographed with a light microscope (magnification, ×200). The
Image-Pro P1us version 6.0 image analysis system (Media
Cybernetics, Inc., Rockville, MD, USA) was used to measure the
optical density of the positive area. The average optical density
was used to represent the expression level of C3aR, FN, Col-I, SCF
and c-kit.
Western blotting analysis
A total of ~50–100 mg of kidney tissues were ground
in liquid nitrogen and homogenized in 500 µl pre-cooled
radioimmunoprecipitation lysis buffer (cat. no. P0013B; Beyotime
Institute of Biotechnology) at 4°C. The lysate was cleared by
centrifugation at 12,000 × g for 20 min at 4°C. Protein
concentration was determined using a bicinchoninic assay kit
(Beyotime Institute of Biotechnology). Subsequently, 20–30 mg
soluble lysates were loaded in each lane and separated by 8 or 10%
SDS-PAGE gel electrophoresis and electrophoretically transferred to
polyvinylidene difluoride membranes. Membranes were blocked with 5%
nonfat dry milk in TBS/0.5% Tween-20 for 1 h at room temperature,
washed with TBS/Tween-20 and incubated with FN (cat. no. ab2413),
Col-I (cat. no. ab34710), SCF (cat. no. ab101072) and c-kit (cat.
no. ab46758) polyclonal antibodies (Abcam, Cambridge, UK) at
1:1,000 dilution overnight at 4°C. Blots were rinsed with
TBS/Tween-20 and subsequently incubated with
horseradish-peroxidase-conjugated secondary antibodies (at room
temperature for 1 h), including goat anti-rabbit IgG (1:4,000; cat.
no. ab6721) and rabbit anti-goat IgG (1:4,000; cat. no. ab97100)
(Abcam, Cambridge, UK). Following washing with TBS/Tween-20, the
blots were developed with enhanced chemiluminescence reagents (GE
Healthcare, Chicago, IL, USA). The density of the identified bands
was quantified by densitometry using ImageQuant software (version
5.2; Molecular Dynamics, Sunnyvale, CA, USA). Values were
normalized with respect to β-actin (1:4,000; cat. no. ab8227;
Abcam, Cambridge, UK) expression.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Tissue expression of FN, Col-I, SCF and c-kit mRNA
was assessed by RT-qPCR. A TRIzol® RNA extraction kit
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was
used to extract total RNA from the renal tissue. Total RNA was used
for cDNA synthesis according to the instructions in the RevertAid
First Strand cDNA Synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc.). Gene sequences were verified in the NCBI GenBank
(https://www.ncbi.nlm.nih.gov/genbank/) and primers
were designed according to primer design principles using Primer
Premier version 5.0 (Premier Biosoft International, Palo Alto, CA,
USA) and purchased from Sangon Biotech Co., Ltd. (Shanghai, China).
The primer sequences are presented in Table I. qPCR amplification of the
products was performed on an ABI 7300 Real Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using SYBR
GreenER® qPCR SuperMix Universal kit (Invitrogen; Thermo
Fisher Scientific, Inc.) under the following reaction conditions:
50°C for 2 min and 95°C for 10 min; 40 cycles of 95°C for 10 sec,
60°C for 60 sec and 72°C for 60 sec; final step for 10 min at 72°C.
The qPCR was repeated three times for each gene. Relative
quantification of gene expression was performed using the
2−ΔΔCq method with β-actin as an endogenous control
(17).
 | Table I.Primer sequences for quantitative
polymerase chain reaction. |
Table I.
Primer sequences for quantitative
polymerase chain reaction.
| Gene | Primer |
|---|
| FN | F:
5′-TGACAACTGCCGTAGACCTGG-3′ |
|
| R:
5′-TACTGGTTGTAGGTGTGGCCG-3′ |
| Col-I | F:
5′-TGGCAAGAACGGAGATGA-3′ |
|
| R:
5′-AGCTGTTCCAGGCAATCC-3′ |
| SCF | F:
5′-AGGCTCATTCGTCTGCTCTG-3′ |
|
| R:
5′-CTACCCATGTCCACCTTTCT-3′ |
| c-kit | F:
5′-GGCCTAGCCAGAGACATCAG-3′ |
|
| R:
5′-GAGAGGCTGTGTGGAAGAGG-3′ |
| β-actin | F:
5′-CCCATCTATGAGGGTTACGC-3′ |
|
| R:
5′-TTTAATGTCACGCACGATTTC-3′ |
Statistical analysis
SPSS software (version 17.0; SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. The results are
presented as mean ± standard deviation. Data were analyzed using
one-way analysis of variance or the Kruskal-Wallis test. Pearson
correlation analysis was performed to determine the correlation
between the expression of SCF and c-kit with the infiltration of
mast cells and indices of renal interstitial fibrosis. P<0.05
was considered to indicate a statistically significant
difference.
Results
The effect of tranilast on the general
condition and biochemical indices of DKD rats
The DKD rats demonstrated polydipsia, polyphagia,
polyuria and other symptoms. Compared with the normal control
group, the body weight of DKD model rats was significantly reduced,
while the kidneys became bigger and heavier, and the kidney
weight/body mass index was significantly increased (P<0.05;
Table II). Blood glucose, UAER
and Scr concentrations were significantly increased in the DKD
model group compared with the normal control group (P<0.05;
Table II). There was no
significant difference in the body weight, the kidney weight/body
mass index, UAER, Scr and blood glucose level among different
tranilast groups and the DKD model group (P>0.05).
 | Table II.The general condition and biochemical
indexes of rats in different groups following 8-week tranilast
treatment. |
Table II.
The general condition and biochemical
indexes of rats in different groups following 8-week tranilast
treatment.
| Groups | Body mass, g | Kidney weight/body
weight, g/kg | Blood glucose,
mmol/l | Scr, µmol/l | UAER, mg/24 h |
|---|
| Control |
715.00±17.48 |
6.24±0.48 |
5.60±0.30 |
60.30±7.20 |
0.38±0.01 |
| DKD |
460.82±40.90a |
10.00±0.92a |
31.10±3.60a |
89.00±20.40a |
1.31±0.25a |
| Low-dose tranilast,
200 mg/kg/day |
460.32±56.50a |
9.43±1.47a |
23.80±6.90a |
76.40±12.90a |
1.27±0.85a |
| High-dose
tranilast, 400 mg/kg/day |
462.13±34.93a |
10.73±1.29a |
30.20±2.50a |
76.90±12.90a |
1.25±0.67a |
Tranilast reduces mast cell
infiltration in the kidneys of DKD rats
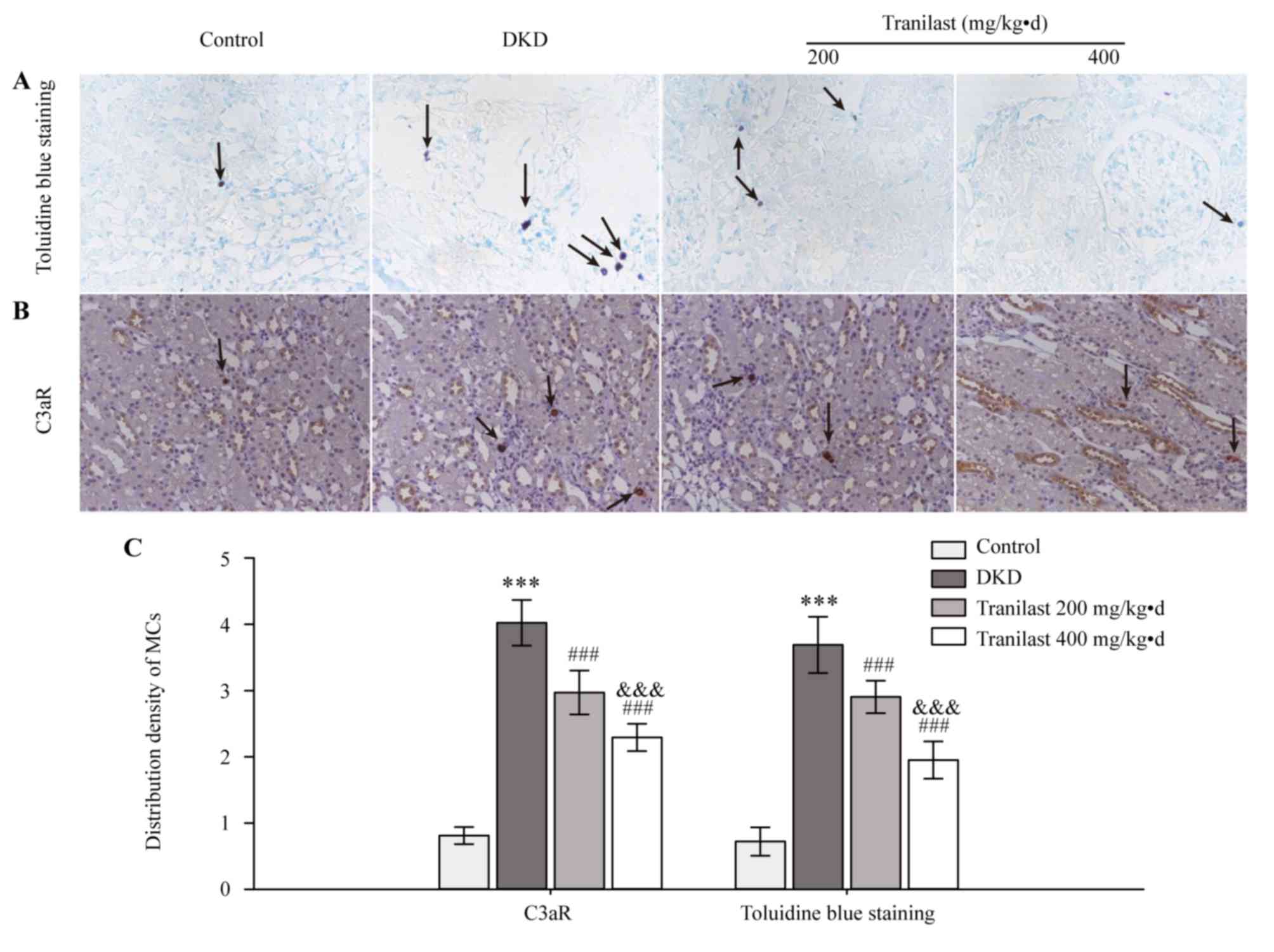
Toluidine blue staining and IHC of C3aR was
performed to detect mast cells in the renal interstitium. Toluidine
blue staining is a classic method for quickly identifying mast
cells, which stains mast cells purple. IHC of C3aR is another
method used for detecting mast cells as mast cells express C3aR. In
normal rats, mast cells, which are stained purple by toluidine blue
or stained brown by IHC, were primarily localized in perivascular
areas and the tubulointerstitium, and only a few mast cells were
detected in the renal interstitium, but not in glomeruli (Fig. 1). However, increased numbers of
mast cells were demonstrated in the renal interstitium of DKD model
rats (P<0.001; Fig. 1).
Tranilast decreased the infiltration of mast cells dose-dependently
(P<0.001; Fig. 1).
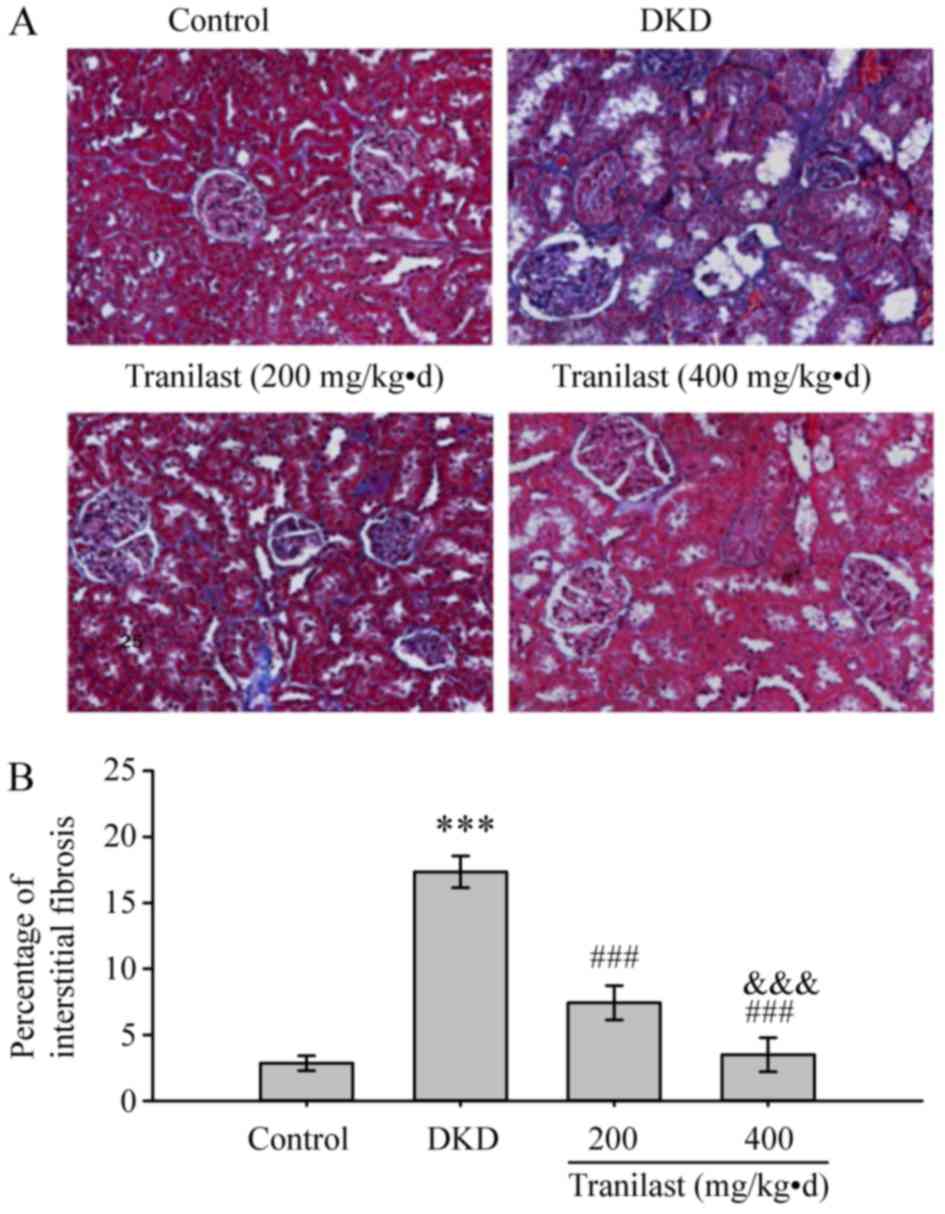
Tranilast ameliorates renal
tubulointerstitial fibrosis in DKD rats
The tubulointerstitial fibrosis of the kidney was
observed using Masson's trichrome staining, which demonstrated that
there was no tubular expansion and fibrous proliferation in the
normal control group (Fig. 2). In
a DKD model, kidneys developed tubular vacuolar degeneration and
atrophy with tubulointerstitial fibrosis (Fig. 2). The fibrotic areas detected by
blue staining were significantly ameliorated in the kidneys treated
with tranilast in a dose-dependent manner as compared with the DKD
group (P<0.001; Fig. 2).
Tranilast reduces the expression of FN
and Col-I in the kidneys of DKD rats
IHC staining demonstrated that FN and Col-I were
primarily deposited in the glomeruli, renal tubular basement
membrane and perivascular areas in the normal control rats
(Fig. 3A and B). However, in the
DKD rats, the expression of FN and Col-I was increased
significantly in the renal interstitium, particularly in
perivascular areas and inflammatory areas (P<0.001; Fig. 3A-C). Tranilast dose-dependently
downregulated the expression of FN and Col-I (P<0.001; Fig. 3A-C). Western blot analysis and
RT-qPCR demonstrated that the mRNA and protein levels of FN and
Col-I were significantly upregulated in the kidneys of DKD rats,
compared with those of normal control rats (P<0.001; Fig. 3D and E, respectively). Tranilast
significantly downregulated the protein and mRNA expression of FN
and Col-I in a dose-dependent manner compared with the DKD rats
(P<0.05; Fig. 3D and E,
respectively).
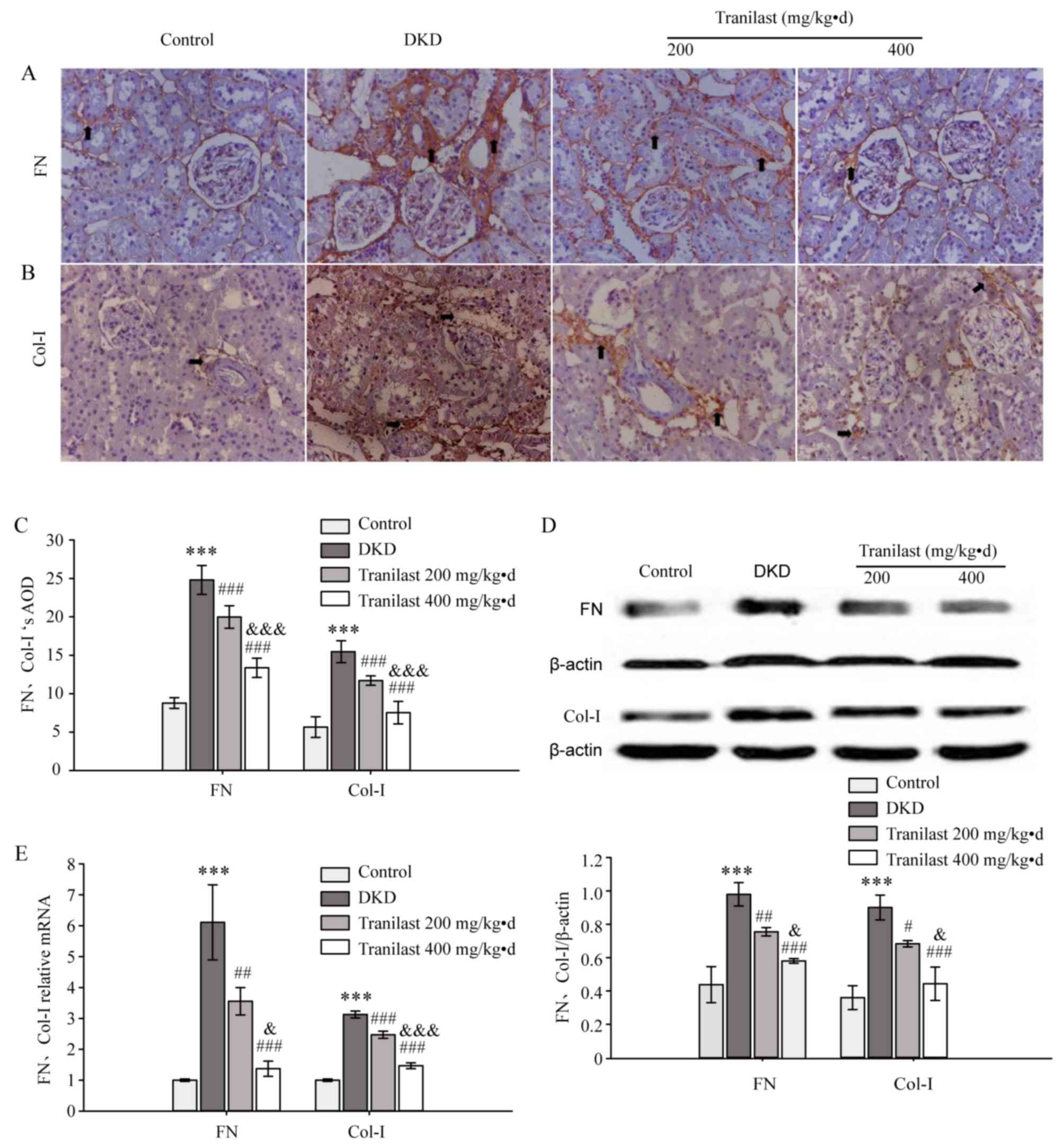 | Figure 3.Tranilast reduced the expression of
FN and Col-I in DKD rat kidneys. (A) Immunohistochemical staining
demonstrated the expression of FN protein in each group.
Magnification, ×200. FN proteins are indicated by black arrows. (B)
Immunohistochemical staining demonstrated the expression of Col-I
protein in each group. Magnification, ×200. Col-I proteins are
indicated by black arrows. (C) AOD value of FN and Col-I protein in
the different groups for immunohistochemical results. (D) Western
blot and densitometric analysis demonstrated the expression of FN
and Col-I proteins in the different groups. (E) Expression of FN
and Col-I mRNA in the different groups according to Cq values
generated by reverse transcription-quantitative polymerase chain
reaction. ***P<0.001 vs. control group; #P<0.05,
##P<0.01, ###P<0.001 vs. DKD model
group; &P<0.05,
&&&P<0.001 vs. low-dose tranilast group
(200 mg/kg.d). FN, fibronectin; Col-I, collagen I; DKD, diabetic
kidney disease; mg/kg.d, mg/kg/day; AOD, adjusted optical density;
Cq, cycle threshold value. |
Tranilast reduces the expression of
SCF and c-kit in the kidneys of DKD rats
A small amount of SCF and c-kit protein was revealed
by IHC staining in the renal tubules and interstitium of normal rat
kidneys. In the DKD model rats, a significantly increased level of
SCF and c-kit protein were observed in renal tubular epithelial
cells and interstitial inflammatory cells, compared with control
rats (P<0.001; Fig. 4A-C).
Tranilast (400 mg/kg.d) downregulated the expression of SCF and
c-kit (P<0.01; Fig. 4A-C).
Compared with the normal control group, the protein and mRNA levels
of SCF and c-kit in the DKD model group were significantly
increased (P<0.001; Fig. 4D and
E, respectively), and tranilast significantly decreased the
protein and mRNA levels of SCF and c-kit in a dose-dependent manner
(P<0.05; Fig. 4D and E,
respectively), as determined by western blotting and RT-qPCR,
respectively.
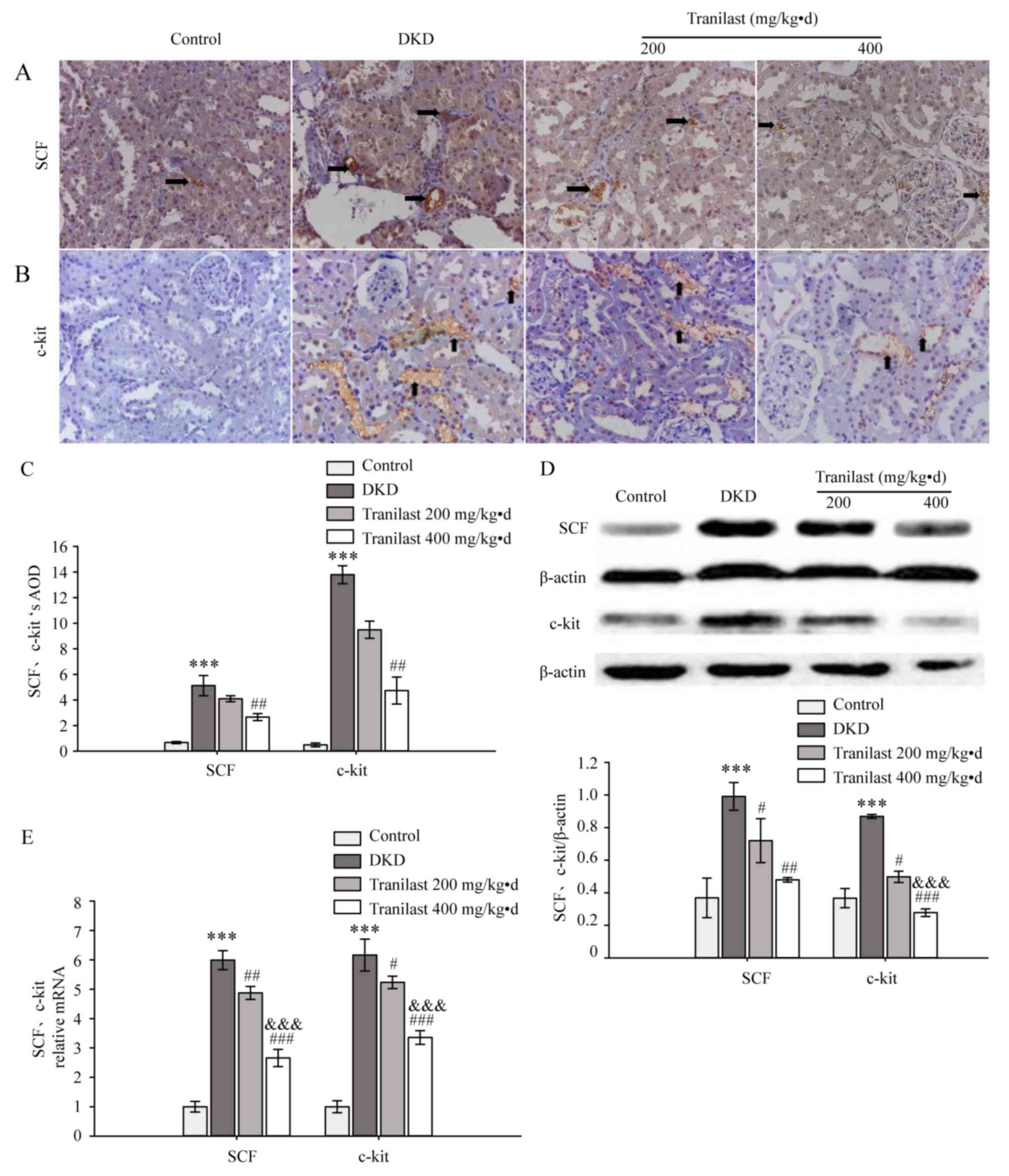 | Figure 4.Tranilast reduced the expression of
SCF and c-kit in DKD rat kidneys. (A) Immunohistochemical staining
demonstrated the expression of SCF protein in each group.
Magnification, ×200. SCF proteins are indicated by black arrows.
(B) Immunohistochemical staining demonstrated the expression of
c-kit protein in the different groups. Magnification, ×200. c-kit
proteins are indicated by black arrows. (C) AOD value of SCF and
c-kit protein in the different groups for immunohistochemical
results. (D) Western blot and densitometric analysis demonstrated
the expression of SCF and c-kit protein in the different groups.
(E) Expression of SCF and c-kit mRNA in the different groups
according to Cq values generated by reverse
transcription-quantitative polymerase chain reaction. ***P<0.001
vs. control group; #P<0.05, ##P<0.01,
###P<0.001 vs. DKD model group;
&&&P<0.001 vs. low-dose tranilast group
(200 mg/kg.d). SCF, stem cell factor; c-kit, proto-oncogene c-kit;
DKD, diabetic kidney disease; mg/kg.d, mg/kg/day; AOD, adjusted
optical density; Cq, cycle threshold value. |
Correlation of SCF and c-kit with the
infiltration of mast cells and the markers of renal
tubulointerstitial fibrosis
Pearson correlation analysis demonstrated a
significant positive correlation between the level of SCF and the
infiltration of C3aR-positive cells or the expression of FN and
Col-I (r=0.941, P<0.01; r=0.896, P<0.01; and r=0.858,
P<0.01, respectively; Table
III). In addition, a strong positive correlation was
demonstrated between the level of c-kit and the infiltration of
C3aR-positive cells or the expression of FN and Col-I (r=0.951,
P<0.01; r=0.976, P<0.01; and r=0.932, P<0.01,
respectively; Table III). These
results indicate that the expression of SCF and c-kit were
positively correlated with the extent of mast cell infiltration and
the expression of FN and Col-I.
 | Table III.Correlation of SCF and c-kit with
mast cell infiltration and markers of renal interstitial
fibrosis. |
Table III.
Correlation of SCF and c-kit with
mast cell infiltration and markers of renal interstitial
fibrosis.
|
| SCF | c-kit |
|---|
|
|
|
|
|---|
|
| r | P-value | r | P-value |
|---|
| C3aR-positive mast
cells | 0.941 | <0.01 | 0.951 | <0.01 |
| FN | 0.896 | <0.01 | 0.976 | <0.01 |
| Col-I | 0.858 | <0.01 | 0.932 | <0.01 |
Discussion
The incidence of chronic kidney disease (CKD) has
increased substantially over the last several decades and was
ranked 27th in 1990 and 18th in 2010 in the list of causes of
global mortality (18). A previous
study indicated that the overall prevalence of CKD was 10.8% in
Chinese adults (19). DKD is
currently a primary cause for CKD to end-stage renal disease (ESRD)
in developed countries and accounts for ~16.4% of all cases of ESRD
in China (1). In DKD, glomerular
sclerosis is observed in the progression from emerging to obvious
nephropathy, whereas currently, there is increasing evidence for
the importance of the interstitial fibrosis of DKD (20). Previous reports have indicated that
tubulointerstitial injury may be important in the progression from
moderate renal insufficiency to ESRD (2,3). In
addition, the underlying mechanism of renal interstitial fibrosis
in DKD has not been fully elucidated, and no favorable drugs that
have the ability to completely reverse renal interstitial fibrosis
of DKD have currently been developed. Therefore, it is of great
importance to elucidate the mechanism of interstitial fibrosis in
DKD and to investigate effective therapeutic measures for slowing
the progression of renal failure. In the present study, it was
demonstrated that tubulointerstitial fibrosis and mast cell
infiltration were upregulated in DKD model rats compared with
normal control rats, and tranilast ameliorated the mast cell
infiltration and the expression of FN, Col-I, SCF and c-kit.
Furthermore, a positive correlation between the expression of
SCF/c-kit and C3aR-positive mast cells or the markers of renal
interstitial fibrosis was observed. These results not only
demonstrated that mast cells served a vital role in renal
interstitial fibrosis of DKD, but also illustrated that mast cells
may promote renal interstitial fibrosis via the SCF/c-kit signaling
pathway and that tranilast may attenuate renal interstitial
fibrosis induced by mast cell infiltration through inhibiting the
SCF/c-kit signaling pathway. Therefore, tranilast may be an
effective therapeutic drug that acts by inhibiting mast cell
infiltration in DKD.
It has previously been demonstrated that
inflammatory cells serve an important role in the development of
DKD, particularly in renal interstitial fibrosis. Macrophages, T
lymphocytes and neutrophil granulocytes are involved in chronic
inflammation and interstitial fibrosis in DKD (21–23).
However, mast cells have also been reported to mediate chronic
inflammation and fibrosis in the kidneys. In humans, only a limited
number of mast cells reside constitutively in normal kidneys,
however, in progressive renal diseases, their numbers increase
substantially and mast cells represent a vital part of the
inflammatory cell infiltration into the renal interstitium
(24). Certain studies have
demonstrated that mast cells infiltrate the renal interstitium in
various nephropathies, including diabetic nephropathy, lupus
nephritis and IgA nephropathy (6,25,26).
Our previous study demonstrated that mast cells increased in the
renal interstitium of rats with protein-overload nephropathy,
indicating that mast cells may serve an important role in renal
interstitial fibrosis induced by proteinuria (27). Consistent with the above studies,
in the present study, it was demonstrated that mast cell
infiltration into the renal interstitium of the DKD model group was
increased compared with the control group, which indicates that
mast cells may serve an important role in the progression of
DKD.
Mast cells release large amounts of cytokines,
chemokines and growth factors, including chymotrypsin, renin,
histamine, preformed tumor necrosis factor-α, interleukin-17 and
TGF-β. Inflammatory mediators lead to the activation of local
inflammation and increased expression of TGF-β, which is involved
in the occurrence of renal interstitial fibrosis (25). Activated mast cells also secrete
chymase, an enzyme that is able to cleave the latent form of TGF-β
from cell membranes to form active TGF-β, resulting in interstitial
fibrosis (25,28). SCF, mast cell growth factor or
steel factor, is a key chemoattractant for mast cell precursor
migration and a key survival and differentiation factor for mast
cells. In addition, SCF was also reported to synergistically
enhance antigen-induced degranulation and cytokine production of
mast cells (29–31). It has been reported that epithelial
cells and fibroblasts produce the soluble form of SCF, which binds
to a receptor of SCF on the membrane of mast cells that is termed
c-kit (32). c-kit, also known as
CD117, is a transmembrane glycoprotein receptor that possesses
tyrosine kinase activity. Additional reports have indicated that
the SCF/c-kit signaling pathway may be a major signaling pathway
involved in mast cell activation (30). It was also demonstrated that the
expression of SCF and c-kit was significantly increased in kidneys
with serum nephrotoxic nephritis (33). The present study demonstrated that
the expression of SCF and c-kit increased in the tissue of the DKD
model group, and the expression of SCF and c-kit was positively
correlated with C3aR-positive mast cell infiltration and the
markers of renal interstitial fibrosis. The above results indicate
that mast cells may promote renal interstitial fibrosis of DKD via
the SCF/c-kit signaling pathway.
Therefore, inhibiting mast cell survival,
differentiation and release of inflammatory mediators via the
SCF/c-kit signaling pathway may be a potential therapeutic target
for renal interstitial fibrosis. Tranilast, an anti-allergic agent,
has been reported to stabilize the mast cell membrane and inhibit
the release of inflammatory granules. It is generally used in the
treatment of inflammatory diseases, including bronchial asthma,
atypical dermatitis, allergic conjunctivitis, keloids and
hypertrophic scars. Furthermore, beneficial effects of tranilast
have also been reported in various diseases, which include fibrotic
and proliferative disorders, tumor development, cardiovascular and
autoimmune disorders, ocular diseases, diabetes and kidney diseases
(7). Kaneyama et al
(8) demonstrated that tranilast
exerted a protective effect on renal interstitial fibrosis in rats
with unilateral ureteral obstruction. Tranilast was also
demonstrated to attenuate renal interstitial fibrosis by reducing
the expression of TGF-β (11),
which may be associated with its role as an antioxidant, inhibiting
the production of inflammatory factors and reducing the synthesis
of collagen.
In the present study, the infiltration of mast
cells, and the expression of FN, Col-I, SCF and c-kit, was reduced
in tranilast treatment groups compared with the DKD model group,
which demonstrates tranilast may attenuate the infiltration of mast
cells to reduce renal interstitial fibrosis by inhibiting the
expression of SCF and c-kit. Therefore, a novel target for the
treatment of renal interstitial fibrosis of DKD using tranilast has
been identified. However, the detailed molecular mechanism of mast
cell-induced renal interstitial fibrosis via the SCF/c-kit
signaling pathway remains to be investigated. In addition, the
mechanism by which tranilast prevents renal interstitial fibrosis
through inhibition of mast cell infiltration mediated by the
SCF/c-kit signaling pathway also requires further
investigation.
Acknowledgements
The authors would like to thank Professor Jun Li,
Professor Lin Sun, Professor Hong Liu and Professor Fu-you Liu, who
have been working in the Department of Nephrology, the Second
Xiangya Hospital, Central South University, for their technical
support for this research and for editing this manuscript.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81100486 and
81370792) and the Hunan science and technology project (grant no.
2017SK2072).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
YL conceived, designed and supervised the study. DDY
made the partial experiment, analyzed and interpreted the data, and
was a major contributor in writing the manuscript. JHL made the
experiment and analyzed the data, and wrote the manuscript. ZZY and
YJL participated in the analysis of the data and the writing of the
manuscript. All the authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animals used in the present study were
appropriately processed following protocols approved in advance by
the Animal Care and Use Committee at Central South University. All
procedures performed in experiments with animals were in accordance
with the ethical standards of the Department of Nephrology, Second
Xiangya Hospital at which the present study was conducted.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Col-I
|
collagen I
|
|
C3aR
|
complement C3a receptor 1
|
|
DKD
|
diabetic kidney disease
|
|
ESRD
|
end-stage renal disease
|
|
FN
|
fibronectin
|
|
IHC
|
immunohistochemical staining
|
|
SCF
|
stem cell factor
|
|
Scr
|
serum creatinine
|
|
TGF
|
transforming growth factor
|
|
UAER
|
urinary albumin excretion rate
|
References
|
1
|
Liu ZH: Nephrology in China. Nat Rev
Nephrol. 9:523–528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tervaert TW, Mooyaart AL, Amann K, Cohen
AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer
E, et al: Pathologic classification of diabetic nephropathy. J Am
Soc Nephrol. 21:556–563. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Najafian B, Alpers CE and Fogo AB:
Pathology of human diabetic nephropathy. Contrib Nephrol.
170:36–47. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elmarakby AA and Sullivan JC: Relationship
between oxidative stress and inflammatory cytokines in diabetic
nephropathy. Cardiovasc Ther. 30:49–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moresco RN, Sangoi MB, De Carvalho JA,
Tatsch E and Bochi GV: Diabetic nephropathy: Traditional to
proteomic markers. Clin Chim Acta. 421:17–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng JM, Yao GH, Cheng Z, Wang R and Liu
ZH: Pathogenic role of mast cells in the development of diabetic
nephropathy: A study of patients at different stages of the
disease. Diabetologia. 55:801–811. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Darakhshan S and Pour AB: Tranilast: A
review of its therapeutic applications. Pharmacol Res. 91:15–28.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaneyama T, Kobayashi S, Aoyagi D and
Ehara T: Tranilast modulates fibrosis, epithelial-mesenchymal
transition and peritubular capillary injury in unilateral ureteral
obstruction rats. Pathology. 42:564–573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kazama I, Baba A, Endo Y, Toyama H, Ejima
Y, Matsubara M and Tachi M: Mast cell involvement in the
progression of peritoneal fibrosis in rats with chronic renal
failure. Nephrology (Carlton). 20:609–616. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
See F, Watanabe M, Kompa AR, Wang BH,
Boyle AJ, Kelly DJ, Gilbert RE and Krum H: Early and delayed
tranilast treatment reduces pathological fibrosis following
myocardial infarction. Heart Lung Circ. 22:122–132. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tao Y, Hu L, Li S, Liu Q, Wu X, Li D, Fu
P, Wei D and Luo Z: Tranilast prevents the progression of chronic
cyclosporine nephrotoxicity through regulation of transforming
growth factor β/Smad pathways. Transplant Proc. 43:pp. 1985–1988.
2011; View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan SM, Zhang Y, Cox AJ, Kelly DJ and Qi
W: Tranilast attenuates the up-regulation of
thioredoxin-interacting protein and oxidative stress in an
experimental model of diabetic nephropathy. Nephrol Dial
Transplant. 26:100–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Chen Q, Liu FY, Peng YM, Hou T, Duan
SB, Li J, Luo JH, Sun L and Ling GH: Norcantharidin attenuates
tubulointerstitial fibrosis in rat models with diabetic
nephropathy. Ren Fail. 33:233–241. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Srinivasan K, Viswanad B, Asrat L, Kaul CL
and Ramarao P: Combination of high-fat diet-fed and low-dose
streptozotocin-treated rat: A model for type 2 diabetes and
pharmacological screening. Pharmacol Res. 52:313–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Katz A, Caramori ML, Sisson-Ross S,
Groppoli T, Basgen JM and Mauer M: An increase in the cell
component of the cortical interstitium antedates interstitial
fibrosis in type 1 diabetic patients. Kidney Int. 61:2058–2066.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Ding J, Jiao H, Honardoust D,
Momtazi M, Shankowsky HA and Tredget EE: Human hypertrophic
scar-like nude mouse model: Characterization of the molecular and
cellular biology of the scar process. Wound Repair Regen.
19:274–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jha V, Garcia-Garcia G, Iseki K, Li Z,
Naicker S, Plattner B, Saran R, Wang AY and Yang CW: Chronic kidney
disease: Global dimension and perspectives. Lancet. 382:260–272.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Wang F, Wang L, Wang W, Liu B,
Liu J, Chen M, He Q, Liao Y, Yu X, et al: Prevalence of chronic
kidney disease in China: A cross-sectional survey. Lancet.
379:815–822. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Slyne J, Slattery C, McMorrow T and Ryan
MP: New developments concerning the proximal tubule in diabetic
nephropathy: In vitro models and mechanisms. Nephrol Dial
Transplant. 4 30 Suppl:iv60–iv67. 2015. View Article : Google Scholar
|
|
21
|
Eddy AA: Overview of the cellular and
molecular basis of kidney fibrosis. Kidney Int Suppl (2011). 4:2–8.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zeisberg M and Kalluri R: Cellular
mechanisms of tissue fibrosis. 1. Common and organ-specific
mechanisms associated with tissue fibrosis. Am J Physiol Cell
Physiol. 304:C216–C225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ribatti D and Crivellato E: Mast cell
ontogeny: An historical overview. Immunol Lett. 159:11–14. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Madjene LC, Pons M, Danelli L, Claver J,
Ali L, Madera-Salcedo IK, Kassas A, Pellefigues C, Marquet F, Dadah
A, et al: Mast cells in renal inflammation and fibrosis: Lessons
learnt from animal studies. Mol Immunol. 63:86–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaczmarczyk K, Kosalka J, Soja J,
Kuzniewski M, Musial J and Okon K: Renal interstitial mast cell
counts differ across classes of proliferative lupus nephritis.
Folia Histochem Cytobiol. 52:218–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu H, Liu F, Peng Y, Liu Y, Li L, Tu X,
Cheng M, Xu X, Chen X, Ling G and Sun L: Role of mast cells, stem
cell factor and protease-activated receptor-2 in tubulointerstitial
lesions in IgA nephropathy. Inflamm Res. 59:551–559. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Zhou L, Liu F, Peng Y, Li J, Sun L,
Duan S, Ling G, Chen X, Jiang W and Xia Y: Mast cell infiltration
is involved in renal interstitial fibrosis in a rat model of
protein-overload nephropathy. Kidney Blood Press Res. 33:240–248.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taipale J, Lohi J, Saarinen J, Kovanen PT
and Keski-Oja J: Human mast cell chymase and leukocyte elastase
release latent transforming growth factor-beta 1 from the
extracellular matrix of cultured human epithelial and endothelial
cells. J Biol Chem. 270:4689–4696. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smrž D, Bandara G, Zhang S, Mock BA,
Beaven MA, Metcalfe DD and Gilfillan AM: A novel KIT-deficient
mouse mast cell model for the examination of human KIT-mediated
activation responses. J Immunol Methods. 390:52–62. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Halova I, Draberova L and Draber P: Mast
cell chemotaxis-chemoattractants and signaling pathways. Front
Immunol. 3:1192012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gilfillan AM and Tkaczyk C: Integrated
signalling pathways for mast-cell activation. Nat Rev Immunol.
6:218–230. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miyamoto K, Kobayashi T, Hayashi Y, Zhang
Y, Hara Y, Higashine M, Shiraishi A and Ohashi Y: Involvement of
stem cell factor and c-kit in corneal wound healing in mice. Mol
Vis. 18:1505–1515. 2012.PubMed/NCBI
|
|
33
|
El Kossi MM, Haylor JL, Johnson TS and El
Nahas AM: Stem cell factor in a rat model of serum nephrotoxic
nephritis. Nephron Exp Nephrol. 108:e1–e10. 2008. View Article : Google Scholar : PubMed/NCBI
|