Introduction
Reperfusion after cardiac ischemia increases cell
death and infarct size (IS), called myocardial ischemia/reperfusion
(I/R) injury, which is the main cause of myocardial injury during
the cardiac surgery particularly in coronary artery bypass graft
surgery (1,2). Hypoxia/reoxygenation (H/R) as well as
hypoxic preconditioning (HPC) is widely used for simulating in
vivo myocardial I/R and ischemic preconditioning (IPC) in a
cell culture model. In addition, inhaled hypoxic air
preconditioning including intermittent hypoxia has been suggested a
potential non-pharmacological therapeutic intervention to improve
some cardiovascular diseases such as hypertension, myocardial
infarction. Such HPC may enhance cellular tolerance to subsequent
hypoxia-induced oxidative stress, simulating the protective effects
of IPC, taking advantage of convenient and non-invasive
intervention (3,4). In recent years, HPC has been
extensively studied and established to be cardioprotective against
H/R injury, which involves many factors including oxygen transport,
energy metabolism, redox balance, stress protein and protein kinase
signaling, adenosine release, ATP-sensitive potassium channels,
mitochondrial function, control of calcium levels, and nitric oxide
production (5–7). However, the detailed mechanisms
underlying HPC are not fully understood.
MicroRNAs (miRNAs/miRs) are endogenous,
single-stranded, small (approximately 22 nucleotides in length)
noncoding RNA molecules that play an important and ubiquitous role
in regulating genes expression. MiRNAs typically bind to the 3′
untranslated region (UTR) of their mRNA targets and downregulate
gene expression via mRNA degradation or translational inhibition
(8–10). So, miRNAs may influence many
cellular activities. Indeed, miRNAs have been shown to be actively
involved in the coordination of almost all major cellular
processes, such as differentiation (11), proliferation (12), metabolism, and apoptosis (13). Also, cumulating evidence suggests
critical roles for miRNAs in various types of heart disease,
including cardiac hypertrophy (14,15),
heart failure (16), and
myocardial infarction (17–19).
miR-133 is one of the most studied and best
characterized miRNAs, specifically expressed in cardiac and
skeletal muscle (20). The miR-133
family contains two members of miR-133a and miR-133b, sharing an
almost identical sequence. miR-133a is known as an important
anti-apoptotic miRNA in myocardial I/R where it attenuates
apoptosis of cardiomyocytes by targeting the apoptosis-related gene
caspase-9 (21,22). The mechanism of miR-133b regulating
apoptosis in the myocardium, however, has yet to be elucidated. In
the present study, we knocked down the expression of miR-133b-5p in
cardiomyocytes by using its specific antagomir, in order to examine
the role of miR-133b-5p in HPC-mediated cardioprotection and the
underlying mechanisms involving caspase apoptotic signaling.
Materials and methods
Animals
All animal experiments were approved by the
Institutional Animal Care and Use Committee of Anhui Medical
University. Adult Sprague-Dawley (SD) rats (250±30 g) were
maintained on a 12 h light/12 h dark cycle at an ambient
temperature of 22±2°C, with food and water available ad libitum.
All animal experimental procedures were performed in accordance
with the National Institutes of Health (NIH) Guide for the Care and
Use of Laboratory Animals (NIH publication no. 85–23, revised
1996).
In vivo I/R injury
Myocardial I/R was performed in vivo by 30
min of ligation of the left anterior descending coronary artery
followed by 2 h of reperfusion, as previously described (23). IPC was performed by three cycles of
5 min of ischemia intermitted by 5 min of reperfusion before I/R
injury. At the end of reperfusion, heart slices were stained by 1%
triphenyl tetrazolium chloride (TTC) for measuring IS. The total
volumes of the left and right ventricles (LV+RV), the area at risk
(AAR), and IS were measured by a blinded investigator using Image J
software (NIH, USA). In other animals, heart tissue samples were
taken from left ventricular myocardium for miRNA detection by
RT-qPCR.
Isolation of neonatal cardiomyocytes
and treatment
Neonatal rat cardiomyocytes were isolated from the
hearts of 1–3 days old Sprague-Dawley (SD) rats. The newborn rats
were killed by cervical dislocation. The isolated cardiomyocytes
were obtained and cultured using the method described previously by
Suh et al (24). Briefly,
hearts excised from the rats were minced into pieces, and then
digested with 0.125% trypsin and 0.1% type II collagenase. The cell
suspensions were collected and incubated for 2 h to reduce
fibroblast contamination. Then, the non-adherent cardiomyocytes
were seeded to dishes and cultured in Dulbecco's modified Eagle's
medium/F12 (DMEM/F12) containing 10% fetal bovine serum (FBS), 100
U/ml penicillin, 100 µg/ml streptomycin. The medium was
supplemented with 100 µmol/l 5-bromo-2′-deoxyuridine (BrdU;
Sigma-Aldrich, St. Louis, MO, USA) in order to increase the purity
of cardiomyocyte.
H/R injury in cardiomyocytes
To induce H/R injury, neonatal rat cardiomyocytes
were incubated in glucose-free Tyrode's solution containing (in
mmol/l) 139 NaCl, 4.7 KCl, 0.5 MgCl2, 1.0
CaCl2, and 5 HEPES, pH 7.4, and exposed to 95%
N2 + 5% CO2 for 4 h using a hypoxia chamber
(Stemcell Technologies, Inc., Vancouver, BC, Canada) followed by 2
h reoxygenation under normal culture conditions. The cells cultured
under a normoxic atmosphere served as a control. HPC was carried
out by 10 min hypoxia and 30 min reoxygenation before the lethal
hypoxia.
Transfection of miR-133b-5p antagomir
in neonatal cardiomyocytes
Neonatal rat myocardial cells were cultured in
DMEM-F12 medium containing 10% FBS, with transfection of
miR-133b-5p antagomir or antagomir negative control (NC; Guangzhou
RiboBio Co., Ltd., Guangzhou, China) using Lipofectamine RNAiMAX
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to procedures the manufacturer recommended (25). After 24 h transfection, the medium
was changed and the cells were then subjected to H/R injury with or
without HPC.
Cell viability and lactate
dehydrogenase (LDH) activity assessment
Neonatal rat cardiomyocytes were plated in the
96-well plates. Cell viability was evaluated by Cell Counting Kit-8
(CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan), and
the extent of cell injury was determined by detecting the release
of LDH in culture medium using a LDH Activity Assay kit (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China). Cell viability
and injury were assayed according to the manufacturer's
instructions.
Annexin V-fluorescein isothiocyanate
(FITC)/ prodium iodide (PI) flow cytometry
Cell apoptosis was detected by Annexin V-FITC/PI
staining kit (Biouniquer Technology, Beijing, China) as described
before (26). After treatment, the
myocytes were collected, washed twice with ice-cold
phosphate-buffered solution (PBS). Then, 500 µl of binding buffer
was added to resuspend the cells and 5 µl Annexin V-FITC and 5 µl
PI were added and mixed. The reaction was conducted in dark at room
temperature for 15 min and analyzed with a flow cytometer (Beckman
Coulter, Inc., Brea, CA, USA) and FCS Express V3.0 software.
RT-qPCR
The total RNA was extracted from the heart tissues
or cardiomyocytes using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.) and quantified by spectrophotometry. miR-133b-5p
level was measured by All-in-One™ miRNA RT-qPCR Detection kit
(GeneCopoeia, Inc., Guangzhou, China) as previously reported
(26). The stably expressed U6 was
used as the endogenous control. The PCR analysis was performed in a
real time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. In each
experimental run, double-distilled H2O was served as the
NC. Relative quantization was expressed as fold-induction compared
with control conditions. Dissociation curves were generated after
each run to confirm amplification of specific transcripts. Relative
gene expression levels were calculated by the 2−∆∆Cq
(27).
Western blot analysis
After treatment, the cells were harvested and the
total protein was extracted by standard procedures and then
quantified by the bicinchoninic acid (BCA) protein assay (Pierce;
Thermo Fisher Scientific, Inc.). A total of 25 µg total protein was
subjected to 10% SDS-PAGE gel followed by electrotransfer onto
polyvinylidene difluoride (PVDF) membranes (EMD Millipore,
Billerica, MA, USA). Nonspecific antibody binding was blocked in
Tris-buffered saline solution/0.1 Tween-20 (TBST) containing 5%
skimmed milk. Membranes were incubated overnight at 4°C with
primary antibodies against β-actin (Abcam, Cambridge, UK), Fas
(Abcam), caspase-3 and cleaved caspase-3 (Cell Signaling
Technology, Inc., Danvers, MA, USA) or caspase-8 (EMD Millipore),
followed by incubation with secondary antibodies for 1 h at room
temperature. After washing three times with TBST, the
immunoreactive proteins were visualized by ECL (Amersham Pharmacia
Biotech, Amersham, UK) and autoradiography. Densitometric analysis
was carried out with Image J2× software (NIH, Bethesda, MD,
USA).
Caspase-8 and caspase-3 activity
assay
The activities of caspase-8 and caspase-3 were
determined by caspase-8 and caspase-3 Colorimetric Assay kit
(RayBiotech, Norcross, GA, USA) as the manufacturer's instructions.
The cells were collected and lysed by cell lysis buffer followed by
centrifuging at 10,000 g for 5 min at 4°C. After assaying protein
concentration, 100 µg of protein was added in 50 µl cell lysis
buffer. Next, the reaction buffer and 5 µl of IETD-ρNA substrate
(caspase-8) or DEVD-ρNA substrate (caspase-3) were added to the
lysis buffer. The reaction mixtures were incubated at 37°C for 2 h
followed by detecting the activities of caspase-8 and caspase-3 at
405 nm in a microtiter plate reader.
Statistical analysis
Data are expressed as mean ± SEM based on at least
three independent experiments. Statistical analysis was performed
using one-way ANOVA with a Tukey's test by SPSS 10.0 for Windows
(SPSS, Inc, Chicago, IL, USA). P<0.05 was considered
significant.
Results
IPC upregulates the expressional level
of miR-133b-5p in rat myocardium after I/R injury
Representative photographs of heart sections stained
by TTC in each group are presented. The non-ischemic area stained
blue and AAR was brick red while IS was white (Fig. 1A). There were no significant
difference in AAR/(LV+RV) between each group in rats (Fig. 1B). IPC significantly reduced IS/AAR
caused by I/R injury (Fig. 1C).
Upon the exposure of rat hearts to I/R, the expression of
miR-133b-5p was decreased in myocardium, while IPC markedly
upregulated the level of miR-133b-5p (Fig. 1D).
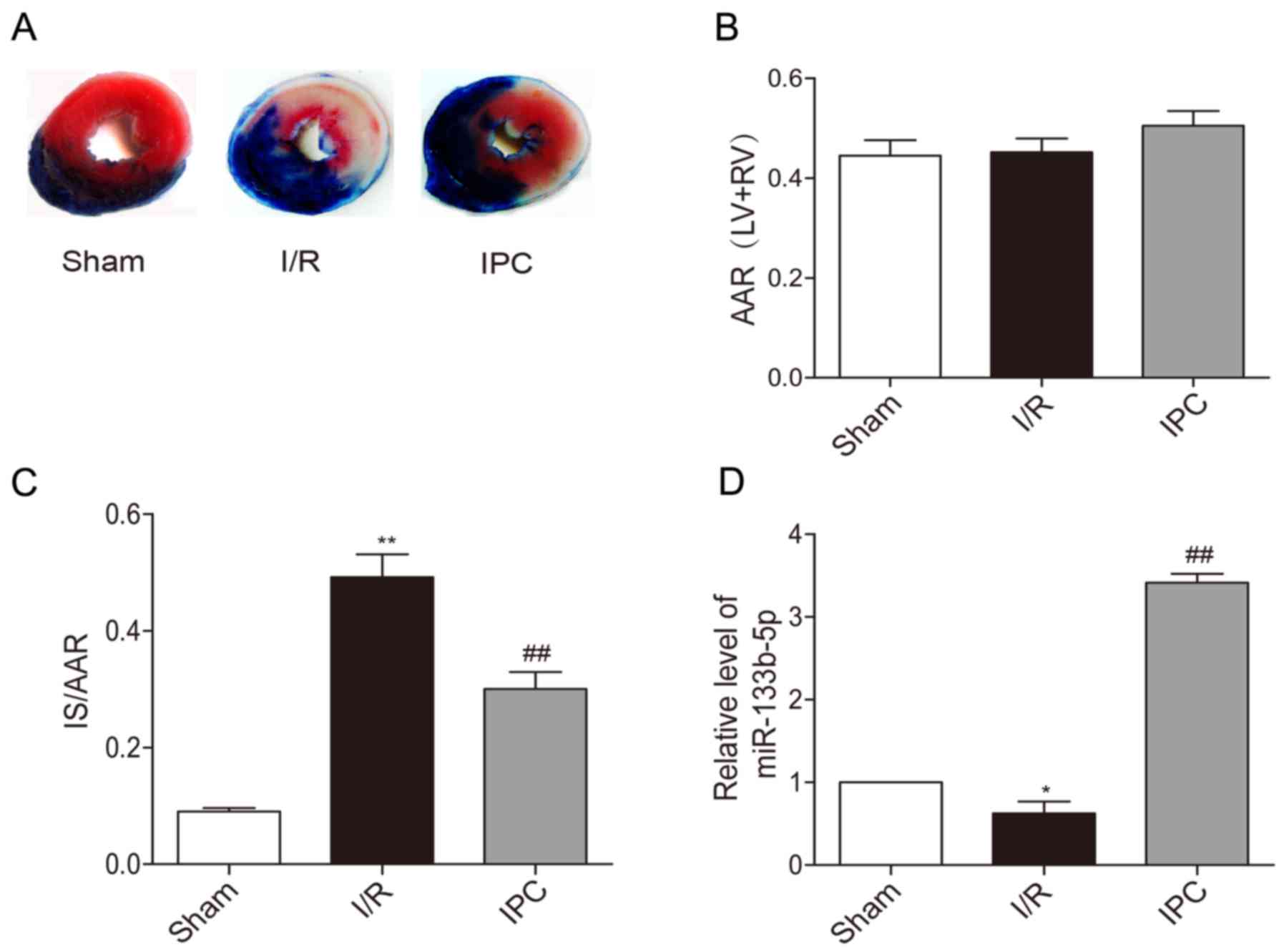 | Figure 1.Effects of IPC on the infarct size
and expressions of miR-133b-5p of in vivo rat hearts. (A)
Heart sections were stained by TTC. (B) AAR/ (LV+RV) and (C) IS/ARR
were calculated by Image J software (n=6). (D) The levels of
miR-133b-5p was detected by quantitative RT-PCR (n=3). Data are
presented as mean ± SEM, *P<0.05, **P<0.01 vs. Sham;
##P<0.01 vs. I/R. IPC, ischemia preconditioning; TTC,
triphenyltetrazolium chloride; AAR, area at risk; LV, left
ventricular; RV, right ventricular; IS, infarct size; I/R,
ischemia/reperfusion; miR, microRNA. |
HPC-mediated cardioprotection against
H/R injury is accompanied with the upregulation of miR-133b-5p
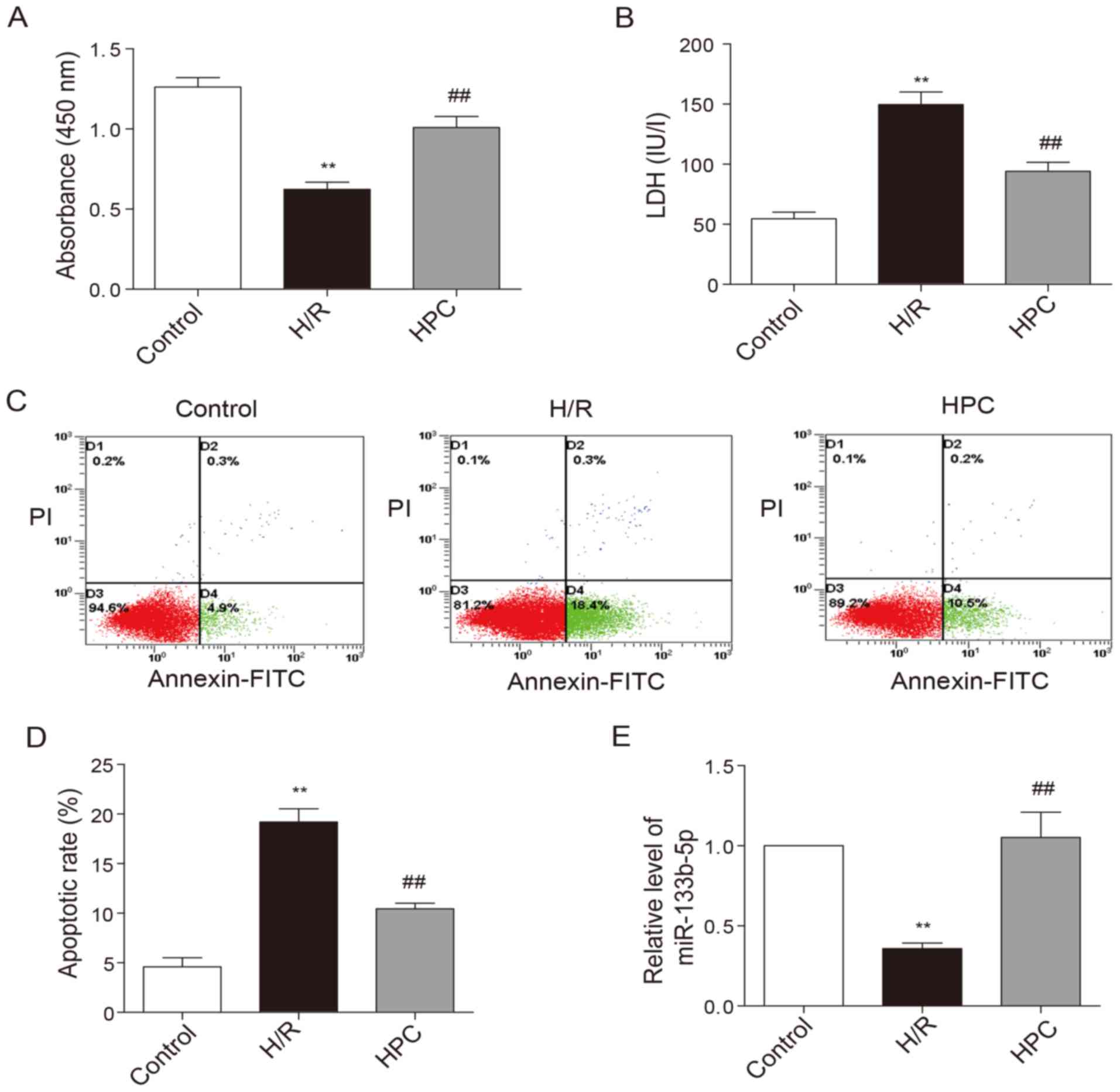
Upon the exposure of neonatal cardiomyocytes to H/R
injury, the viability of cardiomyocytes was noticeably decreased
(Fig. 2A) while the LDH level
elevated (Fig. 2B). In addition,
the percentage of cells at early apoptotic stage as shown in the
lower right quadrant was increased following H/R injury (Fig. 2C and D). HPC significantly
increased cell viability, reduced LDH levels as well as early
apoptotic rate. The level of miR-133b-5p was markedly reduced due
to H/R injury, but restored by HPC (Fig. 2E).
Knockdown of miR-133b-5p blocks the
protective effects of HPC
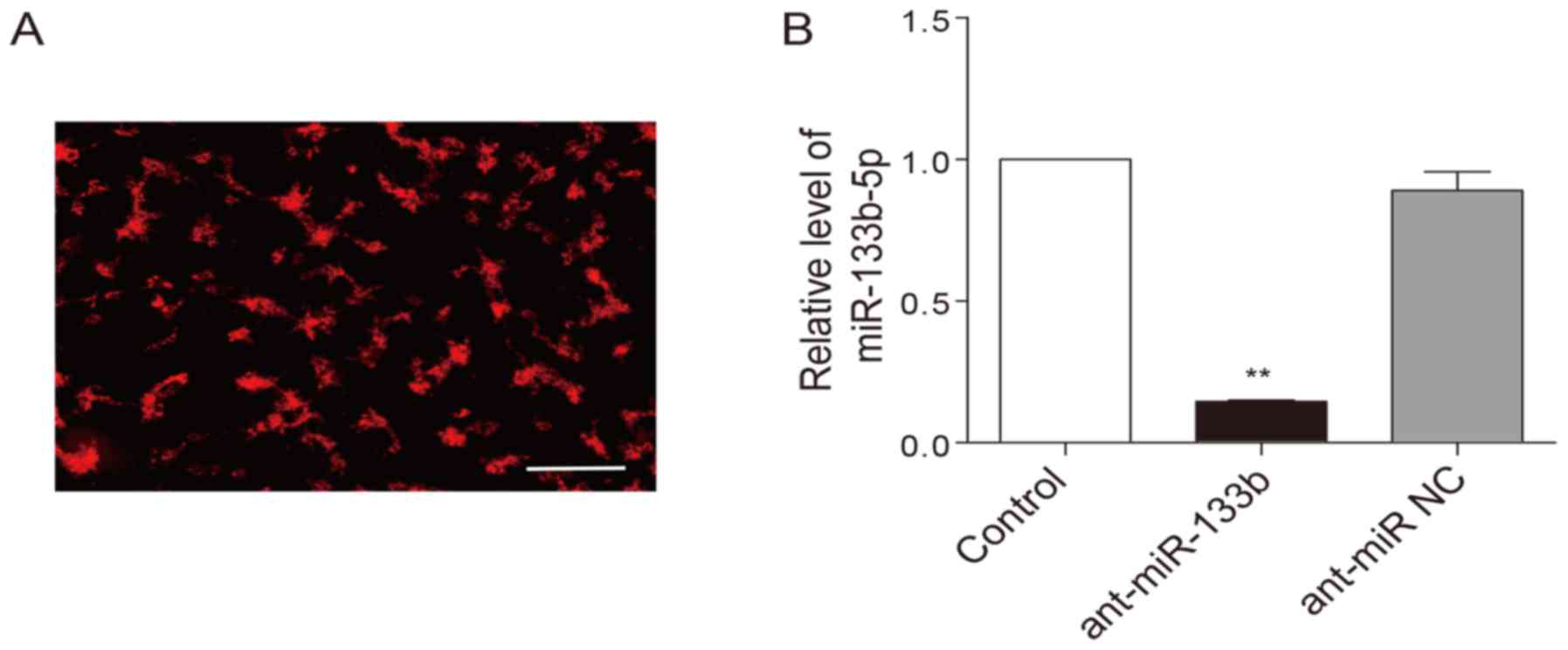
To examine whether HPC-induced cardioprotection is
dependent on miR-133b-5p, the antagomir that mediates specific
silencing of endogenous miR-133b-5p were transfected into neonatal
cardiomyocytes. The transfection efficiency of the antagomir was
above 80%, which was monitored with a Cy3-labelled (red) control
(Fig. 3A). As expected, the
miR-133b-5p antagomir largely decreased the level of miR-133b-5p in
the cardiomyocytes (Fig. 3B). When
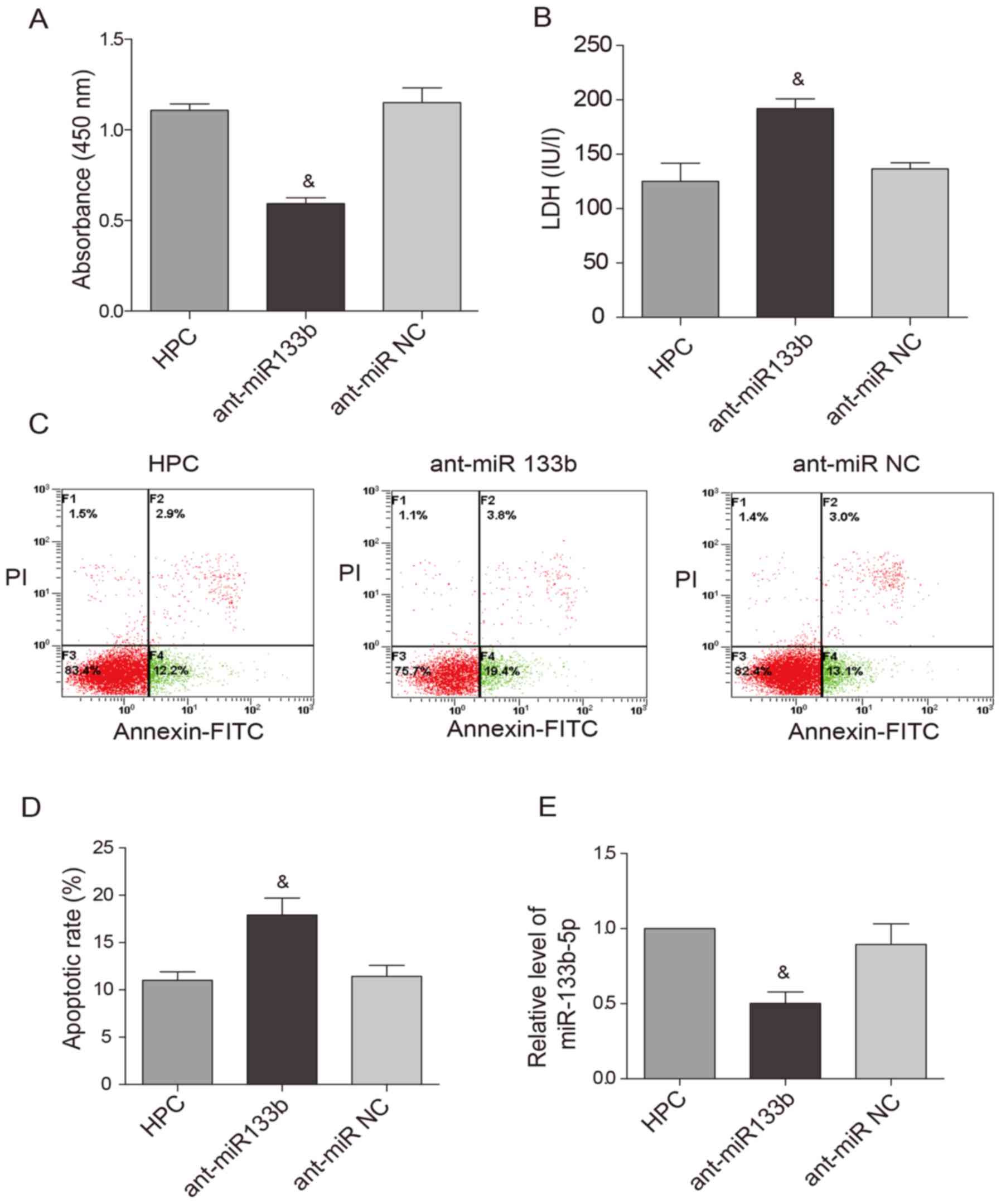
the expression of miR-133b-5p was knocked down by the antagomir,
the cardioprotective effects of HPC were obviously attenuated. Cell
viability was reduced (Fig. 4A)
while LDH level (Fig. 4B) and
apoptotic rate (Fig. 4C and D)
were raised. Meanwhile, the upregulation of miR-133b-5p by HPC was
markedly suppressed by the miR-133b-5p antagomir (Fig. 4E).
Effects of miR-133b-5p antagomir on
the levels of Fas mRNA/protein and the cleavages of caspase-8 and
caspase-3
As one of the target genes of miR-133b-5p, the
expressional levels of Fas mRNA and protein that decreased by HPC
were both reversely raised due to the knockdown of miR-133b-5p
(Fig. 5A and B). We next examined
whether the apoptosis regulators of caspase-8 and caspase-3,
downstream of Fas death receptor, were regulated by miR-133b-5p.
Using western blot method, we detected the cleaved fragments of
caspase-8 and caspase-3, which represents their activation. As
shown in Fig. 5C and D, the full
length caspase-8 (57 kDa) was strongly upregulated upon H/R injury,
while its level was not affected by HPC or miR-133b-5p antagomir.
The enhanced cleavage of caspase-8 (43 kDa) was suppressed by HPC
but reversed by the miR-133b-5p antagomir. Interestingly, the level
of full length caspase-3 (35 kDa) was reduced but the cleaved
caspase-3 (17 kDa) was markedly strengthened in response to H/R
injury. However, the pro-caspase-3 (full length) level was restored
while the activated caspase-3 (cleaved) was weakened by HPC, and
these effects were abolished by knockdown of miR-133b-5p (Fig. 5E and F).
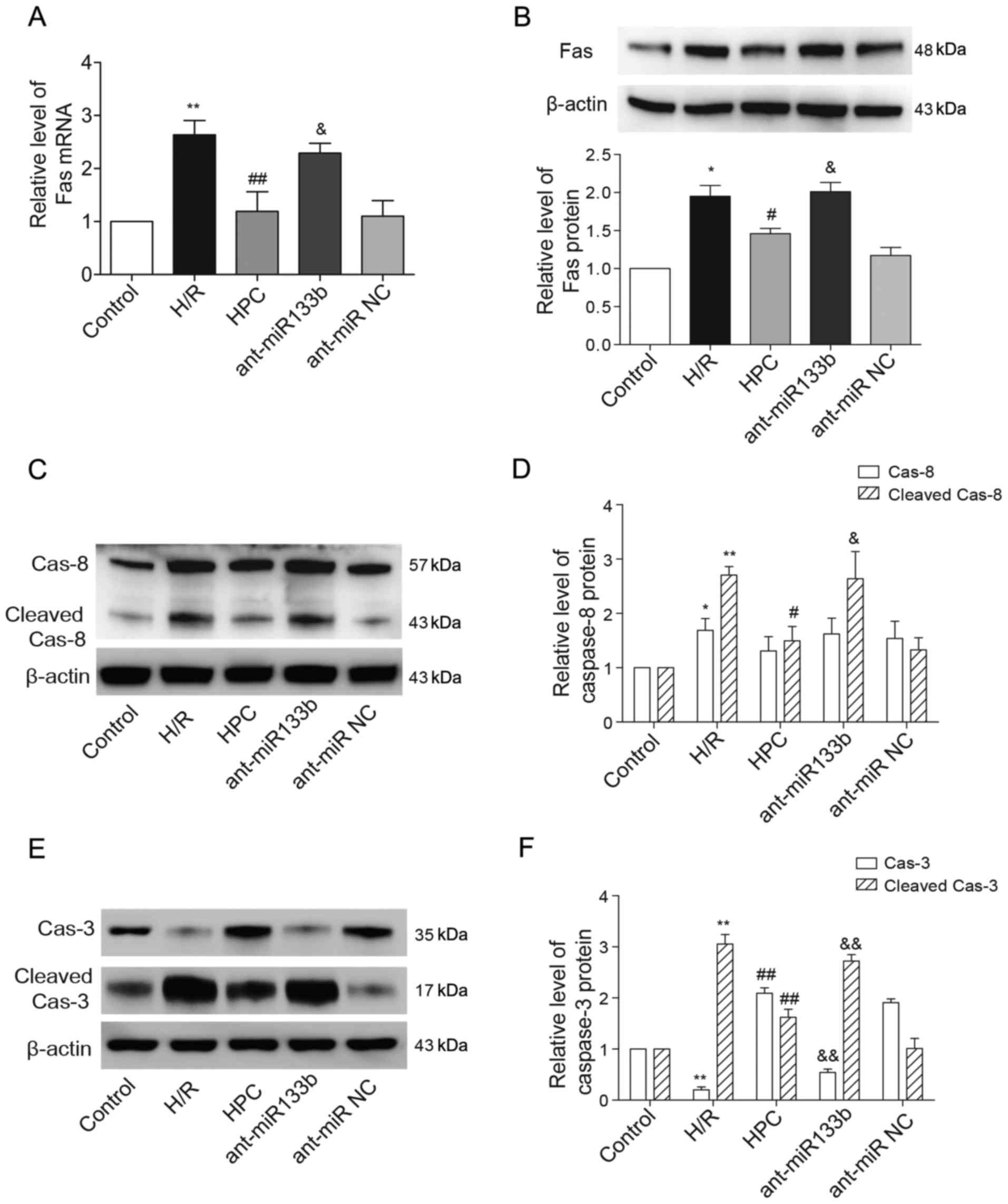 | Figure 5.Effects of miR-133b-5p antagomir on
Fas mRNA/protein expression and the cleavage of caspase-8 and
caspase-3. (A) Fas mRNA detected by quantitative RT-PCR. (B) The
level of Fas protein expression was examined by western blot and
the intensities of Fas protein bands were normalized to β-actin.
(C) The full-length (57 kDa) and cleaved caspase-8 (43 kDa) were
detected by western blot and (D) the intensities of full-length and
cleaved caspase-8 were normalized to β-actin, respectively. (E) The
full-length (35 kDa) and cleaved caspase-3 (17 kDa) were detected
by western blot and (F) the intensities of full-length and cleaved
caspase-3 were normalized to β-actin, respectively. Data are
presented as mean ± SEM (n=3). *P<0.05, **P<0.01 vs. Control;
#P<0.05, ##P<0.01 vs. H/R;
&P<0.05, &&P<0.01 vs. HPC.
miR, microRNA; H/R, hypoxia/reoxygenation; HPC, hypoxic
preconditioning; ant-miR-133b, miR-133b-5p antagomir; ant-miR NC,
antagomir negative control; Cas-8, caspase-8; Cas-3, caspase-3. |
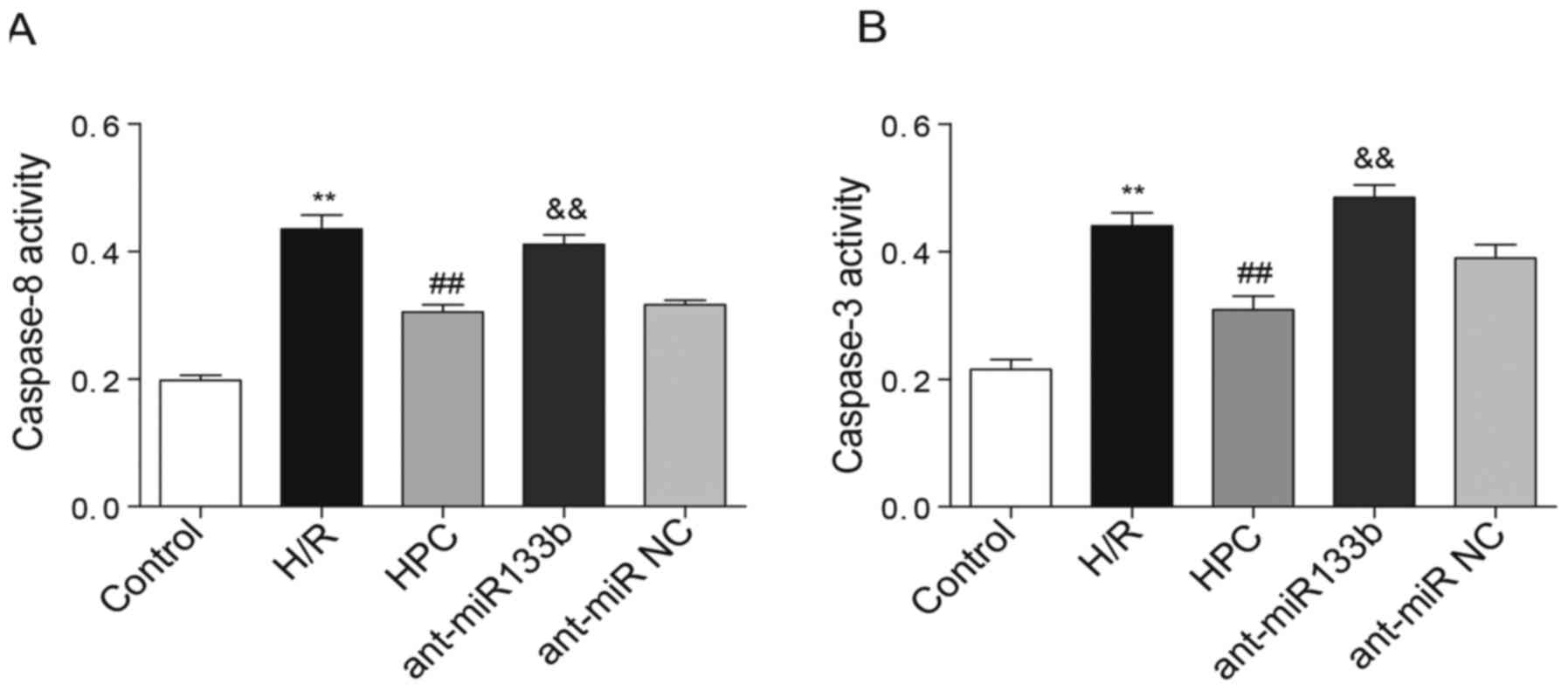
Effects of miR-133b-5p antagomir on
the activities of caspase-8 and caspase-3
The activation of caspase-8 and caspase-3 was
further detected by colorimetric assay in which the activities of
caspase-8 and caspase-3 were determined by their cleavage
capability of the labeled substrates. According to Fig. 6A and B, we found that both
caspase-8 and caspase-3 were activated following H/R injury,
whereas their activities were significantly inhibited by HPC.
However, knockdown of miR-133b-5p recovered the activities of both
caspase-8 and caspase-3 in cardiomyocytes.
Discussion
Cardiomyocyte apoptosis represents a critical event
during myocardial ischemia and reperfusion. Growing evidences show
that a number of miRNAs can promote or inhibit cardiomyocyte
apoptosis via regulating the downstream target genes and signaling
pathways (28). In the present
study, by using miR-133b-5p antagomir, we demonstrated that
miR-133b-5p contributed to HPC-induced cardioprotection against H/R
injury in cardiomyocytes by inhibiting the activation of capase-8
and caspase-3 apoptotic signaling.
We first found that the expressional level of
miR-133b-5p was reduced following in vivo myocardial I/R
injury, while this reduction was restored by IPC treatment, which
suggests a potential role of miR-133b-5p in cardioprotection. In
neonatal rat cardiomyocytes, HPC conferred cardioprotection against
H/R injury by improving cell viability while decreasing LDH release
and cell apoptosis. This protective effect was coupled with the
upregulation of miR-133b-5p, indicating that HPC may exert
cardioprotection, in partly, by upregulating miR-133b-5p. Previous
study reported that the plasma levels of miR-133a and miR-133b were
upregulated following acute myocardial infarction, and this
upregulation was consistent with the increase of cardiac troponin I
(cTnI), representing a novel marker for cardiac damage (29,30).
In addition, the levels of miR-133a and miR-133b in myocardium have
been found changed during cardiac development, myocardial
infarction and heart failure (31–33).
These make both miR-133a and miR-133b truly valuable therapeutic
genes for cardiac injury.
In a previous work, we showed that overexpression of
miR-133b-5p in H9c2 cardiomyoblasts protected the cells against H/R
injury (26). In the present
study, we applied primary neonatal rat cardiomyocytes, which are
more suitable for both simulating in vivo condition and
transfecting the antagomir effectively. The most important
observation of this study is that the cardioprotective effect of
HPC against H/R injury in cardiomyocyte was significantly
attenuated by transfection of the miR-133b-5p antagomir. Thus, it
is reasonable to speculate that HPC induced cardioprotection was
mediated by the upregulation of miR-133b-5p. The finding is in
agreement with the previous observation on a cardiac protective
role of miRNAs (34). Yin et
al (35) reported that an
ischemic stimulus induced the expression of miR-1, miR-21 and
miR-24, and injection of these IPC-derived miRNAs directly into the
left ventricle reduced myocardial IS, indicating that
preconditioning induced miRNAs produced a protective phenotype
following ischemic injury. Inconsistent with our results, however,
miR-133b has been reported to promote apoptosis in several
carcinoma cells including lung cancer, renal carcinoma, and
osteosarcoma (36–38). Moreover, Xia et al (39) recently reported that miR-133b-5p
accelerates neuron apoptosis via targeting heat shock protein 70.
These discrepancies may due to disease-specific circumstances as
well as various cell types applied in different studies.
The activation of caspase-8 and caspapse-3 is
reported directly or indirectly regulated by several miRNAs
including miR-133 in the process of cardiomyocyte apoptosis
(40). Our recent study using dual
luciferase reporter gene technique showed that miR-133b-5p directly
targeted Fas gene by interacting with the 3′ UTR (26). Thus, there is no wonder that
HPC-reduced expression of Fas mRNA/protein was blocked by the
miR-133b-5p antagomir. Fas death pathway has been considered as a
critical mediator for cardiomyocyte death and myocardial infarction
during myocardial I/R (41,42).
It is well known that the activation of Fas by extracellular Fas
ligand (FasL) in turn activates the pro-apoptotic members of
caspase-8 and the downstream apoptosis executors of caspase-3
(43). The cleavage and activities
of caspase-8 and caspase-3 were inhibited by HPC but reversed by
the miR-133b-5p antagomir. These results suggest that miR-133b-5p
antagomir may indirectly influence the activation of caspase-8 and
caspase-3 through regulating Fas gene expression. Otherwise,
miR-133b-5p may directly target the post-transcription of caspase-8
and caspase-3, although the interaction mechanisms need to be
further explored.
The cleavage and activation of caspase-8 and
caspase-3 have been confirmed as critical steps in apoptosis
signaling pathway, resulting in DNA degradation and cell apoptosis.
In this study, the exposure of cardiomyocytes to H/R led to the
cleavage and activation of both caspase-8 and caspase-3, which may
in turn result in cardiomyocytes apoptosis. Interestingly, the
expression of pro-caspase-3 showed a reduction corresponding to its
increased cleavage, while the pro-caspase-8 level was not obviously
changed following cleavage. Caspase-8 is the most upstream caspase
that is well recognized as the initiator caspase, which directly
activates the downstream caspase-3. The effect of caspase-8 on
caspase-3 can also be amplified by the mitochondrial signaling when
initial caspase-8 activation is low (44). This may be the reason why the
cleavage of pro-caspase-8 is markedly less than those of
pro-caspase-3, and thus no apparent change of pro-caspase-8 level
can be observed.
It should be noted that we conducted the experiments
in neonatal rat cardiomyocytes, and the significance and
implications of the results remain need to be verified in an animal
model in vivo. Although we found that the expression of
miR-133b-5p was also changed in an in vivo rat model of
myocardial I/R injury, its role in IPC-mediated cardioprotection
still need to be confirmed in future. In addition, the caspase-8
and caspase-3 apoptotic signaling may be controlled by other miRNAs
during cardiomyocyte apoptosis, while we could only focused on the
miR-133b-5p in the present study.
In summary, our results revealed that HPC induced
cardioprotection in cardiomyocyte was, at least partly, dependent
on the upregulation of miR-133b-5p, and the mechanisms may be
associated with inhibition of caspase-8 and caspase-3 apoptotic
signaling. The finding may provide new insight into potential
therapeutic application for ischemic cardiac injury.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (81471145), and the Natural Science
Foundation of Higher Education Institutions of Anhui Province
(KJ2014ZD16 and KJ2017A172). The authors thank Professor Tak-Min
Wong (University of Hong Kong, Hong Kong, China) for the assistance
in editing and revising the manuscript.
References
|
1
|
Arslan F, Smeets MB, O'Neill LA, Keogh B,
McGuirk P, Timmers L, Tersteeg C, Hoefer IE, Doevendans PA,
Pasterkamp G and de Kleijn DP: Myocardial ischemia/reperfusion
injury is mediated by leukocytic toll-like receptor-2 and reduced
by systemic administration of a novel anti-toll-like receptor-2
antibody. Circulation. 121:80–90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gong D, Zhang Y, Zhang H, Gu H, Jiang Q
and Hu S: Aldehyde dehydrogenase-2 activation during cardioplegic
arrest enhances the cardioprotection against myocardial
ischemia-reperfusion injury. Cardiovasc Toxicol. 12:350–358. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Verges S, Chacaroun S, Godin-Ribuot D and
Baillieul S: Hypoxic conditioning as a new therapeutic modality.
Front Pediatr. 3:582015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barrington JH, Chrismas BCR, Gibson OR,
Tuttle J, Pegrum J, Govilkar S, Kabir C, Giannakakis N, Rayan F,
Okasheh Z, et al: Hypoxic air inhalation and ischemia interventions
both elicit preconditioning which attenuate subsequent cellular
stress In vivo following blood flow occlusion and
reperfusion. Front Physiol. 8:5602017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Das DK and Maulik N: Cardiac genomic
response following preconditioning stimulus. Cardiovasc Res.
70:254–263. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Turer AT and Hill JA: Pathogenesis of
myocardial ischemia-reperfusion injury and rationale for therapy.
Am J Cardiol. 106:360–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hoole SP, Heck PM, Sharples L, Khan SN,
Duehmke R, Densem CG, Clarke SC, Shapiro LM, Schofield PM,
O'Sullivan M and Dutka DP: Cardiac remote ischemic preconditioning
in coronary stenting (CRISP stent) study: A prospective, randomized
control trial. Circulation. 119:820–827. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: Are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gagan J, Dey BK, Layer R, Yan Z and Dutta
A: MicroRNA-378 targets the myogenic repressor MyoR during myoblast
differentiation. J Biol Chem. 286:19431–19438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feng S, Cong S, Zhang X, Bao X, Wang W, Li
H, Wang Z, Wang G, Xu J, Du B, et al: MicroRNA-192 targeting
retinoblastoma 1 inhibits cell proliferation and induces cell
apoptosis in lung cancer cells. Nucleic Acids Res. 39:6669–6678.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan JA, Krichevsky AM and Kosik KS:
MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells.
Cancer Res. 65:6029–6033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carè A, Catalucci D, Felicetti F, Bonci D,
Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, et al:
MicroRNA-133 controls cardiac hypertrophy. Nat Med. 13:613–618.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song XW, Li Q, Lin L, Wang XC, Li DF, Wang
GK, Ren AJ, Wang YR, Qin YW, Yuan WJ and Jing Q: MicroRNAs are
dynamically regulated in hypertrophic hearts, and miR-199a is
essential for the maintenance of cell size in cardiomyocytes. J
Cell Physiol. 225:437–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thum T, Galuppo P, Wolf C, Fiedler J,
Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J,
Haverich A, et al: MicroRNAs in the human heart: A clue to fetal
gene reprogramming in heart failure. Circulation. 116:258–267.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong S, Cheng Y, Yang J, Li J, Liu X, Wang
X, Wang D, Krall TJ, Delphin ES and Zhang C: MicroRNA expression
signature and the role of microRNA-21 in the early phase of acute
myocardial infarction. J Biol Chem. 284:29514–29525. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qian L, Van Laake LW, Huang Y, Liu S,
Wendland MF and Srivastava D: miR-24 inhibits apoptosis and
represses Bim in mouse cardiomyocytes. J Exp Med. 208:549–560.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X, Zhang X, Ren XP, Chen J, Liu H,
Yang J, Medvedovic M, Hu Z and Fan GC: MicroRNA-494 targeting both
proapoptotic and antiapoptotic proteins protects against
ischemia/reperfusion-induced cardiac injury. Circulation.
122:1308–1318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wystub K, Besser J, Bachmann A, Boettger T
and Braun T: miR-1/133a clusters cooperatively specify the
cardiomyogenic lineage by adjustment of myocardin levels during
embryonic heart development. PLoS Genet. 9:e10037932013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He B, Xiao J, Ren AJ, Zhang YF, Zhang H,
Chen M, Xie B, Gao XG and Wang YW: Role of miR-1 and miR-133a in
myocardial ischemic postconditioning. J Biomed Sci. 18:222011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li AY, Yang Q and Yang K: miR-133a
mediates the hypoxia-induced apoptosis by inhibiting TAGLN2
expression in cardiac myocytes. Mol Cell Biochem. 400:173–181.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Irwin MG and Wong TM:
Remifentanil preconditioning protects against ischemic injury in
the intact rat heart. Anesthesiology. 101:918–923. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suh JH, Choi E, Cha MJ, Song BW, Ham O,
Lee SY, Yoon C, Lee CY, Park JH, Lee SH and Hwang KC: Up-regulation
of miR-26a promotes apoptosis of hypoxic rat neonatal
cardiomyocytes by repressing GSK-3β protein expression. Biochem
Biophys Res Commun. 423:404–410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakamura T, Kuroi M, Fujiwara Y, Warashina
S, Sato Y and Harashima H: Small-sized, stable lipid nanoparticle
for the efficient delivery of siRNA to human immune cell lines. Sci
Rep. 6:378492016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He SF, Zhu HJ, Han ZY, Wu H, Jin SY, Irwin
MG and Zhang Y: MicroRNA-133b-5p is involved in cardioprotection of
morphine preconditioning in rat cardiomyocytes by targeting fas.
Can J Cardiol. 32:996–1007. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Louch WE, Sheehan KA and Wolska BM:
Methods in cardiomyocyte isolation, culture, and gene transfer. J
Mol Cell Cardiol. 51:288–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weiss JB, Eisenhardt SU, Stark GB, Bode C,
Moser M and Grundmann S: MicroRNAs in ischemia-reperfusion injury.
Am J Cardiovasc Dis. 2:237–247. 2012.PubMed/NCBI
|
|
29
|
D'Alessandra Y, Devanna P, Limana F,
Straino S, Di Carlo A, Brambilla PG, Rubino M, Carena MC,
Spazzafumo L, De Simone M, et al: Circulating microRNAs are new and
sensitive biomarkers of myocardial infarction. Eur Heart J.
31:2765–2773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cortez-Dias N, Costa MC, Carrilho-Ferreira
P, Silva D, Jorge C, Calisto C, Pessoa T, Robalo Martins S, de
Sousa JC, da Silva PC, et al: Circulating miR-122-5p/miR-133b ratio
is a specific early prognostic biomarker in acute myocardial
infarction. Circ J. 80:2183–2191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boštjančič E, Jerše M, Glavač D and Zidar
N: miR-1, miR-133a/b, and miR-208a in human fetal hearts correlate
to the apoptotic and proliferation markers. Exp Biol Med (Maywood).
240:211–219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bostjancic E, Zidar N, Stajer D and Glavac
D: MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated
in human myocardial infarction. Cardiology. 115:163–169. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sucharov C, Bristow MR and Port JD: miRNA
expression in the failing human heart: Functional correlates. J Mol
Cell Cardiol. 45:185–192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu HJ, Han ZY, He SF, Jin SY, Xu SJ, Fang
XD and Zhang Y: Specific MicroRNAs comparisons in hypoxia and
morphine preconditioning against hypoxia-reoxgenation injury with
and without heart failure. Life Sci. 170:82–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yin C, Salloum FN and Kukreja RC: A novel
role of microRNA in late preconditioning: Upregulation of
endothelial nitric oxide synthase and heat shock protein 70. Circ
Res. 104:572–575. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou W, Bi X, Gao G and Sun L: miRNA-133b
and miRNA-135a induce apoptosis via the JAK2/STAT3 signaling
pathway in human renal carcinoma cells. Biomed Pharmacother.
84:722–729. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pan JY, Sun CC, Bi ZY, Chen ZL, Li SJ, Li
QQ, Wang YX, Bi YY and Li DJ: miR-206/133b Cluster: A weapon
against lung cancer? Mol Ther Nucleic Acids. 8:442–449. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ying B, Huang H, Li H, Song M, Wu S and
Ying H: Procaine inhibits proliferation and migration and promotes
cell apoptosis in osteosarcoma cells by upregulation of
microRNA-133b. Oncol Res. 25:1463–1470. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xia C, Cai Y, Lin Y, Guan R, Xiao G and
Yang J: MiR-133b-5p regulates the expression of the heat shock
protein 70 during rat neuronal cell apoptosis induced by the gp120
V3 loop peptide. J Med Virol. 88:437–447. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang L, Li X, Zhou Y, Shi H, Xu C, He H,
Wang S, Xiong X, Zhang Y, Du Z, et al: Downregulation of miR-133
via MAPK/ERK signaling pathway involved in nicotine-induced
cardiomyocyte apoptosis. Naunyn Schmiedebergs Arch Pharmacol.
387:197–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jeremias I, Kupatt C, Martin-Villalba A,
Habazettl H, Schenkel J, Boekstegers P and Debatin KM: Involvement
of CD95/Apo1/Fas in cell death after myocardial ischemia.
Circulation. 102:915–920. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee P, Sata M, Lefer DJ, Factor SM, Walsh
K and Kitsis RN: Fas pathway is a critical mediator of cardiac
myocyte death and MI during ischemia-reperfusion in vivo. Am
J Physiol Heart Circ Physiol. 284:H456–H463. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kantari C and Walczak H: Caspase-8 and
bid: Caught in the act between death receptors and mitochondria.
Biochim Biophys Acta. 1813:558–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Engels IH, Stepczynska A, Stroh C, Lauber
K, Berg C, Schwenzer R, Wajant H, Jänicke RU, Porter AG, Belka C,
et al: Caspase-8/FLICE functions as an executioner caspase in
anticancer drug-induced apoptosis. Oncogene. 19:4563–4573. 2000.
View Article : Google Scholar : PubMed/NCBI
|