Introduction
Ultraviolet (UV) radiation is one of the major
physical stress factors that affect the human skin. UV radiation
from the sun is composed of a broad spectrum, including short-wave
UVC (200–280 nm), mid-wave UVB (280–320 nm) and long-wave UVA
(320–400 nm). UVC is absorbed by the stratospheric ozone layer,
which means that its contribution to human skin pathogenesis is
minimal. However, UVB is considered to be highly biologically
active and is an established high-risk factor for skin
carcinogenesis, particularly malignant melanoma (1,2).
Accounting for 95% of the solar UV light, UVA is primarily
responsible for photoaging, which is characterized by wrinkles, and
loss of skin tone and elasticity (3). However, certain reports have
demonstrated that UVA is more penetrating than UVB and reaches the
subcutaneous tissue to exert effects on dermal and epidermal skin
structures (4,5). Furthermore, an increasing amount of
evidence indicates that the majority of basal cell carcinomas may
be primarily attributed to UVA irradiation (4,5). To
protect human dermal fibroblasts (HDFs) against the harmful effects
of UVA, natural compounds are often employed, including Coenzyme
Q10, vitamin C, vitamin E and Amaranth Oil (6–8).
Curcumin (Cur), a bioactive photochemical that is
extracted from the rhizome of Curcuma longa Lin., has been
previously employed for the treatment of skin diseases and wound
healing in traditional Chinese medicine (9–11).
Furthermore, increasing scientific evidence has demonstrated that
Cur is able to inhibit chemical-induced carcinogenesis/tumor
promotion and radiation-induced mammary tumorigenesis (12–15).
In addition, a molecular biology-based study demonstrated that Cur
may exert inhibitory effects on the UVB-induced production of
reactive oxygen species (ROS) and expression of matrix
metalloproteinase (MMP) in vitro by blocking the activation
of the UVB-induced mitogen-activated protein kinase, nuclear
factor-κB (NF-κB) and AP-1 transcription factor signal pathways
(16). Tsai et al (17) demonstrated that Cur provided
protection against UVB radiation-induced skin cancer growth in a
mouse model. Li et al (18)
reported that Cur may have potential as a chemoprotective agent
against skin carcinogenesis in vivo and in vitro.
However, research concerning the protective effect of Cur against
UVA is limited and the molecular mechanisms underlying this
protective effect against UVA have not been detailed.
The purpose of the present study was to evaluate the
protective effects and investigate the underlying mechanisms of Cur
against UVA damage in HDFs in vitro. An improved
understanding of the protective properties of Cur may allow the
development of more efficient therapeutic approaches for preventing
the photoaging of skin and promoting UVA-induced fibroblast
repair.
Materials and methods
Reagents and antibodies
Cur (99% purity) was obtained from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany) and dissolved in dimethyl
sulfoxide (DMSO) as a 10 mmol/l stock solution and stored at −20°C.
MTT and DMSO were purchased from Sigma-Aldrich (Merck KGaA).
Dulbecco's modified Eagle medium (DMEM), fetal bovine serum (FBS),
penicillin and streptomycin were purchased from Gibco (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The Annexin
V-Fluorescein isothiocyanate (FITC) Apoptosis Detection kit and was
purchased from BD Biosciences (San Jose, CA, USA). Primary
antibodies against transforming growth factor-β (TGF-β; rabbit;
cat. no. ab50716; 1:400), β-actin (rabbit; cat. no. ab8227;
1:5,000), NF-κB p65 (rabbit; cat. no. ab16502; 1:2,000), MMP-3
(rabbit; cat. no. ab52915; 1:2,000), Bcl-2 (rabbit; cat. no.
ab59348; 1:1,000), caspase-3 (rabbit; cat. no. ab4051; 1:500) and
anti-glucose-regulated protein 78 (GRP78; rabbit; catalog no.
ab21685; 1:1,000) were purchased from Abcam (Cambridge, MA, USA).
The antibody against MMP-1 (human, cat. no. AF901; 1:1,000) was
purchased from Bio-Techne (Minneapolis, MN, USA). The antibody
against Smad2/3 (rabbit; cat. no. 3102; 1:1,000) was purchased from
Cell Signaling Technology, Inc., (Danvers, MA, USA). Smad7
polyclonal antibody (rabbit; cat. no. PA1-41506; 1:200 and
C/EBP-homologous protein (CHOP) monoclonal antibody (9C8; mouse;
cat. no. MA1-250; 1:1,000) were purchased from Thermo Fisher
Scientific, Inc.. Horseradish peroxidase-conjugated anti-rabbit IgG
(cat. no. sc-2357; 1:5,000) and anti-mouse IgG (cat. no. sc-516102;
1:5,000) secondary antibodies were purchased from Santa Cruz
Biotechnology, Inc., (Dallas, TX, USA). N-acetyl cysteine (NAC) and
4-phenylbutyric acid (4-PBA) were obtained from Sigma-Aldrich
(Merck KGaA) and were dissolved in deionized water as a 50 mmol/l
stock solution. The stock solutions (50 mm) were diluted with PBS
to the desired final concentration prior to use and subsequently
added to the cell culture medium.
Cell culture
HDFs were isolated from the foreskin of a 5-year-old
old boy undergoing circumcision at The Third Affiliated Hospital of
Soochow University (Changzhou, China). The study was approved by
the ethics committee of Soochow University and written informed
consent was obtained from the parent of the child. The foreskin was
digested with dispase solution (5 mg/ml) at 4°C. After 16 h of
incubation, the epidermal layer was removed and the dermis was cut
into small pieces (~2 mm3). Dermal explants were allowed
to adhere to 25 cm2 culture flasks containing complete
Dulbecco's modified Eagle's medium (DMEM), which consisted of DMEM
supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin and 50 µg/ml amphotericin solution (all from
Invitrogen; Thermo Fisher Scientific, Inc.), for 30 min at 37°C in
an incubator. Following incubation of cells in a
CO2-regulated incubator at 37°C under 5% CO2
with 95% relative humidity for 2 weeks, fibroblasts reached
confluence and were detached with trypsin-EDTA solution and
passaged. The cells were observed under the inverted microscope
(Olympus IX51; Olympus Inc., Tokyo, Japan) and demonstrated
spindle-shape morphology with bipolar projections and were
refractile. The cultured cells were further identified by
immunocytochemistry. The cover-slips carrying cultured cells were
washed two times with ice-cold PBST (PBS containing 1% Triton
X-100) and then fixed with 4% paraformaldehyde in ice-cold PBS for
10 min at room temperature. The cells were then blocked with 4%
bovine serum albumin (BSA) dissolved in PBST for 30 min at room
temperature and stained with anti-viemtin (rabbit, cat. no.
ab137321; 1:200; Abcam) or anti-cytokeratin 10 (CK10) antibodies
(rabbit, cat. no. ab76318; 1:200; Abcam) at 4°C overnight. After
washed three times with PBS, incubated with secondary antibodies
horseradish peroxidase-conjugated anti-rabbit IgG (cat. no.
sc-2357; 1:5,000; Santa Cruz Biotechnology, Inc.) and anti-mouse
IgG (cat. no. sc-516102; 1:5,000; Santa Cruz Biotechnology, Inc.)
in dark at room temperature for 30 min. Washed with PBS three
times, the DAB staining was performed at temperature according to
the DAB kit (cat. no. AR 0611, 20 x, Beijing Dingguo Changsheng
Biotechnology Co., Ltd., Beijing, China). Following 5 min, the
cells washed with double distilled water to end the DAB staining,
then stained with HE. With inverted microscope (magnification ×400,
Olympus IX51; Olympus Inc., Tokyo, Japan), the immunocytochemistry
demonstrated that the cells were positive for vimentin (dark brown)
and negative for cytokeratin 10 (blue). The cells were confirmed as
fibroblasts. Fibroblasts in the log phase of growth were used in
all experiments in the present study.
UVA irradiation
Fibroblasts were seeded at a density of
3×105 cells in 6-well plates and were incubated in DMEM
complete medium for 24 h at 37°C, followed by incubation in
serum-free DMEM containing a final concentration of 0, 2.5, 5 or 10
µM Cur in DMSO (final DMSO concentration, 0.1%) at 37°C. After 2 h,
the cells were washed twice with PBS and covered with a thin layer
of PBS. Cells were subsequently exposed to various intensities of
UVA irradiation (5 J/cm2 for 17 min, 10 J/cm2
for 33 min or 15 J/cm2 for 51 min). During exposure, the
plate was placed on ice in order to reduce the effect of heat. The
UVA source used in the experiment was a UV phototherapy instrument
(SS-04A; Shanghai Sigma High-tech Co., Ltd., Shanghai, China)
equipped with a 15-W ozone-free UVA lamp (CEL015 W; Philips,
Groningen, The Netherlands) with a peak emission at 350 nm. UVA
radiation was uniformly exposed to samples at a distance of 15 cm
from the cell cultures and the intensity of UVA was calibrated with
a digital radiometer (Shanghai Sigma High-Tech Co., Ltd.). The
cells were divided into the following four groups: Control group,
which was cultured in regular medium and without any treatment; Cur
group, which was only treated with Cur (5 µM for 2 h); UVA
irradiation group, which was treated with UVA alone (10
J/cm2 for 33 min); and Cur + UVA irradiation group,
which was pre-incubated with Cur (5 µM for 2 h) and subsequently
exposed to UVA (10 J/cm2 for 33 min). For the positive
control groups, the cells were pre-incubated either with NAC (5 mM
for 2 h) or 4-PBA (5 mM for 2 h) and then exposed to UVA (10
J/cm2 for 33 min). After UVA irradiation, the MTT,
transmission electron microscopy (TEM), ROS, malondiadehyde (MDA),
catalase (CAT) and glutathione (GSH) assays were performed
immediately. For the apoptosis and western blotting analysis, cells
were further incubated in complete DMEM for 24 h under standard
conditions without rinsing following the irradiation.
MTT assay
Cell viability was examined using MTT assays.
Briefly, cells were seeded in 96-well plates at a density of
1×104 cells/well and incubated at 37°C overnight.
Treatments were performed as described above and the cells were
immediately incubated with 100 µl serum-free DMEM containing 10 µl
MTT (5 mg/ml) at 37°C for 2 h. Subsequently, the medium was
replaced with 100 µl DMSO. Following incubation for 20 min at room
temperature, the absorbance was read by measuring the optical
density (OD) at 490 nm in a microplate reader (Molecular Devices,
LLC, Sunnyvale, CA, USA). The 50% inhibitory concentration
(IC50) was calculated as follows: Inhibition rate
(%)=(OD490Cur-OD490blank)/(OD490control-OD490blank)
×100. Inhibition curves were drawn and the IC50 value of
Cur was calculated. The experiment was repeated three times.
TEM
HDFs in control, Cur, UVA and Cur + UVA groups were
harvested and fixed with 2.5% (v/v) glutaraldehyde for 24 h at 4°C,
collected by centrifugation (200 × g, 4°C for 5 min) and washed
twice with cold PBS. All samples were post-fixed in 1% osmium
tetroxide in 0.1 M phosphate buffer (pH 7.2) at 4°C for 1 h,
dehydrated through a graded ethanol series and embedded in Epon 812
at 60°C for 48 h. Ultrathin sections (70-nm) were cut and stained
with 0.5% uranyl acetate for 15 min and 3% lead citrate for 5 min
at room temperature and examined under a transmission electron
microscope (Tecnai G2; Thermo Fisher Scientific, Inc.).
UVA-induced cellular apoptosis
Cell apoptosis was analyzed by an Annexin V-FITC
Apoptosis Detection kit (BD Biosciences) according to the
manufacturer's protocol. HDF cells (5×105) were seeded
in 60-mm dishes and grew for 24 h, then pre-incubated with or
without Cur (5 µM) for 2 h. After UVA irradiation, cells were
collected by trypsinization and washed twice in cold
phosphate-buffered saline (PBS). Cells (1.0×106/ml) were
added to 1X combination buffer (100 µl). A total of 5 µl Annexin V
and 10 µl propidium iodide were then added. The mixture was
vortex-mixed and incubated in the dark for 15 min at room
temperature. Loading buffer (300 µl) was then added. Flow cytometry
was then employed using a FACS Calibur flow cytometer (BD
Biosciences). A minimum of 10,000 cells per sample was required and
analyzed. All experiments were conducted 3 times. The data was
analyzed by the BD FACStation software (2007; BD Biosciences).
Assessment of the intracellular levels
of ROS
Intracellular ROS levels were measured using the
fluorogenic compound, 2′,7′-dichlorofluorescin diacetate
(H2DCFDA; EMD Millipore, Billerica, MA, USA). The
1.5×104 cells were seeded in 96 well plates and
incubated for 30 min at 37°C with H2DCFDA at a final
concentration of 40 µM in the dark. When the non-fluorescent ester
H2DCFDA penetrates into cells and undergoes
deacetylation to dichloro-dihydro-fluorescein (DCFH) by cellular
esterases, the DCFH probe is rapidly oxidized to the highly
fluorescent compound 2′,7′-dichlorofluorescin by ROS. The
fluorescence intensity was measured using a microplate fluorescence
reader (excitation wavelength 488 nm and emission wavelength 521
nm; SpectraMax M3; Molecular Devices, LLC).
Measurement of the activities of
superoxide dismutase (SOD) and CAT, and the levels of MDA and
GSH
The activities of SOD, CAT, MDA and GSH were
measured by SOD assay kit (WST-1 method; cat. no. A001-3), CAT
assay kit (Ultraviolet method; cat. no. A007-2), Cell MDA assay kit
(colorimetric method; cat. no. A003-4) and reduced GSH assay kit
(colorimetric method; cat. no. A006-2), respectively. All assays
were conducted according to the manufacturer's protocols (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China).
Western blotting
Western blot analysis was performed to investigate
protein expression. Cells cultured in 6-well plates were washed
with PBS twice and harvested on the floor of the plate. Cells were
lysed using radioimmunoprecipitation assay lysis buffer
(Sigma-Aldrich; Merck KGaA) supplemented with a protease inhibitor
cocktail (Roche Diagnostics, Indianapolis, IN, USA). Cell lysates
were centrifuged at 13,000 × g for 15 min at 4°C and protein
concentration was determined with a BCA assay. Samples (10 µg
protein) were loaded onto 10% SDS-PAGE gels. Gel proteins were
electrophoretically transferred to polyvinylidene difluoride
membranes (PVDF; Thermo Fisher Scientific, Inc.). Membranes were
blocked with 3% non-fat dried milk in PBS at 4°C overnight.
Subsequently, PVDF membranes were incubated with specific primary
antibody solutions against antigens for 24 h at 4°C. Detection of
the primary antibodies was performed using secondary, horseradish
peroxidase-conjugated antibodies (Santa Cruz Biotechnology, Inc.).
Details of the antibodies used have been provided earlier in the
manuscript. Finally, the antigen-antibody complexes were visualized
by a Pierce ECL Western Blotting Substrate (Thermo Fisher
Scientific, Inc.; cat. no: 32106) and quantitated using the Versa
DOC system and Quantity One software (#1709600; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Statistical analysis was performed with SPSS version
18.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as the mean
± standard deviation of three independent experiments. The data
were analyzed with one-way analysis of variance, followed by
Dunnett's test and Tukey's test. For all tests, P<0.05 was
considered to indicate a statistically significant difference.
Results
Cur inhibits UVA-induced damage in
HDFs
Pre-experimental results demonstrated that the cell
viability and cell morphology were almost unchanged when treated
with the range of experimental concentrations of Cur, regardless of
the incubation time, indicating that Cur did not affect the growth
of HDFs (data not shown). Subsequently, the cell viability of HDFs
following exposure to UVA irradiation at 5, 10 and 15
J/cm2 intensities was determined. As demonstrated in
Fig. 1A, at the 10
J/cm2 dose of irradiation, cells underwent marked
morphologic alterations observed under inverted microscope,
including the development of a round cell shape, shrinkage and the
presence of blebs, which is an established marker of cellular
damage, on the surface of the cells. At the 15 J/cm2
intensities, the morphological alterations of the cell became more
severe. MTT assays demonstrated that the cell viability decreased
in a dosage-dependent manner in response to UVA irradiation
(Fig. 1B). Compared with the
control group (0 J/cm2), the cell viability of 10
J/cm2 group decreased by ~46% (P<0.01; Fig. 1B). However, 5 µM Cur pretreatment
for 2 h prior to exposure to 10 J/cm2 UVA irradiation
prevented a UVA-induced reduction in cell viability (Fig. 1C) and no evident alterations in
cell morphology were observed (data not shown), indicating that Cur
may protect against photodamage. Therefore, 5 µM Cur pretreatment
for 2 h combined with 10 J/cm2 UVA irradiation were
employed in the following experiments due to the optimal protective
efficiency of this concentration of Cur in HDFs.
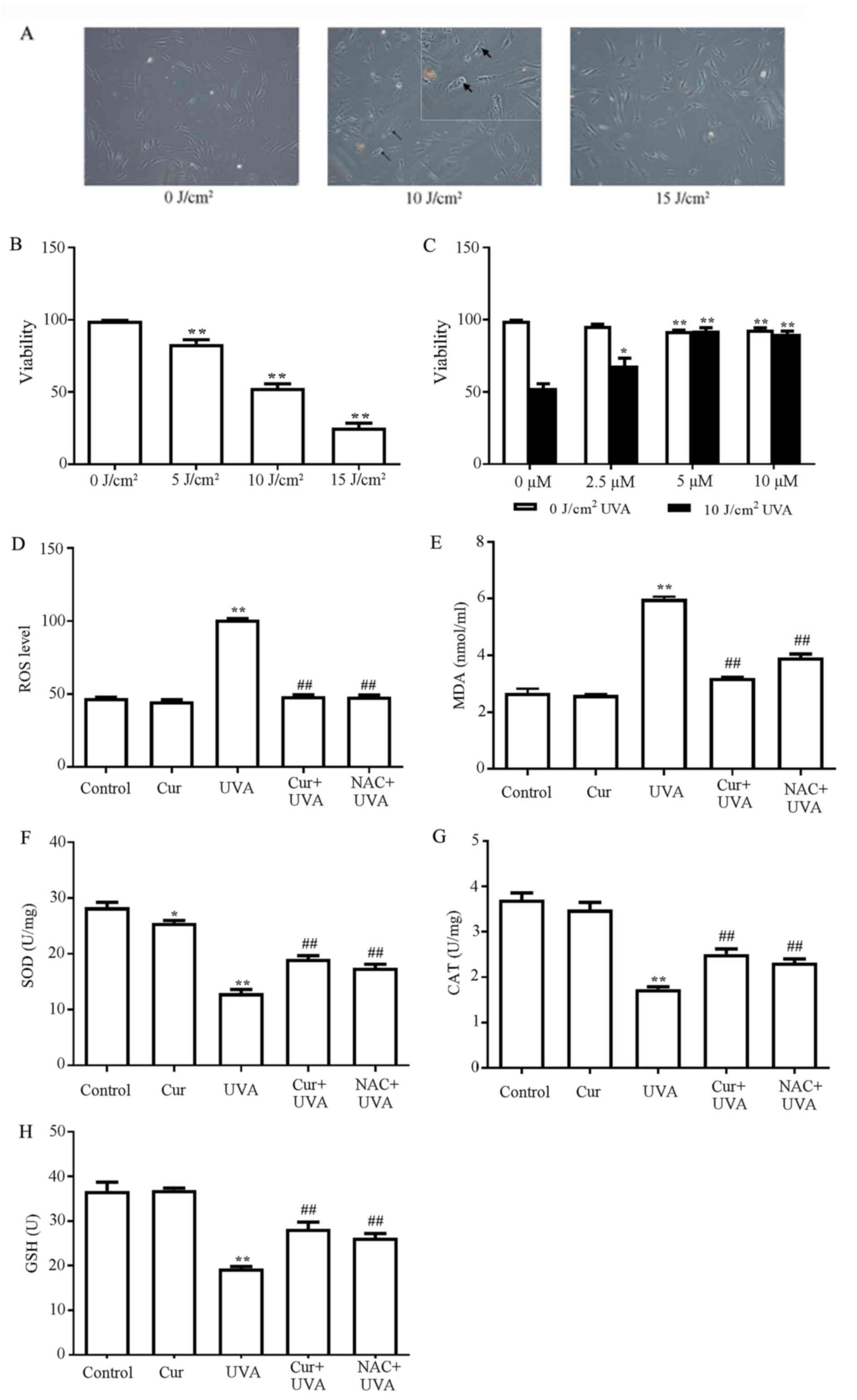 | Figure 1.Cur inhibits UVA-induced damage in
HDFs. (A) Cellular damage to HDFs induced by UVA irradiation at 0,
10 and 15 J/cm2 was observed by inverted microscopy,
Magnification, ×100. Blebs were shown in the 10 J/cm2
group (as indicate by the black arrowheads) and in the smaller
square image on the top right corner of middle image (10
J/cm2, ×400). (B) Cell viability of HDFs treated with
UVA irradiation at 0, 5, 10 and 15 J/cm2. **P<0.01
vs. 0 J/cm2. (C) Cell viability of HDFs that were
pretreated with 0, 2.5, 5 and 10 µM Cur for 2 h, followed by
exposure to 0 or 10 J/cm2 UVA. ##P<0.01
vs. control group without UVA irradiation; *P<0.05 and
**P<0.01 vs. control group with 10 J/cm2 UVA
irradiation (D) Inhibitory effect of Cur on UVA-induced ROS
accumulation in HDFs. The effects of Cur on UVA-induced alterations
in (E) MDA levels, (F) SOD activity, (G) CAT activity and (H) GSH
levels. All data are presented as the mean ± standard deviation,
n=3. *P<0.05 and **P<0.01 vs. control group;
##P<0.01 vs. UVA alone group. Cur, curcumin; UVA,
ultraviolet A; HDFs, human dermal fibroblasts; ROS, reactive oxygen
species; MDA, malondialdehyde; SOD, superoxide dismutase; CAT,
catalase; GSH, glutathione; NAC, N-acetyl cysteine. |
Cur inhibits the accumulation of
UVA-induced ROS in HDFs
As UVA radiation induces phototoxicity by generating
ROS, the present study investigated whether Cur may inhibit the
accumulation of UVA-induced ROS. Cells with pretreatment of 5 mM
NAC were employed as a positive control for ROS protection. The
results demonstrated that UVA irradiation alone led to a marked
increase in the levels of ROS compared with the control group
(P<0.01; Fig. 1D), confirming
that UVA induced the generation of ROS. However, compared with the
control group, no marked alterations in the fluorescent signals
were observed in cells treated with Cur alone, Cur + UVA
irradiation or the NAC positive control (Fig. 1D). These results indicated that Cur
inhibited the accumulation of UVA-induced ROS.
Effects of Cur on MDA levels and
antioxidant proteins
As cells have natural antioxidative systems as a
defense against high levels of ROS, the present study also measured
the levels of MDA, an indicator of ROS, and the activity/levels of
members of the cellular antioxidant defense system, including SOD,
CAT and GSH, in the cells with or without Cur pretreatment. NAC was
employed as the positive control described. The results
demonstrated that the levels of MDA were increased, and the
activities of SOD and CAT, and GSH levels, were significantly
reduced, in the UVA irradiation alone group compared with the
control group (P<0.01; Fig.
1E-G). However, these effects of UVA on MDA, SOD, CAT and GSH
were partially reversed in the Cur + UVA irradiation and NAC
positive control groups compared with the UVA irradiation alone
group (P<0.01; Fig. 1E-G),
indicating that Cur was able to restore the antioxidant capacities
of HDFs subjected to UVA irradiation.
Cur inhibits endoplasmic reticulum
(ER) stress in HDFs
As the accumulation of UVA-induced ROS often leads
to ER stress, the present study further investigated whether the ER
ultrastructure was altered under transmission electron microscopy.
The results demonstrated that the morphological hallmarks of ER
stress were represented by the expansion of the ER compartment, the
shift from membrane-bound ribosomes to free forms and an increase
in the large polysomes in the group treated with UVA irradiation
alone, and such ultrastructural changes of the ER were not observed
in the control, Cur alone or NAC positive control groups (Fig. 2A).
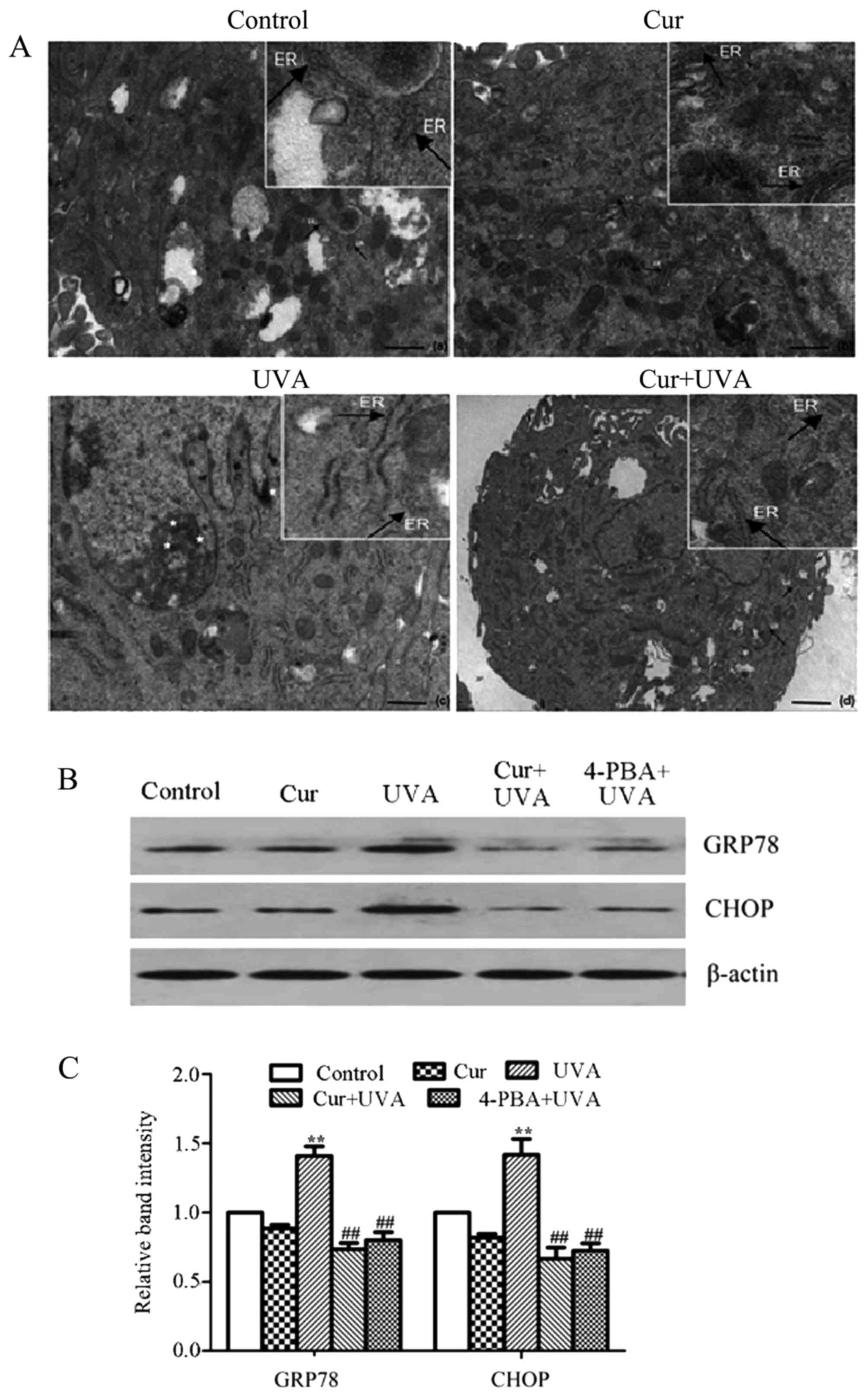 | Figure 2.Cur inhibits UVA-induced ER Stress in
human dermal fibroblasts. (A) Morphological alterations in the ER
were observed by transmission electron microscopy in control, Cur,
UVA and Cur + UVA groups. Scale bars: Control, Cur and UVA groups,
500 nm; Cur + UVA group, 1 µM (a different magnification is
presented for the last group as a clear image could not be attained
at the higher magnification). The areas of increased magnification
within each image were taken directly from the larger image
therefore the precise magnification cannot be stated. (B) Western
blot analysis was performed to investigate the protein expression
of GRP78 and CHOP using specific antibodies. (C) Relative
expression of GRP78 and CHOP proteins was quantified by
densitometry. All data are presented as the mean ± standard
deviation, n=3. **P<0.01 vs. control group;
##P<0.01 vs. UVA group. Cur, curcumin; UVA,
ultraviolet A; ER, endoplasmic reticulum; GRP78, glucose-regulated
protein 78; CHOP, C/EBP-homologous protein; 4-PBA, 4-phenylbutyric
acid. |
Furthermore, ER stress is closely associated with
the activation of the unfolded protein response (UPR), which
includes proteins such as GRP78 and CHOP (19). Therefore, the expression of these
two proteins were determined by western blotting. 4-PBA, an ER
stress inhibitor, was employed as the positive control. The results
of western blotting revealed that the protein expression of GRP78
and CHOP was significantly increased in the group treated with UVA
irradiation alone, compared with the control group (P<0.01;
Fig. 2B and C). However, the
levels of these two proteins were markedly decreased in the Cur +
UVA irradiation group and 4-PBA positive control group, compared
with the UVA irradiation alone group (P<0.01; Fig. 2B and C). These data indicated that
Cur may prevent ER stress in HDFs upon exposure to UVA
irradiation.
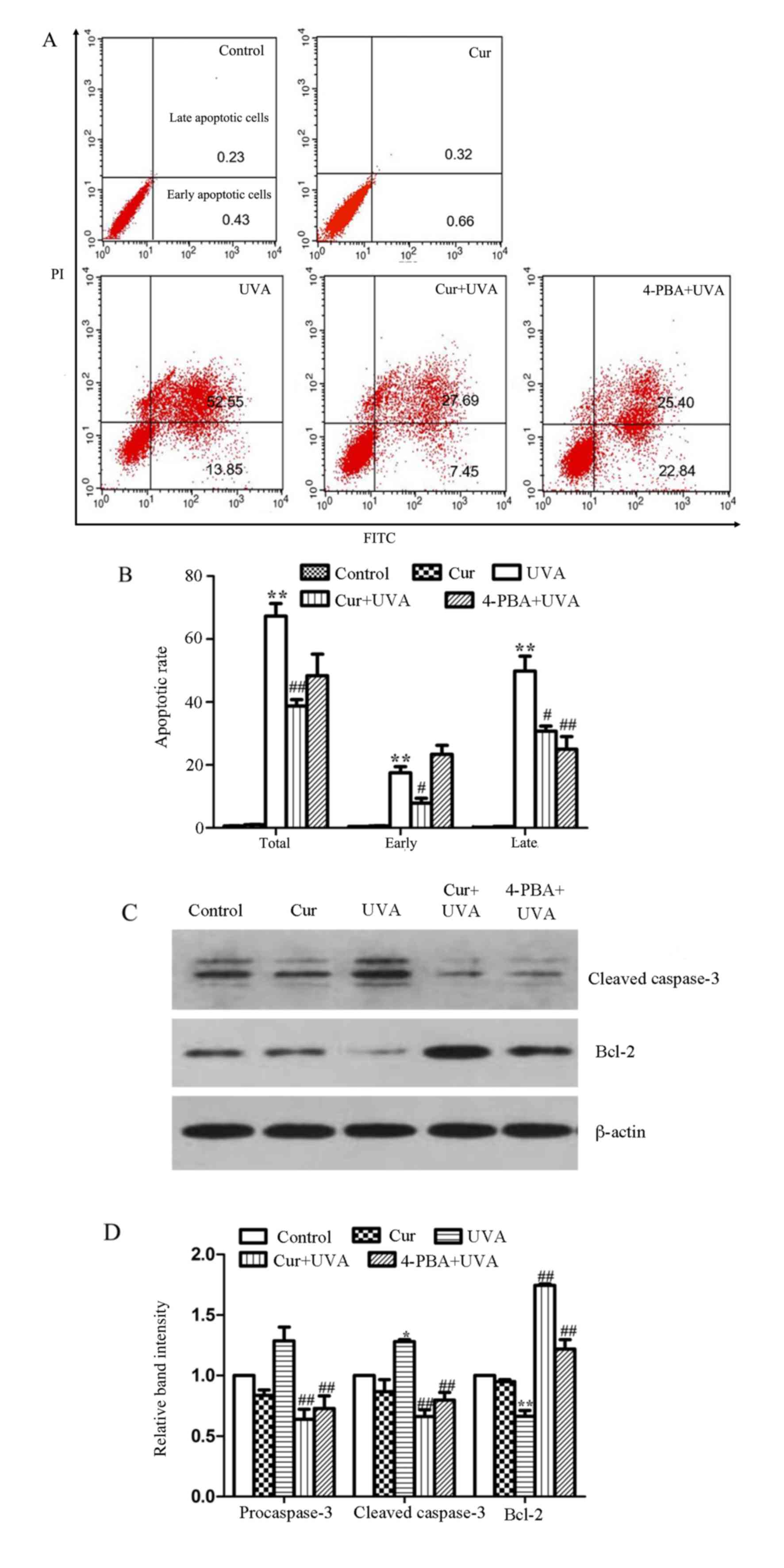
Cur protects HDFs against UVA-mediated
apoptosis
The results of annexin V-FITC/propidium iodide
staining followed by flow cytometry demonstrated that the total
apoptosis rate in the group treated with UVA irradiation alone
increased compared with the control group to ~66.4%; however, the
total values were reduced to ~38.2 and 48.2% in the Cur + UVA
irradiation and 4-PBA positive control groups, respectively, with
the Cur + UVA irradiation group exhibiting a significantly lower
total apoptosis value compared with the UVA irradiation alone group
(P<0.01; Fig. 3A and B).
Furthermore, the expression of proteins associated with apoptosis
was determined by western blotting. The results demonstrated that
the cleaved caspase-3 protein expression was upregulated and the
Bcl-2 expression downregulated in the group treated with UVA
irradiation alone compared with the control group, while the levels
of caspase-3 were decreased and Bcl-2 increased in the Cur + UVA
irradiation and 4-PBA positive control groups, compared with the
UVA irradiation alone group (Fig. 3C
and D). These data confirmed that Cur may attenuate apoptosis
induced by UVA, as demonstrated by increases in the expression of
the antiapoptotic protein, Bcl-2, and reduced caspase-3 levels.
Cur inhibits UVA-induced inflammation
and collagen metabolism
It is established that UVA radiation results in
inflammatory processes and the degradation of the collagen in the
skin. Therefore, the present study also investigated the effects of
Cur on the protein expression of NF-κB, MMP-1 and MMP-3, which are
proteins implicated in inflammation and collagen metabolism in
UVA-irradiated cells (20–22). NAC, which is also an inhibitor of
the NF-κB signaling pathway, was employed as a positive control.
The results of western blot analysis demonstrated that UVA
irradiation alone promoted NF-κB, MMP-1 and MMP-3 expression
compared with the control group (P<0.01; Fig. 4A and B). However, combined
treatment with Cur + UVA irradiation led to a moderate inhibitory
effect on the induction of the expression of these three proteins,
compared with the UVA irradiation alone group (P<0.05; Fig. 4A and B), indicating that Cur may
inhibit inflammation and restore normal collagen metabolism in HDFs
exposed to UVA.
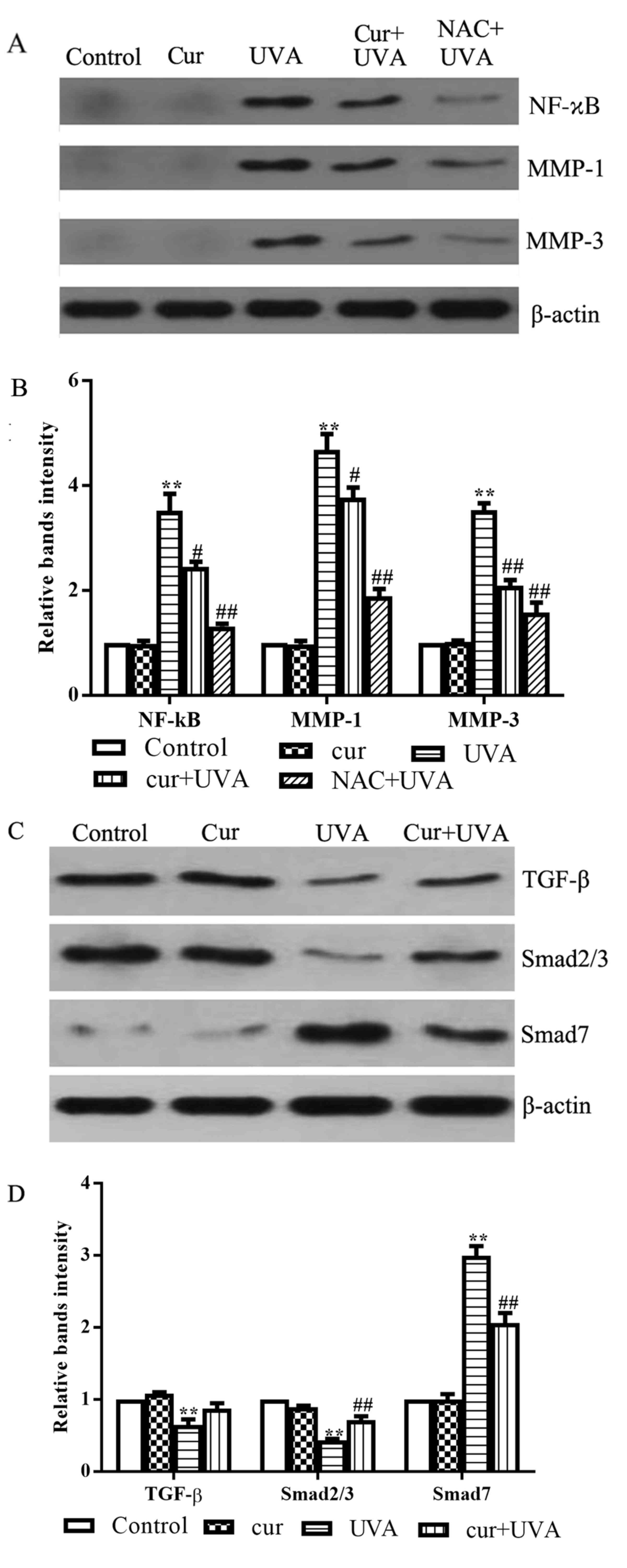 | Figure 4.Effects of Cur on the expression of
inflammation and collagen degradation-associated proteins in
UVA-irradiated human dermal fibroblasts. (A) Western blot analysis
was performed to investigate the protein expression of NF-κB, MMP-1
and MMP-3 using specific antibodies. (B) Relative expression of
NF-κB, MMP-1 and MMP-3 proteins was quantified by densitometry. (C)
Western blot analysis was performed to determine the protein
expression of TGF-β, Smad2/3 and Smad7 using specific antibodies.
(D) Relative expression of TGF-β, Smad2/3 and Smad7 proteins was
quantified by densitometry. All data are presented as the mean ±
standard deviation, n=3. **P<0.01 vs. control group;
#P<0.05 and ##P<0.01 vs. UVA group.
Cur, curcumin; UVA, ultraviolet A; NF-κB, nuclear factor-κB; MMP,
matrix metalloproteinase; TGF-β, transforming growth factor-β; NAC,
N-acetyl cysteine. |
Effects of Cur on TGF-ß signaling
As UVA irradiation damages cells, we hypothesized
that Cur may exert its protective effects on UVA-irradiated cells
by promoting fibrogenesis. Increasing evidence has demonstrated
that TGF-β signaling is a key pathway in the process of wound
repair, particularly for fibroblasts, and Smads are the regulators
of the TGF-β signaling pathway (23–25).
Therefore, the present study further investigated the protein
expression of TGF-β, Smad2/3 and Smad7 in the various treatment
groups. Western blotting results demonstrated that the levels of
TGF-β and Smad2/3 in the UVA irradiation group were lower compared
with the control group (P<0.01), while the levels of Smad7 were
increased compared with the control group (P<0.01; Fig. 4C and D). However, the levels of
Smad2/3 were upregulated, and Smad7 levels were downregulated, in
the Cur + UVA irradiation group compared with the UVA irradiation
alone group (P<0.01; Fig. 4C and
D). Interestingly, the level of TGF-β expression in Cur+UVA
group were not restored to the same degree as that of the control
and Cur group alone, indicating that increase of Smad2 and Smad3
expression might be independent from TGF-β but through another
signaling pathway, for example the mitogen-activated protein kinase
1 pathway (26,27). The detailed mechanism requires
further studies. These data indicated that Cur may stimulate the
repair of UVA-induced damage in HDFs through regulating the TGF-β
pathway and the Smads that regulate the TGF-β signaling pathway
upon exposure to UVA irradiation.
Discussion
UVA-induced damage to cells has been the subject of
numerous detailed studies. It is likely that UVA acts through an
indirect mechanism that involves the absorption of photons by
endogenous photosensitizers, which subsequently cause
photo-oxidation reactions to produce ROS (28–31).
Fortunately, various antioxidant defense systems are present in the
skin, including GSH, CAT and SOD, which protect the skin against
damage caused by UV-induced ROS to a certain extent (32). However, when ROS production exceeds
the capacity of endogenous antioxidant systems to protect the
target cell, oxidative stress develops, which has been associated
with the onset and development of various disease states, including
inflammation, photoaging and skin cancer (33). In the present study, UVA
irradiation caused cell death in a dosage-dependent manner and led
to the generation of increased levels of ROS. Additionally, an
increase in MDA, which is an indicator of ROS, and reduced
activities/levels of antioxidant proteins were also observed, which
may be a result of increased ROS scavenging by the antioxidant
systems upon UVA irradiation. When the cells were treated with Cur
followed by UVA irradiation, the results demonstrated that the rate
of cell death and ROS levels were decreased significantly, compared
with the UVA irradiation alone group. Furthermore, a decrease in
MDA also indicated the reduced levels of ROS following Cur
pretreatment, and the levels of GSH, and CAT and SOD activities,
were restored significantly following Cur pretreatment. These
results indicated that the protective effect of Cur on HDFs may be
due to an ability to scavenge UVA-induced ROS or an ability to
increase the de novo synthesis of GSH, as previously
reported by Sharma et al (34). Therefore, it may be plausible to
employ Cur to inhibit or scavenge UVA-induced ROS so as to minimize
the damage to cells.
ER stress, which may be characterized by the
accumulation of unfolded or misfolded proteins in the ER, may occur
due to alterations in calcium homeostasis, oxidative stress or
inhibition of proteasomal activity in cells (35). To cope with ER stress, cells have
developed a group of signal transduction pathways that are
collectively termed the UPR, which facilitate the adaption of cells
to ER stress (36,37). Generally, the UPR pathways are
mediated through three integral stress receptors, including
inositol-requiring enzyme 1, pancreatic ER kinase-like ER kinase
(PERK) and activating transcription factor 4 (ATF4) (36,37).
For example, under ER stress conditions, GRP78 becomes dissociated
from PERK, which leads to PERK activation. Activated PERK promotes
the translation of ATF4, which subsequently upregulates the
expression of CHOP (38,39). The present study demonstrated that
the levels of the ER stress-associated proteins, GRP78 and CHOP,
were increased significantly after HDFs were subjected to the UVA
irradiation, confirming that ER stress was triggered by UVA.
Furthermore, Cur pretreatment was able to downregulate the
expression of GRP78 and CHOP compared with UVA-exposed cells
without Cur pretreatment, indicating that Cur may protect HDFs
against UVA-induced ER stress. To the best of our knowledge, no
previous reports have demonstrated that Cur may attenuate
UVA-induced ER stress by regulating the expression of proteins
involved in the UPR.
It has been previously demonstrated that UVA
radiation induced inflammatory responses as a result of the
generation of ROS in UVA-exposed skin cells (40). NF-κB has an important role in the
development of inflammation and may serve as a marker of
inflammation. The results of the current study clearly demonstrated
that the expression of NF-κB was increased in HDFs treated with UVA
irradiation alone, but was decreased in UVA-exposed HDFs with Cur
pretreatment, indicating that Cur may inhibit the UVA-induced
inflammation response. Furthermore, the results of flow cytometry
results revealed that the rate of apoptosis was markedly increased
following UVA irradiated alone, indicating that UVA induced cell
damage and apoptosis in HDFs. Western blotting further confirmed
the occurrence of the apoptosis following UVA, as reduced levels of
the anti-apoptotic protein, Bcl-2, and increased levels of cleaved
caspase-3, were observed following UVA treatment alone. However,
pretreatment with Cur followed by UVA irradiation resulted in
reduced apoptosis levels, which was also associated with reduced
caspase-3 and increased Bcl-2 protein expression, compared with the
UVA irradiation alone group, indicating that Cur may inhibit
UVA-induced cell apoptosis by upregulating Bcl-2 and downregulating
caspase-3 enzyme expression.
In addition to those results described above, other
studies have reported that ROS was involved in the UVA-dependent
induction of MMP-1, MMP-2 and MMP-3 mRNA and protein expression
(41–44). In the present study, a significant
decrease in the MMP-1 and MMP-3 levels was observed in the Cur +
UVA irradiation group compared with the group treated with UVA
irradiation alone. This indicates that Cur may exert its protective
effect on the survival of cells by altering the matrix environment
and regulating collagen metabolism. Alterations in collagen have
been thought to be the characteristic alterations of photoaging
(31). MMPs are involved in human
skin photoaging through UV-induced collagen synthesis and
degradation (45). The
upregulation of MMPs, including MMP-1, MMP-2, as well as MMP-3, are
mainly responsible for degrading of the collagen and elastin
(46). TGF-β is the major
regulator that participates in wound repair, particularly within
fibroblasts, in human skin (47,48).
The duration and intensity of the TGF-β signaling pathway has been
reported to be controlled through the phosphorylation of
receptor-regulated Smad proteins (49–52);
TGF-β signaling is positively regulated by Smad2/3 and negatively
regulated by Smad7. The results of the current study demonstrated
that the protein expression of TGF-β and Smad2/3 were reduced,
while Smad7 expression was significantly increased, following UVA
exposure, compared with the control group. However, in cells
pretreated with Cur followed by UVA irradiation, the expression of
TGF-β and Smad2/3 was partially restored and Smad7 levels were
reduced significantly, compared with the UVA irradiation alone
group, indicating that Cur may protect HDFs against UVA-induced
damage by enhancing fibroblast healing. These results demonstrated
that TGF-β, Smad2/3 and Smad7 may have potential as novel targets
through which Cur prevents UVA-induced photoaging in
fibroblasts.
In conclusion, the present study demonstrated that
Cur reduced the accumulation of UVA-induced ROS, restored the
activities of antioxidative enzymes and attenuated ER stress,
inflammation and apoptotic signaling. Additionally, Cur may also
protect cells from photoaging by suppressing collagen degradation
and enhancing collagen synthesis. All of these effects are helpful
in the prevention of skin photoaging, indicating that Cur may be an
effective therapeutic chemical for preventing the occurrence of
UVA-induced skin aging and promoting cell repair. To the best of
the authors' knowledge, the present study is the first to report
that Cur may be beneficial in controlling the development of
photoaging and for the promotion of the repair of UVA-induced
damage. The results of the current study may lead to the
development and application of Cur in the prevention of skin aging
and improving the appearance of skin.
Acknowledgements
Not applicable.
Funding
The present study was supported by the CMA-L'OREAL
China Skin Grant 2014 (grant no. S201411117), the Changzhou
High-Level Medical Talents Training Project (grant no. 2016CZBJ034)
and the Jiangsu Province's Preventive Medicine Science Foundation
(grant no. Y2013020).
Availability of data and materials
All data generated or analyzed during this study is
included in this published article.
Authors' contributions
Conceived and designed the experiments: XLiu, RZ and
XL. Performed the experiments: HS, AT and CX. Analyzed the data:
YL. Wrote and revised manuscript: XL and XLiu.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Soochow University (Changzhou, China) and written
informed consent for the use of the child's foreskin in this study
was obtained from the parent of the child.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Parrish JA, Jaenicke RF and Anderson RR:
Erythema and melanogenesis action spectra of normal human skin.
Photochem Photobiol. 36:187–191. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller DL and Weinstock MA: Nonmelanoma
skin cancer in the United States: Incidence. J Am Acad Dermatol.
30:774–778. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kochevar IE: Molecular and cellular
effects of UV radiation relevant to chronic photodamageBilchrest
BA: Photodamage. Blackwell, MA: pp. 51–58. 1995
|
|
4
|
Jiang Y, Rabbi M, Kim M, Ke C, Lee W,
Clark RL, Mieczkowski PA and Marszalek PE: UVA generates pyrimidine
dimers in DNA directly. Biophys J. 96:1151–1158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heng MC: Curcumin targeted signaling
pathways: Basis for anti-photoaging and anti-carcinogenic therapy.
Int J Dermatol. 49:608–622. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brugè F, Damiani E, Puglia C, Offerta A,
Armeni T, Littarru GP and Tiano L: Nanostructured lipid carriers
loaded with CoQ10: Effect on human dermal fibroblasts under normal
and UVA-mediated oxidative conditions. Int J Pharm. 455:348–356.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Offord EA, Gautier JC, Avanti O, Scaletta
C, Runge F, Krämer K and Applegate LA: Photoprotective potential of
lycopene, beta-carotene, vitamin E, vitamin C and carnosic acid in
UVA-irradiated human skin fibroblasts. Free Radic Biol Med.
32:1293–1303. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wolosik K, Zareba I, Surazynski A and
Markowska A: The possible pre- and post-UVA radiation protective
effect of amaranth oil on human skin fibroblast cells. Pharmacogn
Mag. 13 Suppl 2:S339–S343. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jagetia GC and Aggarwal BB: ‘Spicing up’
of the immune system by curcumin. J Clin Immunol. 27:19–35. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ammon H and Wahl MA: Pharmacology of
Curcuma longa. Planta Med. 57:1–7. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tilak J, Banerjee M, Mohan H and
Devasagayam PA: Antioxidant availability of turmeric in relation to
its medicinal and culinary uses. Phytother Res. 18:798–804. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Azuine MA and Bhide SV: Chemopreventive
effect of turmeric against stomach and skin tumors induced by
chemical carcinogens in Swiss mice. Nutr Cancer. 17:77–83. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang M, Smart RC, Wong CQ and Conney AH:
Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and
ferulic acid on tumor promotion in mouse skin by
12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 48:5941–5946.
1988.PubMed/NCBI
|
|
14
|
Inano H, Onoda M, Inafuku N, Kubota M,
Kamada Y, Osawa T, Kobayashi H and Wakabayashi K: Chemoprevention
by curcumin during the promotion stage of tumorigenesis of mammary
gland in rats irradiated with gamma-rays. Carcinogenesis.
20:1011–1018. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Inano H, Onoda M, Inafuku N, Kubota M,
Kamada Y, Osawa T, Kobayashi H and Wakabayashi K: Potent preventive
action of curcumin on radiation-induced initiation of mammary
tumorigenesis in rats. Carcinogenesis. 21:1835–1841. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hwang BM, Noh EM, Kim JS, Kim JM, You YO,
Hwang JK, Kwon KB and Lee YR: Curcumin inhibits UVB-induced matrix
metalloproteinase-1/3 expression by suppressing the MAPK-p38/JNK
pathways in human dermal fibroblasts. Exp Dermatol. 22:358–379.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsai KD, Lin JC, Yang SM, Tseng MJ, Hsu
JD, Lee YJ and Cherng JM: Curcumin protects against UVB-induced
skin cancers in SKH-1 hairless mouse: Analysis of early molecular
markers in carcinogenesis. Evid Based Complement Alternat Med.
2012:5939522012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li JK and Lin-Shiau SY: Mechanisms of
cancer chemoprevention by curcumin. Proc Natl Sci Counc Repub China
B. 25:pp. 59–66. 2001; PubMed/NCBI
|
|
19
|
Szegezdi E, Logue SE, Gorman AM and Samali
A: Mediators of endoplasmic reticulum stress-induced apoptosis.
EMBO Rep. 7:880–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fisher GJ, Wang Z, Datta SC, Varani J,
Kang S and Voorhees JJ: Pathophysiology of premature skin aging
induced by ultraviolet light. N Engl J Med. 337:1419–1428. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Polte T and Tyrrell RM: Involvement of
lipid peroxidation and organic peroxides in UVA-induced matrix
metalloproteinase-1 expression. Free Radic Biol Med. 36:1566–1574.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brennan M, Bhatti H, Nerusu KC,
Bhagavathula N, Kang S, Fisher GJ, Varani J and Voorhees JJ: Matrix
metalloproteinase-1 is the major collagenolytic enzyme responsible
for collagen damage in UV-irradiated human skin. Photochem
Photobiol. 78:43–48. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu W, Wang DR and Cao YL: TGF-beta: A
fibrotic factor in wound scarring and a potential target for
anti-scarring gene therapy. Curr Gene Ther. 4:123–136. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kopp J, Preis E, Said H, Hafemann B,
Wickert L, Gressner AM, Pallua N and Dooley S: Abrogation of
transforming growth factor-beta signaling by SMAD7 inhibits
collagen gel contraction of human dermal fibroblasts. J Biol Chem.
280:21570–21576. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lan HY: Diverse roles of TGF-β/Smads in
renal fibrosis and inflammation. Int J Biol Sci. 7:1056–1067. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li JH, Huang XR, Zhu HJ, Oldfield M,
Cooper M, Truong LD, Johnson RJ and Lan HY: Advanced glycation end
products activate Smad signaling via TGF-beta-dependent and
independent mechanisms: Implications for diabetic renal and
vascular disease. Faseb J. 18:176–178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chung AC, Zhang H, Kong YZ, Tan JJ, Huang
XR, Kopp JB and Lan HY: Advanced glycation end-products induce
tubular CTGF via TGF-beta-independent Smad3 signaling. J Am Soc
Nephrol. 21:249–260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Douglas RH, Moan J and Rontb G: Light in
Biology and Medicine. Plenum; New York 2: 1991, View Article : Google Scholar
|
|
29
|
Cadet J, Berger M, Douki T, Morin B, Raoul
S, Ravanat JL and Spinelli S: Effects of UV and visible radiation
on DNA-final base damage. Biol. Chem. 378:1275–1286. 1997.
|
|
30
|
Cadet J, Berger M, Decarroz C, Wagner JR,
van Lier JE, Ginot YM and Vigny P: Photosensitized reactions of
nucleic acids. Biochimie. 68:813–834. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fisher GJ, Kang S, Varani J, Bata-Csorgo
Z, Wan Y, Datta S and Voorhees JJ: Mechanisms of photoaging and
chronological skin aging. Arch Dermatol. 138:1462–1770. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Afaq F and Mukhtar H: Effects of solar
radiation on cutaneous detoxification pathways. J Photochem
Photobiol B. 63:61–69. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shindo Y, Witt E and Packer L: Antioxidant
defense mechanisms in murine epidermis and dermis and their
responses to ultraviolet light. J Invest Dermatol. 100:260–265.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sharma RA, Ireson CR, Verschoyle RD, Hill
KA, Williams ML, Leuratti C, Manson MM, Marnett LJ, Steward WP and
Gescher A: Effects of dietary curcumin on glutathione S-transferase
and malonaldehyde-DNA adducts in rat liver and colon mucosa:
Relationship with drug levels. Clin Cancer Res. 7:1452–1458.
2001.PubMed/NCBI
|
|
35
|
Zhang KZ and Kaufman RJ: From
endoplasmic-reticulum stress to the inflammatory response. Nature.
454:455–462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schröder M and Kaufman RJ: The mammalian
unfolded protein response. Annu Rev Biochem. 74:739–789. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lu PD, Harding HP and Ron D: Translation
reinitiation at alternative open reading frames regulates gene
expression in an integrated stress response. J Cell Biol.
167:27–33. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Harding HP, Novoa I, Zhang Y, Zeng H, Wek
R, Schapira M and Ron D: Regulated translation initiation controls
stress-induced gene expression in mammalian cells. Mol Cell.
6:1099–1108. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Soter NA: Sunburn and suntan: Immediate
manifestations of photodamageGilchrest BA: Photodamage. Cambridge
(MA): Blackwell Science; pp. 12–25. 1995
|
|
41
|
Herrmann G, Wlaschek M, Bolsen K, Prenzel
K, Goerz G and Scharffetter-Kochanek K: Pathogenic implication of
matrix-metalloproteinases (MMPs) and their counteracting inhibitor
TIMP-1 in the cutaneous photodamage of human porphyria cutanea
tarda (PCT). J Invest Dermatol. 107:398–403. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wlaschek M, Briviba K, Stricklin GP, Sies
H and Scharffetter-Kochanek K: Singlet oxygen may mediate the
ultraviolet A-induced synthesis of interstitial collagenase. J
Invest Dermatol. 104:194–198. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Scharffetter-Kochanek K, Wlaschek M,
Briviba K and Sies H: Singlet oxygen induces collagenase expression
in human skin fibroblasts. FEBS Lett. 331:304–306. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wenk J, Brenneisen P, Wlaschek M, Poswig
A, Briviba K, Oberley TD and Scharffetter-Kochanek K: Stable
overexpression of manganese superoxide dismutase in mitochondria
identifies hydrogen peroxide as a major oxidant in the
AP-1-mediated induction of matrix-degrading metalloproteinase-1. J
Biol Chem. 274:25869–25876. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pandel R, Poljšak B, Godic A and Dahmane
R: Skin photoaging and the role of antioxidants in its prevention.
ISRN Dermatol. 2013:9301642013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Erman H, Gelisgen R, Cengiz M, Tabak O,
Erdenen F and Uzun H: The association of vascular endothelial
growth factor, metalloproteinases and their tissue inhibitors with
cardiovascular risk factors in the metabolic syndrome. Eur Rev Med
Pharmacol Sci. 20:1015–1022. 2016.PubMed/NCBI
|
|
47
|
Kryger ZB, Sisco M, Roy NK, Lu L,
Rosenberg D and Mustoe TA: Temporal expression of the transforming
growth factor-Beta pathway in the rabbit ear model of wound healing
and scarring. J Am Coll Surg. 205:78–88. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Leask A and Abraham DJ: TGF-beta signaling
and the fibrotic response. FASEB J. 18:816–827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Massagué J: TGF-beta signal transduction.
Annu Rev Biochem. 67:753–791. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Massagué J and Chen YG: Controlling
TGF-beta signaling. Genes Dev. 14:627–644. 2000.PubMed/NCBI
|
|
51
|
Massagué J and Wotton D: Transcriptional
control by the TGF-beta/Smad signaling system. EMBO J.
19:1745–1754. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Piek E, Heldin CH and Ten Dijke P:
Specificity, diversity, and regulation in TGF-beta superfamily
signaling. FASEB J. 13:2105–2124. 1999. View Article : Google Scholar : PubMed/NCBI
|