Introduction
Traumatic brain injury (TBI) is a leading cause of
mortality and morbidity worldwide (1). TBI patients suffer permanent
neurological and psychological disabilities that represent a
significant social and economic burden. TBI-induced deficits are
due to primary (mechanical impact) and secondary (delayed)
injuries. It is essential to elucidate the biological cascades that
drive the delayed secondary phase subsequent to TBI (2). Despite considerable progress being
made in animal models and preclinical research in recent years,
there are currently no available therapeutic strategies in clinical
practice for TBI.
The flavonoid quercetin
(3,5,7,30,40-pentahydroxyflavone), one of the most widely
distributed flavonoids in fruits and vegetables, is known to be a
potent anti-oxidant and free radical scavenger (3). A number of studies have demonstrated
that quercetin possesses anti-inflammatory, anti-coagulation,
anti-ischemic and anti-cancer activities (4–6). In
addition, Yang et al (7)
suggested that in TBI rats, quercetin improves cognitive function
owing to its neuroprotective action via the inhibition of oxidative
stress, leading to a reduced inflammatory response, thereby
reducing neuronal death. It was hypothesized that post-injury
treatment with quercetin may exert a therapeutic effect against
TBI. Our previous study (8)
provided results similar to Yang, however, the specific molecular
mechanisms requires further study. The present study also analyzed
the expression of Akt serine/threonine protein kinase,
phosphorylated (p)-Akt, extracellular signal-regulated kinase
(ERK)1/2 and p-ERK1/2 in injured neurons in the cortex, which
serves an important role in signal transduction following TBI. The
aim of the present study was to investigate the protective effects
of quercetin on neurological impairment and spatial cognitive
function after TBI in rat model. It further examined whether
quercetin could attenuate neuronal apoptosis via PI3K/Akt and
ERK1/2 signaling, thereby reducing brain damage.
Materials and methods
Animals
The Institutional Animal Care and Use Committee of
Hebei Medical University (Shijiazhuang, China) approved all
experiments, which were performed according to the guidelines of
the National Institutes of Health (NIH) Guide for the Care and Use
of Laboratory Animals (NIH Publications no. 80–23, revised 1978;
NIH, Bethesda, MD, USA). All efforts were made to minimize the
number of animals used and their suffering. A total of 75 male
Sprague Dawley rats, weighing 280–320 g (6–8 weeks), were supplied
from the Experimental Animal Center of Hebei Medical University
(Shijiazhuang, Hebei, China). All animals were housed in plastic
boxes at a temperature of 22–24°C, 50% humidity and were provided
food and water ad libitum under a 12-h reversed light-dark
cycle.
Model of TBI
The TBI model was produced using a modified
weight-drop device (9). Following
10% chloral hydrate anesthesia (3 ml/kg), a midline longitudinal
incision was performed to expose the skull between bregma and
lambda suture lines. A steel disk (diameter, 10 mm; thickness, 3
mm) was adhered to the skull using dental acrylic. Animals were
moved onto a foam mattress underneath a weight-drop device where a
weight of 450 g fell freely through a vertical tube from 1.5 m onto
the steel disk. Sham-operated animals underwent the same surgical
procedure without weight-drop impact. Rats were placed on heat pads
(37°C) for 2–4 h to maintain normal body temperature during the
recovery period.
Groups and drug administration
All rats were randomly arranged into 3 groups as
follows: Sham group (n=25); TBI group (n=25); and TBI + quercetin
group (Que; n=25). Quercetin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany; dissolved in 0.9% saline solution) was administered
intraperitoneally at a dose of 50 mg/kg at 30 min, 12 and 24 h
following the TBI insult.
In addition, 15 rats (5/group) underwent behavioral
testing. All investigations were blinded and the animal groupings
were revealed only at the end of the behavioral and histological
analyses.
Evaluation of brain edema
Brain water content was determined at day 1, 3 and 5
following TBI (45 rats, 15/group). In order to reduce the use of
animal population, at 5 days after TBI, 15 rat brains were taken
from rats which had completed the behavioral experiments. Rat
brains were separated and weighed immediately with a chemical
balance to obtain the wet weight (WW). Following drying in a
desiccating oven for 24 h at 100°C, dry tissues were weighed again
to obtain the constant dry weight (DW). The percentage of water in
the tissues was calculated according to the following formula:
brain water (%)=[(WW-DW)/WW] ×100.
Recovery of motor function
The neurobehavioral status of the rats was evaluated
at day 1, 3 and 5 after TBI using a set of 10 tasks, collectively
termed the Neurological Severity Score (NSS) (10), which test reflexes, alertness,
coordination and motor abilities. A point is awarded for failure to
perform a particular task; therefore, a score of 10 reflects
maximal impairment, whereas a healthy rat scores 0. Post-injury,
NSS was evaluated at day 1, 3 and 5. Each animal was assessed by an
observer who was blinded to the treatment group of the animal. The
difference between the initial NSS (performed at day 1) and that at
any subsequent time point was calculated for each rat, and this
value (ΔNSS) reflects the spontaneous or treatment-induced recovery
of motor function.
Hematoxylin and eosin (H&E)
staining and neuron count
At 24 h post-TBI, 15 rats (5/group) were
anesthetized as described above, and perfused intracardially with
isotonic sodium chloride solution, followed by 4% (w/v)
paraformaldehyde in 0.1M sodium phosphate buffer (pH=7.4). The
brains were removed and fixed for 48 h in 4% (w/v) paraformaldehyde
at 22–24°C. Following fixation, brains were embedded in paraffin,
and sliced into 6 µm coronal sections at the level of the bregma
and stained with hematoxylin (20 min) and eosin (3 sec) at 22–24°C.
The staining was visualized by light microscopy at ×400
magnification (Olympus Corporation, Tokyo, Japan). The surviving
and dying neurons per mm2 cortex were quantified (the
nuclei of dead cells were shrunk and thickened).
Immunohistochemical
As for HE staining, the brain tissues were fixed,
embedded and cut into 6 µm slices. Sections were deparaffinized
with xylene and rehydrated at 60°C with graded ethanol (100, 95,
90, 80 and 70%). Endogenous peroxidase activity was blocked using
3% hydrogen peroxide for 30 min at room temperature, followed by 5%
normal goat serum (AR0009; Wuhan Boster Biological Technology,
Ltd., Wuhan, China) for 1 h to block non-specific protein
interactions. The sections were subsequently incubated overnight at
4°C with an anti-activated caspase3 antibody (1:500; AB2302; Abcam,
Cambridge, UK). Following three washes with PBS, the slides were
incubated with a biotinylated goat anti-mouse horseradish
peroxidase conjugated secondary antibody (1:100, BA1051; Wuhan
Boster Biological Technology Ltd.) for 2 h at room temperature. The
sections were washed with PBS again, and incubated with the
kit-provided horseradish peroxidase (HRP)-streptavidin for 30 min
at room temperature. The peroxidase reaction was visualized using
0.05% diaminobenzidine + 0.01% hydrogen peroxide.
Immunohistochemical procedures were performed in accordance with
the manufacturer's protocols. The positive cells were visualized by
a microscope at ×100 magnification.
Western blotting
The rats were deeply anesthetized as described above
24 h following TBI. The cortical region of the rat brain was
rapidly isolated. The segments were immediately stored at −80°C for
further analysis. Total protein samples were extracted from brain
tissues using whole cell lysis buffer (WD2072; Beyotime Institute
of Biotechnology, Shanghai, China) a bicinchoninic acid protein
assay kit (P10310; Beyotime Institute of Biotechnology) was used to
determine the protein concentration of each sample. The homogenate
was heated to 100°C for 10 min and centrifuged again at 15,294 × g
for 1 min at 22–24°C. Equal amounts (80 µg) of protein were
subjected to Tris-HCl SDS-PAGE on 8 and 12% gels (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) for 30 min at 70 V and 60
min at 120 V. Following electrophoresis, the proteins were
transferred onto polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA) at 300 mA for immunoblotting. Following
blocking with 5% skimmed milk for 2 h at room temperature,
membranes were incubated overnight at 4°C with the following
primary antibodies: Rabbit anti-activated Caspase3 (AB2302;
1:1,000; Abcam), rabbit anti-Akt (AB81283; 1:1,000; Abcam), rabbit
anti-p-Akt (AB38449; 1:1,000; Abcam), rabbit anti-ERK1/2 (AB17942;
1:1,000; Abcam), rabbit anti-p-ERK1/2 (AB214362; 1:1,000; Abcam),
and rabbit anti-β-actin (AB227387; 1:5,000; Abcam). Following three
washes in TBS-Tween 20, membranes were incubated with a horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G (BL003A;
1:5,000; EMD Millipore) for 2 h at room temperature. Protein bands
were visualized using an enhanced chemiluminescence kit (Beyotime
Institute of Biotechnology). Band density was quantified via
detection with a DNR Micro Chemi chemiluminescence gel imaging
system (DNR Bio-Imaging Systems Ltd., Neve Yamin, Israel). Each
band density was normalized to the density of β-actin.
Statistical analysis
SPSS software version 16.0 (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. A statistical evaluation of
the data was performed using a one-way analysis of variance,
followed by post hoc comparisons using the least significant
difference or Kruskal-Wallis method. All experimental data are
expressed as the mean ± standard error of the mean, and P<0.05
was considered to indicate a statistically significant
difference.
Results
Quercetin attenuates TBI-induced
cerebral edema
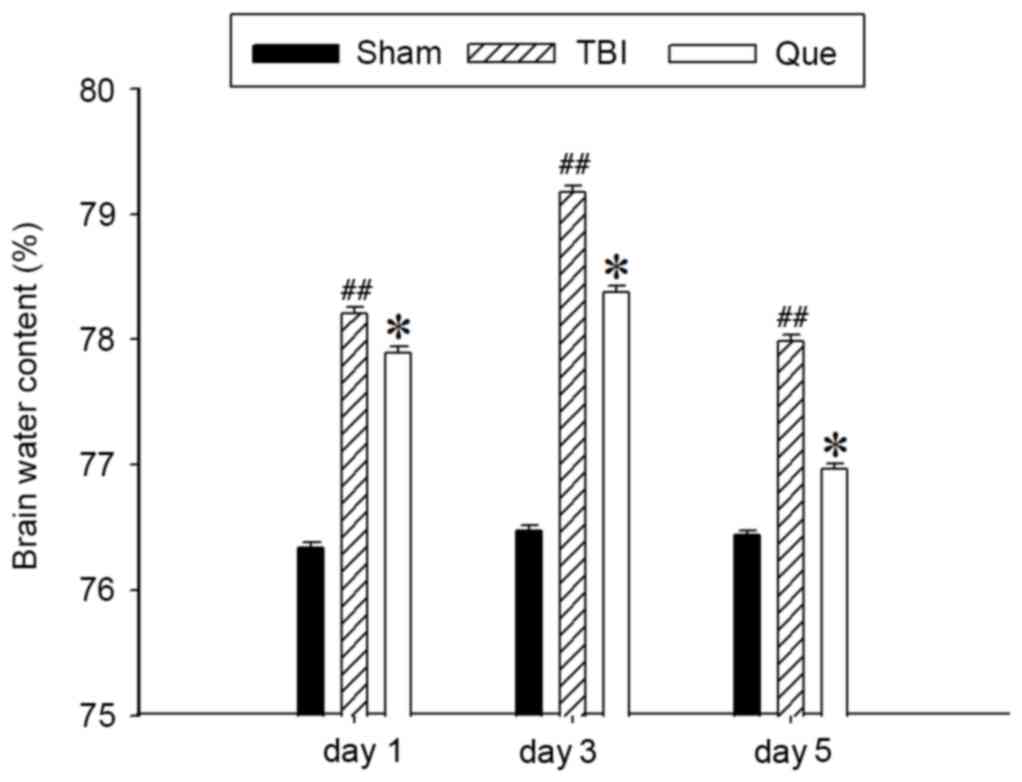
Following injury, brain edema leads to an elevation
in intracranial pressure, reducing cerebral perfusion pressure and
brain oxygenation. Edema is associated with the resultant pathology
following TBI (11). In order to
evaluate the effects of quercetin on brain edema, the wet-dry
weight method was used in the present study to evaluate brain edema
at day 1, 3 and 5 after TBI. As presented in Fig. 1, cerebral water content was
significantly increased at day 1, 3 and 5 after TBI compared with
the sham group (P<0.01). However, treatment with quercetin
attenuated this increased compared with the TBI model group
(P<0.05).
Quercetin attenuates TBI-induced motor
deficits
Motor deficit recovery was expressed as ΔNSS in
present study. Alterations in the functional recovery of rats at
day 1, 3 and 5 are depicted in Fig.
2. It was observed that rats exhibited marked motor deficits
following TBI. Post-injury administration of quercetin
significantly improved the motor function between day 1 and 5 after
trauma compared with the TBI group (P<0.05).
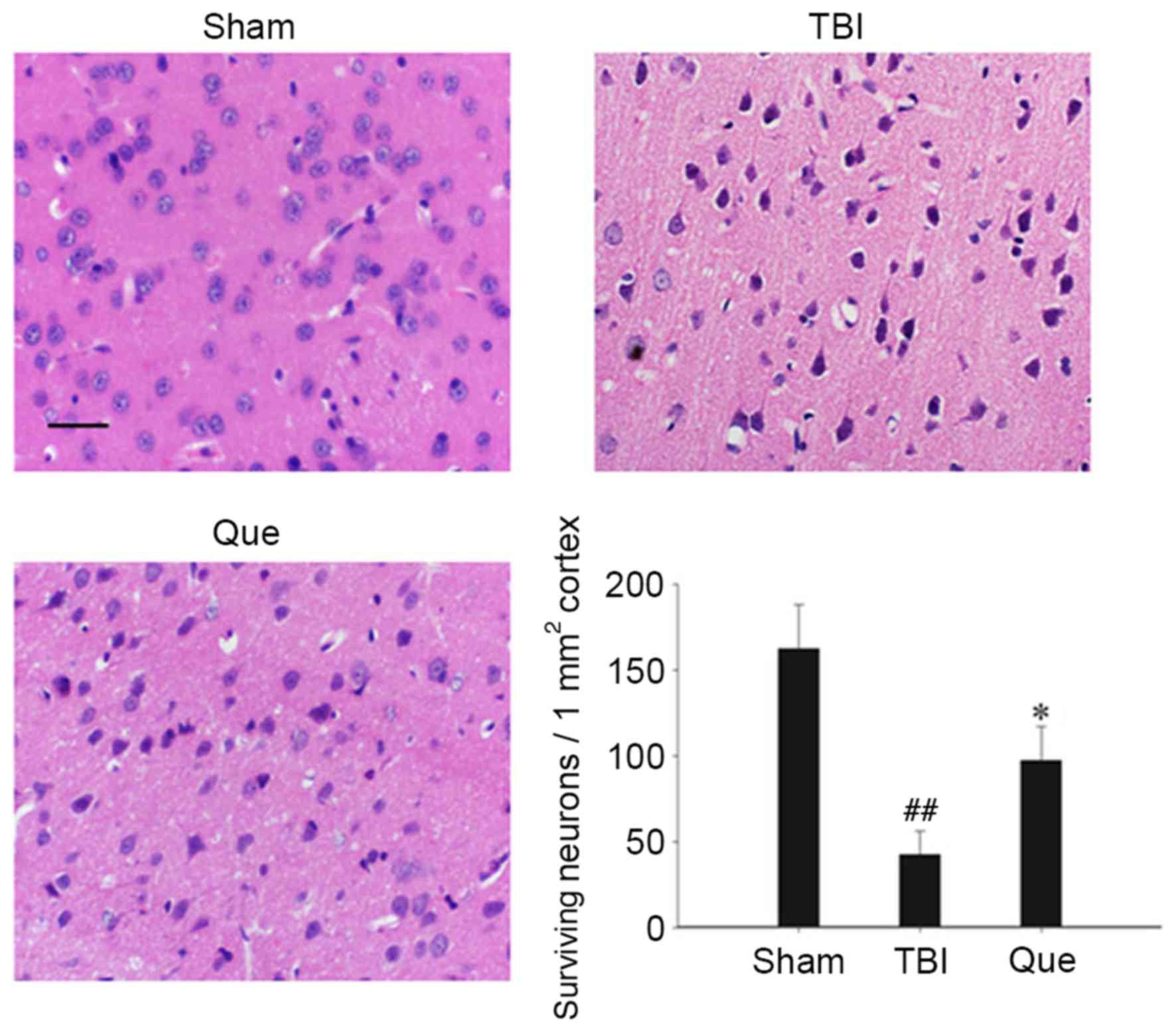
Quercetin increases neuronal survival
in the cortical region of brain
Cortical regions of brains were collected and
neuronal survival was assessed at 24 h via H&E staining. As
presented in Fig. 3, the nuclei of
normal neurons were round and stained pale, whereas nuclei of dying
neurons were pyknotic and darkly stained following TBI. The
survival rate of neurons in the quercetin-treated group was
significantly improved compared with that of the TBI group
(P<0.05).
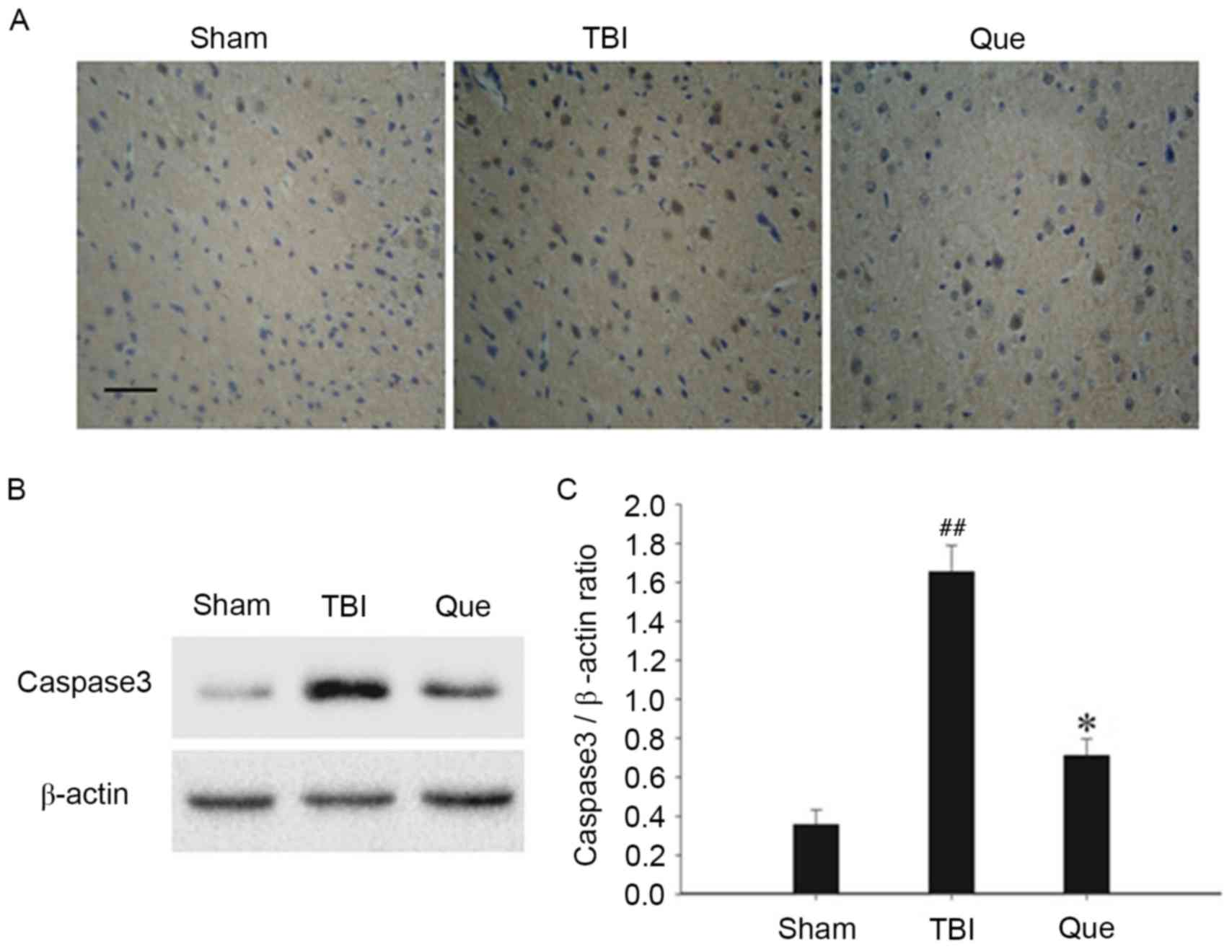
Quercetin attenuates neuronal
apoptosis in the cortex
In order to assess the effect of quercetin on
neuronal apoptosis following TBI, immunohistochemical and western
blot analyses were used to assess alterations in caspase3
expression, respectively. As depicted in Fig. 4A, representative photomicrographs
exhibited a high density of caspase3-positive cells in the TBI
group compared with the sham group at 24 h. However, the expression
of activated caspase3-positive cells notably decreased in the
quercetin treatment group. In addition, western blot analysis
revealed that, compared with the sham group, the protein expression
levels of activated caspase3 increased significantly in the TBI
group at 24 h (P<0.01), and the levels of caspase3 exhibited a
significant downregulation at the same time point following
treatment with quercetin (P<0.05; Fig. 4B and C).
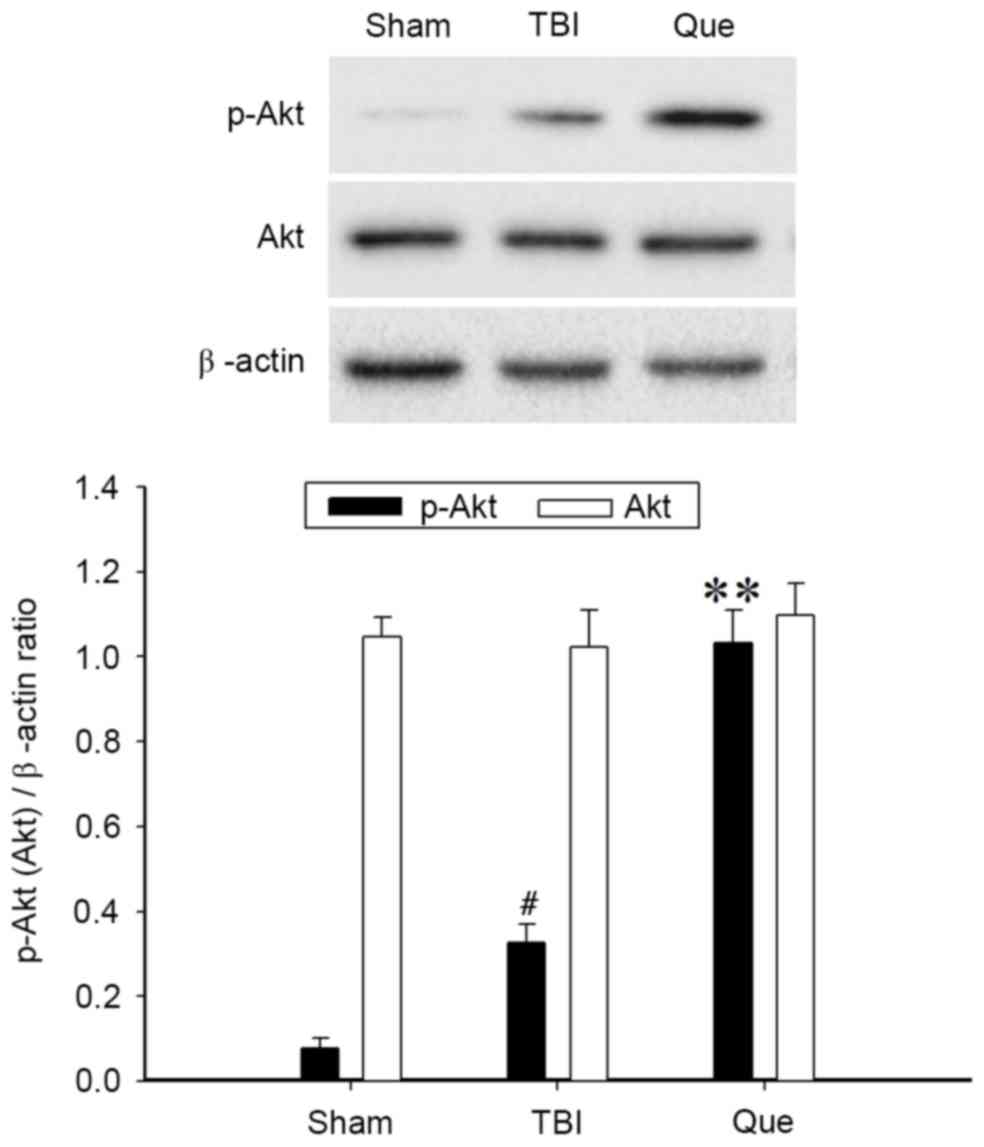
Quercetin induces the activation of
the Akt signaling pathway in the cortex following TBI
Western blot analysis was performed to investigate
the expression of Akt and p-Akt at 24 h after TBI in the 3 groups.
As presented in Fig. 5, the level
of p-Akt was increased following TBI compared with that in the sham
group (P<0.05). Additionally, administration of quercetin
produced a significant elevation of p-Akt (P<0.01). No
significant difference in total Akt protein expression was observed
among the 3 groups.
Quercetin attenuates the ERK1/2
signaling pathway following TBI
Western blot analysis was performed to investigate
the expression of ERK1/2 and p-ERK1/2 at 24 h after TBI in the 3
groups. As presented in Fig. 6,
the level of p-ERK1/2 was increased significantly post-TBI,
compared with the sham group (P<0.01). However, the
administration of quercetin produced a significant attenuation of
p-ERK1/2 levels compared with the TBI group (P<0.01). No
significant difference in total ERK1/2 protein expression was
observed among the 3 groups.
Discussion
The aim of the present study was to investigate the
neuroprotective effects of quercetin on TBI. H&E staining is a
macroscopic and readily available method to assess
histopathological changes. Quercetin treatment notably attenuated
injury. In the Que group, the structure of the brain tissue was
improved and the number of neurons increased compared with the TBI
group. In addition, TBI-induced neurological deficits and brain
edema was suppressed by treatment with quercetin. At the molecular
level, treatment with quercetin significantly inhibited the
TBI-induced expression of cleaved Caspase3. It was additionally
observed that the neuroprotective effects of the drug were
associated with activation of the Akt signaling pathway, and
inhibition of the ERK signaling pathway. The results of the present
study were consistent with previous studies demonstrating that
quercetin may exert neuroprotection in various in vitro and
in vivo models (12–15).
Therefore, it is hypothesized that quercetin may have the potential
to become a novel therapeutic for TBI.
The primary injury occurs at the moment of TBI
impact, with disruption of the blood brain barrier and blood
vessels that contribute to immediate (necrotic) cell death
(16). Subsequently, oxygen free
radical-mediated lipid peroxidation, inflammation and brain edema
appear to be fundamental mechanisms underlying secondary damage in
TBI (17). In the present study,
caspase3 was induced by TBI, which is a key executor in the process
of apoptosis in neurons (18).
However, treatment with quercetin significantly inhibited the
TBI-induced expression of cleaved caspase3. These observations were
consistent with a previous study, which demonstrated that caspase3
immunoreactivity was reduced by quercetin in the cerebral ischemic
penumbra in rats (19). The
neuroprotective effect of quercetin was associated with the
inhibition of neuronal apoptosis.
Akt, also termed protein kinase B, is a
serine/threonine kinase with pro-survival functions in acute brain
injury (20). Extracellular
signals frequently result in the simultaneous activation of the
PI3K/Akt signaling pathway, and a number of reports have suggested
a survival role of the PI3K/Akt signaling pathway through the
suppression of apoptosis (21,22).
Additionally, previous studies have demonstrated that the intrinsic
pathway is characterized by mitochondrial outer membrane
permeabilization, death-inducible signaling complex formation, DNA
fragmentation and caspase3 activation. These events have been
demonstrated to be associated with ERK1/2 signaling pathway
activation (23,24). In the present study, treatment with
quercetin inhibited the TBI-induced activation of the ERK1/2
signaling pathway, and further enhanced the PI3K/Akt pathway in
TBI-injured neurons. Therefore, the neuroprotective effects of
quercetin may be associated with ERK1/2 inhibition in addition to
PI3K/Akt activation.
Previous studies have showed that in TBI rats,
quercetin improves cognitive function due to its neuroprotective
action via the inhibition of oxidative stress, leading to a reduced
inflammatory response and thereby reducing neuronal death (7,8).
Compared with other studies, the present study performed a more
in-depth study on the molecular mechanism of the neuroprotection
effects of quercetin. It was demonstrated that post-TBI
administration of quercetin may attenuate brain edema and improve
motor functions in rats. In addition, quercetin caused marked
ERK1/2 inhibition and PI3K/Akt activation, and thereby attenuation
of neuronal apoptosis. The present study provides novel insight
into the mechanisms through which quercetin may exert its
neuroprotective activity in a rat model of TBI.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All datasets on which the conclusions are based are
provided in the present article.
Authors' contributions
GD, ZZ and YC designed the present study. ZoL, YT
and ZhL performed the experiments. BL and JS analyzed and
interpreted data, and were major contributors in writing the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Institutional Animal Care and Use Committee of
Hebei Medical University (Shijiazhuang, China) approved all
experiments, which were performed according to the guidelines of
the National Institutes of Health (NIH) Guide for the Care and Use
of Laboratory Animals (NIH Publications no. 80–23, revised 1978;
NIH, Bethesda, MD, USA). All efforts were made to minimize the
number of animals used and their suffering.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TBI
|
traumatic brain injury
|
|
NSS
|
neurological severity score
|
|
Que
|
quercetin-treated
|
|
WW
|
wet weight
|
|
DW
|
dry weight
|
|
H&E
|
hematoxylin and eosin
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
Akt
|
Akt serine/threonine protein
kinase
|
|
ERK
|
extracellular signal-regulated
kinase
|
References
|
1
|
Ye X, Asim M and Michael C: Animal models
of traumatic brain injury. Nat Rev Neurosci. 14:128–142. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carroll LJ, Cassidy JD, Cancelliere C,
Côté P, Hincapié CA, Kristman VL, Holm LW, Borg J, Nygren-de
Boussard C and Hartvigsen J: Systematic review of the prognosis
after mild traumatic brain injury in adults: Cognitive,
psychiatric, and mortality outcomes: Results of the international
collaboration on mild traumatic brain injury prognosis. Arch Phys
Med Rehabil. 95 3 Suppl:S152–S173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Verma AK and Pratap R: The biological
potential of flavones. Nat Prod Rep. 27:1571–1593. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Legault J, Perron T, Mshvildadze V,
Girard-Lalancette K, Perron S, Laprise C, Sirois P and Pichette A:
Antioxidant and anti-inflammatory activities of quercetin
7-O-β-D-glucopyranoside from the leaves of Brasenia schreberi. J
Med Food. 14:1127–1134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang H, Zhang M, Yu L, Zhao Y, He N and
Yang X: Antitumor activities of quercetin and
quercetin-5,8-disulfonate in human colon and breast cancer cell
lines. Food Chem Toxicol. 50:1589–1599. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dok-Go H, Lee KH, Kim HJ, Lee EH, Lee J,
Song YS, Lee YH, Jin C, Lee YS and Cho J: Neuroprotective effects
of antioxidative flavonoids, quercetin, (+)-dihydroquercetin and
quercetin 3-methyl ether, isolated from Opuntia ficus-indica var.
saboten. Brain Res. 965:130–136. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang T, Kong B, Gu JW, Kuang YQ, Cheng L,
Yang WT, Xia X and Shu HF: Anti-apoptotic and anti-oxidative roles
of quercetin after traumatic brain injury. Cell Mol Neurobiol.
34:797–804. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du G, Zhao Z, Chen Y, Li Z, Tian Y, Liu Z,
Liu B and Song J: Quercetin attenuates neuronal autophagy and
apoptosis in rat traumatic brain injury model via activation of
PI3K/Akt signaling pathway. Neurol Res. 1–8. 2016.(Epub ahead of
print). PubMed/NCBI
|
|
9
|
Marmarou A, Foda AE, van den Brink W,
Campbell J, Kita H and Demetriadou K: A new model of diffuse brain
injury in rats. Part I: Pathophysiology and biomechanics. J
Neurosurg. 80:291–300. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Constantini S, Trembovler V,
Weinstock M and Shohami E: An experimental model of closed head
injury in mice: Pathophysiology, histopathology, and cognitive
deficits. J Neurotrauma. 13:557–568. 1996.PubMed/NCBI
|
|
11
|
Donkin JJ and Vink R: Mechanisms of
cerebral edema in traumatic brain injury: Therapeutic developments.
Curr Opin Neurol. 23:293–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang ZJ, Cheang LC, Wang MW and Lee SM:
Quercetin exerts a neuroprotective effect through inhibition of the
iNOS/NO system and pro-inflammation gene expression in PC12 cells
and in zebrafish. Int J Mol Med. 27:195–203. 2011.PubMed/NCBI
|
|
13
|
Kumar B, Gupta SK, Nag TC, Srivastava S,
Saxena R, Jha KA and Srinivasan BP: Retinal neuroprotective effects
of quercetin in streptozotocin-induced diabetic rats. Exp Eye Res.
125:193–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pu F, Mishima K, Irie K, Motohashi K,
Tanaka Y, Orito K, Egawa T, Kitamura Y, Egashira N, Iwasaki K and
Fujiwara M: Neuroprotective effects of quercetin and rutin on
spatial memory impairment in an 8-arm radial maze task and neuronal
death induced by repeated cerebral ischemia in rats. J Pharmacol
Sci. 104:329–334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Silva B, Oliveira PJ, Dias A and Malva JO:
Quercetin, kaempferol and biapigenin from Hypericum perforatum are
neuroprotective against excitotoxic insults. Neurotox Res.
13:265–279. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eucker SA, Smith C, Ralston J, Friess SH
and Margulies SS: Physiological and histopathological responses
following closed rotational head injury depend on direction of head
motion. Exp Neurol. 227:79–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng JF, Gurkoff GG, Van KC, Song M, Lowe
DA, Zhou J and Lyeth BG: NAAG peptidase inhibitor reduces cellular
damage in a model of TBI with secondary hypoxia. Brain Res.
1469:144–152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Clark RS, Kochanek PM, Watkins SC, Chen M,
Dixon CE, Seidberg NA, Melick J, Loeffert JE, Nathaniel PD, Jin KL
and Graham SH: Caspase-3 mediated neuronal death after traumatic
brain injury in rats. J Neurochem. 74:740–753. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao RQ, Qi DS, Yu HL, Liu J, Yang LH and
Wu XX: Quercetin attenuates cell apoptosis in focal cerebral
ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling
pathway. Neurochem Res. 37:2777–2786. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Endo H, Nito C, Kamada H, Yu F and Chan
PH: Akt/GSK3beta survival signaling is involved in acute brain
injury after subarachnoid hemorrhage in rats. Stroke. 37:2140–2146.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park KR, Nam D, Yun HM, Lee SG, Jang HJ,
Sethi G, Cho SK and Ahn KS: β-Caryophyllene oxide inhibits growth
and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1
pathways and ROS-mediated MAPKs activation. Cancer Lett.
312:178–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li WX, Chen SF, Chen LP, Yang GY, Li JT,
Liu HZ and Zhu W: Thimerosal-induced apoptosis in mouse C2C12
myoblast cells occurs through suppression of the PI3K/Akt/survivin
pathway. PLoS One. 7:e490642012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan L, Tang Q, Shen D, Peng S, Zheng Q,
Guo H, Jiang M and Deng W: SOCS-1 inhibits TNF-alpha-induced
cardiomyocyte apoptosis via ERK1/2 pathway activation.
Inflammation. 31:180–188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chong YH, Shin YJ, Lee EO, Kayed R, Glabe
CG and Tenner AJ: ERK1/2 activation mediates Abeta oligomer-induced
neurotoxicity via caspase-3 activation and tau cleavage in rat
organotypic hippocampal slice cultures. J Biol Chem.
281:20315–20325. 2006. View Article : Google Scholar : PubMed/NCBI
|