Introduction
Neuropathic pain is defined as pain that arises as a
direct consequence of a lesion or disease affecting the nervous
system, which is caused by an injury to the peripheral or central
nervous system (1,2). Characteristic features of neuropathic
pain include hyperalgesia, allodynia and spontaneous pain (3). Neuropathic pain may develop in
response to sciatic nerve injury. The sciatic nerve is the largest
and longest peripheral nerve in the human body, which is formed
from the anterior and posterior divisions of the L4, L5, S1 and S2
spinal nerves, and the anterior division of the S3 spinal nerve. In
clinical practice, the treatment of neuropathic pain, particularly
sciatic nerve pain, remains a prevalent and persistent challenge
(2,3); therefore, more efficient novel
treatments require further exploration.
Ozone (O3) has been used in pain
treatment for >30 years (4). On
the basis of suggestions made by Wolff (5), ozone therapy has been used by
practitioners in an empirical fashion. The issues regarding how its
toxicity can be controlled and how its therapeutic effects are
exerted have been fully clarified (6–8). At
present, the clinical use of ozone is very popular and is promising
compared with surgical procedures, and it has been used to treat
sciatic nerve injury. It has previously been reported that a single
subcutaneous injection of ozone in mice with spared nerve injury of
the sciatic nerve decreased neuropathic pain-type behavior
(9). The mechanism underlying this
action remains unclear; however, ozone has been observed to
regulate the expression of genes that serve vital roles in the
onset and maintenance of allodynia (9). Another substance that is often used
in clinical pain treatment is Gardenia fruit extracts.
Gardenia fruits are widely used in Chinese traditional
medicine, since they are thought to exert homeostatic,
hepatoprotective, analgesic, antiphlogistic, antipyretic and
hypolipidemic effects (10). In
addition, Gardenia is widely used in modern clinical
medicine for the treatment of numerous diseases, including acute
viral hepatitis, esophagitis, canker sores, coronary heart disease,
neurasthenia and insomnia (11).
Previous studies have confirmed that iridoid constituents and
crocins were the most effective and major chemical components of
Gardenia fruits; among these, gardenoside has been reported
to possess the largest therapeutic effect in sciatic nerve injury
(12,13).
The present study aimed to investigate the
pain-relieving effects of a combination of gardenoside and ozone,
and to elucidate the mechanism underlying their function.
Therefore, a chronic constriction sciatic nerve injury (CCI) rat
model was generated, and the effects of gardenoside and ozone were
examined. A combination of gardenoside and ozone markedly increased
mechanical withdrawal threshold (MWT) and thermal withdrawal
latency (TWL), thus confirming their pain-relieving effects. The
mRNA and protein expression levels of P2X3 and P2X7 purine
receptors were significantly decreased in the dorsal root ganglion
(DRG) following gardenoside and ozone cotreatment, thus suggesting
that the mechanism underlying their pain-relieving effects may be
mediated by inhibiting P2X3 and P2X7 receptors in the DRG. The
present study is the first, to the best of our knowledge, to
demonstrate the mechanism underlying the pain-relieving effects of
gardenoside and ozone cotreatment, and may provide a novel
direction for further studies regarding the treatment of
neuropathic pain.
Materials and methods
Animals
A total of 70 healthy male Sprague-Dawley rats (8
weeks old; weight, 200–250 g) were purchased from the Experimental
Animal Center of Suzhou Aiermaite Technology Co., Ltd. (Suzhou,
China). The rats were housed in a specific pathogen-free animal
facility, at 22–24°C, 50–60% humidity and a 12 h light/dark cycle.
The rats had free access to food and water prior to
experimentation. The animal use protocol was reviewed and approved
by the Institutional Animal Care and Use Committee of Weifang
People's Hospital (Weifang, China).
CCI model construction
A total of 70 male Sprague-Dawley rats were randomly
divided into the following five groups (n=14 rats/group): The
control group (Ctrl), the ozone and gardenoside treatment control
group (Ctrl + Ozo&Gar), the sham surgery group (Sham), the CCI
model group (CCI) and the ozone and gardenoside treatment CCI model
group (CCI + Ozo&Gar). The rats in each group were treated as
follows. After environmental adaptation for ≥5 days, two groups
(CCI and CCI + Ozo&Gar groups; total number of rats, 28) were
used to generate the CCI model. For 24 h prior to surgery, these
rats were provided only water, with no food. The CCI model is well
characterized for the study of neuropathic pain (14,15).
Briefly, rats were anesthetized with 10% chloral hydrate (350
mg/kg, intraperitoneal), fixed in the supine position and were
subjected to an aseptic operation. The right legs were shaved and
sterilized with 70% ethanol and betadine antiseptic solution.
Following exposure of the thigh muscle, the sciatic nerve was
separated and loosely ligated with sterile 4-0 catgut thread at
four consecutive sites, with an interval of ~1 mm. The nerve was
carefully ligated, so as not to completely block peripheral blood
flow in the nerves. In addition, a sham surgery was performed in
the Sham group, in which the sciatic nerve was exposed, but not
ligated. Finally, the wound was sutured in layers and was cleaned
with iodine, followed by an intramuscular injection of penicillin
(40,000 U/mouse). Rats were then maintained in the same manner as
the rats in the control group. The two control groups (Ctrl and
Ctrl + Ozo&Gar) did not undergo surgery.
Drug application
The rats in the Ctrl + Ozo&Gar and CCI +
Ozo&Gar groups were administered an intravenous injection of 30
µg/ml ozone (Chengdu Must Biotechnology Co., Ltd., Chengdu, China)
and 300 µmol/l gardenoside (Chengdu Must Biotechnology Co., Ltd.).
The rats in the Ctrl, Sham and CCI groups were administered the
same volume of saline. The drugs were delivered intravenously once
daily for 14 days, beginning on day 1 after CCI. Specimens were
collected from the L4-L5 DRG on the 15th day for analysis.
Evaluation of pain behavior
The five groups of rats were raised in separate
cages after the operation. MWT and TWL were measured prior to the
operation (day 0), and 1, 3, 5, 7, 9, 11 and 14 days after the
operation. The time of each daily measurement, the room temperature
and other conditions were similarly maintained across all
measurements.
Detection of mechanical
hyperalgesia
BME-403 Von Frey fine thread (Beijing Jinuotai
Technology Development Co. Ltd., Beijing, China) was used to
measure MWT. The rats were maintained in a transparent plexiglass
box (20×30×30 cm); the bottom of the plexiglass box was made of
wire netting (1×1 cm). The rats were maintained in the plexiglass
box for 15 min prior to the measurement. The bending forces of the
wires inserted through the wire netting used were as follows: 0.13,
0.20, 0.33, 0.60, 1.30, 3.60, 5.00, 7.30, 9.90 and 20.1 g, and
tolerance of bending forces >20.1 g were recorded as 20.1 g. The
interval between two stimulations was 20 sec or until the
stimulation-induced reactions, such as licking feet and paw
withdrawal. The test was stopped once paw withdrawal was induced.
Each test was performed from the smallest to the largest bending
force until the frequency of withdrawal was ≥50%. From this, the
MWT value was determined. The test was repeated three times and the
mean was calculated.
Detection of thermal hyperalgesia
Thermal hyperalgesia was measured using an automatic
thermalgia stimulator system (BME-410C; Institute of
Bioengineering, Chinese Academy of Sciences, Beijing, China) to
irradiate the paws. The plexiglass box was placed on a glass plate
(3 mm thick), and the rats were maintained in the box for 30 min
prior to the experiment. The time it took for the rats to withdraw
their paws from the thermal stimulus was considered the TWL. The
cut-off time was 30 sec, in order to prevent tissue damage. Each
rat was measured three times and the mean value was taken as the
threshold value.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
To measure the mRNA expression levels of P2X3 and
P2X7 receptors, rats were anesthetized with an intraperitoneal
injection of 10% chloral hydrate (350 mg/kg) and the L4 and L5 DRG
was harvested on the 15th day post-surgery, and was used for total
RNA isolation using TRIzol (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's protocol.
Subsequently, 1,000 ng total RNA was used as a template for RT
using the Applied Biosystems RT kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) according to the manufacturer
instructions. β-actin was used as the internal reference gene. The
primer sequences are listed in Table
I. The SYBR-Green master kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) PCR products were amplified using the following
cycling parameters: 94°C for 5 min, followed by 35 cycles at 94°C
for 45 sec, 53°C for 30 sec and 72°C for 40 sec, and a final step
at 72°C for 5 min. Calculated relative expression of the target
gene expression was calculated with the 2−ΔΔCq method
(16).
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primers | Primer length
(bp) |
|---|
| P2X3 receptor | F:
5′-CAACTTCAGGTTTGCCAAA-3′ | 519 |
|
| R:
5′-TGAACAGTGAGGGCCTAGAT-3′ |
|
| P2X7 receptor | F:
5′-GTTTGACATCATCCAGTTGGTTGT-3′ | 566 |
|
| R:
5′-ATCTTACTGAAGAGCTCAGAGGTA-3′ |
|
| β-actin | F:
5′-TAAAGACCTCTATGCCAACACAGT-3′ | 240 |
|
| R:
5′-CACGATGGAGGGGCCGGACTCATC-3′ |
|
Western blot analysis
For western blot analysis, rats were deeply
anesthetized with diethyl ether and were sacrificed by
decapitation. The L4 and 5 DRG was rapidly removed and lysed with
20 mM Tris-HCl buffer (pH 8.0), containing 1% NP-40, 150 mM NaCl, 1
mM EDTA, 10% glycerol, 0.1% β-mercaptoethanol, 0.5 mM
dithiothreitol, and a mixture of proteinase and phosphatase
inhibitors (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Protein
concentration was determined using the bicinchoninic acid protein
assay method with bovine serum albumin (Beyotime Institute of
Biotechnology, Shanghai, China) as a standard. β-actin served as an
internal control. Protein samples (60 µg/lane) from DRG were
separated by 8% SDS-PAGE and were then electrotransferred onto
polyvinylidene fluoride membranes. The membranes were blocked with
5% non-fat dry milk in Tris-buffered saline containing Tween 20 (50
mM Tris, 150 mM NaCl and 0.1% Tween 20 v/v, pH 7.4) for 2 h at room
temperature, and were then incubated at 4°C overnight with P2X3
(cat no. sc-12215) and P2X7 (cat no. sc-25698) receptor antibodies
(1:2,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), and
β-actin antibody (cat no. sc-130656; 1:1,000; Santa Cruz
Biotechnology, Inc.). The membrane was subsequently incubated with
horseradish peroxidase-conjugated goat anti-mouse IgG secondary
antibody (cat no. sc-2005; Santa Cruz Biotechnology, Inc.)
overnight at 4°C. The signal was detected using the Amersham
enhanced chemiluminescence system (Amersham; GE Healthcare,
Chicago, IL, USA). The relative expression levels of P2X3 and P2X7
receptors were densitometrically semi-quantified using Image-Pro
Plus (Media Cybernetics, Inc., Rockville, MD, USA), and were
calculated according to the reference bands of β-actin.
Statistical data analysis
Statistical analysis was performed using SPSS
statistical software, version 19.0 (IBM Corp., Armonk, NY, USA).
Data are expressed as the mean ± standard deviation. Experiments
were repeated three times. Statistical analyses were performed
using one-way analysis of variance followed by a least significant
difference post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Mechanical hyperalgesia
measurement
MWT was determined prior to the operation (day 0),
and 1, 3, 5, 7, 9, 11 and 14 days after the operation. On days 0
and 1, there was no significant difference between the rats in all
five groups (P>0.05; Table
II). However, from day 3, MWT values were significantly lower
in the CCI and CCI + Ozo&Gar groups compared with in the
control groups (P<0.01). At all time points, there was no
significant difference between the Ctrl, Ctrl + Ozo&Gar and
Sham groups. MWT remained significantly reduced in the CCI group
between days 3 and 14, thus confirming that the CCI model was
successfully generated.
 | Table II.MWT measurement of the rats in each
group. |
Table II.
MWT measurement of the rats in each
group.
|
| MWT (g) |
|---|
|
|
|
|---|
|
| Day |
|---|
|
|
|
|---|
| Group | 0 | 1 | 3 | 5 | 7 | 9 | 11 | 14 |
|---|
| Ctrl | 20.1 | 20.1 | 20.1 | 20.1 | 20.1 | 20.1 | 20.1 | 20.1 |
| Ctrl +
Ozo&Gar | 20.1 | 20.1 | 20.1 | 20.1 | 20.1 | 20.1 | 20.1 | 20.1 |
| Sham | 20.1 | 20.1 | 18.0±1.4 | 18.6±1.4 | 19.0±1.3 | 19.1±1.4 | 19.4±1.7 | 19.5±1.5 |
| CCI | 20.1 | 18.9±1.3 |
9.9±1.3a |
8.9±1.4a |
7.1±1.5a |
6.7±1.2a |
7.5±1.9a |
8.9±1.2a |
| CCI +
Ozo&Gar | 20.1 | 18.8±1.5 |
10.9±1.7a |
12.9±1.5b |
14.2±1.4c |
15.3±1.5c |
16.9±1.4c |
18.5±1.6c |
Compared with in the CCI group, MWT was
significantly increased in the CCI + Ozo&Gar group between days
5 and 14 (P<0.05; Table II).
The MWT in the CCI + Ozo&Gar group was lowest on day 3, and
continued to increase on the following days, until it was similar
to that in the Ctrl, Ctrl + Ozo&Gar and Sham groups (Table II). These findings indicated that
gardenoside and ozone cotreatment was able to increase MWT in rats
suffering from neuropathic pain.
Thermal hyperalgesia measurement
TWL was measured on day 0, and on days 1, 3, 5, 7,
9, 11 and 14 after the operation. On days 0 and 1, there was no
significant difference between the rats in all five groups
(P>0.05; Table III). However,
from day 3, TWL values were significantly lower in the CCI and CCI
+ Ozo&Gar groups compared with in the control groups
(P<0.01). At all time points, there was no significant
difference between the Ctrl, Ctrl + Ozo&Gar and Sham groups.
TWL was significantly lower in the CCI group between days 3 and 14,
thus confirming that the CCI model was successfully generated.
 | Table III.TWL measurement of rats in each
group. |
Table III.
TWL measurement of rats in each
group.
|
| TWL (sec) |
|---|
|
|
|
|---|
|
| Day |
|---|
|
|
|
|---|
| Group | 0 | 1 | 3 | 5 | 7 | 9 | 11 | 14 |
|---|
| Ctrl | 23.3±2.3 | 22.4±2.5 | 23.7±1.9 | 24.2±1.8 | 23.4±2.1 | 22.5±2.4 | 21.9±2.5 | 23.2±2.6 |
| Ctrl +
Ozo&Gar | 22.7±1.8 | 21.8±1.7 | 21.9±2.1 | 22.7±1.9 | 22.5±1.8 | 21.4±1.9 | 22.4±1.8 | 22.6±2.1 |
| Sham | 22.1±1.8 | 20.3±2.1 | 19.3±2.5 | 20.8±2.9 | 21.2±2.4 | 22.6±2.3 | 23.1±2.1 | 22.7±1.9 |
| CCI | 23.4±2.4 | 22.4±1.9 |
14.3±2.3a |
12.7±2.4a |
10.2±1.9a |
11.3±2.4a |
11.7±2.3a |
11.1±2.2a |
| CCI +
Ozo&Gar | 22.7±2.1 | 21.7±1.9 |
15.3±2.1a |
17.2±2.2b |
18.7±2.0c |
19.4±2.1c |
21.2±1.7c |
21.8±1.8c |
Compared with in the CCI group, TWL was
significantly increased in the CCI + Ozo&Gar groups between
days 5 and 14 (P<0.05; Table
III). The TWL in the CCI + Ozo&Gar group was lowest on day
3, after which it continued to increase, until it was similar to
that in the Ctrl, Ctrl + Ozo&Gar and Sham groups (Table III). These findings indicated
that gardenoside and ozone cotreatment was able to increase TWL in
rats suffering from neuropathic pain.
Alterations in the mRNA expression
levels of P2X3 receptor in the DRG
RT-qPCR was used to detect the mRNA expression level
of P2X3 receptor in the DRG on day 15 post-surgery (Fig. 1). Compared with in the Ctrl group,
no significant alterations in the mRNA expression levels of P2X3
receptor were detected in the Ctrl + Ozo&Gar and Sham groups
(P>0.05). However, the mRNA expression levels of P2X3 receptor
were significantly higher in the CCI (P<0.01) and CCI +
Ozo&Gar groups (P<0.05) compared with in the other three
groups. Compared with in the CCI group, the CCI + Ozo&Gar group
exhibited a significant reduction in P2X3 receptor mRNA expression
(P<0.05). These results suggested that the mRNA expression
levels of P2X3 receptor were increased in the CCI group, but were
decreased following gardenoside and ozone cotreatment.
Alterations in the mRNA expression
levels of P2X7 receptor in the DRG
RT-qPCR was also used to detect the mRNA expression
levels of P2X7 receptor in the DRG on day 15 post-surgery (Fig. 2). Compared with in the Ctrl group,
no significant alterations in mRNA expression levels of P2X7
receptor were observed in the Ctrl + Ozo&Gar and Sham groups
(P>0.05). However, the mRNA expression levels of P2X7 receptor
were significantly higher in the CCI (P<0.01) and CCI +
Ozo&Gar groups (P<0.05) compared with in the other three
groups. Compared with in the CCI group, the CCI + Ozo&Gar group
exhibited a significant reduction in P2X7 receptor mRNA expression
(P<0.01). These results suggested that the mRNA expression
levels of P2X7 receptor were increased in the CCI group, but were
decreased following gardenoside and ozone cotreatment.
Alterations in the protein expression
levels of P2X3 receptor in the DRG
Western blotting was used to detect the protein
expression levels of P2X3 receptor on day 15 post-surgery. As
presented in Fig. 3, there was no
significant difference in the protein expression levels of P2X3
receptor between the Ctrl, Ctrl + Ozo&Gar and Sham groups
(P>0.05). However, the expression levels of P2X3 receptor in the
CCI group were significantly higher compared with in the three
control groups. Conversely, the protein expression levels of P2X3
receptor were significantly reduced in the CCI + Ozo&Gar group
compared with in the CCI group. These results indicated that P2X3
receptor protein expression was increased in the CCI group, but was
decreased following cotreatment with gardenoside and ozone.
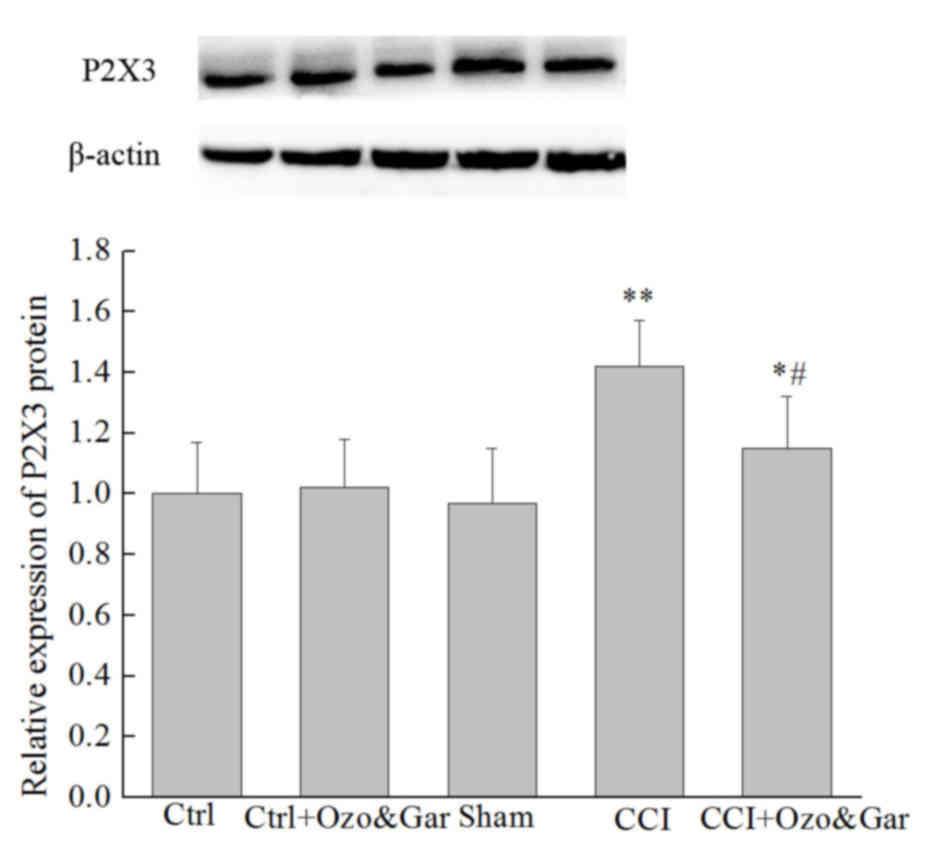 | Figure 3.Protein expression levels of P2X3
receptor in the rats of the five groups with or without CCI and/or
gardenoside and ozone cotreatment, as determined by western
blotting. β-actin was used as a control. P2X3 receptor expression
was significantly higher in the CCI group compared with in the
Ctrl, Ctrl + Ozo&Gar and Sham groups. However, in the CCI +
Ozo&Gar group, P2X3 receptor expression was significantly lower
than in the CCI group. *P<0.05, **P<0.01 vs. Ctrl, Ctrl +
Ozo&Gar and Sham groups; ##P<0.01 vs. the CCI
group. CCI, chronic constriction sciatic nerve injury; Ctrl,
control; Gar, gardenoside; Ozo, ozone. |
Alterations in the protein expression
levels of P2X7 receptor in the DRG
Western blotting was used to detect the protein
expression levels of P2X7 receptor on day 15 post-surgery. As shown
in Fig. 4, there was no
significant difference in the protein expression levels of P2X7
receptor between the Ctrl, Ctrl + Ozo&Gar and Sham groups
(P>0.05). However, the expression levels of P2X7 receptor in the
CCI group were significantly higher compared with in the three
control groups. Conversely, the protein expression levels of P2X7
receptor were significantly lower in the CCI + Ozo&Gar group
compared with in the CCI group. These results indicated that P2X7
receptor protein expression was increased in the CCI group, but was
decreased following cotreatment with gardenoside and ozone.
 | Figure 4.Protein expression levels of P2X7
receptor in the rats of the five groups with or without CCI and/or
gardenoside and ozone cotreatment, as determined by western
blotting. β-actin was used as a control. P2X7 receptor expression
was significantly higher in the CCI group compared with in the
Ctrl, Ctrl + Ozo&Gar and Sham groups. However, in the CCI +
Ozo&Gar group, P2X7 receptor expression was significantly lower
than in the CCI group. *P<0.05, **P<0.01 vs. Ctrl, Ctrl +
Ozo&Gar and Sham groups; ##P<0.01 vs. CCI group.
CCI, chronic constriction sciatic nerve injury; Ctrl, control; Gar,
gardenoside; Ozo, ozone. |
Discussion
The present study used gardenoside combined with
ozone to treat rats following the generation of a CCI model. The
treatment proved to be effective with regards to pain relief, and
decreased the mRNA and protein expression levels of P2X3 and P2X7
receptors in the DRG. The DRG is an anatomically discrete structure
that forms part of the peripheral nervous system, and is located
laterally to the spine. The DRG is recognized as one of the organs
that may be damaged in response to peripheral sensory neuropathic
pain (17). The results of the
present study indicated that the mechanism underlying the
pain-relieving effects of gardenoside and ozone cotreatment may be
associated with the inhibition of the mRNA and protein expression
levels of P2X3 and P2X7 receptors in the DRG. The present study is
the first, to the best of our knowledge, to experimentally
demonstrate the mechanism underlying the effects of gardenoside and
ozone on neuropathic pain.
P2X receptors are a family of cation-permeable
ligand-gated ion channels that open in response to the binding of
extracellular adenosine 5′-triphosphate, which results in the
generation and transmission of pain and inflammatory nociceptive
signals (18,19). It has been suggested that P2X3
homotrimers are responsible for acute pain, whereas P2X2/3
heterotrimers mediate chronic pain (20); therefore, P2X receptors have long
been considered potential therapeutic targets for the treatment of
inflammatory pain (21). P2X7
receptor is the most intensively investigated, and numerous
pharmaceutical companies have synthesized small molecules that
potently and selectively block its expression (21). In addition, there has also been
significant progress in the specific targeting of the P2X3
homotrimeric and P2X2/3 heterotrimeric receptors (22). The findings of the present study
suggested that novel chemicals, including gardenoside and ozone,
may potentially target P2X3 and P2X7 receptors.
As an exploratory experiment, the present study may
be considered successful. However, there are numerous questions
that must be addressed and require follow-up studies. For example,
it remains unclear whether gardenoside was oxidized into geniposide
during treatment. Gardenoside and geniposide exist in
Gardenia fruits (23,24);
geniposide also exhibits a wide range of pharmacological
activities, including hepatoprotective (25), hypoglycemic (26), insulin resistance-alleviating
(27), antiproliferative (28), antioxidant (29), and antioxidant and neuroprotective
effects (30). It is also used as
a cross-linker to generate polymeric material in biomedical
applications (31). In addition,
it remains unclear as to whether the pain-relieving effects were
mainly induced by ozone or gardenoside, or whether both were
equally important. It also remains unclear as to whether ozone or
gardenoside inhibited the mRNA and protein expression levels of
P2X3 and P2X7 receptors in the DRG. Therefore, more experiments are
required to address these questions.
Taken together, the present study confirmed the
pain-relieving effects of gardenoside and ozone cotreatment, and
also revealed a possible mechanism underlying this effect, which
may be mediated by the inhibition of P2X3 and P2X7 receptors in the
DRG. Therefore, gardenoside and ozone may be considered novel drug
candidates that target P2X3 and P2X7 receptors.
In conclusion, the present study demonstrated that
gardenoside combined with ozone was able to increase the MWT and
TWL of rats that suffered from neuropathic pain, thus suggesting
that this treatment could alleviate chronic neuropathic pain. The
effects of gardenoside and ozone may be mediated by the inhibition
of P2X3 and P2X7 receptor expression in the DRG.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
IASP: Classification of chronic pain:
Descriptions of chronic pain syndromes and definitions of pain
termsTask Force on Taxonomy of the IASP. Merskey H and Bogduk N:
2nd edition. IASP Press; Seattle, WA: 1994
|
|
2
|
Chou R, Atlas SJ, Stanos SP and Rosenquist
RW: Nonsurgical interventional therapies for low back pain: A
review of the evidence for the American Pain Society clinical
practice guideline. Spine (Phila Pa 1976). 34:1078–1093. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hayden JA, van Tulder MW, Malmivaara AV
and Koes BW: Meta-analysis: Exercise therapy for nonspecific low
back pain. Ann Intern Med. 142:765–775. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bocci V, Borrelli E, Zanardi I and
Travagli V: The usefulness of ozone treatment in spinal pain. Drug
Des Devel Ther. 9:2677–2685. 2015.PubMed/NCBI
|
|
5
|
Wolff HH: Medical ozone: theoretical
bases, therapeutic applications. Verlag für Medizin, Heidelberg:
1979, (In German).
|
|
6
|
Sagai M and Bocci V: Mechanisms of action
involved in ozone therapy: Is healing induced via a mild oxidative
stress? Med Gas Res. 1:292011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bocci V: How a calculated oxidative stress
can yield multiple therapeutic effects. Free Radic Res.
46:1068–1075. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bocci V and Valacchi G: Free radicals and
antioxidants: How to reestablish redox homeostasis in chronic
diseases? Curr Med Chem. 20:3397–3415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fuccio C, Luongo C, Capodanno P, Giordano
C, Scafuro MA, Siniscalco D, Lettieri B, Rossi F, Maione S and
Berrino L: A single subcutaneous injection of ozone prevents
allodynia and decreases the over-expression of pro-inflammatory
caspases in the orbito-frontal cortex of neuropathic mice. Eur J
Pharmacol. 603:42–49. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee IA, Lee JH, Baek NI and Kim DH:
Antihyperlipidemic effect of crocin isolated from the fructus of
Gardenia jasminoides and its metabolite crocetin. Biol Pharm
Bull. 28:2106–2110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang CX, Cheng JG and Luo T: Nutritional
value analysis of medicinal and edible plant. J Anhui Agri Sci.
43:282–284. 2015.
|
|
12
|
Meng XL, Li HW, Li Y, Yu Q, Wan LL and Guo
C: Advances in studies on chemical constituents and pharmacological
activities of Gardenia jasminoides. Chin J New Drug.
20:959–967. 2011.
|
|
13
|
Chen H, Xiao YQ, Li L and Zhang C: Studies
on chemical constituents in fruit of Gardenia jasminoides.
Zhongguo Zhong Yao Za Zhi. 32:1041–1043. 2007.(In Chinese).
PubMed/NCBI
|
|
14
|
Bennett G and Xie YK: A peripheral
mononeuropathy in rat that produces disorders of pain sensation
like those seen in man. Pain. 33:87–107. 1998. View Article : Google Scholar
|
|
15
|
Austin PJ, Wu A and Moalem-Taylor G:
Chronic constriction of the sciatic nerve and pain hypersensitivity
testing in rats. J Vis Exp. 61:e33932012.
|
|
16
|
Rao X, Huang X, Zhou Z and Lin X: An
improvement of the 2ˆ(-delta delta CT) method for quantitative
real-time polymerase chain reaction data analysis. Biostat
Bioinforma Biomath. 3:71–85. 2013.PubMed/NCBI
|
|
17
|
Ezquerra L, Alguacil LF, Nguyen T, Deuel
TF, Silos-Santiago I and Herradon G: Different pattern of
pleiotrophin and midkine expression in neuropathic pain:
Correlation between changes in pleiotrophin gene expression and rat
strain differences in neuropathic pain. Growth Factors. 26:44–48.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burnstock G: Purinergic mechanisms and
pain-an update. Eur J Pharmacol. 716:24–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murrell-Lagnado RD and Qureshi OS:
Assembly and trafficking of P2X purinergic receptors (review). Mol
Membr Biol. 25:321–331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jarvis MF: Contributions of P2X3 homomeric
and heteromeric channels to acute and chronic pain. Expert Opin
Ther Targets. 7:513–522. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
North RA and Jarvis MF: P2X receptors as
drug targets. Mol Pharmacol. 83:759–769. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ford AP: In pursuit of P2X3 antagonists:
Novel therapeutics for chronic pain and afferent sensitization.
Purinerg Signal. 8 Suppl 1:3–26. 2012. View Article : Google Scholar
|
|
23
|
Oshima T, Sagara K, Yoshida T, Tong YY,
Zhang GD and Chen YH: Determination of geniposide, gardenoside,
geniposidic acid and genipin-1-beta-gentiobioside in Gardenia
jasminoides by high-performance liquid chromatography. J
Chromatogr. 455:410–414. 1998. View Article : Google Scholar
|
|
24
|
Nagatoshi M, Terasaka K, Nagatsu A and
Mizukami H: Iridoid-specific glucosyltransferase from Gardenia
jasminoides. J Biol Chem. 286:32866–32874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma T, Huang C, Zong G, Zha D, Meng X, Li J
and Tang W: Hepatoprotective effects of geniposide in a rat model
of nonalcoholic steatohepatitis. J Pharm Pharmacol. 63:587–593.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu SY, Wang GF, Liu ZQ, Rao JJ, Lü L, Xu
W, Wu SG and Zhang JJ: Effect of geniposide, a hypoglycemic
glucoside, on hepatic regulating enzymes in diabetic mice induced
by a high-fat diet and streptozotoci. Acta Pharmacol Sin.
30:202–208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kojima K, Shimada T, Nagareda Y, Watanabe
M, Ishizaki J, Sai Y, Miyamoto K and Aburada M: Preventive effect
of geniposide on metabolic disease status in spontaneously obese
type 2 diabetic mice and free fatty acid-treated HepG2 cells. Biol
Pharm Bull. 34:1613–1618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim ES, Jeong CS and Moon A: Genipin, a
constituent of Gardenia jasminoides Ellis, induces apoptosis
and inhibits invasion in MDA-MB-231 breast cancer cells. Oncol Rep.
27:567–572. 2012.PubMed/NCBI
|
|
29
|
Yin F, Liu J, Zheng X, Guo L and Xiao H:
Geniposide induces the expression of heme oxygenase-1 via
PI3K/Nrf2-signaling to enhance the antioxidant capacity in primary
hippocampal neurons. Biol Pharm Bull. 33:1841–1846. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo LX, Liu JH and Xia ZN: Geniposide
inhibits CoCl2-induced PC12 cells death via the mitochondrial
pathway. Chin Med J (Engl). 122:2886–2892. 2009.PubMed/NCBI
|
|
31
|
Kamiński K, Zazakowny K, Szczubiałka K and
Nowakowska M: pH-sensitive genipin-cross-linked chitosan
microspheres for heparin removal. Biomacromolecules. 9:3127–3132.
2008. View Article : Google Scholar : PubMed/NCBI
|


















