Introduction
Nasopharyngeal carcinoma (NPC) primarily occurs in
the epithelial lining of the nasopharynx and is one of the primary
head and neck tumor types (1). It
is prevalent in Southern China and East Asia, Greenland (native),
Canada (Northwest Territories), and Alaska (2,3). The
health status of patients under intensity-modulated radiation
therapy and concurrent chemoradiotherapy were improved, however,
the prognosis of patients with NPC with distant metastasis remains
poor (4). Therefore, further
investigation of the molecular mechanisms of NPC metastasis and
development of effective treatment strategies is required in order
to develop individualized treatment for patients with the
disease.
A previous study demonstrated that cancer-associated
mortalities caused by malignant tumors are associated with
metastasis (5). The tumor cell
microenvironment serves a role during tumor progression, including
metastasis (6). Transforming
growth factor-β (TGF-β) is a key cytokine in the tumor
microenvironment, which serves an important regulatory role in
tumorigenesis and development (7).
The best-known epithelial-mesenchymal transition (EMT) pathway is
induced by TGF-β and causes epithelial cells to generate
mesenchymal phenotypes and subsequently enables them to promotes
their development (8,9). It has been demonstrated that an
increase in the expression of miR-9-3p suppresses the metastasis of
NPC via inhibition of the EMT process, thereby providing a series
of therapeutic targets for the treatment of NPC (10). Pigment epithelium-derived factor
functions as a tumor-suppressor gene in the occurrence of EMT and
metastasis in NPC (11). Thus,
investigation of therapeutic approaches targeting EMT pathways may
be promising for patients with NPC.
Traditional Chinese medicine (TCM) has been used for
the treatment of cancer for more than 2,000 years (12). Curcumol is extracted from the roots
of the medicinal plant Rhizoma curcumae as a guaiane-type
sesquiterpenoid hemiketal (13).
There is a previous study on tumor treatment in clinical or
scientific research focusing on TCM treatment with low toxicity and
drug resistance (14). Previous
studies have also demonstrated that Curcumol possesses antitumor,
anti-hepatic fibrosis, antiproliferation, anti-inflammatory,
antioxidant and antimicrobial activities (15,16).
In addition, Curcumol suppresses human cancer cell growth and
promotes apoptosis activity in several types of cancer, including
cervical cancer, breast cancer, lung cancer, gastric cancer and
hepatocarcinoma (17,18). Chen et al (19) demonstrated that Curcumol induces
HSC-T6 apoptosis at a concentration of 300 µM and has few side
effects on normal liver cell line BRL-3A. Although Curcumol
exhibits antitumor activity, the mechanism of action remains
unknown. Furthermore, the effect of Curcumol on the occurrence and
development of NPC remains unclear. Therefore, the current study
investigated the effect of Curcumol on NPC with regards to
metastasis.
Materials and methods
Cell culture and animals
All experiments were performed in the Central
Laboratory of the North Sichuan Medical College (Sichuan, China).
Human NPC 5–8F cells were provided by the Shanghai Institute for
Biological Sciences (Shanghai, China). Cells were cultured in RPMI
1640 medium (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
with 10% fetal bovine serum (FBS; Hyclone; GE Healthcare Life
Sciences) and maintained with 5% CO2 at 37°C. A total of
20 female BALB/C athymic nude mice, aged 4–6 weeks and weighing
18–20 g, were purchased from the Institute of Zoology, Chinese
Academy of Sciences (Beijing, China) and housed in a sterile
environment at 25°C with a 12-h light/dark cycle and 40% humidity,
and food and water sterilized by high pressure used to feed the
mice ad libitum. Curcumol was dissolved to a concentration
of 10 mg/ml in absolute ethyl alcohol and then diluted with the 10%
FBS RPMI-1640 medium (adjustment based on the required
concentration of the drug). Cells without Curcumol treatment (0
µΜ/ml) served as a control group. Animal experiments were approved
by the Affiliated Hospital of North Sichuan Medical College ethics
committee (CBYXY0022).
Drugs and reagents
Curcumol (cat. no. 100185-200506) was purchased from
MedChemExpress (Monmouth Junction, NJ, USA). A cell counting kit-8
(CCK-8) assay kit was obtained from Dojindo Molecular Technologies,
Inc. (Kumamoto, Japan). Dimethyl sulfoxide (DMSO) was purchased
from Beyotime Institute of Biotechnology (Haimen, China) and used
for the storage of cells. The purified recombinant human TGF-β1
powder (R&D Systems, Inc., Minneapolis, MN USA) was dissolved
in 4 mM HCl containing 1 mg/ml bovine serum albumin (Shanghai Yi
Sheng Biotechnology Co., Ltd., Shanghai, China; storage
concentration: 50 ng/ml). Curcumol was diluted with media to
different concentrations prior to use. Matrigel was purchased from
BD Biosciences (Franklin Lakes, NJ, USA). Transwell inserts were
provided by Corning Costar Co., Ltd. (Corning, NY, USA). Rabbit
polyclonal antibodies against E-cadherin (cat. no. 3195),
N-cadherin (cat. no. 13116) and β-actin (cat. no. 3700) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Goat anti-TGF-β1 (cat. no. TA130016), rabbit monoclonal antibody
for β-actin (cat. no. TA130008), horseradish peroxidase
(HRP)-conjugated goat anti-mouse (cat. no. TA130010) and goat
anti-rabbit (cat. no. TA130020) immunoglobulin G were purchased
from OriGene Technologies, Inc. (Rockville, MD, USA). The enhanced
chemiluminescence (ECL) reagent was purchased from EMD Millipore
(Billerica, MA, USA). The reagents for quantitative polymerase
chain reaction (qPCR) were obtained from Takara Biotechnology Co.,
Ltd. (Dalian, China). Other chemicals were of standard analytical
grade.
Curcumol treatment in vitro
RPMI-1640 culture medium without FBS was used as the
primary solvent with the following treatments: i) Control group,
cells without Curcumol (0 µM/ml) treatment; ii) 0.1 µM/ml Curcumol
group; iii) 0.2 µM/ml Curcumol group; iv) 0.4 µM/ml Curcumol group.
Following mixing of the medium, serum and Curcumol according to the
concentration requirement, cells were cultured in 37°C with 5%
CO2.
Cell viability assay
Cell viability was detected using a CCK-8 assay.
Cells were seeded in 100 µl complete medium in 96-well plates at a
density of 5×105/ml and triplicate wells were used for
each group. Following 8 h incubation at 37°C, cells were exposed to
different doses of Curcumol for 48 h in 96-well plates. A solution
of 10 µl CCK-8 and 90 µl FBS-free medium was added to each well and
incubated for a further 10 min at 37°C. A wavelength of 450 nm was
then used to measure cell viability.
Apoptosis detected by flow cytometry
(FCM)
Curcumol-induced cell apoptosis was detected by FCM
using an Annexin V-fluorescein isothiocyanate (FITC) apoptosis
detection kit (Beyotime Institute of Biotechnology). Briefly,
following conditions of cell culture described above, all cells
were harvested and washed with cold PBS twice and then treated with
different concentrations of Curcumol for 48 h at 37°C. Cells were
stained with Annexin propidium iodide and V-FITC following
centrifugation (100 × g for 5 min at 25°C) and using a flow
cytometer and BD CellQuest Pro software version 5.1 (BD
Biosciences, Franklin Lakes, NJ, USA).
Cell invasion and migration assay
A wound-healing assay was performed using 5–8F
treated cells. A total of 8×105 cells were incubated in
6-well plates and grown to confluence. An artificial homogenous
wound was created in the cell monolayer with a sterile 100 µl tip
and was then washed with PBS to remove cell debris. Cells were then
treated with different concentrations of Curcumol for 24 and 48 h.
The wound healing rate following treatment was evaluated using
ImageJ software version k1.45 (National Institutes of Health,
Bethesda, MD, USA). Transwell chambers (8 µm pore size; EMD
Millipore) with Matrigel were used to detected cell invasion. Cells
(1×104) were treated with Curcumol for 48 h and were
then plated in the top chambers with an 8 µm membrane pore in
FBS-free medium. The medium in the bottom chamber was supplemented
with 10% FBS to allow for invasion for 24 h at 37°C. At the end of
the experiment, cells at the bottom of the membrane were fixed in
4% paraformaldehyde for 10 min at 37°C and stained with 1% crystal
violet at 25°C (Sigma-Aldrich; Merck KGaA, Darmstadt Germany) for
10 min. Images were captured using a light microscope at a
magnification of ×100. The specific number of cells per filter in
five predetermined fields was then counted and analyzed.
Reverse transcription-qPCR
(RT-qPCR)
Total RNA was extracted from 5–8F cells using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
according to the manufacturer's protocol. RNA was then reverse
transcribed into cDNA using an M-MLV kit (Takara Biotechnology Co.,
Ltd.). All PCR primers were as follows: E-cadherin, forward,
5′-GGTTATTCCTCCCATCAGCT-3′ and reverse, 5′-CTTGGCTGAGGAGGGTGTA-3′;
N-cadherin, forward, 5′-GGTGGAGGAGAAGAAGACCAG-3′ and reverse,
5′-GGCATCAGGCTCCACAGT-3′; GAPDH, forward, 5′-TTCGTCATGGGTGTGAAC-3′
and reverse, 5′-AGTGAGCTTCCCGTTCAGC-3′. The qPCR assay (95°C for 30
sec, then 40 cycles of 95°C for 5 sec and 60°C for 30 sec) was
performed as the instruction in triplicate using SYBR Green PCR
Master mix and Applied Biosystems Prism 7500 (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The relative fold change among
groups was analyzed using 2−ΔΔCq quantitative analysis
(20).
Western blot analysis
Cells treated with Curcumol were lysed in
radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology) on ice for 30 min. Cells were then centrifuged at
100 × g for 15 min at 4°C and the protein concentration was
determined using a BCA protein assay kit (Beyotime Institute of
Biotechnology). Equal amounts (25 µg) of total protein samples were
separated using 10% SDS-PAGE and different protein bands were
transferred to polyvinylidene fluoride membranes (EMD Millipore).
Membranes were blocked using 0.1% TBST with 5% non-fat dry milk at
room temperature for 1 h and incubated with E-cadherin (dilution:
1:1,000), N-cadherin (dilution: 1:1,000) and anti-β-actin
(dilution: 1:4,000) at 4°C overnight. Membranes were then washed
using 0.1% TBST four times and incubated with HRP-conjugated
secondary antibodies for 1.5 h at room temperature. The target
protein was detected by ECL and the bands were quantified using
ImageJ software version k1.45.
ELISA
Pretreated serum samples and cell supernatants were
dissolved on ice and a TGF-β1 ELISA kit (cat. no. ab100647; Abcam,
Cambridge, UK) was used to measure the TGF-β1 concentrations in
accordance with the manufacturer's protocols. Each sample was run
in triplicate. Standards, serum samples or cell supernatants were
added to 96-well plates, respectively. Subsequently, the enzyme
conjugation solutions were added and the mixtures were co-incubated
for 60 min at 37°C. Every well was washed 5 times with wash buffer.
Substrate I and II were then added to each well respectively and
the plate was kept away from light at room temperature for 15 min.
The plate was then read at a wavelength of 450 nm using an ELISA
reader.
Xenograft tumor models
NPC 5–8F cells (1×107) were resuspended
with 200 µl PBS and Matrigel and subcutaneous injections were
performed on both sides of the nude mice following anesthesia with
ether. Mice were randomly assigned to a control group and a treated
group when the tumors reached an average volume of 50
mm3 (volume=length × width 2/2). The treated group were
administered with Curcumol at 15 µg/kg crude drug (sterile water
solution, equivalent to the human dosage) by gavage twice a day for
35 days and the control group mice were given an equal volume of
sterile water. The tumor volume was measured every 7 days. Mice
were sacrificed by cervical dislocation and tumors and peripheral
blood were collected. Following weighing of the tumor, the tumors
were stored at −8°C. In addition, blood samples were centrifuged at
100 × g for 15 min at 25°C in order to obtain the serum and were
then stored at −80°C.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Each experiment was repeated at least three times. Data
were analyzed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). A
Student's t-test was used to analyze differences between two
groups, while the differences between >2 groups were detected
using one-way analysis of variance followed by Student-Newman-Keuls
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Curcumol suppresses NPC cell
proliferation and promotes NPC cell apoptosis
The effect of Curcumol on NPC 5–8F cell viability
was investigated by determining the growth of 5–8F cells using a
CCK-8 assay. The results demonstrated that Curcumol significantly
inhibited cell growth at doses of 0.1, 0.2 and 0.4 µM/ml (Fig. 1A). 5–8F cell apoptosis was
quantified using FCM. The results indicated that the rate of
apoptosis was significantly increased following Curcumol treatment
in a dose-dependent manner (Fig. 1B
and C).
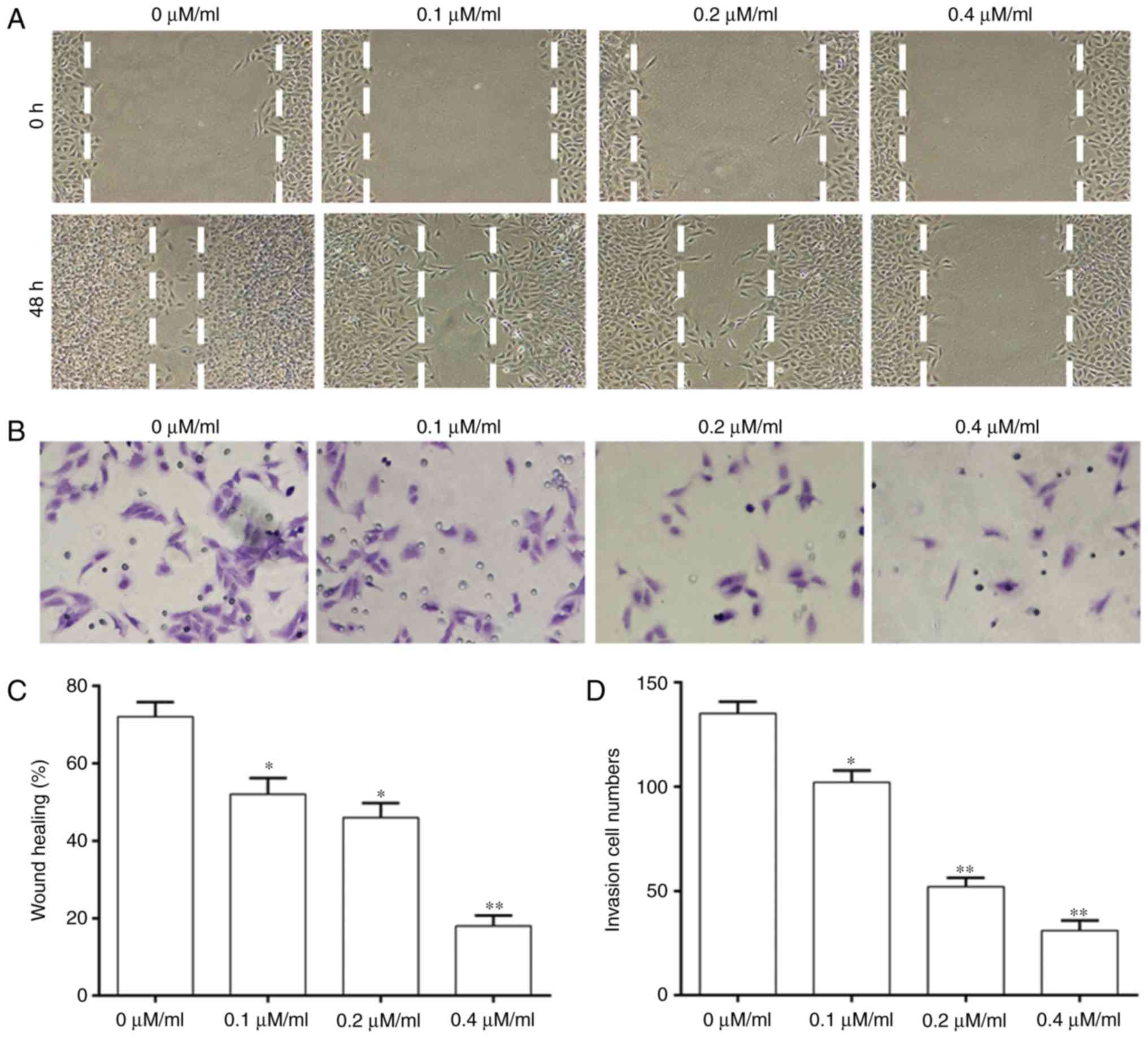
Curcumol inhibits 5–8F cell invasion
and migration
A wound-healing assay and Transwell assays were
performed to investigate whether Curcumol treatment affected the
migration and invasion ability of NPC cells (Fig. 2). The migration ability of cells
was significantly decreased with an increase of the concentration
of Curcumol compared with that in the control group (Fig. 2A and C). Cell invasion was
significantly decreased in a dose-dependent manner at different
concentrations of Curcumol treatment (Fig. 2B and D).
Effect of Curcumol on expression of
EMT-associated genes
The mechanism of action of Curcumol on inhibition of
5–8F cell development was investigated by performing RT-qPCR and
western blotting (Fig. 3). The
results of RT-qPCR suggested that the expression of E-cadherin mRNA
was significantly increased in 5–8F cells treated with Curcumol at
doses of 0.1, 0.2 and 0.4 µM/ml (Fig.
3A). By contrast, the expression of N-cadherin was
significantly inhibited in 5–8F cells treated with Curcumol in a
dose-dependent manner (Fig. 3B).
The results of western blotting were consistent with this (Fig. 3C-F). Thus, this suggests that
Curcumol may regulate EMT-associated proteins in a dose-dependent
manner.
Curcumol inhibits the growth of
transplanted tumors with 5–8F cells in vivo
Based on the results of experiments on 5–8F cells,
further investigation was performed into the effect of Curcumol on
NPC in vivo. The results indicated that the tumor volume of
the Curcumol group on day 35 was significantly decreased compared
with that in the control group (Fig.
4A and B). The tumor weight of the control group was also
significantly increased compared with that in the Curcumol group
(Fig. 4C), suggesting that
Curcumol significantly inhibited the growth of tumors in
vivo.
Curcumol effected the expression of
EMT-associated genes and the secretion of TGF-β1 in vivo
Tumors and serum were extracted from nude mice and
western blotting and ELISA assays were performed to detect the
expression of EMT-associated proteins and the secretion of TGF-β1.
The expression of E-cadherin was significantly increased in the
Curcumol group compared with the control group (Fig. 5A), while the expression of
N-cadherin was significantly decreased in the Curcumol group
(Fig. 5B). The expression of
TGF-β1 in tumors and serum was measured by western blotting and
ELISA. The concentration of TGF-β1 was significantly decreased in
the Curcumol group compared with the control group (Fig. 5C) and the results of tumor analysis
were consistent with this (Fig.
5D).
Discussion
NPC is the most common malignant tumor of the
nasopharynx mucosa and has regional distribution characteristics;
the incidence in developing countries is higher compared with
developed countries (21). The
application of intensity-modulated radiotherapy and platinum-based
chemotherapy, which are used alone or in combination, has led to
significant improvements in tumor control and long-term survival of
patients with NPC (22). However,
metastasis remains an important factor in the treatment and
prognosis of patients with NPC (23). TCM has been developed for cancer
research and many Chinese herbal medicines have been demonstrated
to inhibit cancer. Celastrol is a triterpene from TCM, which
demonstrates anti-proliferative activity in HONE-1 and NPC-039 cell
lines and induces apoptosis through the death receptor and
mitochondrial pathway in human NPC cells, which makes it a
promising candidate in the development of drugs for treating NPC
(24). Evodiamine inhibits the
migration and invasive ability of NPC cells and metastasis
targeting matrix metalloproteinase-2 (25). The aim of the current study was to
identify a Chinese herbal medicine that targets the EMT pathway in
order to inhibit metastasis of NPC.
EMT is one of the key factors serving a role in
tumor progression (26). It is a
biological process in which epithelial cells lose their epithelial
morphology, thereby improving their migration and invasive ability
and giving them status as mesenchymal cells (27). Thus, this suggests that the EMT
pathway is vital in the invasion and metastasis ability of
malignant tumors (28). It has
been suggested that TGF-β1 is the most important factor in the
induction of EMT during development, cancer and other pathological
conditions (26). It has also been
previously demonstrated that in HepG2 cell lines, simple TGF-β1
stimulation induces EMT (29). In
addition, the molecular hallmarks of EMT are mesenchymal and
epithelial phenotypes, including the expression of E-cadherin and
N-cadherin (30). The metastasis
of NPC is closely associated with the occurrence of EMT and
previous study has indicated that inhibition of EMT may
significantly suppress metastasis of NPC (25). Therefore, investigation of
EMT-associated medicine is required to improve the prognosis of
patients with NPC.
Due to the lack of side effects, an increasing
number of studies have focused on the biological activities of
Curcumol. Curcumol exhibits multiple biological activities,
including antitumor activity (31). In addition, the mechanism of the
anticancer activity of Curcumol was by inducing the expression of
anti-oncogenes or inhibiting the expression of oncogenes. The
results of the current study indicated that the viability of NPC
5–8F cells was significantly decreased following treatment with
Curcumol and the rate of apoptosis was significantly increased in a
dose-dependent manner, which provides basis for further study of
the effect of Curcumol on NPC. The results of the wound-healing and
Transwell assays confirmed that Curcumol reduced the migration and
invasion ability of 5–8F cells. Furthermore, the expression of the
primary regulatory proteins serving a role in the EMT pathway are
also regulated by Curcumol. The results of the in vivo
experiments also indicated that Curcumol significantly inhibited
tumor growth and the inhibitory effect of the 0.4 µM/ml group was
significantly higher compared with that of the control group.
Furthermore, the results of in vivo experiments
investigating the expression of EMT associated proteins were
consistent with those on 5–8F cells. In addition, Curcumol
inhibited the expression of TGF-β1 in tumors in nude mice and also
inhibits the secretion of TGF-β1 in the serum of nude mice.
In conclusion, the current study investigated the
effect of Curcumol in arresting cell migration against the human
NPC5-8F cell line and also showed that the inhibition of TGF-β1 led
to the decrease of EMT occurrence, which was considered a result of
the anticancer activity of Curcumol. These results were also
confirmed in vivo using a xenograft mouse model.
However, there are some limitations to the current
study. Due to the funding of the project and the time schedule of
the authors, only one cell line was selected for the experiment.
Therefore, further studies using a greater number of cell lines are
required to supplement the experimental results. However, the
results of the current study did indicate the molecular mechanism
of Curcumol, which may potentially improve the quality of life of
patients with NPC in the future.
Acknowledgements
Not applicable.
Funding
This study was funded by Sichuan Provincial Science
and Technology Department of key projects (14ZA0198).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DY did the majority of the experiments and wrote the
paper. SD, WG, SL and YL helped perform the cell experiments. DY
performed the statistical analysis.
Ethics approval and consent to
participate
Animal experiments were approved by Affiliated
Hospital of North Sichuan Medical College hospital ethics committee
(CBYXY0022). All procedures performed in studies were in accordance
with the ethical standards.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hu C, Wei W, Chen X, Woodman CB, Yao Y,
Nicholls JM, Joab I, Sihota SK, Shao JY, Derkaoui KD, et al: A
global view of the oncogenic landscape in nasopharyngeal carcinoma:
An integrated analysis at the genetic and expression levels. PLoS
One. 7:e410552012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu N, Chen NY, Cui RX, Li WF, Li Y, Wei
RR, Zhang MY, Sun Y, Huang BJ, Chen M, et al: Prognostic value of a
microRNA signature in nasopharyngeal carcinoma: A microRNA
expression analysis. Lancet Oncol. 13:633–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sarmiento MP and Mejia MB: Preliminary
assessment of nasopharyngeal carcinoma incidence in the
Philippines: A second look at published data from four centers.
Chin J Cancer. 33:159–164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang HZ, Cao CN, Luo JW, Yi JL, Huang XD,
Zhang SP, Wang K, Qu Y, Xiao JP, Li SY, et al: High-risk factors of
parotid lymph node metastasis in nasopharyngeal carcinoma: A
case-control study. Radiat Oncol. 11:1132016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yousefi M, Bahrami T, Salmaninejad A,
Nosrati R, Ghaffari P and Ghaffari SH: Lung cancer-associated brain
metastasis: Molecular mechanisms and therapeutic options. Cell
Oncol (Dordr). 40:419–441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu D: Innate and adaptive immune cell
metabolism in tumor microenvironment. Adv Exp Med Biol.
1011:211–223. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He X, Guo X, Zhang H, Kong X, Yang F and
Zheng C: Mechanism of action and efficacy of LY2109761, a TGF-β
receptor inhibitor, targeting tumor microenvironment in liver
cancer after TACE. Oncotarget. 9:1130–1142. 2017.PubMed/NCBI
|
|
8
|
Fuxe J and Karlsson MC: TGF-β-induced
epithelial-mesenchymal transition: A link between cancer and
inflammation. Semin Cancer Biol. 22:455–461. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grelet S, McShane A, Geslain R and Howe
PH: Pleiotropic roles of non-coding RNAs in TGF-β-mediated
epithelial-mesenchymal transition and their functions in tumor
progression. Cancers (Basel). 9:E752017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ding Y, Pan Y, Liu S, Jiang F and Jiao J:
Elevation of MiR-9-3p suppresses the epithelial-mesenchymal
transition of nasopharyngeal carcinoma cells via down-regulating
FN1, ITGB1 and ITGAV. Cancer Biol Ther. 18:414–424. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang T, Yin P, Zhang Z, Xu B, Che D, Dai
Z, Dong C, Jiang P, Hong H, Yang Z, et al: Deficiency of pigment
epithelium-derived factor in nasopharyngeal carcinoma cells
triggers the epithelial-mesenchymal transition and metastasis. Cell
Death Dis. 8:e28382017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiao LJ and Tao R: Traditional chinese
medicine (TCM) therapy. Adv Exp Med Biol. 1010:261–280. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Huang F, Bai Z, Chi B, Wu J and
Chen X: Curcumol inhibits growth and induces apoptosis of
colorectal cancer LoVo cell line via IGF-1R and p38 MAPK pathway.
Int J Mol Sci. 16:19851–19867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mun SH, Kim SB, Kong R, Choi JG, Kim YC,
Shin DW, Kang OH and Kwon DY: Curcumin reverse methicillin
resistance in Staphylococcus aureus. Molecules. 19:18283–18295.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang Y, Li ZS, Jiang FS, Deng X, Yao CS
and Nie G: Effects of different ingredients of zedoary on gene
expression of HSC-T6 cells. World J Gastroenterol. 11:6780–6786.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dang YY, Li XC, Zhang QW, Li SP and Wang
YT: Preparative isolation and purification of six volatile
compounds from essential oil of Curcuma wenyujin using
high-performance centrifugal partition chromatography. J Sep Sci.
33:1658–1664. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang W, Wang Z and Chen T: Curcumol
induces apoptosis via caspases-independent mitochondrial pathway in
human lung adenocarcinoma ASTC-a-1 cells. Med Oncol. 28:307–314.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo P, Wang YW, Weng BX, Li XK, Yang SL
and Ye FQ: Synthesis, anti-tumor activity, and structure-activity
relationships of curcumol derivatives. J Asian Nat Prod Res.
16:53–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen G, Wang Y, Li M, Xu T, Wang X, Hong B
and Niu Y: Curcumol induces HSC-T6 cell death through suppression
of Bcl-2: Involvement of PI3K and NF-kappaB pathways. Eur J Pharm
Sci. 65:21–28. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fles R, Bos ACRK, Supriyati, Rachmawati D,
Waliyanti E, Tan IB, Haryana SM, Schmidt MK and Dewi FST: The role
of Indonesian patients' health behaviors in delaying the diagnosis
of nasopharyngeal carcinoma. BMC Public Health. 17:5102017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Twu CW, Wang WY, Chen CC, Liang KL, Jiang
RS, Wu CT, Shih YT, Lin PJ, Liu YC and Lin JC: Metronomic adjuvant
chemotherapy improves treatment outcome in nasopharyngeal carcinoma
patients with postradiation persistently detectable plasma
Epstein-Barr virus deoxyribonucleic acid. Int J Radiat Oncol Biol
Phys. 89:21–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chee J, Loh KS, Tham I, Ho F, Wong LC, Tan
CS, Goh BC and Lim CM: Prognostic stratification of patients with
metastatic nasopharyngeal carcinoma using a clinical and
biochemical scoring system. J Cancer Res Clin Oncol. 143:2563–2570.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin HF, Hsieh MJ, His YT, Lo YS, Chuang
YC, Chen MK and Chien SY: Celastrol-induced apoptosis in human
nasopharyngeal carcinoma is associated with the activation of the
death receptor and the mitochondrial pathway. Oncol Lett.
14:1683–1690. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peng X, Zhang Q, Zeng Y, Li J, Wang L and
Ai P: Evodiamine inhibits the migration and invasion of
nasopharyngeal carcinoma cells in vitro via repressing MMP-2
expression. Cancer Chemother Pharmacol. 76:1173–1184. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Runa F, Hamalian S, Meade K, Shisgal P,
Gray PC and Kelber JA: Tumor microenvironment heterogeneity:
Challenges and opportunities. Curr Mol Biol Rep. 3:218–229. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu A, Wu K, Li J, Mo Y, Lin Y, Wang Y,
Shen X, Li S, Li L and Yang Z: Let-7a inhibits migration, invasion
and epithelial-mesenchymal transition by targeting HMGA2 in
nasopharyngeal carcinoma. J Transl Med. 13:1052015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taparra K, Tran PT and Zachara NE:
Hijacking the hexosamine biosynthetic pathway to promote
EMT-mediated neoplastic phenotypes. Front Oncol. 6:852016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang M, Sui C, Dai B, Shen W, Lu J and
Yang J: PEG10 is imperative for TGF-β1-induced
epithelialmesenchymal transition in hepatocellular carcinoma. Oncol
Rep. 37:510–518. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Heldin CH, Vanlandewijck M and Moustakas
A: Regulation of EMT by TGFβ in cancer. FEBS Lett. 586:1959–1970.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu JJ, Dang YY, Huang M, Xu WS, Chen XP
and Wang YT: Anti-cancer properties of terpenoids isolated from
Rhizoma Curcumae-a review. J Ethnopharmacol. 143:406–411. 2012.
View Article : Google Scholar : PubMed/NCBI
|