Introduction
Intraductal papillary mucinous neoplasms of the
pancreas (IPMN) are one of the most important precancerous lesions
associated with pancreatic cancers. IPMN are slow-growing
intraductal tumors with a favorable prognosis (1). With the development of radiological
technology, intraductal lesions of the pancreas have been diagnosed
increasingly frequently. IPMN currently accounts for 8–20% of all
resected pancreatectomy specimens (2–6), and
the incidence is increasing.
IPMN are characterized by intraductal papillary
proliferation of mucin-producing cells, which causes cystic
dilation of the pancreatic ducts. IPMN are different from
pancreatic intraepithelial neoplasias (PanINs). IPMN are visible
and detectable lesions with a diameter >1 cm, whereas PanINs are
diagnosed microscopically and are usually <0.5 cm in diameter
(7). Similar to the
adenoma-carcinoma sequence that is observed in colon cancer, IPMN
stand to progress from adenomatous lesions (IPMN with low- or
intermediate-grade dysplasia) to high-grade dysplasia and finally
to invasive carcinomas (IPMN-associated carcinomas), which exhibit
the most severe architectural and cytological atypia.
Histologically, IPMN may be divided into gastric-type,
intestinal-type, pancreatobiliary-type and oncocytic-type,
according to the epithelial lining. Each histological type has
specific histopathological features and biological behaviors.
IPMN-associated carcinomas primarily comprise pancreatic ductal
adenocarcinomas (PDAs) and colloid carcinomas. It has been
previously demonstrated that intestinal-type IPMNs usually progress
to pancreatic carcinomas via a colloidal pattern, while
pancreatobiliary-type progress via a tubular pattern. Minimally
invasive IPMN is defined by Nara et al (8) as a lesion that invades slightly
beyond the ductal wall.
KRAS, a member of the ras gene family, is one of the
most important and commonly mutated oncogenes observed in
pancreatic neoplasms (9). Numerous
reports have confirmed that KRAS mutations serve a dominant and
important role in the tumorigenesis of PDAs and IPMN (10–14).
Previous studies have discussed the association between KRAS
mutations and survival in patients with PDAs, and demonstrated that
mutations were not associated with poor prognosis (15–18).
Previous studies have reported that point mutations are located
primarily at codon 12 in exon 2 in 31–86% of patients with IPMN
(10–14,19–22).
There have been few studies elaborating upon the alteration of KRAS
mutation frequency among different subtypes (gastric, intestinal or
pancreatobiliary type) or different grades (low, intermediate and
high grade). Few articles have discussed the correlation between
prognosis and KRAS mutation status in patients with IPMN (23).
In China, to the best of our knowledge, there is no
available research regarding the incidence of IPMN or other
associated data. A total of 56 IPMN cases were collected for the
present study, clinicopathological analysis was performed and KRAS
mutation status was detected. The objective of the present study
was to discuss the clinical meaning of KRAS mutations and their
association with histological classification, and subtypes,
invasion and survival of IPMN.
Materials and methods
Study population
All of the pancreatic specimens were obtained from
Peking Union Medical College Hospital, Beijing, China. Between
January 2000 and December 2009, a total of 900 pancreatic tumors
were resected and confirmed by pathology analysis. A total of two
expert pathologists reviewed all slides and classified the cases
according to the new World Health Organization (WHO) classification
system. A total of 61 patients were diagnosed with IPMN, and 29
exhibited invasive carcinomas associated with IPMN. Of the 61 IPMN
cases, there was a failure to extract sufficient genomic DNA to
assess KRAS genetic alterations in five cases. All IPMN lesions
were associated with the pancreatic duct system, and microscopic
observation did not reveal any ‘ovarian-type’ hypercellular stroma
surrounding the neoplasms. The present study was approved by the
Ethics Committee of Peking Union Medical College Hospital, and
informed consent was obtained from all cases.
Pathological examination of IPMN
Histological grading of the IPMN was performed
according to the criteria defined by the 2010 edition of the WHO
classification system for digestive neoplasms (24). Infiltrative growth accompanied by
desmoplastic fibrosis was used as the only criterion to determine
infiltration. A small number of samples exhibited evidence of mucin
leaking into the interstitial space and forming a simple mucous
lake around the pancreatic duct in the absence of floating cancer
cells, and this was not regarded as invasion in the present study.
Invasive depth was measured from the duct to the deepest
infiltrating cancer cells. A cut-off point of 0.5 cm was selected
for the depth of invasion and the cases were divided into two
groups: Cases with an invasive depth ≤0.5 cm was designated as
IPMN-associated minimally invasive carcinoma (IPMN-MI), and
invasive depth >0.5 cm was designated as IPMN-associated
advanced invasive carcinoma (IPMN-AI). All sections were observed
at a magnification of ×40, 100 and 200 by Olympus BX53 microscope
(Olympus Corporation, Tokyo, Japan).
Mutation analysis
Neutral 10% formalin-fixed (at room temperature,
overnight) and paraffin-embedded pancreatic neoplasm tissue
sections were cut (6 µm), and subsequently microdissected manually
to ensure the highest purity and the largest amount of tumor cells
in each sample. The resection margins with non-tumorous tissue
served as normal controls. Genomic DNA was extracted using the
QIAmp DNA Mini kit (Qiagen Inc., Valencia, CA, USA).
A DxS ARMS KRAS mutation test kit (DXS International
PLC, Farnham, UK) was used to detect point mutations in codons 12
and 13 of exon 2 of the KRAS gene. This ARMS assay was a
quantitative polymerase chain reaction (qPCR) assay that detected a
total of eight genetic mutations, including 35G>A, 35G>T,
35G>C, 34G>A, 34G>T, 34G>C, 37G>C and 38G>A. The
mutation test was performed according to the manufacturer's
protocol. qPCR was performed using an internal Scorpion reporter
probe and primers specific for wild and mutant KRAS on the ABI 7500
Real-time polymerase chain reaction system (ABI; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The following thermocycling
conditions were used for the qPCR: 50°C for 2 min, initial
denaturation at 95°C for 10 min; 40 cycles of 95°C for 15 sec and
60°C for 10 sec. Data analysis was performed using the ABI SDS
software v1.4.0.25 (Thermo Fisher Scientific, Inc.). The
differences between the cycle threshold (CT) values of the mutant
and wild types were calculated. When the difference of CT value
between wild and mutant type was less than the cut-off value, we
determined that there was a corresponding mutation in the sample
(25,26). Subsequently, the mutation of KRAS
in codon 61 was detected via direct sequencing of the PCR
amplification product with an automated ABI 3730 sequencer (ABI;
Thermo Fisher Scientific, Inc.). The primers for codon 61 of KRAS
were 5′-TCCCTTCTCAGGATTCCTACA-3′ and 5′-CAAAGAAAGCCCTCCCCAGT-3′.
The following thermocycling conditions were used for the PCR
amplification of codon 61: Initial denaturation at 94°C for 5 min;
40 cycles of 94°C for 30 sec, 58°C for 30 sec and 72°C for 30 sec;
and final extension for 10 min at 72°C. The amplification was
performed using TaKaRa Ex Taq polymerase (Takara Bio Inc., Otsu,
Japan).
Statistical analysis
All statistical analyses were performed using SPSS
version 12.0 (SPSS Inc., Chicago, IL, USA) software. Data are
presented as the mean value. P<0.05 was considered to indicate a
statistically significant difference. The comparison between
continuous variables was calculated using a Student's t-test. The
correlation between categorical variables was acquired by
χ2 test or Fisher's exact test. The χ2 test
was used in the IPMN constituent ratio analysis. The Kaplan-Meier
method was used to assess survival time, and the log-rank test was
applied to determine significance.
Results
IPMNs subtypes and classification
Of the 56 cases of IPMN, 33 were in the head of the
pancreas, 12 in the tail of the pancreas, and 11 in the whole
pancreas. The age of patients ranged between 39 and 79 years, and
the average was 61.8 years. The onset manifestation was abdominal
discomfort (abdominal pain, abdominal distention and diarrhea) in
34 cases. A total of 13 cases were detected accidentally with no
clinical symptoms. The remaining 9 cases represented symptoms with
jaundice (n=4), weight loss (n=2), back pain (n=1), a high level of
cancer antigen 199 (n=1) and a high creatinine level (n=1). Sex,
age and tumor location were not associated with invasion. However,
21/56 (37.5%) patients had a history of acute or chronic
pancreatitis with a high amylase level in the blood on at least one
occasion prior to the surgery, and 17 patients smoked for >10
years. However, pancreatitis (P=0.408) and smoking (P=0.771) were
not associated with KRAS mutations.
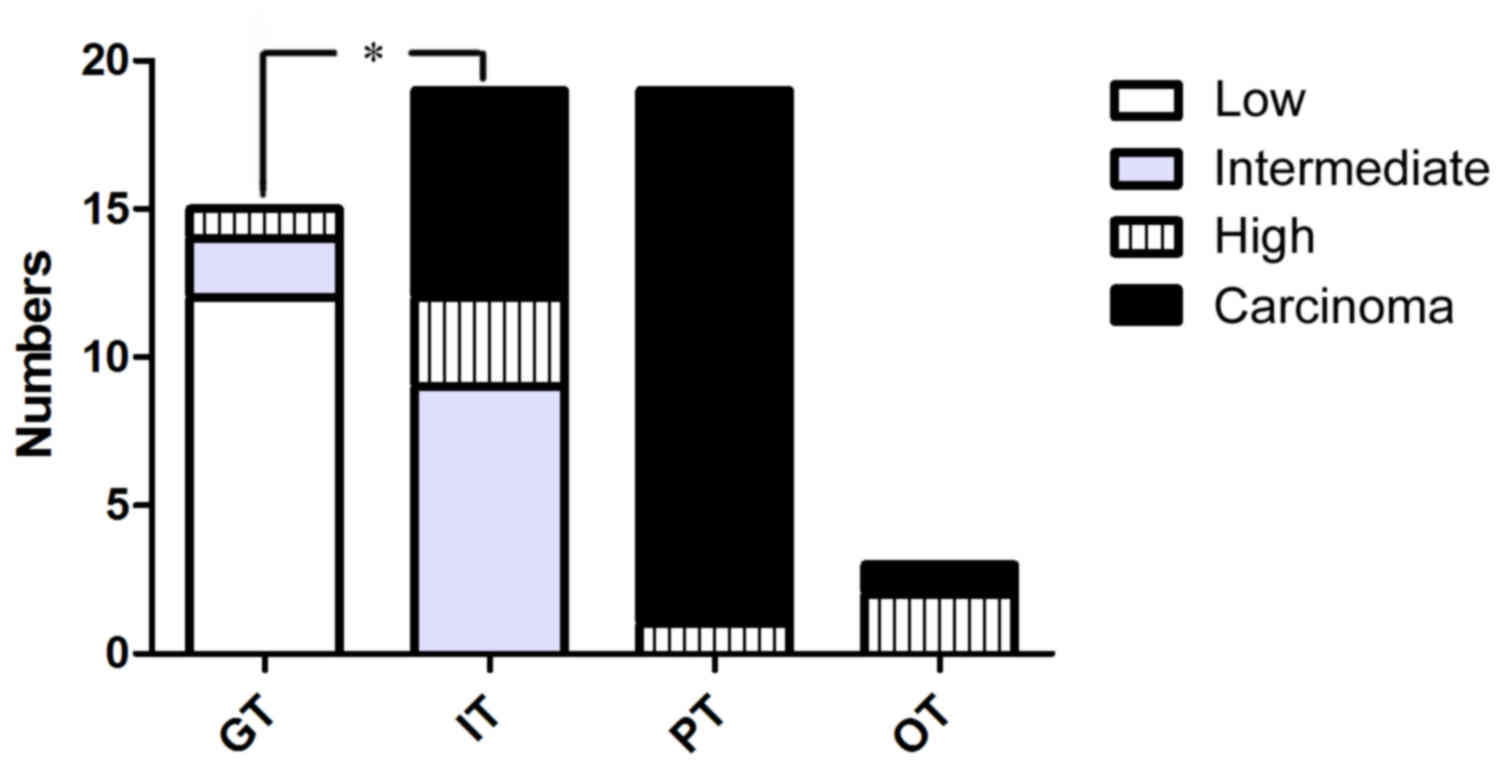
IPMN were subclassified into four histological
subtypes: gastric-type (15/56; 26.8%), intestinal-type (19/56;
33.9%), pancreatobiliary-type (19/56; 33.9%) and oncocytic-type
(3/56; 5.3%). Based on the highest degree of architectural and
cytological atypia, the present cohort comprised 12 (21.4%) IPMN
cases with low-grade dysplasia, 11 (19.6%) IPMN cases with
intermediate-grade dysplasia, 7 (12.5%) IPMN cases with high-grade
dysplasia and 26 (46.4%) IPMN-associated invasive carcinomas
(Table I). The majority of the
gastric-type IPMN cases (14/15) were classified as IPMN with low-
and intermediate-grade dysplasia (Fig.
1). Almost all of the pancreatobiliary-type IPMN cases (18/19)
were IPMN-associated carcinomas. This suggested that
pancreatobiliary-type IPMN are significantly associated with high
grade dysplasia and invasive adenocarcinoma, while gastric-type
IPMN were associated with low and intermediate grade dysplasia
(P=0.004). IPMN-associated carcinomas were primarily PDAs (21/26;
80.8%), colloid carcinomas (4/26; 15.4%) and, rarely,
undifferentiated carcinomas (1/26; 3.8%). A total of 11 cases
belonged to the IPMN-microinvasive group, including 10 tubular
adenocarcinomas and one colloid carcinoma.
 | Table I.Association between KRAS mutation and
the clinicopathological features of IPMN. |
Table I.
Association between KRAS mutation and
the clinicopathological features of IPMN.
| Feature | KRAS+ (%) | KRAS- (%) | P-value |
|---|
| Sex |
|
| 0.408a |
|
Male | 19 (76.0) | 16 (64.0) |
|
|
Female | 9 (42.9) | 12 (57.1) |
|
| Age, years |
|
|
>0.9a |
|
≥65 | 12 (50.0) | 12 (50.0) |
|
|
<65 | 16 (50.0) | 16 (50.0) |
|
| Mean size, cm | 3.50 | 3.71 | 0.718b |
| Pancreatitis |
|
| 0.408a |
|
Yes | 12 (57.1) | 9 (42.9) |
|
| No | 16 (64.0) | 19 (76.0) |
|
| Smoking |
|
| 0.771a |
|
Yes | 9 (52.9) | 8 (47.1) |
|
| No | 19 (48.7) | 20 (51.3) |
|
| Macroscopic
type |
|
| 0.695c |
| Main
duct type | 14 (45.2) | 17 (54.8) |
|
| Branch
duct type | 4 (50.0) | 4 (50.0) |
|
| Mixed
type | 10 (58.8) | 7 (41.2) |
|
| Histological
classification |
|
| 0.605a |
| Low
grade | 7 (58.3) | 5 (41.7) |
|
|
Intermediate grade | 3 (27.3) | 8 (72.7) |
|
| High
grade | 4 (57.1) | 3 (42.9) |
|
| With
associated carcinoma | 14 (53.8) | 12 (46.2) |
|
| Histological
subtype |
|
| 0.159c |
|
Gastric-type | 9 (60.0) | 6 (40.0) |
|
|
Intestinal-type | 10 (52.6) | 9 (47.4) |
|
|
Pancreatobiliary-type | 9 (47.4) | 10 (52.6) |
|
|
Oncocytic-type | 0 (0) | 3 (100) |
|
| IPMN/associated
carcinoma |
|
| 0.592a |
|
Yes | 14 (53.8) | 12 (46.2) |
|
| No | 14 (46.7) | 16 (53.3) |
|
Survival of patients with IPMNs
A total of five out of 56 patients were lost to
follow-up, 35 patients were alive and tumor-free, four patients
were alive with IPMN, and 12 patients succumbed to IPMN-associated
invasive carcinoma. The mean postoperative follow-up period was
40.8 months (range, 12–112 months) for living patients. The overall
5-year survival rate was 76%. The 5-year survival rate of the
patients with non-invasive IPMN was 100%. The 5-year survival rate
of the patients with invasive IPMN was not available; instead, the
3-year survival rate of the patients with invasive IPMN was 55%. Of
the 12 patients who succumbed, eight presented with
pancreatobiliary-type IPMN, three had intestinal-type IPMN and one
had oncocytic-type IPMN. A patient diagnosed with colloid carcinoma
succumbed due to a complication of Trousseau syndrome 11 months
post-surgery. Of the 12 patients who succumbed, all of them
presented with IPMN-associated carcinomas and succumbed 7–41 months
post-surgery; 11 of these patients succumbed within 2 years. A
total of four patients developed recurrent tumors in the remnant
pancreas, and these patients presented with gastric-type IPMN with
low-grade dysplasia, intestinal-type IPMN with intermediate-grade
dysplasia, and intestinal-type and oncocytic-type IPMN with
high-grade dysplasia, respectively. Recurrent tumors remained
within the pancreatic ducts and exhibited the same histological
features as their origin tumors. The first two patients had
positive surgical margins in the first segmental pancreatectomy
operation.
Disease-specific survival rates were analyzed using
the Kaplan-Meier method. Upon comparison of the survival stages of
IPMN-MI and IPMN-AI, it was identified that the survival stage of
IPMN-AI tumors was significantly shorter compared with that of
IPMN-MI tumors (log-rank test; P=0.0006; Fig. 2A). Among the three histological
subtypes, gastric-type IPMN had the best prognosis,
pancreatobiliary-type IPMN had the worst, and intestinal-type IPMN
lay in between (log-rank test; P<0.0001; Fig. 2B). Pancreatobiliary-type IPMN were
more likely to develop into infiltrative carcinomas.
KRAS mutations
KRAS mutations were identified in 28 of the 56
tested patients, with a rate of 50%. No mutation was detected in
the matching normal tissues. The frequency of KRAS mutations was
60% (9/15 cases) in gastric-type IPMN, 52.6% (10/19 cases) in
intestinal-type, and 47.3% (9/19 cases) in pancreatobiliary-type.
KRAS mutations were not present in oncocytic-type IPMN. Except for
oncocytic-type IPMN, the frequencies of KRAS mutations in IPMN with
low-, intermediate- and high-grade dysplasia, and IPMN-associated
carcinomas were 58.3% (7/12 cases), 27.3% (3/11 cases), 80% (4/5
cases) and 56% (14/25 cases), respectively. KRAS mutation rates in
low-grade (58.3%) and high-grade IPMNs (80%) were increased
compared with intermediate-grade IPMN (27%). There was no
association between KRAS mutations and histological subtype
(P=0.159), or invasive cancer (P=0.592) (Table I).
There were certain differences in KRAS mutations
among the three histological subtypes. Of the 19 intestinal-type
IPMN cases, 6/7 invasive intraductal papillary mucinous carcinoma
exhibited KRAS mutations, and 2/9 borderline IPMN exhibited KRAS
mutations. With the increased degree of dysplasia and invasion of
intestinal-type IPMN, KRAS mutation rates increased (P=0.012)
(Table II). Compared with
intestinal-type IPMN with low- and intermediate-grade dysplasia,
gastric-type IPMN with low- and intermediate-grade dysplasia had
increased KRAS mutations (57.1 and 22.2%). Intestinal-type IPMN
with associated carcinomas had a higher KRAS mutation prevalence
compared with pancreatobiliary-type IPMN with associated carcinomas
(86.7 and 44.4%). Due to the small number of cases, it was not
possible to calculate a P-value.
 | Table II.V-Ki-ras 2 Kirsten rat sarcoma viral
oncogene homolog mutational ratio of gastric-type, intestinal-type
and pancreatobiliary-type IPMN in IPMN with low and intermediate
grade dysplasia, high grade dysplasia, and associated
carcinoma. |
Table II.
V-Ki-ras 2 Kirsten rat sarcoma viral
oncogene homolog mutational ratio of gastric-type, intestinal-type
and pancreatobiliary-type IPMN in IPMN with low and intermediate
grade dysplasia, high grade dysplasia, and associated
carcinoma.
|
| Gastric-type
(n=15) | Intestinal-type
(n=19) |
Pancreatobiliary-type (n=19) |
|---|
| Low and
intermediate grade | 8/14 (57.1%) | 2/9 (22.2%) | 0 |
| High grade | 1/1 | 2/3 | 1/1 |
| Associated
carcinoma | 0 | 6/7 (85.7%) | 8/18 (44.4%) |
The present study compared the histopathological
features of the invasive group (26 cases) with KRAS mutation
occurrence, and demonstrated that the infiltrative depth (P=0.753),
differentiation (P=0.909), lymph node metastasis (P=0.177),
infiltrative extent (P=0.419) and the type of carcinoma (P=0.468)
had no association with KRAS mutation occurrence (Table III). The Kaplan-Meier survival
curve demonstrated that survival was not associated with KRAS
mutation (log-rank test; P=0.308; Fig.
2C).
 | Table III.Association between KRAS mutation,
and pathological features and prognosis of intraductal papillary
mucinous neoplasms of the pancreas-associated carcinoma. |
Table III.
Association between KRAS mutation,
and pathological features and prognosis of intraductal papillary
mucinous neoplasms of the pancreas-associated carcinoma.
|
| KRAS+ | KRAS- | P-value |
|---|
| Infiltrative depth,
cm |
|
|
|
|
≤0.5/>0.5 | 4/10 | 7/5 | 0.753a |
|
Differentiation |
|
|
|
|
Well-differentiated | 7 | 7 |
|
|
Moderately-differentiated | 4 | 3 |
|
|
Poorly-differentiated | 3 | 2 | 0.909a |
| Lymph node
metastasis |
|
|
|
|
Yes/no | 4/10 | 2/10 | 0.470a |
| Infiltrative
scope |
|
|
|
|
Limited/adipose tissue | 8/6 | 8/4 | 0.619a |
| AJCC |
|
|
|
| Stage
I | 6 | 5 |
|
| Stage
II | 7 | 7 |
|
| Stage
III | 1 | 0 |
|
| Stage
IV | 0 | 0 | N/A |
| Carcinoma
types |
|
|
|
|
Tubular/non-tubular | 10/4 | 10/2 | 0.468a |
The mutated site exhibited single-amino-acid
substitutions, including G12D (13 cases; 41%), G12V (12 cases;
38%), G12S (two cases), G12A (one case), G12C (two cases), G12R
(one case) and 61 CAA>CGA (one case), and double-amino-acid
substitutions, including G12V/G12S (two cases), G12D/G12S (one
case) and G12D/G12V (one case). Among the four cases with double
mutations, three were invasive carcinomas and the other was a
benign tumor. A total of eight out of 13 cases with G12D mutation
were IPMN with high-grade dysplasia and/or IPMN-associated
carcinomas, and seven out of 12 cases with G12V mutation were IPMN
with high-grade dysplasia and/or IPMN-associated carcinomas. The
altered amino acids were not associated with IPMN classification.
No mutations were identified within codon 13 of exon 2.
Discussion
IPMN are a common cystic neoplasm located in the
main and/or branch ducts of the pancreas. The histological features
and classification of IPMN have been well characterized (2,27–32).
The results of the present study indicated that histological
subtype may suggest prognosis, similar to the reports by
Mino-Kenudson et al (33),
Furukawa et al (34) and
Kang et al (35).
Gastric-type IPMN have an overall favorable prognosis, exhibiting a
very low risk of developing into carcinomas and with a longer
survival compared with other subtypes (33–35).
Almost all IPMN-associated colloid carcinomas progress from
intestinal-type IPMN (36), which
have a better prognosis compared with IPMN-associated tubular
adenocarcinomas (37,38). Pancreatobiliary-type IPMN
frequently appear with high-grade dysplasia and usually progress
into tubular adenocarcinomas (39). A total of 18 of the 19
pancreatobiliary-type IPMN were invasive IPMN in the present study.
Furthermore, the incidence rates of the pancreatobiliary-type and
intestinal-type lesions were equal, and tubular adenocarcinoma was
the predominant type of neoplasm, rather than colloid carcinoma.
Therefore, pancreatobiliary-type IPMN have the worst prognosis.
In accordance with previous reports, the mutation
rates of the KRAS gene in PDAs and undifferentiated carcinomas were
high, up to 90–100% (40,41). Mutations in the KRAS gene have been
identified in 36–81% of IPMN. In the present study, the mutation
rate of the KRAS gene was 50% in IPMN and 54% in IPMN-associated
carcinomas, significantly lower compared with PDAs and PanINs. This
result suggested that IPMN may employ a different carcinogenic
pathway compared with the PanIN-PDA sequence (11,14,42,43).
The KRAS mutation foci of IPMN were primarily G12D and G12V,
similar to PDAs (22,42–45).
Wu et al (22) identified a
high prevalence of KRAS mutations at codon 12. In accordance with
the present results, the most commonly altered amino acid was G12D
and G12V. The other amino acid alterations, including G12S, G12A,
G12C and G12R, occurred at a low rate. No mutations were detected
in codon 13 of exon 2 in the present study. Nikiforova et al
(45) reported a 4% mutation rate
in codon 13 of pancreatic cysts (G13D); it may be considered that
the small number of cases and the Chinese cohort used in the
present study may account for this difference. In addition,
previous studies have demonstrated that Chinese cohorts have a
lower KRAS mutation rate compared with European or Japanese cohorts
(46,47).
In the present study, the rate of mutation in IPMN
with low-grade dysplasia was 58%, which suggested that KRAS
mutation is a very early event during tumorigenesis and serves an
important role in the development of IPMNs, similar to what has
been observed in PanIN (47). KRAS
gene mutations have been demonstrated to be increased in proportion
to the degree of dysplasia, and were previously identifies in 36,
44 and 87% of PanIN1A, PanIN1B and PanIN-2/3 lesions, respectively
(48). KRAS mutation has been
observed to serve an important role in the PanIN-ductal
adenocarcinoma sequence of the pancreas (49,50).
Regarding the association between KRAS mutations and subtypes of
IPMN, the previous studies in the literature are controversial
(10–14,19–21).
A particular study reported that KRAS mutation was not associated
with classification or histological subtype (43), while another study suggested that
KRAS mutation correlated with malignancy in IPMN (51). The present results were similar to
those of Wu et al (22) and
Nikiforova et al (45),
demonstrating that higher KRAS mutation rates were identified in
low- and high-grade IPMN compared with intermediate-grade IPMN.
This suggested that KRAS mutations were early onset and had no
association with the degree of dysplasia in IPMN.
KRAS mutations were present in all histological
subtypes except oncocytic IPMN. Wu et al (22) demonstrated that KRAS mutations had
the highest frequency in pancreatobiliary-type IPMN and the lowest
frequency in intestinal-type IPMN. Nikiforova et al
(45) reported that gastric-type
IPMN had the highest mutation prevalence. The results of the
present study demonstrated that low-grade IPMN and gastric-type
IPMN tended to have KRAS mutations, although it was not possible to
acquire statistical data due to the low case numbers. Typically,
intestinal-type IPMN had the lowest KRAS mutant prevalence. This
may explain why IPMN with intermediate-grade dysplasia had the
lowest mutation prevalence. However, no KRAS mutations were
detected in the three oncocytic-type IPMN cases, consistent with
other reports in the literature (52). KRAS mutation prevalence was
markedly low in intestinal-type IPMN with low- and
intermediate-grade dysplasia, and high in gastric-type IPMN. Almost
no low- or intermediate-grade dysplasia was identified in
pancreatobiliary type IPMN, while this type of dysplasia was
frequently identified in intestinal and gastric type IPMN. KRAS
mutation frequency differed between gastric-, intestinal- and
pancreatobiliary type of IPMN and therefore the overall KRAS
mutation rate depended on the relative proportion of each type of
IPMN in the studied cohort. The higher the proportion of gastric
type IPMN in the studied cohort, the higher the frequency of KRAS
mutation. In a similar way, KRAS mutation prevalence in
IPMN-associated carcinoma was determined to a great extent by the
ratio of intestinal- and pancreatobiliary-type IPMN. The
heterogeneity of IPMN may explain the marked difference in various
studies into KRAS mutation prevalence.
In the present study, intestinal type IPMN was the
only type with low-grade to high-grade dysplasia and infiltrating
adenocarcinoma. There was no gastric-type associated
adenocarcinoma, or pancreatobiliary-type with low- and intermediate
dysplasia. Therefore, intestinal type IPMN was selected to study
the association between KRAS mutation and dysplasia. It was
demonstrated that the frequency of KRAS mutation increased with the
increase of the degree of dysplasia and invasion. Intestinal-type
IPMN-associated carcinomas exhibited higher KRAS mutation
frequencies compared with non-invasive IPMN.
Previously, a number of studies have discussed the
association between KRAS mutation and survival in patients with
pancreatic carcinomas, and reported that KRAS mutations were not
associated with poor prognosis in patients with PDA (15,17,53),
and a KRAS mutant allele-specific imbalance was associated with
worse prognosis in pancreatic cancer and progression to
undifferentiated carcinoma of the pancreas (9). From the data in the present study, it
was suggested that there was no significant difference in KRAS
mutation frequencies among the different histological subtypes,
associated invasive carcinoma incidence, infiltrative depth,
differentiation, lymph node metastasis or invasive scope.
The Kaplan-Meier survival curve demonstrated that
the survival rate had no association with KRAS mutation. Therefore,
the detection of KRAS mutations in a biopsy from a patient with
IPMN may not be used to draw the conclusion that the lesion had
high dysplasia, infiltration or a bad prognosis. KRAS mutations do
not serve a role in predicting the prognosis or survival of
patients with IPMN.
The present study elaborated upon the clinical
significance of KRAS mutations. However, the present study had a
relatively small sample size. A future study is required with a
larger sample size. Furthermore, further studies may focus on other
molecular alterations in addition to KRAS.
In conclusion, KRAS mutation prevalence in
intestinal-type IPMN was the lowest, and the incidence increases
with the degree of dysplasia and invasion in intestinal-type IPMNs.
KRAS mutation in IPMN does not correlate with histological subtype,
dysplasia grade, invasion or survival.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Foundation of
Pathologic Research Centre of the China Academy of Medical Sciences
(grant nos. 2015PT320002 and 2016ZX310176-3).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC contributed to the conception of the idea. XC and
YL contributed to the literature search and drafting of the
manuscript. YW performed the molecular genetic studies. JW
contributed to the statistical work and language editing. All
authors critically reviewed and accepted the final version of the
manuscript. JC will accept full responsibility for the present
study.
Ethics approval and consent to
participate
This study was approved by Ethics Committee of
Peking Union Medical College Hospital and informed consents were
obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IPMN
|
intraductal papillary mucinous
neoplasms of the pancreas
|
|
IPMN-MI
|
IPMN with an associated micro-invasive
carcinoma
|
|
IPMN-AI
|
IPMN with an associated advanced
invasive carcinoma
|
|
IPMN-GT
|
gastric type IPMN
|
|
IPMN-IT
|
intestinal type IPMN
|
|
IPMN-PT
|
pancreatobiliary type IPMN
|
|
PDA
|
pancreatic ductal adenocarcinomas
|
|
KRAS
|
V-Ki-ras 2 Kirsten rat sarcoma viral
oncogene homolog
|
|
ABI
|
Applied Biosystems
|
|
ARMS
|
amplified refractory mutation
system
|
|
qPCR
|
quantitative polymerase chain
reaction
|
References
|
1
|
Fernández-del Castillo C and Adsay NV:
Intraductal papillary mucinous neoplasms of the pancreas.
Gastroenterology. 139(708–713): e1–2. 2010.
|
|
2
|
Adsay NV, Conlon KC, Zee SY, Brennan MF
and Klimstra DS: Intraductal papillary-mucinous neoplasms of the
pancreas: An analysis of in situ and invasive carcinomas in 28
patients. Cancer. 94:62–77. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fernández-del Castillo C, Targarona J,
Thayer SP, Rattner DW, Brugge WR and Warshaw AL: Incidental
pancreatic cysts: Clinicopathologic characteristics and comparison
with symptomatic patients. Arch Surg. 138:427–434. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andrejevic-Blant S, Kosmahl M, Sipos B and
Klöppel G: Pancreatic intraductal papillary-mucinous neoplasms: A
new and evolving entity. Virchows Arch. 451:863–869. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kosmahl M, Pauser U, Peters K, Sipos B,
Lüttges J, Kremer B and Klöppel G: Cystic neoplasms of the pancreas
and tumor-like lesions with cystic features: A review of 418 cases
and a classification proposal. Virchows Arch. 445:168–178. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Balzano G, Zerbi A and Di Carlo V:
Intraductal papillary mucinous tumors of the pancreas: Incidence,
clinical findings and natural history. JOP. 6 1 Suppl:S108–S111.
2005.
|
|
7
|
Hruban RH and Klimsra DS: Intraductal
neoplasms. Hruban RH, Pitman MB and Klimsstra DS: AFIP Atlas of
Tumor Pathology, Series 4: Tumors of the Pancreas. 2007.
|
|
8
|
Nara S, Shimada K, Kosuge T, Kanai Y and
Hiraoka N: Minimally invasive intraductal papillary-mucinous
carcinoma of the pancreas: Clinicopathologic study of 104
intraductal papillary-mucinous neoplasms. Am J Surg Pathol.
32:243–255. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krasinskas AM, Moser AJ, Saka B, Adsay NV
and Chiosea SI: KRAS mutant allele-specific imbalance is associated
with worse prognosis in pancreatic cancer and progression to
undifferentiated carcinoma of the pancreas. Mod Pathol.
26:1346–1354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sessa F, Solcia E, Capella C, Bonato M,
Scarpa A, Zamboni G, Pellegata NS, Ranzani GN, Rickaert F and
Klöppel G: Intraductal papillary-mucinous tumours represent a
distinct group of pancreatic neoplasms: An investigation of tumour
cell differentiation and K-ras, p53 and c-erbB-2 abnormalities in
26 patients. Virchows Arch. 425:357–367. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tada M, Omata M and Ohto M: Ras gene
mutations in intraductal papillary neoplasms of the pancreas.
Analysis in five cases. Cancer. 67:634–637. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Satoh K, Shimosegawa T, Moriizumi S,
Koizumi M and Toyota T: K-ras mutation and p53 protein accumulation
in intraductal mucin-hypersecreting neoplasms of the pancreas.
Pancreas. 12:362–368. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Satoh K, Sawai T, Shimosegawa T, Koizumi
M, Yamazaki T, Mochizuki F and Toyota T: The point mutation of
c-Ki-ras at codon 12 in carcinoma of the pancreatic head region and
in intraductal mucin-hypersecreting neoplasm of the pancreas. Int J
Pancreatol. 14:135–143. 1993.PubMed/NCBI
|
|
14
|
Uemura K, Hiyama E, Murakami Y, Kanehiro
T, Ohge H, Sueda T and Yokoyama T: Comparative analysis of K-ras
point mutation, telomerase activity, and p53 overexpression in
pancreatic tumours. Oncol Rep. 10:277–283. 2003.PubMed/NCBI
|
|
15
|
Oliveira-Cunha M, Hadfield KD, Siriwardena
AK and Newman W: EGFR and KRAS mutational analysis and their
correlation to survival in pancreatic and periampullary cancer.
Pancreas. 41:428–434. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim ST, Lim DH, Jang KT, Lim T, Lee J,
Choi YL, Jang HL, Yi JH, Baek KK, Park SH, et al: Impact of KRAS
mutations on clinical outcomes in pancreatic cancer patients
treated with first-line gemcitabine-based chemotherapy. Mol Cancer
Ther. 10:1993–1999. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schultz NA, Roslind A, Christensen IJ,
Horn T, Høgdall E, Pedersen LN, Kruhøffer M, Burcharth F, Wøjdemann
M and Johansen JS: Frequencies and prognostic role of KRAS and BRAF
mutations in patients with localized pancreatic and ampullary
adenocarcinomas. Pancreas. 41:759–766. 2012.PubMed/NCBI
|
|
18
|
Bournet B, Muscari F, Guimbaud R,
Cordelier P and Buscail L: KRAS mutations and their correlation
with survival of patients with advanced pancreatic cancer.
Pancreas. 42:543–544. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Z'Graggen K, Rivera JA, Compton CC, Pins
M, Werner J, Fernández-del Castillo C, Rattner DW, Lewandrowski KB,
Rustgi AK and Warshaw AL: Prevalence of activating K-ras mutations
in the evolutionary stages of neoplasia in intraductal papillary
mucinous tumors of the pancreas. Ann Surg. 226:491–500. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schönleben F, Qiu W, Ciau NT, Ho DJ, Li X,
Allendorf JD, Remotti HE and Su GH: PIK3CA mutations in intraductal
papillary mucinous neoplasm/carcinoma of the pancreas. Clin Cancer
Res. 12:3851–3855. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshizawa K, Nagai H, Sakurai S, Hironaka
M, Morinaga S, Saitoh K and Fukayama M: Clonality and K-ras
mutation analyses of epithelia in intraductal papillary mucinous
tumor and mucinous cystic tumor of the pancreas. Virchows Arch.
441:437–443. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu J, Matthaei H, Maitra A, Dal Molin M,
Wood LD, Eshleman JR, Goggins M, Canto MI, Schulick RD, Edil BH, et
al: Recurrent GNAS mutations define an unexpected pathway for
pancreatic cyst development. Sci Transl Med. 3:92ra662011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Furukawa T, Kuboki Y, Tanji E, Yoshida S,
Hatori T, Yamamoto M, Shibata N, Shimizu K, Kamatani N and
Shiratori K: Whole-exome sequencing uncovers frequent GNAS
mutations in intraductal papillary mucinous neoplasms of the
pancreas. Sci Rep. 1:1612011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Swerdlow SH, Campo E and Harris NL: WHO
classification of tumors of the digestive system. 4th edition.
IARC; pp. 304–313. 2010
|
|
25
|
Bando H, Tsuchihara K, Yoshino T, Kojima
M, Ogasawara N, Fukushima H, Ochiai A, Ohtsu A and Esumi H: Biased
discordance of KRAS mutation detection in archived colorectal
cancer specimens between the ARMS-Scorpion method and direct
sequencing. Jpn J Clin Oncol. 41:239–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Franklin WA, Haney J, Sugita M, Bemis L,
Jimeno A and Messersmith WA: KRAS mutation: Comparison of testing
methods and tissue sampling techniques in colon cancer. J Mol
Diagn. 12:43–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Adsay NV, Merati K, Basturk O,
Iacobuzio-Donahue C, Levi E, Cheng JD, Sarkar FH, Hruban RH and
Klimstra DS: Pathologically and biologically distinct types of
epithelium in intraductal papillary mucinous neoplasms: Delineation
of an ‘intestinal’ pathway of carcinogenesis in the pancreas. Am J
Surg Pathol. 28:839–848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hruban RH, Takaori K, Klimstra DS, Adsay
NV, Albores-Saavedra J, Biankin AV, Biankin SA, Compton C,
Fukushima N, Furukawa T, et al: An illustrated consensus on the
classification of pancreatic intraepithelial neoplasia and
intraductal papillary mucinous neoplasms. Am J Surg Pathol.
28:977–987. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lüttges J, Zamboni G, Longnecker D and
Klöppel G: The immunohistochemical mucin expression pattern
distinguishes different types of intraductal papillary mucinous
neoplasms of the pancreas and determines their relationship to
mucinous noncystic carcinoma and ductal adenocarcinoma. Am J Surg
Pathol. 25:942–948. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Furukawa T, Klöppel G, Adsay Volkan N,
Albores-Saavedra J, Fukushima N, Horii A, Hruban RH, Kato Y,
Klimstra DS, Longnecker DS, et al: Classification of types of
intraductal papillary-mucinous neoplasm of the pancreas: A
consensus study. Virchows Arch. 447:794–799. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakamura A, Horinouchi M, Goto M, Nagata
K, Sakoda K, Takao S, Imai K, Kim YS, Sato E and Yonezawa S: New
classification of pancreatic intraductal papillary-mucinous tumour
by mucin expression: Its relationship with potential for
malignancy. J Pathol. 197:201–210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tanaka M, Chari S, Adsay V, Fernandez-del
Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K and Matsuno
S: International Association of Pancreatology: International
consensus guidelines for management of intraductal papillary
mucinous neoplasms and mucinous cystic neoplasms of the pancreas.
Pancreatology. 6:17–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mino-Kenudson M, Fernández-del Castillo C,
Baba Y, Valsangkar NP, Liss AS, Hsu M, Correa-Gallego C, Ingkakul
T, Johnston Perez R, Turner BG, et al: Prognosis of invasive
intraductal papillary mucinous neoplasm depends on histological and
precursor epithelial subtypes. Gut. 60:1712–1720. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Furukawa T, Hatori T, Fujita I, Yamamoto
M, Kobayashi M, Ohike N, Morohoshi T, Egawa S, Unno M, Takao S, et
al: Prognostic relevance of morphological types of intraductal
papillary mucinous neoplasms of the pancreas. Gut. 60:509–516.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kang MJ, Lee KB, Jang JY, Han IW and Kim
SW: Evaluation of clinical meaning of histological subtypes of
intraductal papillary mucinous neoplasm of the pancreas. Pancreas.
42:959–966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Poultsides GA, Reddy S, Cameron JL, Hruban
RH, Pawlik TM, Ahuja N, Jain A, Edil BH, Iacobuzio-Donahue CA,
Schulick RD and Wolfgang CL: Histopathologic basis for the
favorable survival after resection of intraductal papillary
mucinous neoplasm-associated invasive adenocarcinoma of the
pancreas. Ann Surg. 251:470–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Adsay NV, Pierson C, Sarkar F, Abrams J,
Weaver D, Conlon KC, Brennan MF and Klimstra DS: Colloid (mucinous
noncystic) carcinoma of the pancreas. Am J Surg Pathol. 25:26–42.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sadakari Y, Ohuchida K, Nakata K, Ohtsuka
T, Aishima S, Takahata S, Nakamura M, Mizumoto K and Tanaka M:
Invasive carcinoma derived from the nonintestinal type intraductal
papillary mucinous neoplasm of the pancreas has a poorer prognosis
than that derived from the intestinal type. Surgery. 147:812–817.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takasu N, Kimura W, Moriya T, Hirai I,
Takeshita A, Kamio Y and Nomura T: Intraductal papillary-mucinous
neoplasms of the gastric and intestinal types may have less
malignant potential than the pancreatobiliary type. Pancreas.
39:604–610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Moskaluk CA, Hruban RH and Kern SE: p16
and K-ras gene mutations in the intraductal precursors of human
pancreatic adenocarcinoma. Cancer Res. 57:2140–2143.
1997.PubMed/NCBI
|
|
41
|
Rozenblum E, Schutte M, Goggins M, Hahn
SA, Panzer S, Zahurak M, Goodman SN, Sohn TA, Hruban RH, Yeo CJ and
Kern SE: Tumor-suppressive pathways in pancreatic carcinoma. Cancer
Res. 57:1731–1734. 1997.PubMed/NCBI
|
|
42
|
Schonleben F, Qiu W, Remotti HE,
Hohenberger W and Su GH: PIK3CA, KRAS, and BRAF mutations in
intraductal papillary mucinous neoplasm/carcinoma (IPMN/C) of the
pancreas. Langenbecks Arch Surg. 393:289–296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chadwick B, Willmore-Payne C, Tripp S,
Layfield LJ, Hirschowitz S and Holden J: Histologic,
immunohistochemical, and molecular classification of 52 IPMNs of
the pancreas. Appl Immunohistochem Mol Morphol. 17:31–39. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Izeradjene K, Combs C, Best M, Gopinathan
A, Wagner A, Grady WM, Deng CX, Hruban RH, Adsay NV, Tuveson DA and
Hingorani SR: Kras(G12D) and Smad4/Dpc4 haploinsufficiency
cooperate to induce mucinous cystic neoplasms and invasive
adenocarcinoma of the pancreas. Cancer Cell. 11:229–243. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nikiforova MN, Khalid A, Fasanella KE,
McGrath KM, Brand RE, Chennat JS, Slivka A, Zeh HJ, Zureikat AH,
Krasinskas AM, et al: Integration of KRAS testing in the diagnosis
of pancreatic cystic lesions: A clinical experience of 618
pancreatic cysts. Mod Pathol. 26:1478–1487. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kwon MJ, Jeon JY, Park HR, Nam ES, Cho SJ,
Shin HS, Kwon JH, Kim JS, Han B, Kim DH and Choi YL: Low frequency
of KRAS mutation in pancreatic ductal adenocarcinomas in Korean
patients and its prognostic value. Pancreas. 44:484–492.
2015.PubMed/NCBI
|
|
47
|
Kanda M, Matthaei H, Wu J, Hong SM, Yu J,
Borges M, Hruban RH, Maitra A, Kinzler K, Vogelstein B and Goggins
M: Presence of somatic mutations in most early-stage pancreatic
intraepithelial neoplasia. Gastroenterology. 142(730–733):
e92012.
|
|
48
|
Löhr M, Klöppel G, Maisonneuve P,
Lowenfels AB and Lüttges J: Frequency of K-ras mutations in
pancreatic intraductal neoplasia associated with pancreatic ductal
adenocarcinoma and chronic pancreatitis: A meta-analysis.
Neoplasia. 7:17–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shi C, Hong SM, Lim P, Kamiyama H, Khan M,
Anders RA, Goggins M, Hruban RH and Eshleman JR: KRAS2 mutations in
human pancreatic acinar-ductal metaplastic lesions are limited to
those with PanIN: Implications for the human pancreatic cancer cell
of origin. Mol Cancer Res. 7:230–236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
di Magliano MP and Logsdon CD: Roles for
KRAS in pancreatic tumor development and progression.
Gastroenterology. 144:1220–1229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jang JY, Park YC, Song YS, Lee SE, Hwang
DW, Lim CS, Lee HE, Kim WH and Kim SW: Increased K-ras mutation and
expression of S100A4 and MUC2 protein in the malignant intraductal
papillary mucinous tumor of the pancreas. J Hepatobiliary Pancreat
Surg. 16:668–674. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chung SM, Hruban RH, Iacobuzio-Donahue CA,
Adsay NV, Zee SY and Klimstra DS: Analysis of molecular alterations
and differentiation pathways in intraductal oncocytic papillary
neoplasm of the pancreas. Mod Pathol. 18:277A–288A. 2005.
|
|
53
|
Kim J, Jang KT, Park Mo S, Lim SW, Kim JH,
Lee KH, Lee JK, Heo JS, Choi SH, Choi DW, et al: Prognostic
relevance of pathologic subtypes and minimal invasion in
intraductal papillary mucinous neoplasms of the pancreas. Tumour
Biol. 32:535–542. 2011. View Article : Google Scholar : PubMed/NCBI
|