Introduction
Spinal cord injury (SCI) results from the breakdown
of spinal cord tissues due to mechanical stress and is a severe
health problem worldwide. The primary acute phase of SCI consists
of direct tissue lesion and is accompanied by hemorrhage, whereas
the secondary phase consists of neuronal apoptosis at the sites of
lesion formation (1,2). During the secondary phase of SCI, the
generation of reactive oxygen species, formation of edema,
expression of cytokines leading to inflammation, and neuronal
apoptosis occur (3–5). The onset of inflammation is
considered to be the most serious factor resulting in secondary
injury (3–5). Neuronal cells do not possess the
ability to regenerate following SCI, which requires the treatment
strategy be free from any harmful side effects. Therefore, studies
have investigated the effect of inhibitors of apoptosis on
neurological injuries, including SCI, with the aim of preventing
the secondary phase of injury (6).
Cytokines, also known as inflammatory factors,
including, tumor necrosis factor (TNF)-α), interleukin (IL)-6 and
IL-18, are vital in various pathological and physiological process
in cells. In patients with acute kidney injury, it has been
observed that IL-18 acts as a marker for the level of acute kidney
injury, and its expression determines patient survival rates
(7). The expression of TNF-α and
IL-6 mediated by IL-18 enhance the rate of inflammatory reactions
leading to organ injury (8).
Therefore, inhibiting the expression of inflammatory factors is
important in the prevention of organ injury.
Dexmedetomidine is known for its role as a selective
α-2 adrenoceptor agonist and it exhibits inhibitory effect on
sympathetic activity (9,10). It has been reported that
dexmedetomidine inhibits the apoptosis of neurons and protects
various other organs from damage (11,12).
Dexmedetomidine treatment has also been found to prevent liver and
intestinal injury in patients following hepatectomy through the
inhibition of cell apoptosis (13,14).
In the present study, the effect of dexmedetomidine treatment on
SCI in a rat model was investigated. It was observed that
dexmedetomidine improved locomotor activity in the rats with SCI by
inhibiting edema formation, reducing cytokine expression and
inhibiting the induction of neuronal apoptosis.
Materials and methods
Experimental animals
A total of 30 adult male Sprague-Dawley rats
(weight, 240–260 g) were obtained from the Experimental Animal
Center (Xiamen University, Zhangzhou, China). The animals were
housed in well-ventilated rooms with ~60% humidity, a temperature
maintained at 23°C and a 12/12-h light/dark cycle for 15 days prior
to the start of experiments. The experimental procedures were
performed in accordance with the Guide for the Care and Use of
Laboratory Animals of the National Institute of Health (15). The procedures were approved by the
Government Animal Care Committee of the Medical College of Xiamen
University under the reference no. 011/MCXU/2013. The animals were
sacrificed following anesthetization with an injection of 400 mg/kg
of chloral hydrate (Beyotime Institute of Biotechnology, Haimen,
China) intraperitoneally. Following SCI, the animals were placed in
their cages with free access to standard food and water.
Preparation of the traumatic SCI
animal model using the weight-drop method
The SCI rat model was prepared using the weight-drop
method. The rats were divided randomly into three groups of 10
rats: Sham injury, SCI and treatment groups. The rats were
anesthetized with an injection of 400 mg/kg chloral hydrate and the
spinal cord of each animal was exposed by T12 spinal laminectomy.
Following exposure, an impactor with a diameter of 2.0 mm was used
to perform the weight-drop injury to establish the traumatic SCI
animal model. The animals in the sham group were not subjected to
the weight-drop step.
Treatment strategy
Following SCI, the animals in the treatment group
were injected with a single 50 mg/kg dose of dexmedetomidine
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) intraperitoneally
immediately after the incision was closed. The animals in the sham
and SCI groups received the same volume of normal saline.
Analysis of edema formation in the
spinal cord of rats with SCI
The accumulation of water (edema formation) in the
spinal cord tissues of the rats with SCI was determined by
measuring the spinal cord weight. The wet weight of the spinal cord
immediately following extraction was recorded. The spinal cord was
then dried for 24 h at 75°C and the dry weight was measured. The
difference in the wet and dry weight of the spinal cord was used
for the determination of edema.
Determination of the activity of
myeloperoxidase (MPO)
The activity of MPO was analyzed for the
determination of neutrophil accumulation in the spinal cord tissues
of the rats. Briefly, the spinal cord lysates were treated with the
o-dianisidine, 50 mM potassium phosphate buffer and 20 mM
H2O2 mixture. Absorbance of the solution was
recorded spectrophotometrically at 460 nm to determine the rate of
change. The quantity of enzymes (U/g) consumed to quench the
reactive oxygen species was measured.
Behavioral assessments
Improvements in the locomotor activity of rats
following dexmedetomidine treatment were analyzed using the method
described by Basso et al (16). Three independent observers analyzed
the locomotor activities of the rats using the Basso, Beattie and
Bresnahan (BBB) locomotor rating scale. The scale is graded into 21
points with the lowest point indicating complete paralysis and the
highest point representing normal locomotion. On day 1 post-SCI and
every week following SCI, the rats were allowed to walk on an
irregularly distributed horizontal wire grid. When walking, the
observers carefully recorded the locomotor activity of the
rats.
Determination of expression levels of
TNF-α and IL-1β in rats
As the rate of inflammatory reactions resulting in
organ injury is mediated by the increased expression of TNF-α and
IL-6 (8), the present study
analyzed the expression levels of TNF-α and IL-6. At 24 h post-SCI,
the rats in the treatment groups were weighed, anesthetized, placed
in a prone position and then sacrificed via injecting air through
the ear vein. A small incision was made in the back of each rat,
and the skin was carefully removed, followed by the subcutaneous
tissues, to expose the spinal cord. The injured spinal cords were
collected, placed into lysis buffer (5 mg/100 µl) and homogenized
on ice. The commercially available TNF-α and IL-1β colorimetric
kits (Calbiochem; EMD Millipore, Billerica, MA, USA) were used for
determination of the expression levels of TNF-α and IL-1β
expression according to the manufacturer's protocol. The
determination was performed in triplicate.
Western blot analysis
At 24 h post-SCI, the rats were weighed,
anesthetized, placed in a prone position and then sacrificed via
injecting air through the ear vein. A small incision was made
carefully in the back of rat, and the skin was removed, followed by
subcutaneous tissues, to expose the spinal cord. The injured spinal
cord was collected, placed into lysis buffer (5 mg/100 µl) and
homogenized on ice. The homogenized spinal cord tissues were
transferred into centrifuge tubes and lysed for 10 min, followed by
centrifugation for 20 min at 4,000 × g at 4°C. The supernatants
were decanted into the pre-cooled centrifuge tubes. The
concentration of proteins was determined by Bicinchoninic Acid
protein assay kit (Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China) according to the manufacturer instructions. A
total of 2 µg protein samples were loaded onto 15%
SDS-polyacrylamide gel and separated by electrophoresis. The
separated proteins were then transferred onto nitrocellulose
membranes. The membranes were blocked with 5% non-fat-milk solution
and then blotted with appropriate primary antibodies against Bax
(1:1,000; 5023S) and Bcl-2 (1:1,000; 15071S; both from Cell
Signaling Technology, Inc., Danvers, MA, USA). The incubation with
primary antibodies was performed overnight at 4°C followed by 1 h
incubation with peroxidase-labeled secondary antibody (1:2,000
dilution; 7077S; Cell Signaling Technology, Inc.) at room
temperature. The bands were visualized using an enhanced
chemiluminescence detection technique (GE Healthcare Life Sciences,
Chalfont, UK) using Quantity One Software v4.2 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Immunohistochemical assay and H&E
staining
After 10 days post-treatment with dexmedetomidine
following SCI, two animals from each group were anesthetized using
Nembutal. The thoracic cavities of the rats after opening were
intracardially perfused using normal saline. Then, ~400 ml fixative
containing paraformaldehyde (4%) in 0.1 M PBS (pH 7.4) was used for
perfusion of the rats and subsequently T6-14 segment was extracted
from the animal spinal cord. The spinal cord tissues were treated
with phosphate-buffered sucrose (30%) following 3 h of fixing
paraformaldehyde (4%) in 0.1 M PBS at room temperature. The
paraffin embedded tissues were mounted on the slides coated with
0.02% poly-L-lysine. The chromagen used contained a combination of
avidin-biotin-peroxidase complex (Invitrogen; Thermo Fisher
Scientific, Inc.) and 3,3′-diaminobenzidine hydrochloride (DAB;
Sigma-Aldrich; Merck KGaA). For this, the tissues following PBS
washing were incubated for 45 min at room temperature with 1%
bovine serum albumin (Gibco; Thermo Fisher Scientific, Inc.). The
tissues were then incubated at 4°C for overnight with primary
antibodies against platelet-derived growth factor subunit B
(PDGF-B; dilution, 1:100; cat. no. 8912; Cell Signaling Technology,
Inc.). Following incubation, tissues were washed with PBS and then
incubated with biotinylated goat anti-rabbit secondary antibody
(diluted to 1:200 in PBS) at room temperature for 1 h.
Visualization was performed using DAB (0.05%) and hydrogen peroxide
(0.3%) in combination. Dehydration of tissues using ethanol and
xylene was followed by mounting under coverslips. For pathological
changes 2 µm thick tissue sections were stained with hematoxylin
for 5 min at 25°C and with eosin for 2 min at 25°C. Stained
sections were imaged using a Olympus BX51 light microscope (Olympus
Corporation, Tokyo, Japan) (magnification, ×400).
Statistical analysis
For statistical analysis, Statistical Package for
Social Sciences (SPSS) version 13.0 for Windows (SPSS, Inc.,
Chicago, IL, USA) was used. One-way analysis of variance was used
for the comparison of BBB scores. P<0.05 was considered to
indicate a statistically significant difference. Data are expressed
as the mean ± standard error of the mean.
Results
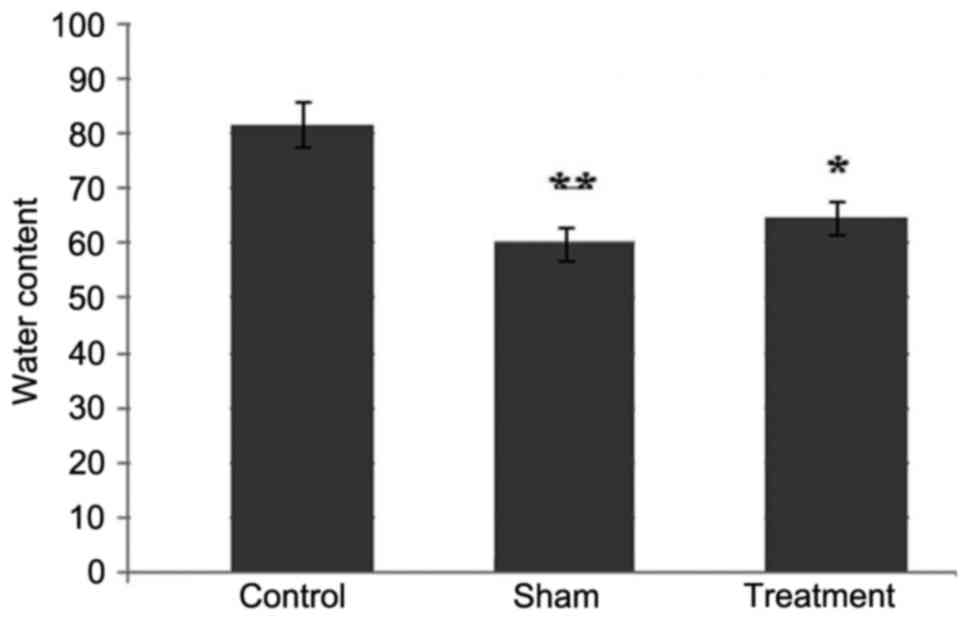
Dexmedetomidine prevents the formation
of edema in rat spinal cord tissues following SCI
The examination of the rats 24 h following SCI
showed the formation of edema due to the accumulation of water
content in the spinal cord tissues. However, treatment of the rats
with dexmedetomidine at a dose of 50 mg/kg significantly decreased
the formation of edema in the tissues of the spinal cord (Fig. 1). Compared with the rats in the
sham and dexmedetomidine treatment groups, edema formation was
higher in the spinal cord of rats in the control group.
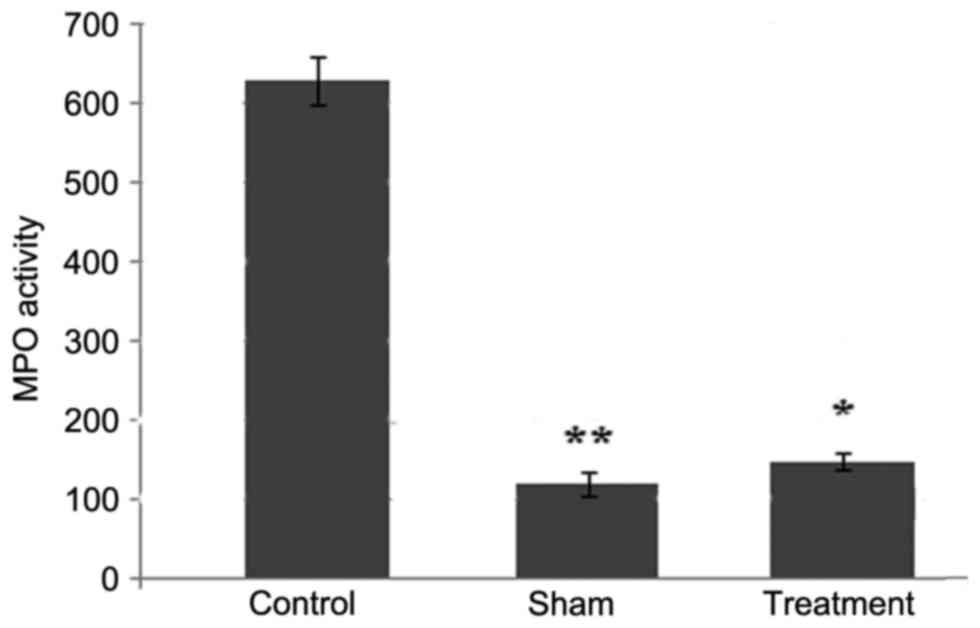
Dexmedetomidine inhibits the
infiltration of neutrophils in the spinal cord tissues
At 24 h post-SCI, the accumulation of neutrophils
was increased, which was evident by a significant increase in the
activity of MPO. Treatment of the rats with dexmedetomidine at a
dose of 50 mg/kg exhibited an inhibitory effect on the SCI-induced
increase in the accumulation of neutrophils (Fig. 2). Compared with the animals in the
control group, the accumulation of neutrophils was significantly
(P<0.05) decreased in the dexmedetomidine and sham groups
(Fig. 2).
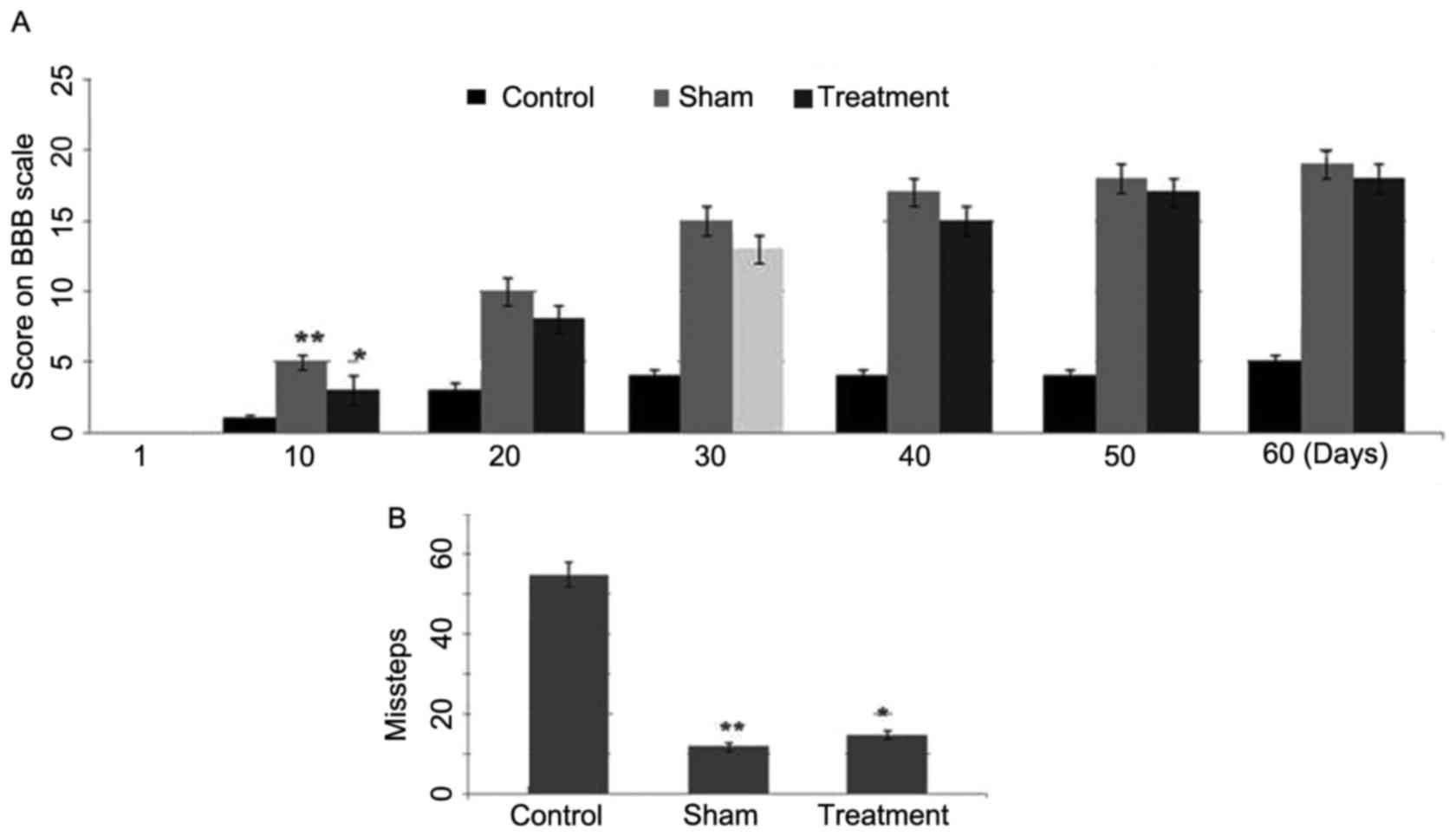
Effect of dexmedetomidine on
functional recovery following SCI in rats
The results from the BBB locomotor test revealed a
significant (P<0.001) increase in locomotor performance 10 days
following SCI in the dexmedetomidine treatment group (Fig. 3A). Compared with the first day
following SCI, the BBB scores were significantly increased in the
rats of the dexmedetomidine group 10 days post-SCI. The comparison
of BBB locomotor scores showed the highest score in the sham group,
followed by the dexmedetomidine group and then the control group.
However, the scores were closer in the animals of the
dexmedetomidine and sham groups (Fig.
3A). In the grid walking test, the animals in the control group
showed a higher number of missteps, compared with those in the
dexmedetomidine treatment group (Fig.
3B). Therefore, a significant improvement was observed in the
locomotion performance of the animals with SCI treated with
dexmedetomidine.
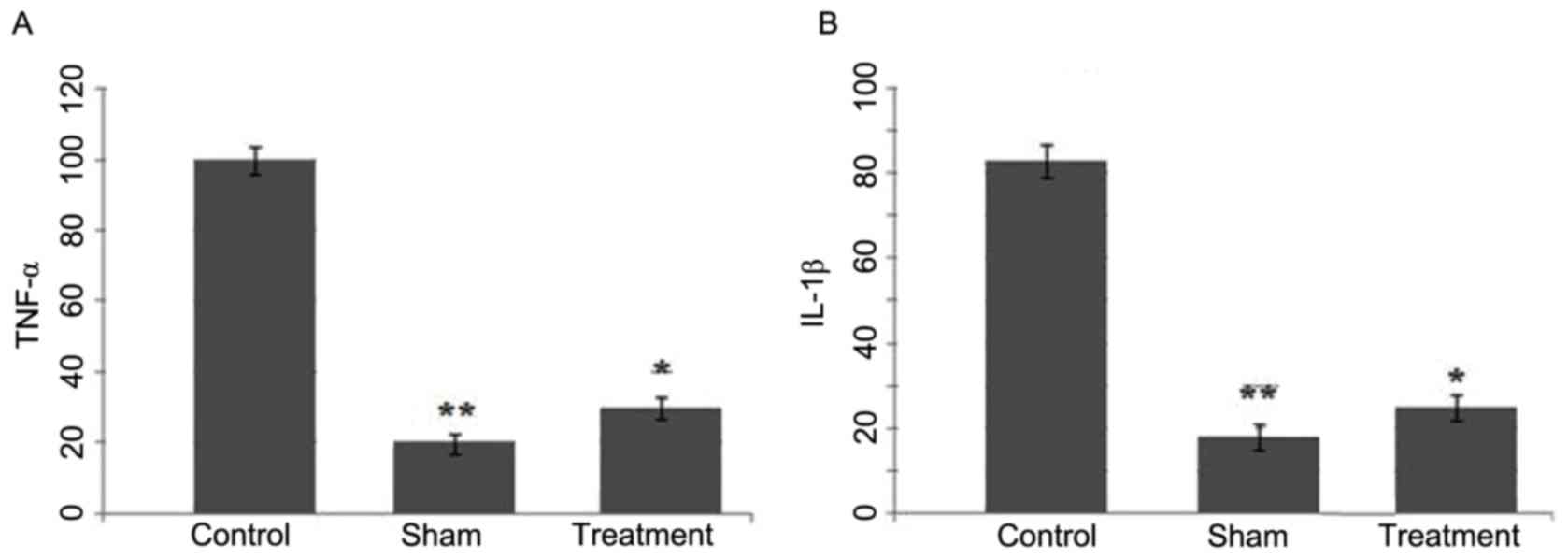
Effect of the dexmedetomidine on the
expression levels of TNF-α and IL-1β following SCI
The expression levels of TNF-α and IL-1β in the
spinal cord tissues of SCI rats treated with dexmedetomidine were
analyzed using western blot analysis. SCI led to a marked increase
in the expression of levels of TNF-α and IL-1β at 24 h (control
group), compared with the sham group (Fig. 4A and B). However, in the rats
treated with dexmedetomidine following SCI, no such increase in the
expression levels of TNF-α or IL-1β were observed in the spinal
cord tissues (Fig. 4).
Effect of dexmedetomidine on the
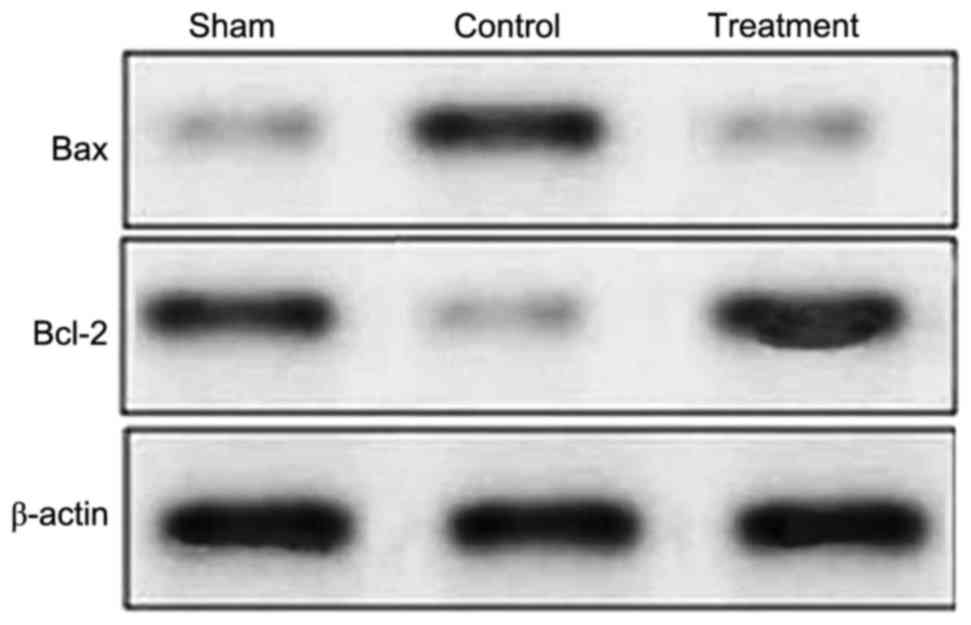
protein expression levels of Bax and Bcl-2
Compared with the rats in the sham group, the rats
in the control group revealed a marked increase in the expression
of Bax following SCI (Fig. 5).
Treatment with dexmedetomidine following SCI had an inhibitory
effect on the expression of Bax in the tissues of the spinal cord.
The expression of Bax in the dexmedetomidine treatment group was
similar to that of the control group (Fig. 5). Compared with the sham group, the
expression of Bcl-2 was significantly reduced in the rats 24 h
following SCI, however, the expression of Bcl-2 was significantly
increased in the rats with SCI treated with dexmedetomidine
(Fig. 5).
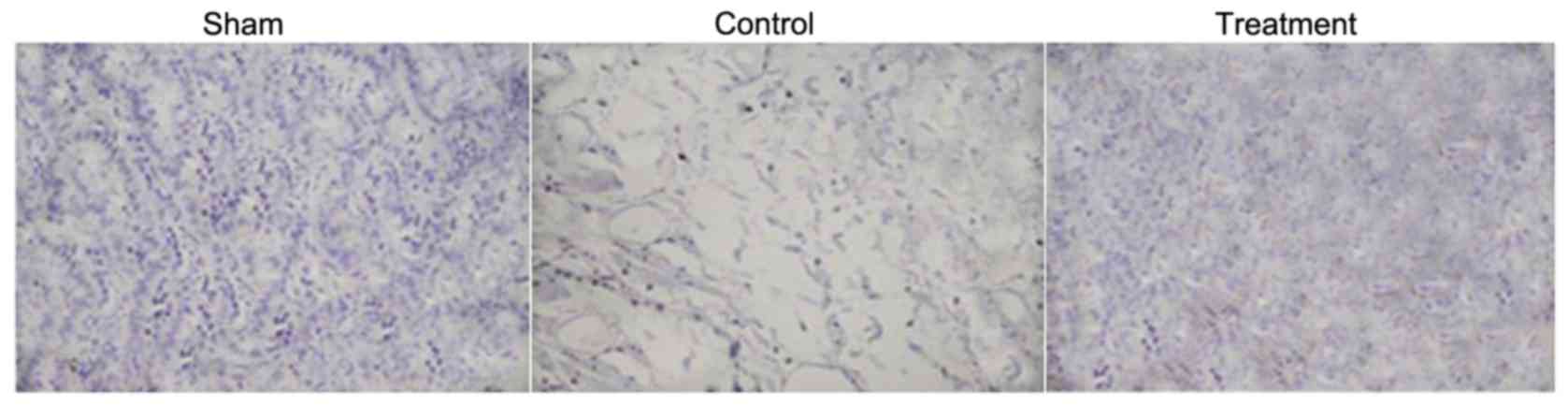
Hematoxylin and eosin staining
The examination of spinal cord tissues using light
microscopy revealed the presence of large cavities. The number of
neuronal cells undergoing apoptosis and gliocytes increased
markedly in the rats with SCI. However, treatment of the rats with
dexmedetomidine prevented the formation of cavities, induction of
neuronal cell apoptosis and increased number of gliocytes in the
injured region (Fig. 6). The
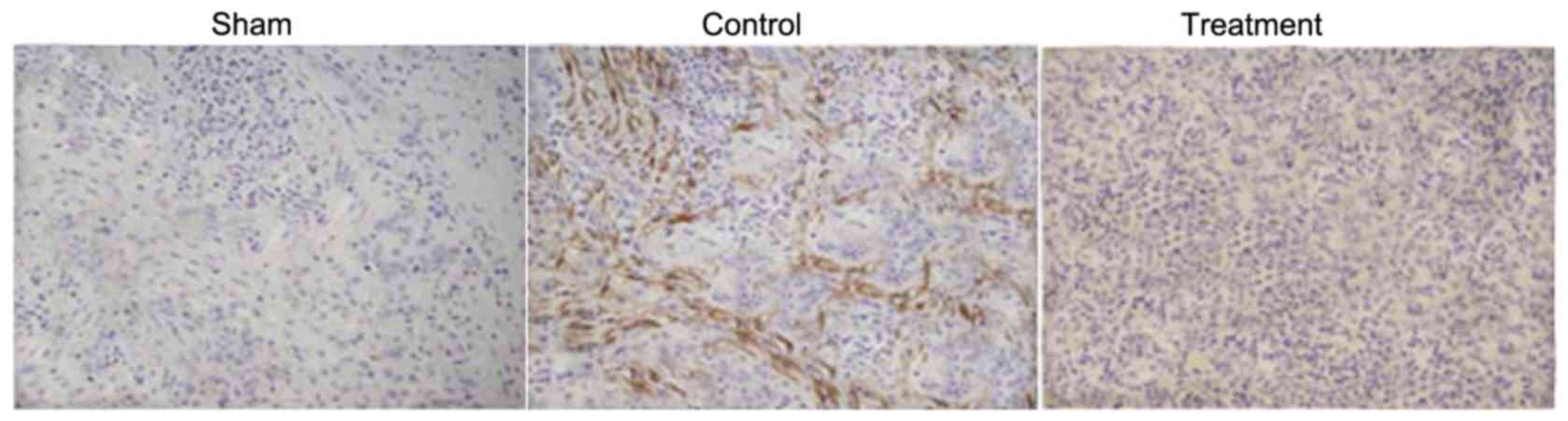
results from the immunohistochemical examination showed reductions
in the percentage of PDGF-B-positive cells and porosis formation in
the spinal cord of rats with SCI. Treatment of the SCI rats with
dexmedetomidine increased the migration of PDGF-B-positive cells to
the injured region of spinal cord and prevented porosis formation
(Fig. 7).
Discussion
In the present study, the effect of dexmedetomidine
on locomotor activity in the rats following SCI was investigated.
The study demonstrated a significant improvement in the locomotor
activity of rats with SCI treated with dexmedetomidine through the
inhibition of edema formation, reduction in neutrophil
accumulation, decrease in the expression of cytokines and
inhibition of cell apoptosis.
Studies have demonstrated that SCI leads to the
development of edema and production of reactive oxygen species, and
inhibits the potential of neurons to undergo repair, which is
associated with worsening of the disorder (17). These factors are responsible for
mediating the onset of secondary processes in cases of SCI. In the
present study, dexmedetomidine exhibited an inhibitory effect on
the accumulation of neutrophils, which was evident by a significant
decrease in the activity of MPO. Treatment of the rats suffering
from SCI with dexmedetomidine led to a significant decrease in the
expression of factors involved in inflammation, including TNF-α and
IL-1β. Treatment of mice with SCI with certain chemotherapeutic
agents has been shown enhance the regenerative potential of the
affected neurons, prevent neuronal apoptosis and induce
improvements in motor function (18,19).
In the present study, the expression of Bax was reduced, whereas
that of Bcl-2 was increased in the spinal cord tissues of SCI rats
treated with dexmedetomidine. This suggested that dexmedetomidine
inhibited the induction of neuronal apoptosis. Treatment of the SCI
rats with dexmedetomidine also led to improvements in the functions
of limbs in the rats.
SCI is followed by the development of inflammation
and the subsequent accumulation of neutrophils into the tissues
(20). Inflammatory factors are
secreted and reactive oxygen species are produced, resulting in
neuronal apoptosis and necrosis (21,22).
Therefore, the inhibition of cytokine secretion by the spinal cord
tissues following SCI can prevent neuronal cell damage. The results
from the present study demonstrated a significant decrease in the
expression of cytokines TNF-α and IL-1β in the rats with SCI
treated with dexmedetomidine.
In conclusion, the results of the present study
demonstrated that dexmedetomidine improved the locomotor function
of rats with SCI through the inhibition of edema formation,
reduction of neutrophil accumulation, inhibited expression of
cytokines and inhibited induction of apoptosis. Therefore,
dexmedetomidine may be used for the improvement of locomotor
function in cases of SCI.
Acknowledgements
The authors are highly thankful to The First
Affiliated Hospital of Shandong University of Traditional Chinese
Medicine (Jinan, China) for the facilities to complete the research
of the present study.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZHJ and BX performed the experimental work, whereas
ZWX made substantial contributions to the analysis of the obtained
data. WGW and LW made substantial contributions to the conception
and design of the study and wrote the paper. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The experimental procedures were performed in
accordance with Guide for the Care and Use of Laboratory Animals of
the National Institute of Health (15). The procedures were approved by the
Government Animal Care Committee of the Medical College of Xiamen
University under the reference no. 011/MCXU/2013.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schmitt C, Miranpuri GS, Dhodda VK,
Isaacson J, Vemuganti R and Resnick DK: Changes in spinal cord
injury-induced gene expression in rat are strain-dependent. Spine
J. 6:113–119. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hong Z, Chen H, Hong H, Lin L and Wang Z:
TSP-1 expression changes in diabetic rats with spinal cord injury.
Neurol Res. 31:878–882. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qiao F, Atkinson C, Kindy MS, Shunmugavel
A, Morgan BP, Song H and Tomlinson S: The alternative and terminal
pathways of complement mediate post-traumatic spinal cord
inflammation and injury. Am J Pathol. 177:3061–3070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beattie MS: Inflammation and apoptosis:
Linked therapeutic targets in spinal cord injury. Trends Mol Med.
10:580–583. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beck KD, Nguyen HX, Galvan MD, Salazar DL,
Woodruff TM and Anderson AJ: Quantitative analysis of cellular
inflammation after traumatic spinal cord injury: Evidence for a
multiphasic inflammatory response in the acute to chronic
environment. Brain. 133:433–447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grasso G, Sfacteria A, Meli F, Fodale V,
Buemi M and Iacopino DG: Neuroprotection by erythropoietin
administration after experimental traumatic brain injury. Brain
Res. 1182:99–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nisula S, Yang R, Poukkanen M, Vaara ST,
Kaukonen KM, Tallgren M, Haapio M, Tenhunen J, Korhonen AM and
Pettilä V: FINNAKI Study Group: Predictive value of urine
interleukin-18 in the evolution and outcome of acute kidney injury
in critically ill adult patients. Br J Anaesth. 114:460–468. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang Y, Zhang ZX, Lian D, Haig A,
Bhattacharjee RN and Jevnikar AM: IL-37 inhibits IL-18-induced
tubular epithelial cell expression of pro-inflammatory cytokines
and renal ischemia-reperfusion injury. Kidney Int. 87:396–408.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miranda ML, Balarini MM and Bouskela E:
Dexmedetomidine attenuates the microcirculatory derangements evoked
by experimental sepsis. Anesthesiology. 122:619–630. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Geloen A, Chapelier K, Cividjian A,
Dantony E, Rabilloud M, May CN and Quintin L: Clonidine and
dexmedetomidine increase the pressor response to norepinephrine in
experimental sepsis: A pilot study. Crit Care Med. 41:e431–e438.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duan X, Li Y, Zhou C, Huang L and Dong Z:
Dexmedetomidine provides neuroprotection: Impact on
ketamine-induced neuroapoptosis in the developing rat brain. Acta
Anaesthesiol Scand. 58:1121–1126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Turan A, Bashour CA, You J, Kirkova Y,
Kurz A, Sessler DI and Saager L: Dexmedetomidine sedation after
cardiac surgery decreases atrial arrhythmias. J Clin Anesth.
26:634–642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang ZX, Huang CY, Hua YP, Huang WQ, Deng
LH and Liu KX: Dexmedetomidine reduces intestinal and hepatic
injury after hepatectomy with inflow occlusion under general
anaesthesia: A randomized controlled trial. Br J Anaesth.
112:1055–1064. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Si YN, Bao HG, Xu L, Wang XL, Shen Y, Wang
JS and Yang XB: Dexmedetomidine protects against
ischemia/reperfusion injury in rat kidney. Eur Rev Med Pharmacol
Sci. 18:1843–1851. 2014.PubMed/NCBI
|
|
15
|
Guide for the Care and Use of Laboratory
Animals: National Research Council (US) Committee for the Update of
the Guide for the Care and Use of Laboratory Animals. 8th edition.
National Academies Press; Washington (DC): 2011
|
|
16
|
Basso DM, Beattie MS and Bresnahan JC:
Graded histological and locomotor outcomes after spinal cord
contusion using the NYU weight-drop device versus transection. Exp
Neurol. 139:244–256. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Loane DJ and Byrnes KR: Role of microglia
in neurotrauma. Neurotherapeutics. 7:366–377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin Y, Sun W, Li Z, Zhang B, Cui H, Deng
L, Xie P, Xiang J and Zou J: Effects of combining
methylprednisolone with rolipram on functional recovery in adult
rats following spinal cord injury. Neurochem Int. 62:903–912. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Genovese T, Mazzon E, Crisafulli C,
Esposito E, Di Paola R, Muià C, Di Bella P, Meli R, Bramanti P and
Cuzzocrea S: Combination of dexamethasone and etanercept reduces
secondary damage in experimental spinal cord trauma. Neuroscience.
150:168–181. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McTigue DM, Tani M, Krivacic K, Chernosky
A, Kelner GS, Maciejewski D, Maki R, Ransohoff RM and Stokes BT:
Selective chemokine mRNA accumulation in the rat spinal cord after
contusion injury. J Neurosci Res. 53:368–376. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao W, Xie W, Le W, Beers DR, He Y,
Henkel JS, Simpson EP, Yen AA, Xiao Q and Appel SH: Activated
microglia initiate motor neuron injury by a nitric oxide and
glutamate-mediated mechanism. J Neuropathol Exp Neurol. 63:964–977.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morino T, Ogata T, Horiuchi H, Takeba J,
Okumura H, Miyazaki T and Yamamoto H: Delayed neuronal damage
related to microglia proliferation after mild spinal cord
compression injury. Neurosci Res. 46:309–318. 2003. View Article : Google Scholar : PubMed/NCBI
|