Introduction
Neuroblastomas affect 10.2 children <15 years of
age in every million in the United States and constitute a major
cause of cancer-associated mortality (1,2). A
number of factors, including the stage of disease, age at
diagnosis, and cellular and genetic features of the tumor,
determined whether the tumor will spontaneously regress, or
metastasize and become refractory to therapy (3,4). In
previous decades, a number of approaches have been developed to
treat neuroblastoma, including surgery, radiotherapy, high-dose
multi-agent chemotherapy, autologous stem cell transplantation and
targeted therapy using anti-disialoganglioside GD2 monoclonal
antibodies (5–7). However, there is no effective
therapeutic approach for treating neuroblastoma due to its
complexity and heterogeneity. Chimeric antigen receptor T cell
immunotherapy is one of the most promising therapeutic methods but
remains problematic (8).
Therefore, it is desirable to further reveal the molecular
mechanisms underlying carcinogenesis in order to identify novel
prognostic markers or molecular targets, which may facilitate the
development of effective therapeutic strategies against
neuroblastoma.
There is increasing evidence that a plethora of
protein involved in the nuclear factor κ-B (NF-κB) signalling
pathway, including NF-κB, inhibitor of NF-κB (IκB), and TRAF family
member-associated NF-κB activator (TANK), contribute to the
proliferation, apoptosis and metastasis of multiple types of
cancer, including neuroblastoma (9,10).
Although NF-κB signaling serves an important role in neuroblastoma
tumorigenesis and cellular proliferation, its upstream regulators
remain to be elucidated. As well as tumor cell proliferation, tumor
cell metastasis is a complex phenomenon, which accounts for 90% of
cancer-associated mortality worldwide (11). Studies of the molecular mechanism
have revealed that matrix metalloproteinases (MMPs) and
zinc-dependent endopeptidases (ZDEs) serve vital functions in tumor
metastatic progression (12).
However, the molecular expression of MMPs and ZDEs, particularly in
neuroblastoma, remains to be elucidated.
Zinc is a fundamental dietary element and has a
critical role in a range of cellular processes, including cellular
proliferation and tumor cell metastasis (13). Zinc metabolism is primarily
coordinated by zinc transporters distributed across the cell
membrane (14). The zinc
transporters in mammals are encoded by two solute-linked carrier
(SLC) gene families that include fourteen SLC39 (also known as Zip)
family members and ten SLC30 (also known as ZnT) family members
(15). Zinc transporter ZIP8
(Zip8) belongs to the SLC39 family and serves an important role in
increasing cytosolic zinc content, through the promotion of
extracellular uptake or subcellular organelle zinc release
(16,17). It has been demonstrated that zinc
is involved in tumor regulation and that Zip8is vital to
controlling the zinc concentration. Therefore, there is
accumulating interest in theinvestigation of Zip8 function in
cancer cell progression, proliferation and migration (18–20).
In the present study, Zip8 was observed to regulate the
proliferation and migration of neuroblastoma cells, and the
molecular mechanisms of Zip8 in neuroblastoma cells were further
investigated.
Materials and methods
Cell lines and cell culture
The human neuroblastoma SH-SY5Y cell line was
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China). SH-SY5Y cells were cultured at 37°C
in a 5% CO2 atmosphere in Dulbecco's modified Eagle's
medium-F12 supplemented with 10% fetal bovine serum (FBS), 100 U/ml
streptomycin and 100 U/ml penicillin (all Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
Cell viability assay
The colorimetric water-soluble tetrazolium salt
assay was applied to detect cell proliferation using a Cell
Counting kit-8 (CCK8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan), according to the manufacturer's protocol. SH-SY5Y
cells were seeded into 96-well plates at a density of
1×103 cells/well, and cell proliferation was evaluated
at 12, 24, 48, 72 and 96 h. The number of viable cells was assessed
by measuring the absorbance at 450 nm using a microplate reader
(Thermo Fisher Scientific, Inc.).
Colony formation assay
SH-SY5Y cells were maintained in complete minimum
essential medium supplemented with 10% FBS and 1%
penicillin/streptomycin (all Gibco; Thermo Fisher Scientific, Inc.)
and the cells were plated in 6-well plates at a density of 1,000
cells/well followed by incubation at 37°C overnight. The media was
replaced every 2 days, and after 2 weeks each well was washed with
1 ml PBS followed by adding 1 ml of crystal violet (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) solution (1% crystal violet and 10%
ethanol) into each well. After 10 min incubation at room
temperature, the colonies were washed out with PBS three times and
were counted by eye.
Cell cycle assay
Cell cycle analysis of SH-SY5Y cells was performed
using the BD Cycle assay kit (BD Biosciences, Franklin Lakes, NJ,
USA), according to the manufacturer's protocol. Different groups of
SH-SY5Y cells were seeded into flasks at a density of
7×104 cells (T25 flasks; Thermo Fisher Scientific, Inc.)
and incubated at 37°C for 48 h. Following incubation, the cells
were harvested and fixed at 4°C in 90% methanol for ~30 min. The
cells were centrifuged at 4°C for 5 min at 1,000 × g and washed
twice with PBS. The pellets were then resuspended in propidium
iodide and incubated at 37°C for 1 h. A fluorescence-activated cell
sorting machine (FACS Calibur flow cytometer; BD Biosciences) was
used and data were analyzed using BD CellQuest Pro software version
4.0.2 (BD Biosciences).
Cell migration assay
The culture inserts (ibidi GmbH, Munich, Germany),
consisting of 2 reservoirs separated by a 500-µm-thick wall, were
placed in a 24-well plate. According to the manufacturer's
protocol, an equal amount (70 µl) of SH-SY5Y cell suspension
(1×106 cells/ml) was added to each reservoir followed by
incubation at 37°C. Following complete attachment of the cells (10
h), the culture inserts were gently removed and the medium was
replaced with serum-free DMEM-F12 medium containing 0.2% bovine
serum albumin (BSA; Beyotime Institute of Biotechnology, Haimen,
China). The gap between the two cell layers was observed at 0 and
20 h after Zip8 knockdown under an inverted microscope (Advanced
Microscopy Group, Mill Creek, WA, USA).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
RT-qPCR was performed to detect the mRNA expression
levels of Zip8, glycogen synthase kinase-3 β (Gsk-3β), β-catenin,
NF-κ-B essential modulator (Ikbkg), TANK, MMP2, MMP3, MMP9 and
MMP14, according to the manufacturer's protocol. Total cellular RNA
was extracted from SH-SY5Y cells by lysing cells with TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) reagent followed by
centrifugation at 12,000 × g for 15 min at 4°C with chloroform (5:1
ratio). The supernatant was centrifuged with isopropanol (1:1
ratio) at 10,000 × g at 4°C for 10 min. The RNA pellet was washed
with 75% ethanol and solubilized with DNase and RNase free water.
The RNA was quantified by measuring absorbance at 260 nm using a
NanoDrop ND-1000 (Thermo Fisher Scientific, Inc.). Single-stranded
cDNA was prepared using a Reverse Transcription system (Takara
Biotechnology Co., Ltd., Dalian, China) according to the
manufacturer's protocol. Briefly, 1 µg total RNA was added into the
mixture and incubated at 37°C for 15 min, and then kept at 85°C for
5 sec. The mRNA expression of the target gene was determined by
SYBR-Green assays. The SYBR-Green qPCR kit was purchased from Roche
Diagnostics GmbH (Mannheim, Germany). qPCR was performed using an
Applied Biosystems 7300 sequence detection system. The
thermocycling conditions were as follows: Initial denaturation at
95°C for 10 min, followed by 45 cycles at 95°C for 15 sec and at
60°C for 25 sec. All experiments were performed in triplicate. The
relative gene expression levels were calculated using the
2−ΔΔCq method (21) and
normalized to GAPDH. The following primers were used: Zip8 forward,
5′-ATGCTACCCAAATAACCAGCTC-3′ and reverse,
5′-ACAGGAATCCATATCCCCAAACT-3′; Gsk3β forward,
5′-AGACGCTCCCTGTGATTTATGT-3′ and reverse,
5′-CCGATGGCAGATTCCAAAGG-3′; β-catenin forward,
5′-CATCTACACAGTTTGATGCTGCT-3′ and reverse,
5′-GCAGTTTTGTCAGTTCAGGGA-3′; Ikbkg forward,
5′-CGGCAGAGCAACCAGATTCT-3′ and reverse,
5′-CCTGGCATTCCTTAGTGGCAG-3′; TANK forward,
5′-AGCAGAGAATACGTGAACAACAG-3′ and reverse,
5′-CAGAAGCAATGTCTACCTTTGGT-3′; MMP2 forward,
5′-CTGCGGTTTTCTCGAATCCATG-3′ and reverse,
5′-GTCCTTACCGTCAAAGGGGTATCC-3′; MMP3 forward,
5′-CTGGACTCCGACACTCTGGA-3′ and reverse,
5′-CAGGAAAGGTTCTGAAGTGACC-3′; MMP9 forward,
5′-GAGGCGCTCATGTACCCTATGTAC-3′ and reverse,
5′-GTTCAGGGCGAGGACCATAGAG-3′; MMP14 forward,
5′-CTTCCGTGGAAACAAGTACTACCGT-3′ and reverse,
5′-ATCCCTTCCCAGACTTTGATGTTC-3′; and GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′.
Nuclear protein isolation and western
blot analysis
The protein expression levels of ZIP8, NF-κB, IκB,
phosphorylated (p)-IκB, and MMP2, 3, 9 and 13, histone H1 and GAPDH
in SH-SY5Y cells were evaluated by western blot analysis. Total
proteins were extracted using the Nuclear and Cytoplasmic Protein
Extraction kit (Beyotime Institute of Biotechnology) according to
the manufacturer's protocol. The cells were washed twice with
ice-cold PBS and lysed using a radioimmunoprecipitation assay lysis
buffer (Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. Total cell lysates were then centrifuged
at 12,000 × g for 15 min at 4°C and the supernatants were used for
further processing. The bicinchoninic acid protein assay kit
(Beyotime Institute of Biotechnology) was used to determine the
protein concentration. Total proteins (20 µg) were separated by 10%
SDS-PAGE and electroblotted onto a polyvinylidene difluoride
membrane (EMD Millipore, Billerica, MA, USA). The membranes were
blocked with 5% BSA for 2 h at room temperature and incubated
overnight at 4°C with different primary antibodies: Anti-Zip8 (cat.
no. ab106576; dilution 1:1,000), anti-NF-κB (cat. no. ab32360;
dilution 1:1,000), anti-IκB (cat. no. ab32518; dilution 1:1,000),
anti-p-IκB (cat. no. ab12135; dilution 1:1,500), anti-MMP2 (cat.
no. 37150; dilution 1:1,000), anti-MMP3 (cat. no. 52915; dilution
1:2,000), anti-MMP9 (cat. no. 38898; dilution 1:1,000), anti-MMP14
(cat. no. 51074; dilution 1:5,000), anti-histone H1 (cat. no.
61177; dilution 1:1,000) and anti-GAPDH antibody (cat. no.
ab181602; dilution 1:10,000; all Abcam, Cambridge, UK). Following
incubation with primary antibodies, membranes were incubated with
anti-rabbit horseradish peroxidase-conjugated secondary antibodies
(cat. no. 7074; dilution 1:1,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA) for 1 h at room temperature. Protein bands were
visualized using an enhanced chemiluminescent assay kit (Beyotime
Institute of Biotechnology) and a luminescent image analyzer.
Construction of recombinant lentivirus
and cell infection
The short hairpin (sh)RNA sequences that were used
were as follows: Zip8-shRNA1 forward,
5′-GCCAAGTTCATGTACCTGTTTCTCGAGAAACAGGTACATGAACTTGGCTTTTT-3′ and
reverse,
5′-AAAAAGCCAAGTTCATGTACCTGTTTCTCGAGAAACAGGTACATGAACTTGGC-3′;
Zip8-shRNA2 forward,
5′-CCTTGCTATTCAACTTCCTTTCTCGAGAAAGGAAGTTGAATAGCAAGGTTTTT-3′ and
reverse,
5′-AAAAACCTTGCTATTCAACTTCCTTTCTCGAGAAAGGAAGTTGAATAGCAAGG-3′;
scramble control shRNA forward,
5′-CTCTTCTACTCATCCACTTTTCTCGAGAAAAGTGGATGAGTAGAAGAGTTTTT-3′ and
reverse,
5′-AAAAACTCTTCTACTCATCCACTTTTCTCGAGAAAAGTGGATGAGTAGAAGAG-3′. The
sequences were synthesized (Shanghai GeneChem Co., Ltd., Shanghai,
China) and inserted into the lentiviral vector PLVX-IRES-ZsGreen1
by Shanghai GeneChem Co., Ltd., through standard molecular cloning
methods and the recombinant plasmids were confirmed by sequencing.
The lentiviral plasmids including PLVX-IRES-ZsGreen1 vector, pVSVG,
pREV and pRRE were purchased from Takara Biotechnology Co., Ltd.
HEK293T cells (Shanghai GeneChem Co., Ltd.) were cultured in 10-cm
dishes at a concentration of 6×105 cells/ml for 24 h at
37°C and 5% CO2 in a humidified incubator. Recombinant
lentiviral particles were then generated by co-transfecting the
packaging plasmids (5 µg for each plasmids), including packaging
plasmids (pVSVG, pREV and pRRE), and the shRNA1-, shRNA2- or
scramble shRNA-containing vector plasmids, into HEK293T cells using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) as
the transfection reagent. Following incubation for 48 h at 37°C and
5% CO2 in a humidified incubator, the lentiviral
particles were harvested and centrifuged at 50,000 × g for 2 h at
4°C. SH-SY5Y cells were divided into 3 groups: Scramble control
shRNA, Zip8 shRNA1 and Zip8 shRNA2. For the lentiviral infection,
the SH-SY5Y cells were seeded onto 6-well plates at a density of
2×104 cells/well. A total of 1 ml of medium containing
polybrene (5 µg/ml) and 100 µl lentivirus (virus titer,
109 TU/ml) were added into each well. After 24 h, the
medium was replaced with fresh medium (DMEM-F12 supplemented with
10% fetal bovine serum, 100 U/ml streptomycin and 100 U/ml
penicillin; Gibco; Thermo Fisher Scientific, Inc.) and incubated
for another 48 h. The cells were then examined under an inverted
fluorescence microscope (IX71; Olympus Corporation, Tokyo, Japan)
and the green fluorescence emitted by the green fluorescent protein
in successfully infected cells was observed.
Statistical analysis
All the results represented at least three
independent experiments and the data are expressed as the mean ±
standard deviation. Differences between the control and treatment
groups were analyzed using one-way analysis of variance followed by
Fisher's least significant difference test and SPSS software
(version 17.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Zip8 knockdown inhibits the
proliferation of neuroblastoma cancer cells
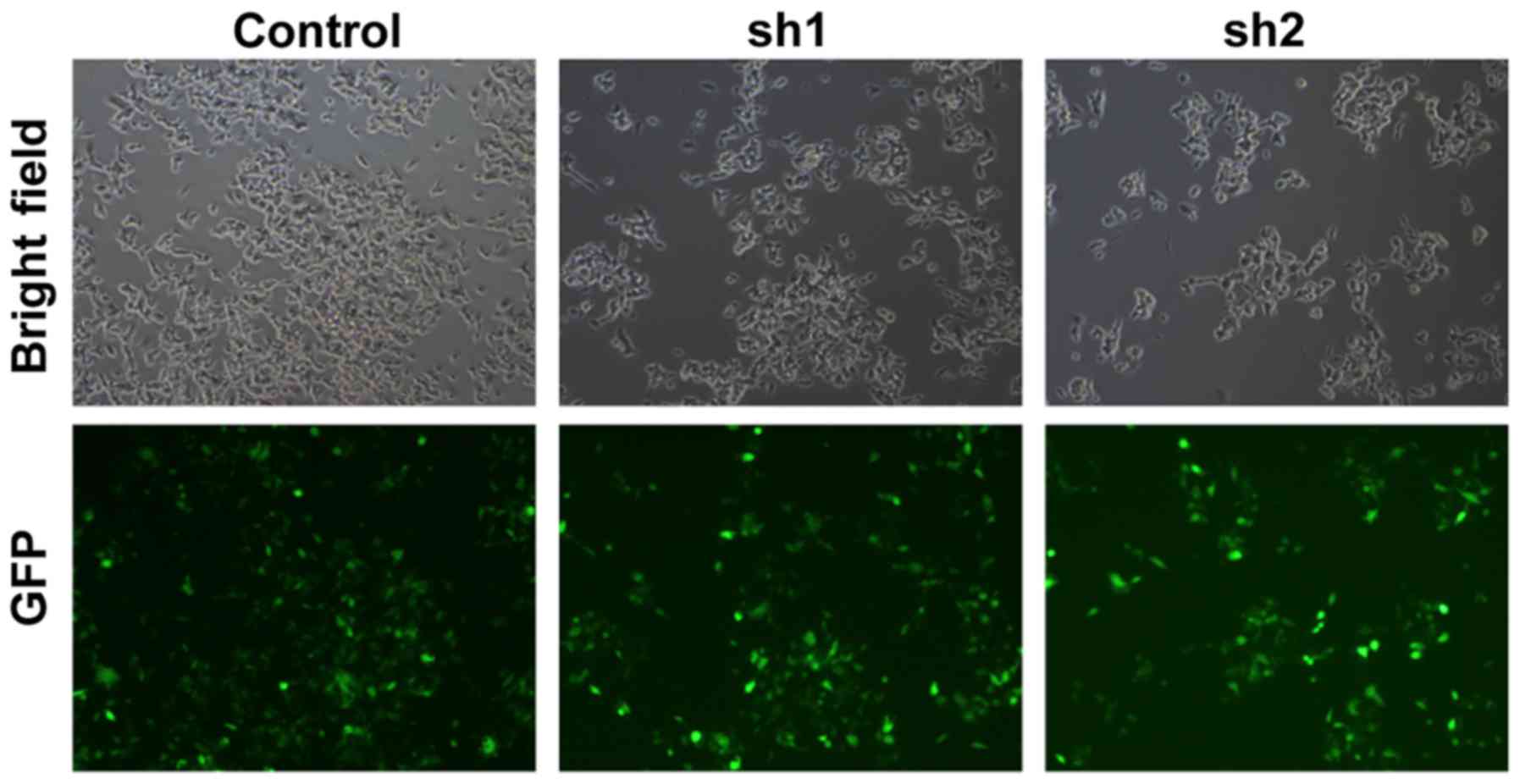
To investigate the function of Zip8 in
neuroblastoma, we knockdown the Zip8 expression in SH-SY5Y cells
using lentiviral-mediated RNA interference (RNAi). Zip8 shRNA
targets were cloned into lentivirus vectors and the lentivirus was
further packaged to infect SH-SY5Y cells. The infecting efficiency
was more than 90% as assessed by GFP florescence suggestion of the
successful lentivirus infection in neuroblastoma cancer cells
(Fig. 1). Quantification analysis
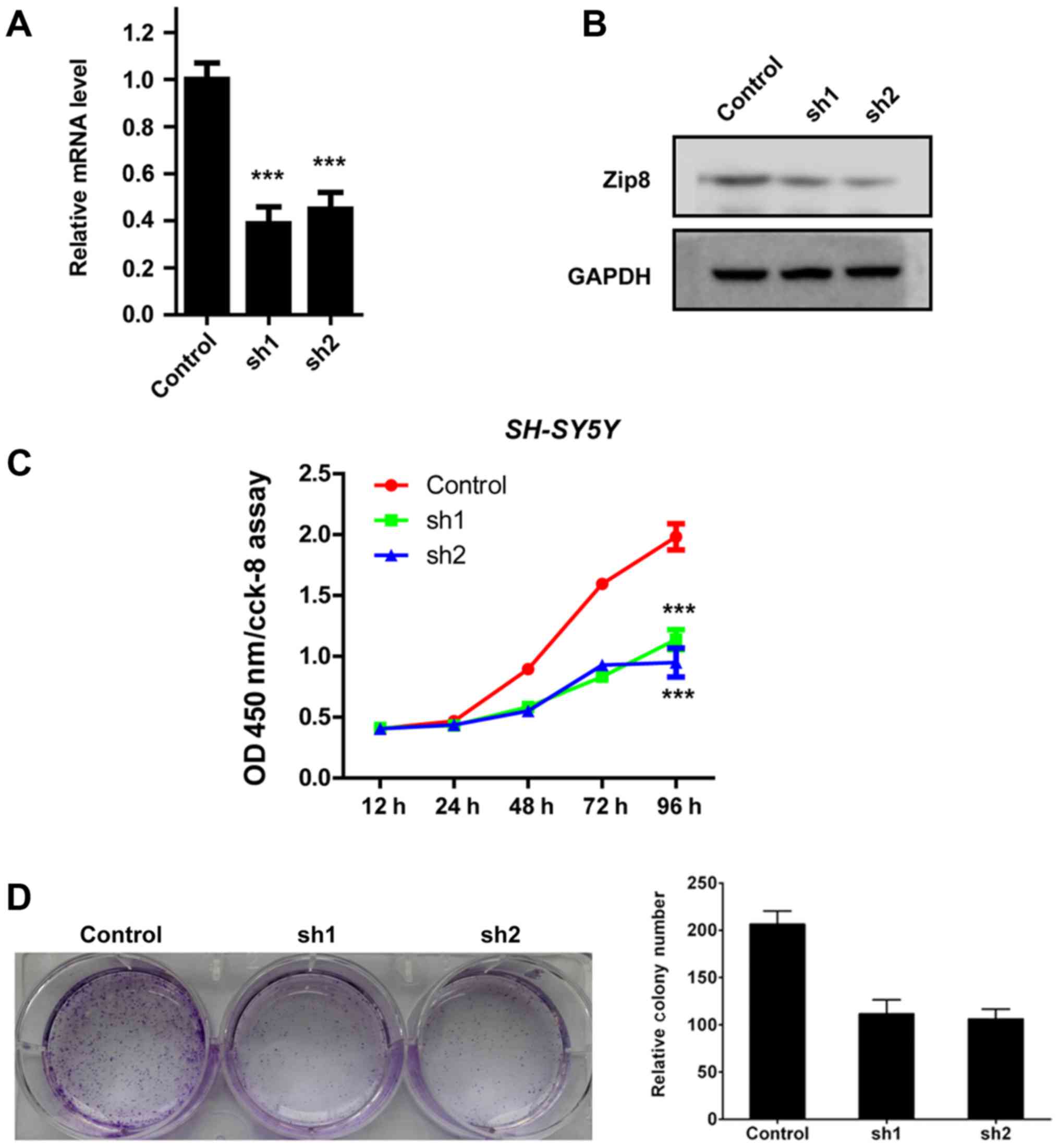
by RT-qPCR assay revealed that lentivirus-mediated RNAi obviously
reduced the Zip8 mRNA expression levels in SH-SY5Y cells (Fig. 2A). The protein levels of Zip8 were
also decreased significantly in SH-SY5Y cells infected by Zip8
shRNA lentivirus (Fig. 2B). Hence,
the results demonstrated that the expression of Zip8 was
effectively silenced by Zip8 shRNA lentivirus in neuroblastoma cell
lines.
The effect of Zip8 on neuroblastoma cell
proliferation was assessed by CCK-8 assay. According to the
results, the proliferation rates of Zip8 silenced SH-SY5Y cells
were both obviously reduced compared with that of the scramble
control group (P<0.001; Fig.
2C). In addition, the colony formation assay was also performed
to evaluate the effect of Zip8 knockdown on the colony formation
ability of SH-SY5Y cells. Compared to the scramble control group,
the number of cell colonies by crystal violet staining in the Zip8
silenced groups was obviously reduced (Fig. 2D and E). Therefore, these results
indicated that Zip8 played an important role in the regulation of
neuroblastoma cells proliferation.
Zip8 suppression affects the cell
cycle of neuroblastoma cells
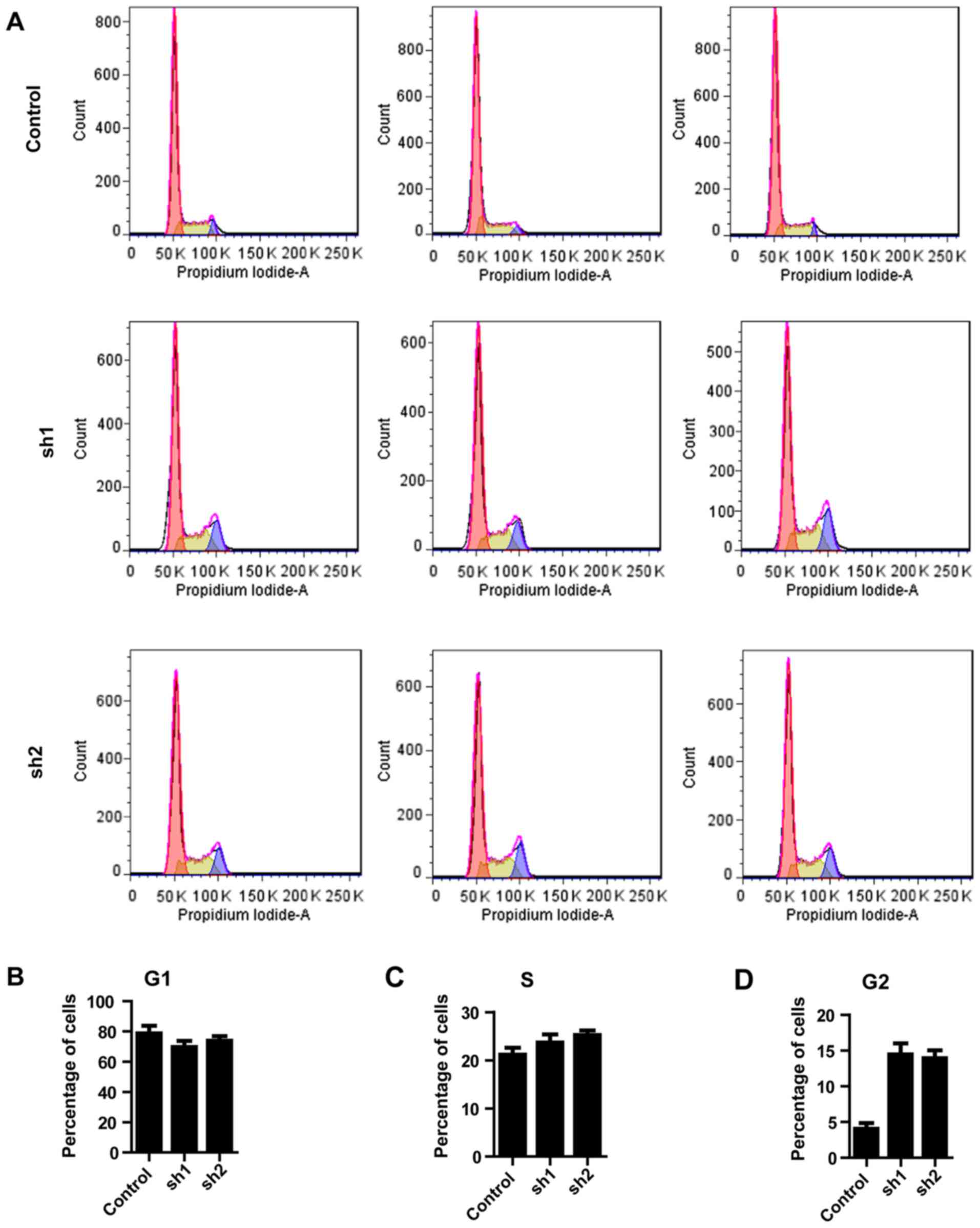
The observation that Zip8 knockdown inhibited the
proliferation of neuroblastoma cells led to the investigation of
whether Zip8 participates in regulation of the cell cycle. The cell
cycle distribution was analyzed by flow cytometry following
silencing of Zip8 (Fig. 3A). In
the G0/G1 phase of cell cycle, the percentages of SH-SY5Y cells
accumulated following Zip8 knockdown by lentiviral-mediated RNAi
and the scramble control group presented no difference (Fig. 3B). Similarly, there was no
significantly difference between each groups in the S phase
(Fig. 3C). In the G2/M phase of
cell cycle the percentages of SH-SY5Y cells following silencing
Zip8 were increased, suggesting G2/M phase arrest following Zip8
depletion (Fig. 3D). Therefore,
the results revealed that knockdown of Zip8 suppressed the growth
of neuroblastoma cells possibly through induction of cell cycle
arrest.
Zip8 modulates NF-κB signaling
pathway
To reveal the essential molecular mechanism involved
in the inhibition of neuroblastoma cells proliferation induced by
Zip8 silencing, a number of known key genes which serve key roles
in regulating cancer cells proliferation, including Gsk-3β,
β-catenin, Ikbkg and TANK, were evaluated through RT-qPCR assays in
SH-SY5Y cells. As the results demonstrated, the alterations in the
mRNA levels of Gsk-3β and β-catenin in each group were not
significant (Fig. 4A and B). The
mRNA levels of Ikbkg and TANK involved in the NF-κB signaling
pathway decreased markedly following Zip8 silencing (Fig. 4C and D). Therefore, western
blotting was subsequently performed to evaluate the effect of Zip8
silencing on the NF-κB signaling pathway in each group. The protein
levels of nuclear NF-κB were reduced following Zip8 knockdown
(Fig. 4E). The NF-κB and IκB
levels of Zip8-silenced groups in the cytoplasm were increased;
however, p-IκB levels were decreased in cytoplasm (Fig 4F). These results suggested that
silencing of Zip8 had an essential effect on the role of the NF-κB
signaling pathway in neuroblastoma cell proliferation.
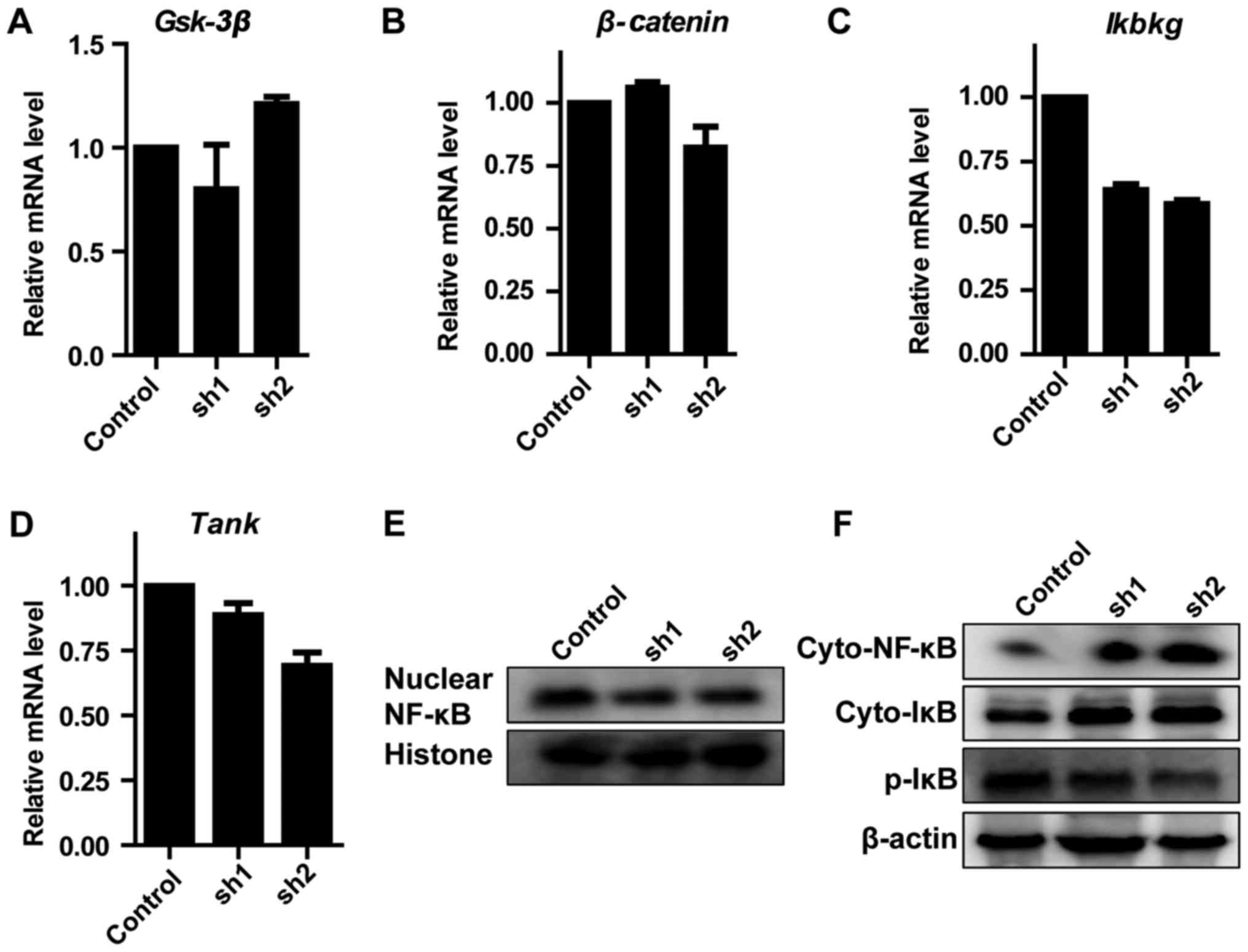 | Figure 4.Effect of Zip8 knockdown on NF-κB
signaling pathway. The mRNA levels of (A) Gsk-3β, (B) β-Catenin,
(C) Ikbkg and (D) Tank in SH-SY5Y cells were detected by the
reverse transcription-quantitative polymerase chain reaction (n=3).
(E) The nuclear protein levels of NF-κB were detected by western
blotting. (F) The cytoplasmic protein levels of NF-κB, IκB and
p-IκB were detected by western blotting. Values are presented as
the mean ± standard error of the mean. Zip8, zinc transporter ZIP8;
NF-κB, nuclear factor κ-B; Gsk-3β, glycogen synthase kinase-3 β;
Ikbkg, NF-κ-B essential modulator; Tank, TRAF family
member-associated NF-κB activator; IκB, inhibitor of NF-κB; p,
phosphorylated; sh, short hairpin RNA; cyto, cytoplasmic. |
Zip8 silencing decreases the migration
of neuroblastoma cells
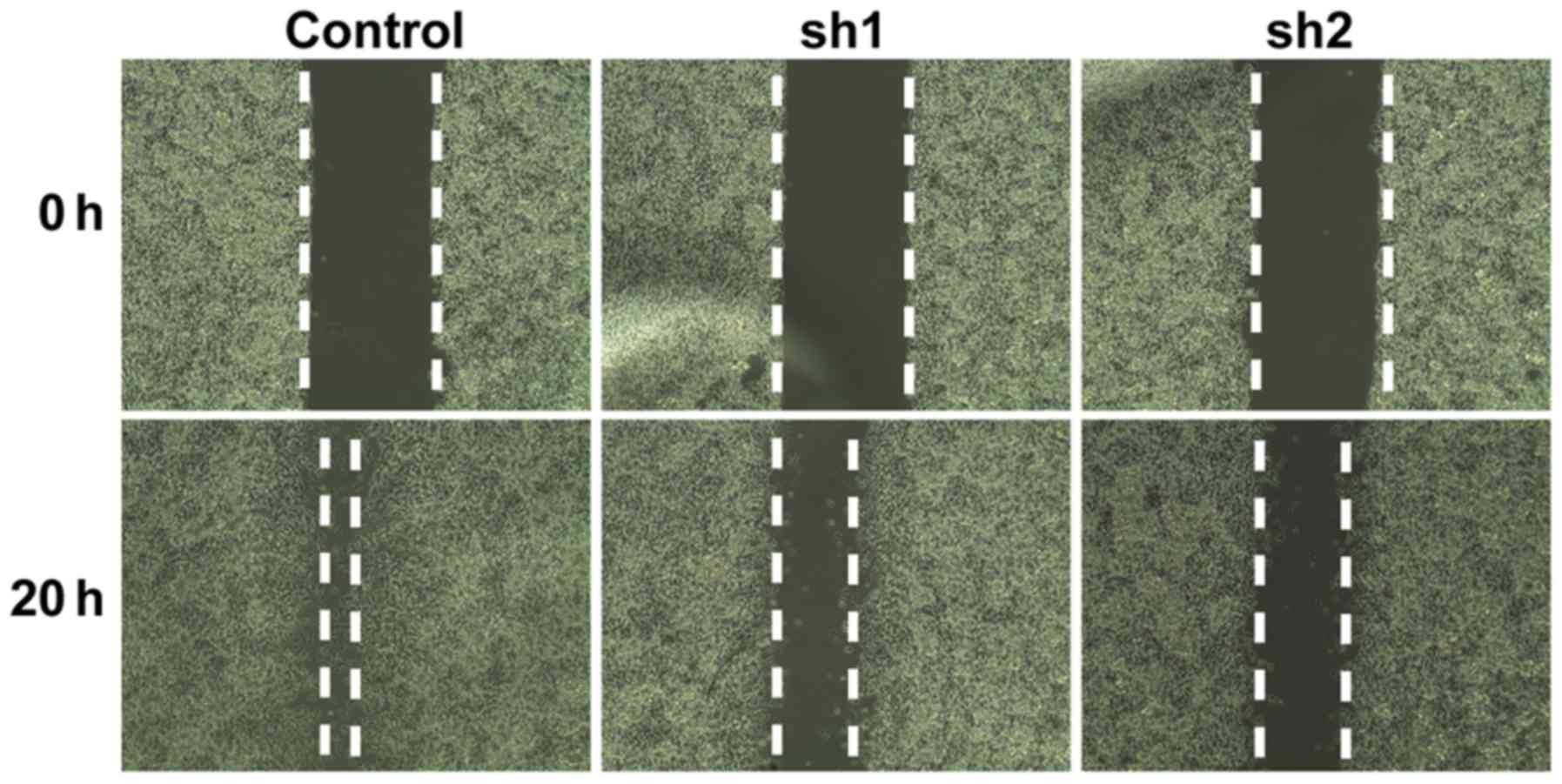
To examine whether Zip8 serves a role in regulating
the migration of SH-SY5Y cells, a cell migration assay was
performed. The gap between 2 cell layers was observed and recorded
at 0 h and 20 h, respectively. The control group of SH-SY5Y cells
migrated towards to the empty area after culturing for 20 h.
However, the cells of the Zip8-silenced groups demonstrated a
marked reduction in their migratory ability, as the gaps between
the two cell layers were obviously increased compared with that of
the control group after 20 h, suggesting that Zip8 knockdown
reduced cell migration (Fig. 5).
These data indicated that Zip8 could affect the migration of
neuroblastoma cells.
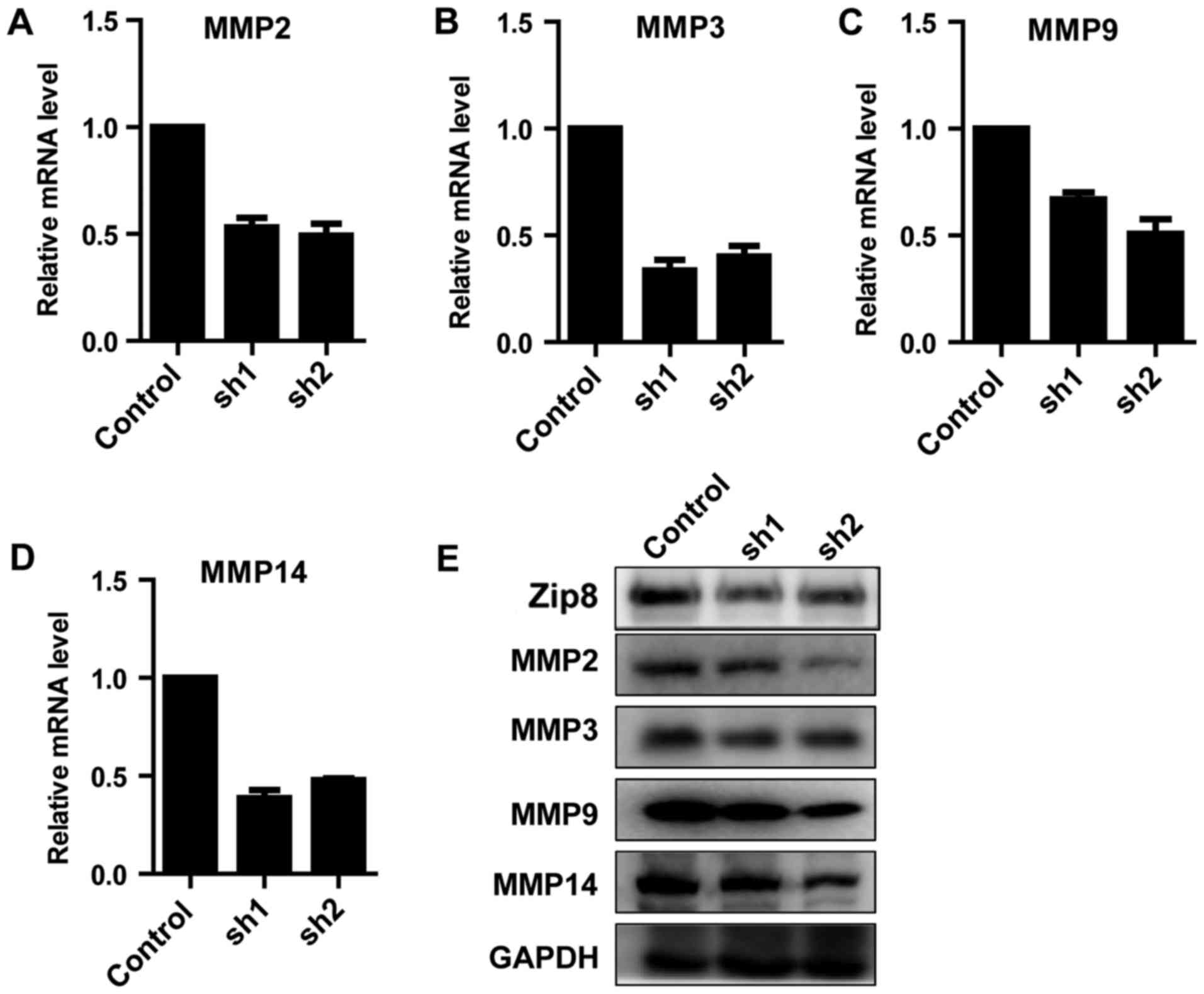
Zip8 regulates the expression of
MMPs
Based on the above data demonstrating that knockdown
of Zip8 inhibited the migratory ability of neuroblastoma cells, the
key molecular mechanism involved in the phenomenon was
investigated. The gene expression levels of migratory-associated
proteins, including MMP2, 3, 9 and 14, were detected by RT-qPCR.
The results suggested that, the expression levels of all the four
MMPs decreased markedly (Fig.
6A-D). The western blot analysis further reconfirmed that the
expression levels of MMP2, 3, 9 and 14 were reduced following Zip8
knockdown in SH-SY5Y cells (Fig.
6E). The results demonstrated that Zip8 could regulate the
expression of MMPs to affect the migratory ability of neuroblastoma
cells.
Discussion
Carcinogenesis is a complex and multifactorial
process, resulting from a number of environmental effects.
Neuroblastoma is commonly lethal due to its aggressive metastasis,
migration and invasion. However, the molecular mechanism underlying
neuroblastoma metastasis remains unclear. Zinc, which participates
in multiple enzymatic and metabolic functions, is required for the
activity of >300 enzymes (13).
A total of >2,000 transcription factors involved in gene
expression require zinc for maintaining structural integrity and
DNA binding properties (22,23).
Therefore, zinc serves an important role in protein function and
regulation of zinc metabolism affects a range of cellular progress,
including cellular proliferation. There is evidence suggesting that
zinc concentrations maybe associated with the risk of cancer
(24–26). Conversely, zinc transport proteins,
including Zip8, are known to control intracellular zinc
concentrations and metabolic homeostasis in mammalian cells,
including cancer cells (27).
Therefore, it was hypothesized that inhibiting the metabolic
homeostasis of zinc by silencing Zip8 in neuroblastoma cancer cells
may affect cell progression, including proliferation and migration.
The results of the present study revealed that Zip8 knockdown could
suppress the proliferation of neuroblastoma cells in vitro
and reduce the migratory ability of neuroblastoma cells. These
results suggested that Zip8 may be a potential therapeutic target
of neuroblastoma. There are studies demonstrating that the
expression levels of Zip8 are high in several types of cancer cells
(28–30).
Cellular proliferation assays in the present study
demonstrated that Zip8 could effectively regulate the proliferation
of neuroblastoma cells. However, the molecular mechanism of Zip8
modulation remained unclear and a further study to elucidate the
Zip8 modulation mechanism is required. The NF-κB signaling pathway
is involved in several aspects of tumorigenesis, including cancer
cell survival and proliferation, the prevention of apoptosis, and
an increase in the metastatic potential of tumor cells (31). In addition, activation of the NF-κB
signaling pathway serves an essential role in the modulation of
apoptosis and migration in neuroblastoma cells (32,33).
Therefore, whether Zip8 modulated the proliferation of
neuroblastoma cancer cells through the NF-κB signaling pathway
requires investigation. As demonstrated in the present study, Zip8
silencing in neuroblastoma cancer cells exhibited an essential
effect on the expression of key genes involved in the NF-κB
signaling pathway.
Metastasis, a complex phenomenon mediated by a large
number of signaling cascades, accounted for >90% of
cancer-associated mortalities worldwide (34). The progression of metastasis
involves cellular migration from the primary tumor site to
secondary sites through the blood vascular system. In the present
study, it was observed that Zip8 silencing decreased the migratory
potential of neuroblastoma cells. MMPs, including the key members
MMP2, 3, 9 and 14, perform vital functions in the regulation the
cancer cell metastasis by modifying the cell microenvironment
(35). Therefore, whether Zip8
regulates the expression of MMPs in neuroblastoma cells was
investigated in the present study. The gene expression levels of
migratory-associated proteins, including MMP2, 3, 9 and 14,
decreased following Zip8 knockdown, suggesting that Zip8 could
regulate the expression of MMPs to further affect the migratory
ability of neuroblastoma cells.
In conclusion, the present study identified an
important protein, Zip8, which serves an important role in the
regulation of neuroblastoma cell proliferation. Further study
revealed that Zip8 depletion inhibited cancer cell proliferation by
modulating the expression of key genes involving in the NF-κB
signaling pathway. Additionally, Zip8 depletion decreased the
migratory potential of neuroblastoma cancer cells by modulating the
expression of MMP2, 3, 9 and 14. The present study demonstrated
that Zip8 serves important roles in the regulation of the
proliferation of neuroblastoma cells and the modulation of the
migration of neuroblastoma cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from
Guangzhou Education Bureau (grant no. 1201421151).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
ZM and FH designed the experiments. YW and SL
conducted experiments. PY analyzed and interpreted data.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Zip8
|
zinc transporter ZIP8
|
|
RNAi
|
RNA interference
|
|
MMP
|
matrix metalloproteinase
|
|
NF-κB
|
nuclear factor κ-B
|
|
FBS
|
fetal bovine serum
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
References
|
1
|
Cheung NK and Dyer MA: Neuroblastoma:
Developmental biology, cancer genomics and immunotherapy. Nat Rev
Cancer. 13:397–411. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maris JM: Recent advances in
neuroblastoma. N Engl J Med. 362:2202–2211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brodeur GM: Neuroblastoma: Biological
insights into a clinical enigma. Nat Rev Cancer. 3:203–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Molenaar JJ, Koster J, Zwijnenburg DA, van
Sluis P, Valentijn LJ, van der Ploeg I, Hamdi M, van Nes J,
Westerman BA, van Arkel J, et al: Sequencing of neuroblastoma
identifies chromothripsis and defects in neuritogenesis genes.
Nature. 483:589–593. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnson E, Dean SM and Sondel PM:
Antibody-based immunotherapy in high-risk neuroblastoma. Expert Rev
Mol Med. 9:1–21. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Navid F, Armstrong M and Barfield RC:
Immune therapies for neuroblastoma. Cancer Biol Ther. 8:874–882.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang RK and Sondel PM: Anti-GD2 strategy
in the treatment of neuroblastoma. Drugs Future. 35:6652010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Louis CU, Savoldo B, Dotti G, Pule M, Yvon
E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, et al: Antitumor
activity and long-term fate of chimeric antigen receptor-positive T
cells in patients with neuroblastoma. Blood. 118:6050–6056. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karin M and Greten FR: NF-kappaB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ho E: Zinc deficiency, DNA damage and
cancer risk. J Nutr Biochem. 15:572–578. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prasad AS and Kucuk O: Zinc in cancer
prevention. Cancer Metastasis Rev. 21:291–295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liuzzi JP and Cousins RJ: Mammalian zinc
transporters. Annu Rev Nutr. 24:151–172. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aydemir TB, Liuzzi JP, McClellan S and
Cousins RJ: Zinc transporter ZIP8 (SLC39A8) and zinc influence
IFN-gamma expression in activated human T cells. J Leukoc Biol.
86:337–348. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Besecker B, Bao S, Bohacova B, Papp A,
Sadee W and Knoell DL: The human zinc transporter SLC39A8 (Zip8) is
critical in zinc-mediated cytoprotection in lung epithelia. Am J
Physiol Lung Cell Mol Physiol. 294:L1127–L1136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang C, Cui X, Sun X, Yang J and Li M:
Zinc transporters are differentially expressed in human non-small
cell lung cancer. Oncotarget. 7:66935–66943. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jeong J and Eide DJ: The SLC39 family of
zinc transporters. Mol Aspects Med. 34:612–619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taylor KM, Morgan HE, Smart K, Zahari NM,
Pumford S, Ellis IO, Robertson JF and Nicholson RI: The emerging
role of the LIV-1 subfamily of zinc transporters in breast cancer.
Mol Med. 13:396–406. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cousins RJ: A role of zinc in the
regulation of gene expression. Proc Nutr Soc. 57:307–311. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dreosti IE: Zinc and the gene. Mutat Res.
475:161–167. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Franklin RB and Costello LC: Zinc as an
anti-tumor agent in prostate cancer and in other cancers. Arch
Biochem Biophys. 463:211–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leitzmann MF, Stampfer MJ, Wu K, Colditz
GA, Willett WC and Giovannucci EL: Zinc supplement use and risk of
prostate cancer. J Natl Cancer Inst. 95:1004–1007. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu T, Sempos CT, Freudenheim JL, Muti P
and Smit E: Serum iron, copper and zinc concentrations and risk of
cancer mortality in US adults. Ann Epidemiol. 14:195–201. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He L, Girijashanker K, Dalton TP, Reed J,
Li H, Soleimani M and Nebert D: ZIP8, member of the
solute-carrier-39 (SLC39) metal-transporter family:
Characterization of transporter properties. Mol Pharmacol.
70:171–180. 2006.PubMed/NCBI
|
|
28
|
Franklin RB, Milon B, Feng P and Costello
LC: Zinc and zinc transporters in normal prostate and the
pathogenesis of prostate cancer. Front Biosci. 10:2230–2239. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kolenko V, Teper E, Kutikov A and Uzzo R:
Zinc and zinc transporters in prostate carcinogenesis. Nat Rev
Urol. 10:219–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang LY, Wang XL, Sun DX, Liu XX, Hu XY
and Kong F: Regulation of zinc transporters by dietary flaxseed
lignan in human breast cancer xenografts. Mol Biol Rep. 35:595–600.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luo JL, Kamata H and Karin M:
IKK/NF-kappaB signaling: Balancing life and death-a new approach to
cancer therapy. J Clin Invest. 115:2625–2632. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gatsinzi T and Iverfeldt K: Sensitization
to TRAIL-induced apoptosis in human neuroblastoma SK-N-AS cells by
NF-κB inhibitors is dependent on reactive oxygen species (ROS). J
Neurooncol. 104:459–472. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ammann JU, Haag C, Kasperczyk H, Debatin
KM and Fulda S: Sensitization of neuroblastoma cells for
TRAIL-induced apoptosis by NF-kappaB inhibition. Int J Cancer.
124:1301–1311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fidler IJ: The pathogenesis of cancer
metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|