Introduction
Breast cancer is one of the most common cancers in
women worldwide. Rapid proliferation ensures the growth and the
further invasion of breast cancer (1). Determination of the regulatory
mechanism of breast cancer proliferation, eparticularly the cell
cycle regulation, is important for diagnosis earlier and clinical
therapy.
Previous studies showed that sevoflurane, a common
anesthetic, could repress the tumorigenesis of some types of
cancers. For example, sevoflurane could inhibit the migration and
invasion of glioma cells by upregulating the level of miR-637
(2). MMP-2 expression could be
also repressed by sevoflurane to attenuate the migration of glioma
cells (3). Sevoflurane inhibited
hypoxia-induced growth and metastasis in lung cancer cells
(4). Exposure to sevoflurane (1%)
for 6 h promoted the proliferation of human colon cancer (5). These studies showed the inhibitory
function of sevoflurane on cancer initiation and development.
However, whether or how sevoflurane regulates the breast cancer
growth and the downstream regulatory signaling pathway remain
largely unknown.
MicroRNAs (miRNAs) are small non-coding RNAs, which
regulate gene expression by binding to the 3-untranslated regions
(3′UTRs) of mRNAs (6). miRNAs
participate in regulating many biological processes including the
embryonic development, initiation of many diseases (6–10).
Increasing studies have showed that miRNAs affect the
proliferation, metastasis, invasion of cancer cells. Additionally,
the expression level of miRNAs can also determine the pathogenesis
classification, diagnosis and prognosis of cancer (11–13).
Among these miRNAs, miR-29 family have been reported to be the
tumor regulator and biomarker (14,15).
miR-106 promoted colorectal cancer cell migration and invasion by
repressing the expression of DLC1 (16). Although more and more miRNAs were
identified to be involved in cancer and other diseases, the
particular roles of miRNA in different context of disease remain
largely unknown. miR-203 has been also reported to be as the
potential marker of the early detection of cervical cancer lymph
node metastases. The low level of miR-203 was related to lymph node
metastasis (17). A previous study
showed that miR-203 repressed proliferation and induced apoptosis
of colorectal cancer cells by post transcriptionally deregulating
CPEB4 (18). However, prostate
carcinoma patients with high miR-203 level showed lower average
survival time than those with low miR-203 level (19). Although miR-203 showed different
regulatory function in different cancers, miR-203 can be the
critical regulator of some cancers and even the potential marker of
diagnose. However, the function of miR-203 on regulating the
proliferation of breast cancer cells remains largely unknown.
In the present study we made the hypothesis that
sevoflurane repressed the proliferation of breast cancer. In order
to investigate the mechanism of proliferation regulation, we
performed the cell cycle analysis and detected the miRNAs function
and related regulatory signaling. This study may uncover the
function of sevoflurane on regulating breast cancer cell
proliferation and suggested the potential therapeutic target of
miRNA for cancer prevention and treatment.
Materials and methods
Cell culture and sevoflurane
treatment
Breast cancer cells were purchased from the Cell
Bank Type Culture Collection of Chinese Academy of Sciences
(CBTCCCAS; Shanghai, China) and cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) with
fetal bovine serum (10%) (Gibco; Thermo Fisher Scientific, Inc.) at
37°C, in a humidified atmosphere containing CO2 (5%).
The culture medium was added 100 U/ml of penicillin sodium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), and
100 mg/ml of streptomycin sulfate (Invitrogen; Thermo Fisher
Scientific, Inc.). Cells were exposed to 2% sevoflurane for 6 h as
previously described (5,20). 2% sevoflurane is clinically
relevant and regulates the proliferation of human hepatocellular
carcinoma cells (21).
CO2, O2 and sevoflurane concentration was
monitored by A Drager Vamos gas analyzer (Drager, Lübeck, Germany).
The cells used for each experiments were culture in the 6 wells
dish and seeded at the concentration of 1×105/well.
Overexpression and inhibition of
miRNA
Pre-miR-203, miRNA-203 inhibitor and the control
miRNA were synthetic nucleic acids (Biotend, Shanghai, China).
Pre-miRNAs can be processed by enzymes to become mature miRNAs.
miRNA inhibitor has reverse complementary sequence of the miRNA.
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) was
used for transfection of the pre-miRNA or inhibitor into cells
which grown to approximately confluence (80%).
MTS proliferation assay
Proliferation was detected by CellTiter
96®. A Queous One Solution Cell Proliferation Assay kit
(Promega Corporation, Madison, WI, USA) was used to perform the
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sul-fophenyl)-2H-tetrazolium
(MTS) assay by following the instruction. The record absorbance at
490 nm was detected by microplate reader. We performed the
detection every 24 h during 3 days.
Bromodeoxyuridine (BrdU) incorporation
assay
Cells were seeded in 96-wells (2×103
cells/well) in the culture medium with BrdU incubation. After 2 h,
phosphate-buffered saline (PBS) solution with paraformaldehyde
(PFA) (4%) was used to fix cells for 20 min. Cells were washed by
PBS and treated by DNase for 20 min at room temperature. Then, BrdU
primary antibody (Abcam, Cambridge, MA, USA) was added and
incubated at 4°C for 12 h. After washing by PBS, cells were
incubated with secondary antibody at room temperature for 60 min.
Cell nucleus were dyed by 4′,6-diamidino-2-phenylindole (DAPI)
(Sigma-Aldrich; Merck KGaA). BrdU-positive cells were counted by
microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNAiso (Takara Bio, Inc., Otsu, Japan) was used for
isolating total RNA. For reverse-transcription of miRNA, miRNA
specific stem-loop reverse-transcription primer (Ribobio, China)
was used. The amount of gene expression (2-ΔΔCt) was normalized
tothe endogenous nuclear RNA U6 using miRNA-specific primers
(RiboBio Co., Ltd., Guangzhou, China). For mRNA RT-qPCR, cDNA was
reverse-transcribed from mRNA by M-MLV Reverse Transcriptase
(Takara) using random primers and oligo (dT) primers. The
quantification of level of target gene expression
(2−ΔΔCt) was normalized to the endogenous GAPDH mRNA
(7,22). The reaction conditions of PCR were
according to the SYBR-Green qPCR Mix instruction (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The primers sequences are
as follows: Cyclin E1, PF, 5′-ACTCAACGTGCAAGCCTCG-3′, PR,
5′-GCTCAAGAAAGTGCTGATCCC-3′; Cyclin D1, PF,
5′-CAATGACCCCGCACGATTTC-3′, PR, 5′-CATGGAGGGCGGATTGGAA-3′; P21, PF,
5′-TGTCCGTCAGAACCCATGC-3′, PR, 5′-AAAGTCGAAGTTCCATCGCTC-3′; P27,
PF, 5′-TAATTGGGGCTCCGGCTAACT-3′, PR, 5′-TGCAGGTCGCTTCCTTATTCC-3′;
RB1, PF, 5′-TTGGATCACAGCGATACAAACTT-3′, PR,
5′-AGCGCACGCCAATAAAGACAT-3′. The primers of miRNA RT-qPCR were
purchased from the RiboBio Co., Ltd. The thermocycling condition
for mRNA is as follows: For mRNA: 95°C for 30 sec followed by 40
cycles of 95°C for 10 sec, 60°C for 30 sec, 70°C for 30 sec. For
miRNA: 95°C for 30 sec followed by 40 cycles of 95°C for 5 sec,
60°C for 20 sec, 70°C for 10 sec.
Western blot analysis
Proteins from MDA-MB-231 cells were lysed and then
transfered onto PVDF membranes (What man, England). Then incubated
the membranes with the primary antibodies diluted by TBST
(Beyotime, China) at room temperature for 2 h. The primary
antibodies are as follows: Anti-GAPDH (ab9485, Abcam, USA),
anti-cyclin E (ab135380, Abcam), anti-cyclin D (ab134175, Abcam),
anti-P21 (ab109520, Abcam), anti-P27 (ab32034, Abcam), anti-Rb1
(ab181616, Abcam). Then incubated the membranes with secondary
antibodies diluted by TBST (Beyotime) according to the primary
antibodies at room temperature for 1 h. Protein signals were
detected using enhanced chemiluminescence (ECL).
Statistical analyses
For statistical analyses, student's t-test or one
way ANOVA with Boferroni's correction was used. The values were
showed as the mean ± SD. P<0.05 was defined as statistically
significant.
Results
Sevoflurane inhibits the proliferation
of breast cancer cells
In order to determine the function of sevoflurane on
breast cancer cells proliferation, MDA-MB-231 or MCF-7 cells were
treated with sevoflurane (2%) for 6 h and cultured for another 24,
48 and 72 h without sevoflurane. The proliferation capacity was
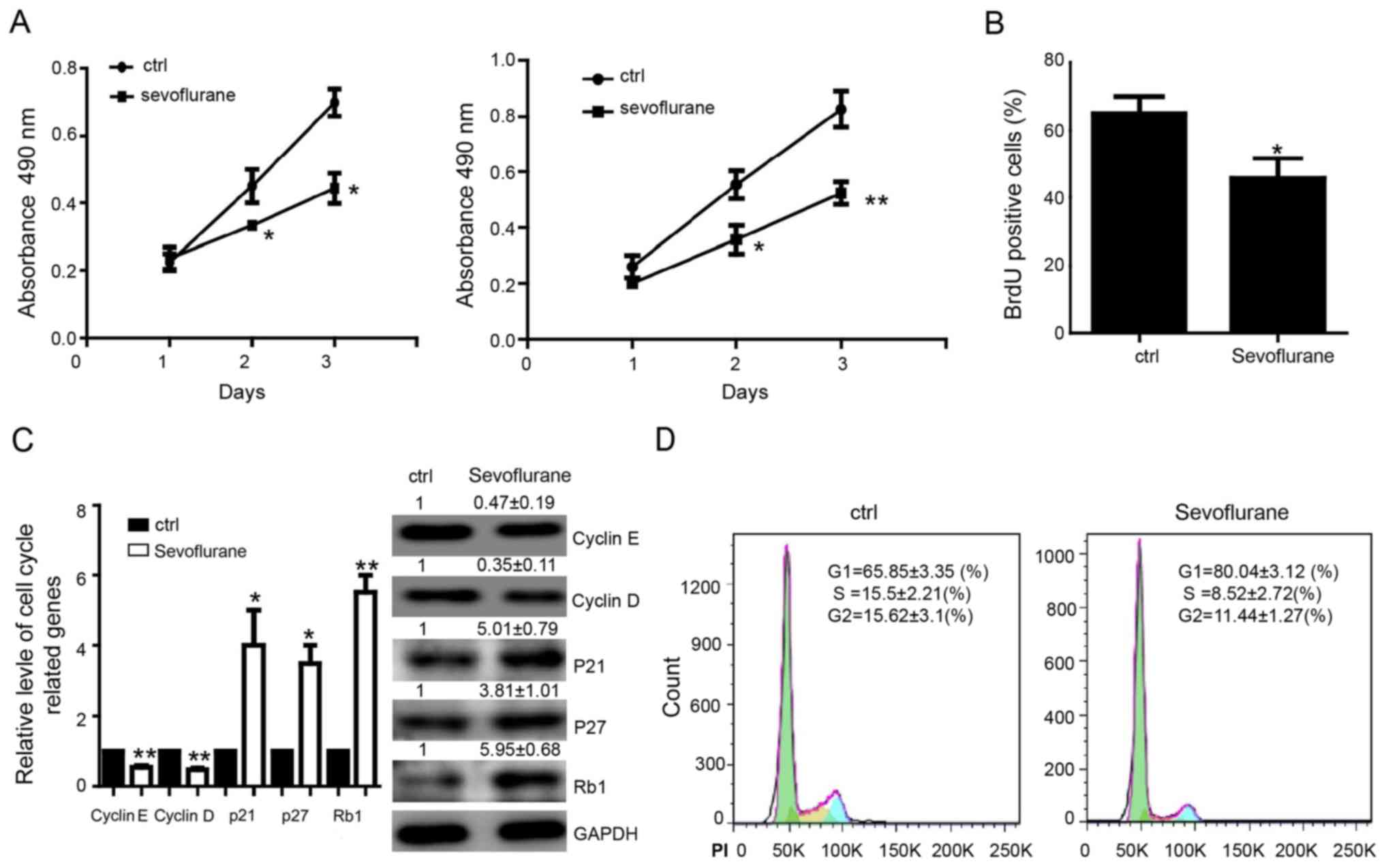
detected by MTS assay. The results showed that sevoflurane
decreased proliferation of MDA-MB-231 or MCF-7 cells (Fig. 1A). The BrdU incorporation assay in
MDA-MB-231 cells was performed and cells treated with sevoflurane
(2%) for 6 h and then cultured for another 24 h without sevoflurane
were found showing decreased proliferation capacity (Fig. 1B). We further detected the
cycle-related genes in MDA-MB-231 cells and found that the level of
cyclin D, cyclin E, which have been reported to be the critical
regulatory genes of G1 phase, were significantly downregulated. In
contrary, the expression of Rb1, P21, P27, the cell
cycle-inhibiting genes, were upregulated compared with control
group on both mRNA and protein levels (Fig. 1C). Flow cytometry assay showed that
proportion of cells at G1 phase was increased and cells at the S,
G2/M phase was decreased after treating by sevoflurane in
MDA-MB-231 cells (Fig. 1D).
miR-203 is upregulated by sevoflurane
and repressed the proliferation of breast cancer cells
Using RT-qPCR, we detected that miR-203 could be
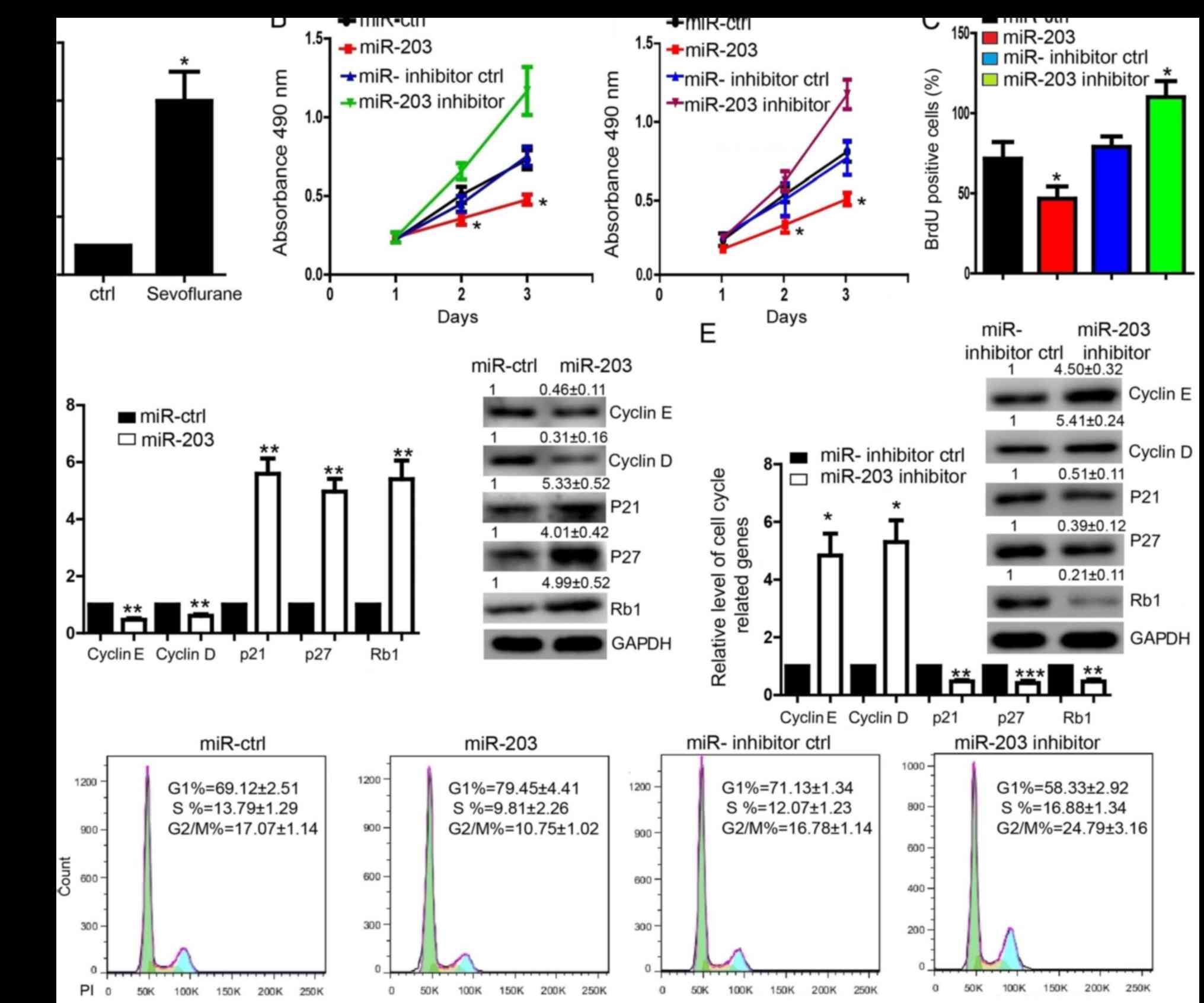
upregulated in MDA-MB-231 cells treated with sevoflurane (Fig. 2A). To determine the effect of
miR-203, we transfected the pre-miR-203 into MDA-MB-231 cells and
found that miR-203 significantly repressed the proliferation in
both MDA-MB-231 and MCF-7 cells (Fig.
2B and C). In contrast, transfection of the miR-203 inhibitor
whose efficiency was determined by detecting the target gene SNAI2
level (23) promoted the
proliferation of MDA-MB-231 and MCF-7 cells detected by MTS assay
(Fig. 2B). BrdU incorporation
assay in MDA-MB-231 cells also indicated the similar results
(Fig. 2C). Expression level of
cyclin D, cyclin E was downregulated in cells transfected with
pre-miR-203 (Fig. 2D) and
upregulated in the MDA-MB-231 cells transfected with miR-203
inhibitor (Fig. 2E). The
expression of P21, P27, Rb1 was upregulated in cells transfected
with pre-miR-203, while downregulated in cells transfected with
miR-203 inhibitor (Fig. 2D and E).
Flow cytometry assay showed that proportion of cells was increased
at G1 phase and decreased at the S and G2/M phase in MDA-MB-231
cells transfected with pre-miR-203 (Fig. 2F). Whereas, the proportion of cells
was decreased at G1 phase and increased at the S and G2/M phase in
MDA-MB-231 cells transfected with miR-203 inhibitor (Fig. 2F).
miR-203 inhibitor rescues the effects
of sevoflurane in breast cancer cells
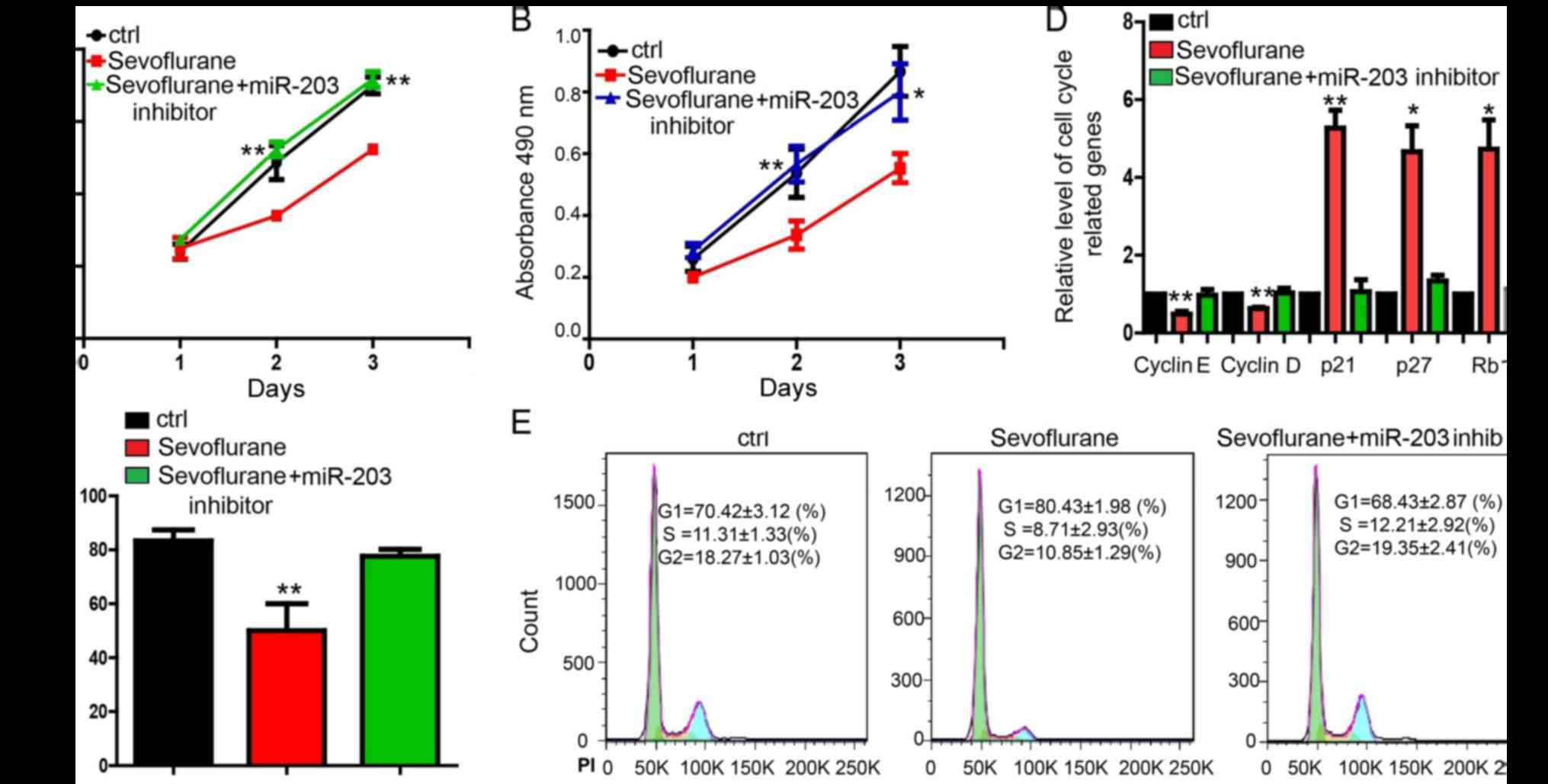
In order to determine whether miR-203 actually
mediated the function of sevoflurane, we performed the rescue
experiment (Fig. 3). Results
indicated that miR-203 inhibitor significantly attenuated the
proliferation repression caused by sevoflurane treatment in both
MDA-MB-231 (Fig. 3A and C) and
MCF-7 cells (Fig. 3B). miR-203
inhibitor also resisted the effect of sevoflurane on the expression
of cell cycle-related genes in MDA-MB-231 cells (Fig. 3D). Flow cytometry assay showed
that, compared with MDA-MB-231 cells treated with sevoflurane, the
increase of cells at G1 phase and the decrease of cells at the S
and G2/M phase can be reversed by miR-203 inhibitor in MDA-MB-231
cells (Fig. 3E).
Discussion
Breast cancer is the world's first serious malignant
neoplasm which threaten the health of female, it had exceeded the
cervical cancer and became the first factor of cancer deaths in
women. Proliferation, invasion and metastasis are the important
characteristic of breast cancer. Disclosing the underlying
mechanism of breast cancer development will be helpful to find
potential therapeutic approaches to treat breast cancer. Although
sevoflurane is commonly used as an inhalational anesthetic during
the surgery of cancer patients, evidences have demonstrated its
effects on cell growth. A previous study reported that volatile
anaesthetics, such as isoflurane, sevoflurane or desflurane,
enhanced the metastasis-related cellular signaling including CXCR2
in ovarian cancer cells (24).
Sevoflurane could induce the apoptosis of lung alveolar epithelial
cells (25). Sevoflurane also was
reported to regulate the expression of cyclin D1, but not p27Kip1
in the hippocampus (26). These
studies suggested the complicated effects of sevoflurane on
regulating the proliferation and cell cycle. It is significative to
investigate the mechanism of regulatory effect of sevoflurane on
different cells. In our study, we found that sevoflurane could
significantly suppress the proliferation of MDA-MB-231 and MCF-7
breast cancer cells. Cells treated with sevoflurane arrested at the
G1 phase. Our results showed that sevoflurane could be a potential
therapeutic agent to prevent or treat breast cancer by regulating
the cell cycle. Some other studies showed that sevoflurane
possessed the opposite function, which suggested the complex
effects of the sevoflurane on different cancer cells.
Among tumor-related miRNAs, miR-203 is one of the
critical miRNAs that plays important roles in regulating
proliferation of several types of cells. miR-203 reduced the
PDGF-stimulated proliferation in human airway smooth muscle (HASM)
cells (27), and formed the
JNK-miR-203-p63 signaling to regulate keratinocyte proliferation
and differentiation (28). miR-203
also plays important roles in cancerigenesis. miR-203 has been
reported to be the tumor suppressor in osteosarcoma (29). miR-203 also directly targeted
oncogene ADAM9 and long non-coding RNA HULC to inhibit the
hepatocellular carcinoma cell proliferation and metastasis
(30). In this study, we found
that overexpression of miR-203 could suppress the proliferation and
inhibition of miR-203 promoted the proliferation of MDA-MB-231 and
MCF-7 breast cancer cells. Notably, miR-203 could be upregulated by
sevoflurane treatment in breast cancer cells. The miR-203 inhibitor
blocked the repression the proliferation of sevoflurane in breast
cancer cells.
In this study, we uncovered the function of
sevoflurane in regulating the breast cancer proliferation. Abnormal
cell proliferation is most associated with influence of cell cycle
regulation (31). The cell cycle
was arrested at G1 phase, which is indicated by change of some
important cell cycle-related genes expression.
It is the first time to determine the function and
mechanism of sevoflurane in breast cancer cells. miR-203 is the
downstream target of sevoflurane to mediate the proliferation and
cell cycle. We will further study whether there are transcription
factors involved in the expression of miR-203 regulated by
sevoflurane. Additionally, we will also investigate the target
genes of miR-203, which regulates cell cycle. We will also need
further study about more similar types of breast cancers to
validate the present results to confirm the mechanism of the
miR-203 mediated the function of sevoflurane. Additionally, in
vitro and in vivo studies will be conducted in order to
validate the current results. In summary, we demonstrated a new
functional role of sevoflurane/miR-203 signaling pathway on
suppressing breast cancer cell proliferation and further confirmed
the influence of cell cycle regulation of G1 phase and cell
cycle-related genes expression.
Acknowledgements
Not applicable.
Funding
This research was supported by the Science and
Technology Research Project of Henan Province (grant no.
142102310459), the Science and Technology Research Project of Henan
Province (grant no. 162102310228) and the Medical Co construction
Project of the Department of Health and Henan Province (grant no.
201701035).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JYL and LY contributed to the study idea, analyzed
the majority of the data, and wrote the initial draft of the paper.
XG performed cell cycle analysis, GJ performed the cell culture and
some of the BrdU experiments, QW was involved in the experimental
operation of sevoflurane-treated cells and DL treated cells with
sevoflurane. JLL performed the transfection experiments, QC
designed the RT-qPCR primers and performed some of the RT-qPCR
experiments, QS is one of the chief directors of this study and
performed most of the statistical analysis and BL designed and
guided the majority of the experiments and gave final approval of
the manuscript. All authors discussed the results and contributed
to the writing and revisions.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yi W, Li D, Guo Y, Zhang Y, Huang B and Li
X: Sevoflurane inhibits the migration and invasion of glioma cells
by upregulating microRNA-637. Int J Mol Med. 38:1857–1863. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hurmath FK, Mittal M, Ramaswamy P, Rao
Umamaheswara GS and Nanjaiah Dalavaikodihalli N: Sevoflurane and
thiopental preconditioning attenuates the migration and activity of
MMP-2 in U87MG glioma cells. Neurochem Int. 94:32–38. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liang H, Yang CX, Zhang B, Wang HB, Liu
HZ, Lai XH, Liao MJ and Zhang T: Sevoflurane suppresses
hypoxia-induced growth and metastasis of lung cancer cells via
inhibiting hypoxia-inducible factor-1α. J Anesth. 29:821–830. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sugimoto H, Kawaraguchi Y, Nomura Y,
Nishiwada T, Uemura K, Furuya H and Kawaguchi M: Exposure to 1%
sevoflurane for 6 hours enhances proliferation of human colon
cancer cells. Masui. 64:357–361. 2015.(In Japanese). PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Q, Wang G, Chen Y, Li G, Yang D and
Kang J: A miR-590/Acvr2a/Rad51b axis regulates DNA damage repair
during mESC proliferation. Stem Cell Reports. 3:1103–1117. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu N, Papagiannakopoulos T, Pan G, Thomson
JA and Kosik KS: MicroRNA-145 regulates OCT4, SOX2, and KLF4 and
represses pluripotency in human embryonic stem cells. Cell.
137:647–658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ivey KN, Muth A, Arnold J, King FW, Yeh
RF, Fish JE, Hsiao EC, Schwartz RJ, Conklin BR, Bernstein HS and
Srivastava D: MicroRNA regulation of cell lineages in mouse and
human embryonic stem cells. Cell Stem Cell. 2:219–229. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singaravelu R, O'Hara S, Jones DM, Chen R,
Taylor NG, Srinivasan P, Quan C, Roy DG, Steenbergen RH, Kumar A,
et al: MicroRNAs regulate the immunometabolic response to viral
infection in the liver. Nat Chem Biol. 11:988–993. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shukla GC, Singh J and Barik S: MicroRNAs:
Processing, maturation, target recognition and regulatory
functions. Mol Cell Pharmacol. 3:83–92. 2011.PubMed/NCBI
|
|
12
|
Laios A, O'Toole S, Flavin R, Martin C,
Kelly L, Ring M, Finn SP, Barrett C, Loda M, Gleeson N, et al:
Potential role of miR-9 and miR-223 in recurrent ovarian cancer.
Mol Cancer. 7:352008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Georges SA, Biery MC, Kim SY, Schelter JM,
Guo J, Chang AN, Jackson AL, Carleton MO, Linsley PS, Cleary MA, et
al: Coordinated regulation of cell cycle transcripts by
p53-inducible microRNAs, miR-192 and miR-215. Cancer Res.
68:10105–10112. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang D, Fan Z, Liu F and Zuo J: Hsa-miR-21
and Hsa-miR-29 in tissue as potential diagnostic and prognostic
biomarkers for gastric cancer. Cell Physiol Biochem. 37:1454–1462.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Braconi C, Kogure T, Valeri N, Huang N,
Nuovo G, Costinean S, Negrini M, Miotto E, Croce CM and Patel T:
microRNA-29 can regulate expression of the long non-coding RNA gene
MEG3 in hepatocellular cancer. Oncogene. 30:4750–4756. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang GJ, Li JS, Zhou H, Xiao HX, Li Y and
Zhou T: MicroRNA-106b promotes colorectal cancer cell migration and
invasion by directly targeting DLC1. J Exp Clin Cancer Res.
34:732015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seifoleslami M, Khameneie MK, Mashayekhi
F, Sedaghati F, Ziari K, Mansouri K and Safari A: Identification of
microRNAs (miR-203/miR-7) as potential markers for the early
detection of lymph node metastases in patients with cervical
cancer. Tumour Biol. Oct 22–2015.(Epub ahead of print).
|
|
18
|
Zhong X, Xiao Y, Chen C, Wei X, Hu C, Ling
X and Liu X: MicroRNA-203-mediated posttranscriptional deregulation
of CPEB4 contributes to colorectal cancer progression. Biochem
Biophys Res Commun. 466:206–213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang Z, Zhang L, Yi X and Yu X:
Diagnostic and prognostic values of tissue hsa-miR-30c and
hsa-miR-203 in prostate carcinoma. Tumour Biol. 37:4359–4365. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Y, Hu R, Yan J, Chen Z, Lu Y, Jiang J
and Jiang H: Sevoflurane inhibits the malignant potential of head
and neck squamous cell carcinoma via activating the
hypoxia-inducible factor-1α signaling pathway in vitro. Int J Mol
Med. 41:995–1002. 2018.PubMed/NCBI
|
|
21
|
Nishiwada T, Kawaraguchi Y, Uemura K,
Sugimoto H and Kawaguchi M: Effect of sevoflurane on human
hepatocellular carcinoma HepG2 cells under conditions of high
glucose and insulin. J Anesth. 29:805–808. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X, Zhang Y, Fan C, Wang L, Liu Y, Li
A, Jiang G, Zhou H, Cai L and Miao Y: Noxin promotes proliferation
of breast cancer cells via P38-ATF2 signaling pathway. Tumour Biol.
39:10104283177055152017.PubMed/NCBI
|
|
23
|
Zhang Z, Zhang B, Li W, Fu L, Fu L, Zhu Z
and Dong JT: Epigenetic silencing of miR-203 upregulates SNAI2 and
contributes to the invasiveness of malignant breast cancer cells.
Genes Cancer. 2:782–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iwasaki M, Zhao H, Jaffer T, Unwith S,
Benzonana L, Lian Q, Sakamoto A and Ma D: Volatile anaesthetics
enhance the metastasis related cellular signalling including CXCR2
of ovarian cancer cells. Oncotarget. 7:26042–26056. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei GH, Zhang J, Liao DQ, Li Z, Yang J,
Luo NF and Gu Y: The common anesthetic, sevoflurane, induces
apoptosis in A549 lung alveolar epithelial cells. Mol Med Rep.
9:197–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fang F, Lin W, Ling X, Song R, Liu Q, Lai
B and Cang J: The hippocampal cyclin D1 expression is involved in
postoperative cognitive dysfunction after sevoflurane exposure in
aged mice. Life Sci. 160:34–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liao G, Panettieri RA and Tang DD:
MicroRNA-203 negatively regulates c-Abl, ERK1/2 phosphorylation,
and proliferation in smooth muscle cells. Physiol Rep. 3:pii:
e12541. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen HL, Chiang PC, Lo CH, Lo YH, Hsu DK,
Chen HY and Liu FT: Galectin-7 regulates keratinocyte proliferation
and differentiation through JNK-miR-203-p63 signaling. J Invest
Dermatol. 136:182–191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang D, Liu G and Wang K: miR-203 acts as
a tumor suppressor gene in osteosarcoma by regulating RAB22A. PLoS
One. 10:e01322252015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wan D, Shen S, Fu S, Preston B, Brandon C,
He S, Shen C, Wu J, Wang S, Xie W, et al: miR-203 suppresses the
proliferation and metastasis of hepatocellular carcinoma by
targeting oncogene ADAM9 and oncogenic long non-coding RNA HULC.
Anticancer Agents Med Chem. 16:414–423. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gérard C and Goldbeter A: The balance
between cell cycle arrest and cell proliferation: Control by the
extracellular matrix and by contact inhibition. Interface Focus.
4:201300752014. View Article : Google Scholar : PubMed/NCBI
|