Introduction
Lumbar spinal stenosis (LSS) refers to the lower or
buttock extremity pain caused by the reduced space for vascular and
neural elements in the lumbar spine (1). LSS causes severe pain that can
seriously affect the quality of life of patients, particularly in
elderly patients with risks falling, disability and depression
(2). In addition, the lack of
satisfactory treatment outcomes and the costs of surgery places
heavy economic and psychological burden on patients and their
families (3). LSS mainly affects
patients older than 65 years. It has been reported that the
proportion of people older than 65 years will increase from 8 to
14% in next several decades, indicating a potential increase in
incidence rate of LSS (4). A
variety of methods, including imaging techniques, have been
developed to diagnose LSS; however, the application of most of
those methods is limited by unsatisfactory accuracy (5). Therefore, the development of
diagnostic methods than can be used to accurately identify LSS is
required.
The pathogenesis of LSS remains to be fully
elucidated. As a group small noncoding RNAs containing 22–24
nucleotides, microRNAs (miRs) have been observed to participate in
a variety of biological processes, including cell proliferation,
differentiation and fibrosis (6–8).
Previous studies have demonstrated that the development of LSS is
associated with the function of various miRNAs (9,10).
As a member of miR-29 family, miR-29a has been reported to be
involved in the deterioration of synovitis in patients with
end-stage knee arthritis (11);
however, the role of miR-29a in the development of LSS requires
further investigation.
In the present study, the expression of miR-29a in
plasma and intervertebral disc tissue of patients with LSS and
lumbar intervertebral disc herniation (LDH) were detected. In
addition, the possibility of the application of miR-29a as a
biomarker of LSS was investigated.
Materials and methods
Patients
A total of 30 patients with LSS, including 21 males
and 9 females were selected in the Beijing Shi Ji Tan Hospital
(Beijing, China) between January 2015 and January 2017; the average
age of the patients was 60±4.3-years-old. Simultaneously, 27
patients with LDH, including 20 males and 7 females were also
selected, and average age of those patients was 57±6.1-years-old.
In addition, 27 healthy individuals with similar age distribution
(19 males and 8 females with a mean age of 56±7.7 years) were
selected to collected plasma samples, and 7 patients that had
succumbed to mortality (5 males and 2 females with a mean age of
58±5.2 years) were selected to collect normal intervertebral disc
tissue. All patients were diagnosed by pathological examination and
medical imaging. Patients with lumbar spondylolisthesis, cancer,
infection, hypertension, heart disease and diabetes were excluded.
All patients with LSS underwent laminectomy for decompression and
surgical resection of intervertebral disc tissue. The present study
was approved by the Ethics Committee of Beijing Shi Ji Tan
Hospital. All patients provided written informed consent.
Specimen collection
Blood (5 ml) was extracted from middle vein of the
elbow of each participant. Red blood cells were removed by
centrifugation at 106 × g for 20 min at room temperature, and
plasma was transferred to 0.5 ml sterile Eppendorf tubes
(Eppendorf, Hamburg, Germany) and stored at −20°C until use.
Intervertebral disc tissue was collected from patients with LSS and
LDH. Normal intervertebral disc tissue was collected from patients
without lumbar diseases that had succumbed to mortality. Tissues
were washed with saline and stored at −80°C prior to use.
Enzyme-linked immunosorbent assay
(ELISA)
Human MMP-9 Quantikine ELISA kit DMP900 (R&D
Systems, Inc., Minneapolis, MN, USA) and Human ADAMTS5 DuoSet ELISA
kit (R&D Systems, Inc.) were used according to the
manufacture's protocols.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Intervertebral disc tissue was sectioned into pieces
and ground in liquid nitrogen. TRIzol (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) was used to extract RNA from plasma and
intervertebral disc tissue. RNA samples were dissolved in diethyl
pyrocarbonate water. Only RNA samples with an optical density ratio
(260/280 nm) between 1.8 and 2.0 were used. RT was performed to
synthesize cDNA using Oligo (dT) 15 (Sangon Biotech Co., Ltd.,
Shanghai, China) and AMV reverse transcriptase (Gibco; Thermo
Fisher Scientific, Inc.). Reaction conditions were: 25°C for 5 min,
55°C for 45 min and 80°C for 20 min. SYBR® Green
Real-Time PCR Master Mix (Thermo Fisher Scientific, Inc.) was used
to prepare the qPCR reaction system. Primers used were: miR-29a
forward, 5′-ATAGGATCCCGACCTTCTGTGACCCCTTA-3′, reverse,
5′-CGCAAGCTTACCACATGCAATTCAGGTCA-3′ and GAPDH forward,
5′-GAGTCAACGGATTTGGTCGT-3′ and reverse, 5′-TTGATTTTGGAGGGATCTCG-3′.
qPCR was performed on a Bio-Rad iCycler (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The reaction conditions were: 94°C for 2
min, followed by 40 cycles of 94°C for 10 sec, 55°C for 30 sec and
72°C for 25 sec. Cq values were processed using 2−ΔΔCq
method (12), and relative
expression levels of miR-29a were normalized to that of the
endogenous control, GAPDH.
Western blotting
Protein was extracted from intervertebral disc
tissue using RIPA buffer (Thermo Fisher Scientific, Inc.). A
Bicinchoninic Acid protein method was used to determine the
concentration of protein; 20 µg of protein from each sample was
subjected to 10% SDS-PAGE, followed by transfer onto polyvinylidene
difluoride membranes (Bio-Rad Laboratories, Inc.). Membranes were
blocked with 5% skimmed milk at room temperature for 2 h. Following
washing with tris-buffered saline with Tween-20 (0.3%), membranes
were incubated with primary antibodies including anti-matrix
metalloproteinase 9 (MMP9; 1:1,000; cat. no. EP1255Y; Abcam,
Cambridge, UK), anti-a disentigrin and metalloproteinase with
thrombospondin motifs 5 (ADAMTS5; 1:1,000; cat. no. ab41037;
Abcam), and anti-β-actin (1:1,000; cat. no. ab8227; Abcam)
overnight at 4°C. Following washing with tris-buffered saline with
Tween-20 (0.3%), membranes were incubated with goat anti-rabbit
immunoglobulin G horseradish peroxidase secondary antibody (cat.
no. sc-2004; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at
37°C for 1 h. An enhanced chemiluminescence assay was performed
according to the protocols of the Pierce ECL Western Blotting
Substrate (Pierce; Thermo Fisher Scientific, Inc.) and iBright
Imaging Systems (Thermo Fisher Scientific, Inc.) was used to
visualize the bands. Data was analyzed by Image J version 1.48
(National Institutes of Health, Bethesda, MD, USA).
Determination of the stability of
Plasma miR-29a
Plasma samples were kept at room temperature or at
4°C, and sampling was performed 2, 4, 6, 8, 10 and 12 h later.
Plasma samples were also subjected to freeze-thaw cycles (−80°C for
20 min and room temperature for 20 min). The amount of miR-29a
retained in plasma samples was measured by RT-qPCR.
Statistical analysis
SPSS software version 19.0 (IBM Corp., Armonk, NY,
USA) was used to analyze the data. Each experiment was performed 3
times and data were expressed as mean ± standard deviation.
Comparisons between groups were performed by an unpaired Student's
t-test, and comparisons among multiple groups were performed by one
way-analysis of variance followed by the Least Significant
Difference test. Correlations between the expression levels of
miR-29 and the expression levels of MMP9 and ADAMTS5, and the
correlation between expression levels in plasma and intervertebral
disc tissue were performed by Spearman's correlation coefficient
analysis. Receiver operating characteristic (ROC) curve analysis
was performed to evaluate the diagnostic value of miR-29 for LSS
and LDH. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels of miR-29a in plasma
of different groups of patients
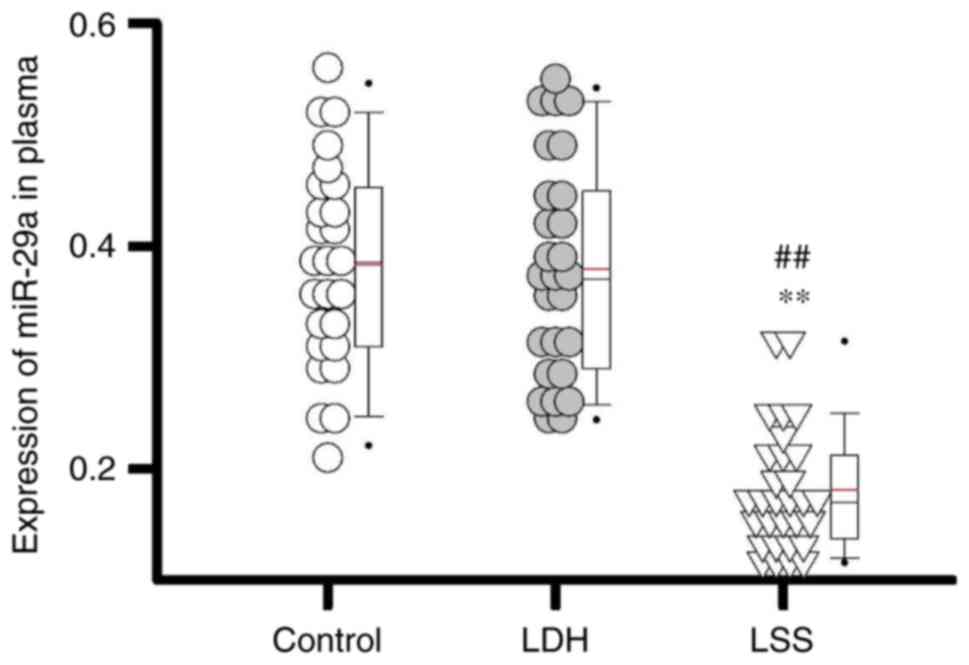
As presented in Fig.
1, the expression levels of miR-29a were significantly lower in
patients with LSS compared with in patients with LDH and healthy
individuals (both P<0.01). The data of the present study
suggested that the expression levels of miR-29a were downregulated
in patients with LSS.
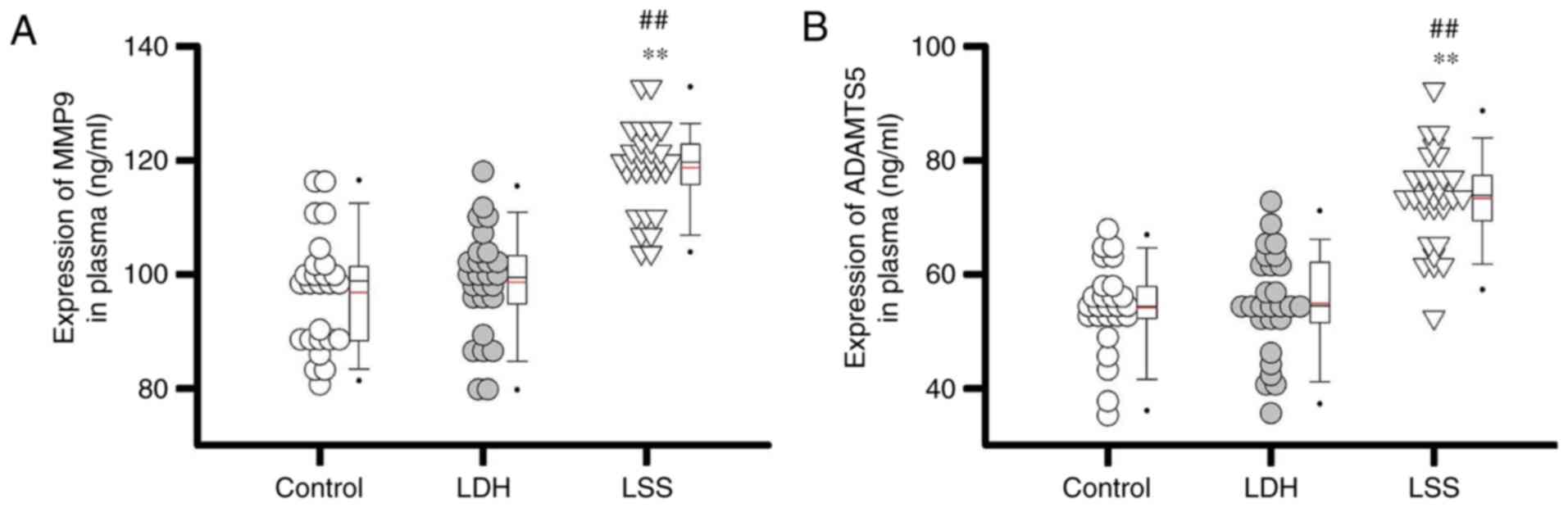
Comparison of plasma levels of MMP9
and ADAMTS5 between different patient groups
miR-29a expression is positively (13) or negatively (14) correlated with the expression of
MMP9 in a variety of disease models; however, the effects of
miR-29a expression on MMP9 in LSS require further investigation. A
recent study revealed that miR-29a targeted ADAMTS5 to reduce its
expression levels in patients with knee osteoarthritis (11). Therefore, the plasma expression
levels of MMP9 and ADAMTS5 were detected in a variety of patient
groups. The results of the present study revealed that plasma
expression levels of MMP9 (Fig.
2A) and ADAMTS5 (Fig. 2B) in
patients with LSS were significantly higher compared with patients
with LDH and healthy controls (all P<0.01). The data suggested
that the expression levels of miR-29a were inhibited in patients
with LSS, which in turn may have upregulated plasma expression
levels of MMP9 and ADAMTS5.
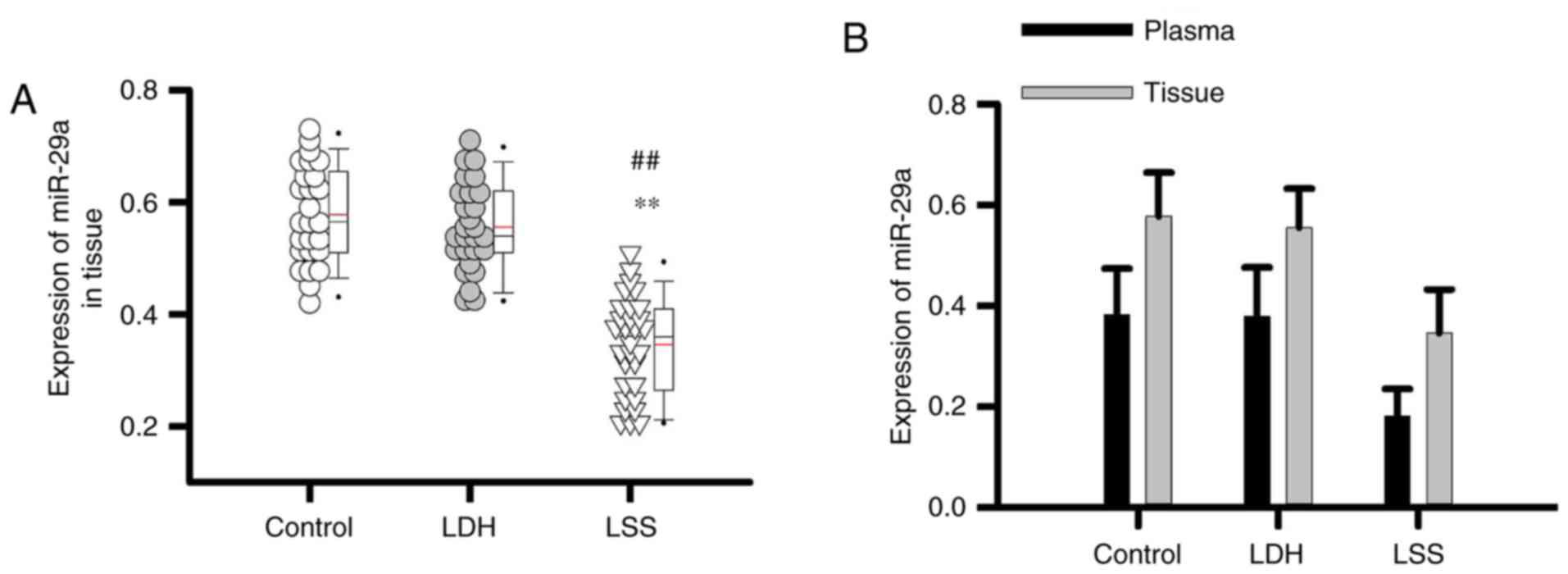
Comparison of the expression of
miR-29a in intervertebral disc tissue of different groups of
patients
The expression levels of miR-29a in intervertebral
disc tissue of patients with LSS were significantly lower than that
of patients with LDH, as well as healthy controls (P<0.01;
Fig. 3A). In addition, the
expression levels of miR-29a in plasma revealed similar expression
profiles to that in intervertebral disc tissue (Fig. 3B). The data indicated that the
plasma expression levels of miR-29a may reflect its expression in
intervertebral disc tissue.
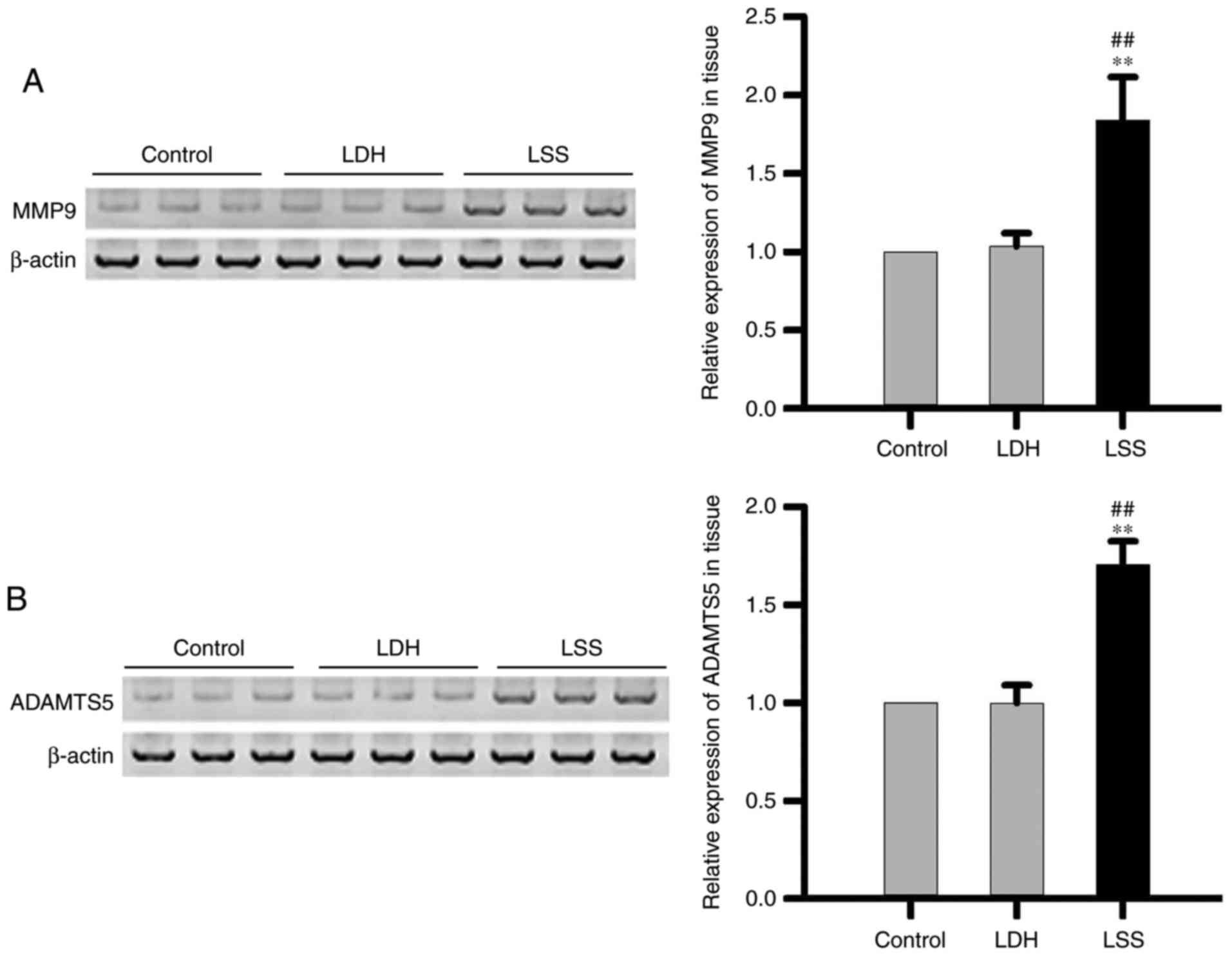
Comparison of MMP9 and ADAMTS5
expression levels in intervertebral disc tissue of different groups
of patients
The expression levels of MMP9 (Fig. 4A) and ADAMTS5 (Fig. 4B) in intervertebral disc tissue of
patients with LSS were significantly higher compared with patients
with LDH, as well as healthy controls. The data suggested that the
expression levels of MMP9 and ADAMTS5 were increased in
intervertebral disc tissue of patients with LSS.
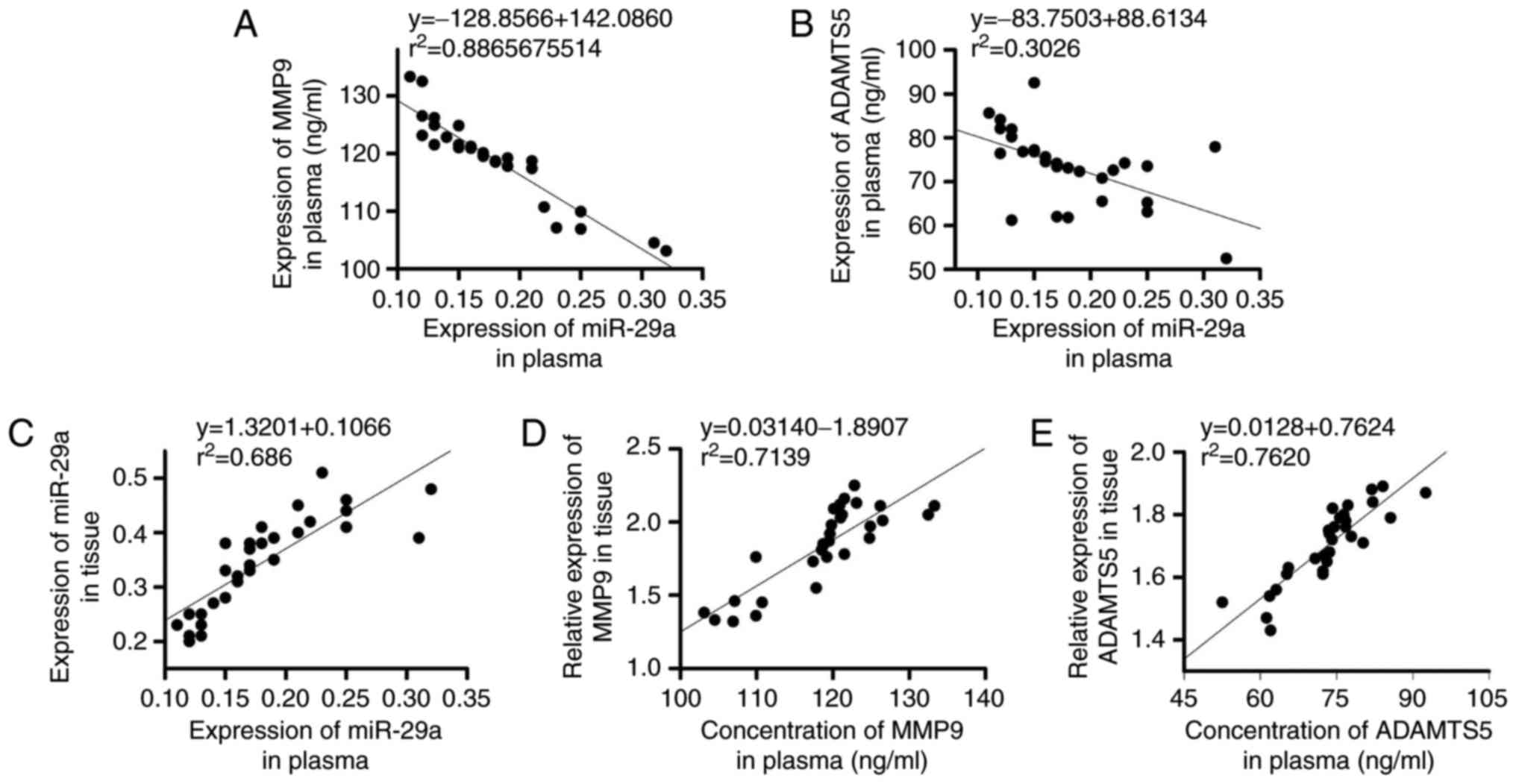
Correlations between the expression
levels of miR-29 and the expression levels of MMP9 and ADAMTS5, and
the correlation between expression levels in plasma and
intervertebral disc tissue
The expression levels of miR-29 were negatively
correlated with that of MMP9 (Fig.
5A) and ADAMTS5 (Fig. 5B). In
addition, the expression levels of miR-29 (Fig. 5C), MMP9 (Fig. 5D) and ADAMTS5 (Fig. 5E) in plasma were positively
correlated with their expression levels in intervertebral disc
tissue of LSS patients (P<0.05).
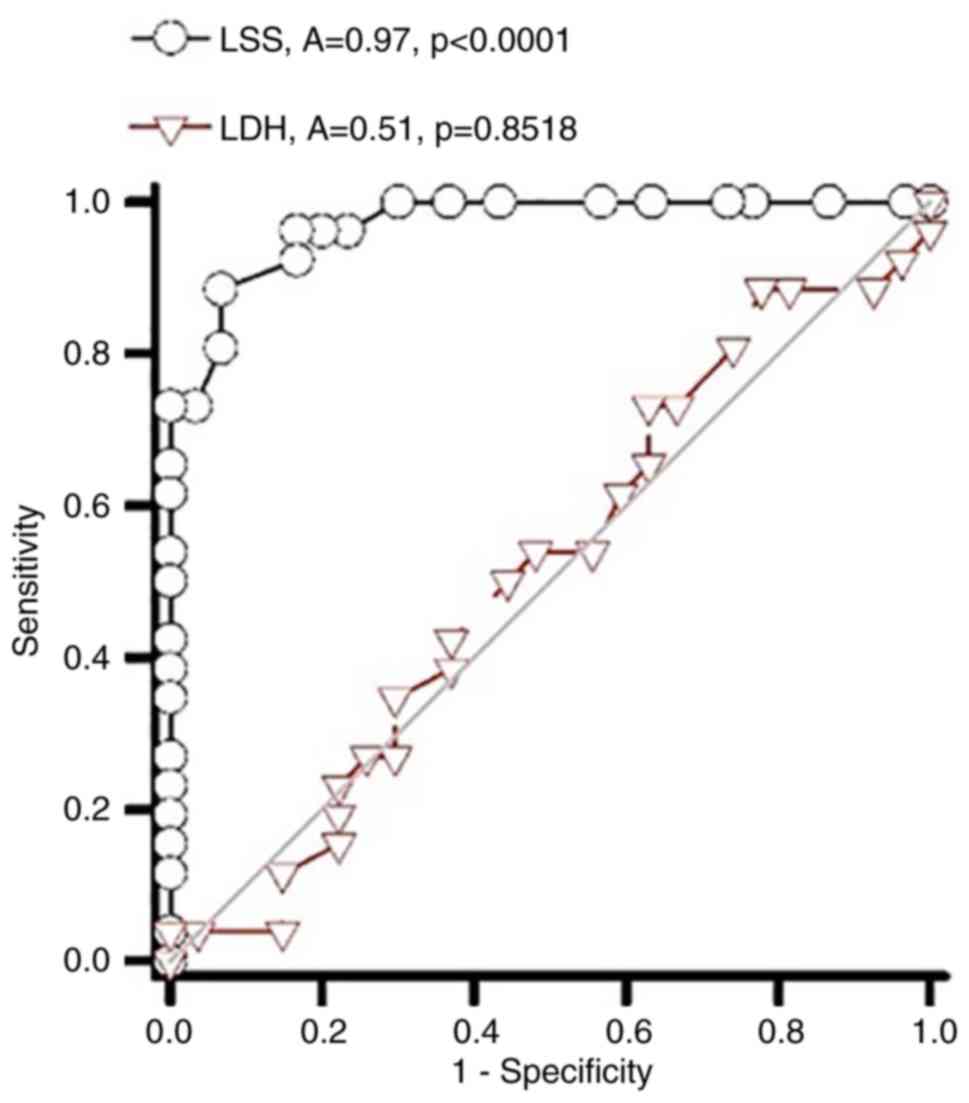
ROC curve analysis of the prediction
of LSS and LDH by miR-29
ROC curve analysis was performed to investigate the
diagnostic value of plasma miRNA-29 for LSS and LDH. The area under
the curve of miR-29 associated with LSS was 0.97, while the area
under the curve of miR-29 in predicting LDH was only 0.51 (Fig. 6). The findings of the present study
indicated that miR-29 may be used to accurately diagnose LSS.
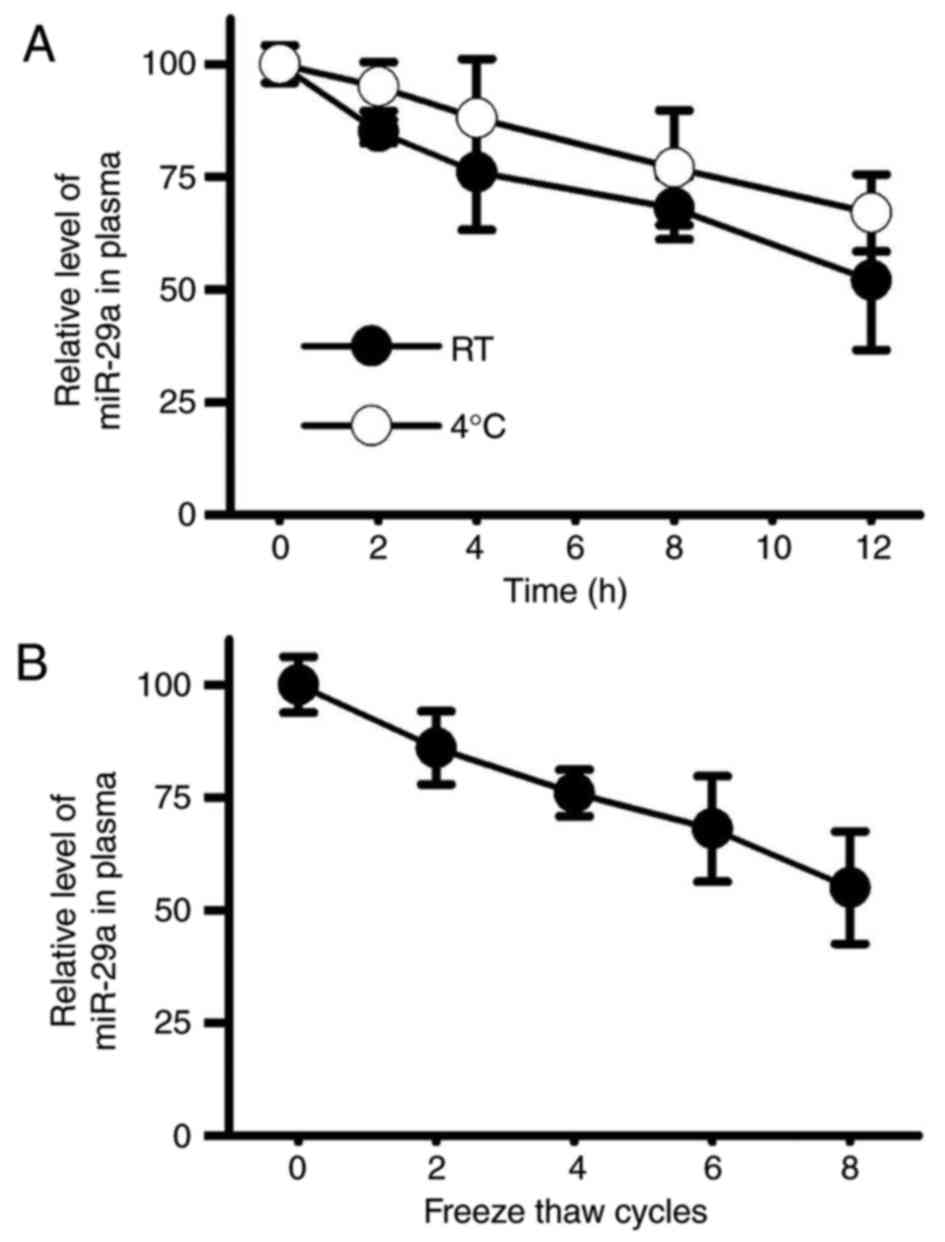
Stability of miR-29a in the plasma of
patients with LSS
The present study revealed that miR-29a may be used
to accurately diagnose LSS but not LDH; however, the application of
miR-29a as a biomarker of LSS also requires high RNA stability.
Therefore, temperature sensitivity and freeze-thaw stability of
miR-29a were detected by RT-qPCR. The results demonstrated that
miR-29a under room temperature showed similar stability to that at
4°C (Fig. 7A). In addition,
miR-29a in plasma also exhibited high freeze-thaw stability
(Fig. 7B), and about 50% of the
RNA was retained in plasma after 5 cycles of freeze-thaw. These
findings suggested that miR-29a may be easily detected under a
variety of conditions, which in turn may reduce the cost and the
requirement of equipment for the diagnosis test. Therefore, miR-29a
may be considered to be a promising biomarker for LSS.
Discussion
Development of LSS is a complex process with various
internal and external factors involved. At present, pathogenesis of
LSS remains to be investigated; however, various clinical and
experimental studies have reported that overweight and obese
individuals usually have high risk of LSS due to relatively high
incidences of inflammatory responses (15,16).
Inflammation is closely associated with the progression of LSS
(16). Angiopoietin-like protein 2
can accelerate the progression of lumbar spinal canal stenosis by
promoting inflammatory responses via the activation of interleukin
(IL)-6, which serves a role as both an proinflammatory and
anti-inflammatory factor (17).
miRNAs constitute a group of small noncoding RNAs that serve
pivotal roles in a variety biochemical and physiological processes.
Involvement of miRNAs in the progression of intervertebral
disc-associated diseases has also been reported recently (18,19).
miRNA-146a, as a member of the miRNA-146 family, was reported to
limit inflammatory responses by inhibiting the expression of IL-10
in intervertebral discs (18).
Conversely, miR-515 and −194 were demonstrated to promote the
development of intervertebral disc degeneration by interrupting the
biosynthesis of chondroitin sulfate in humans (19). In the study of LSS, Xu et al
(10) reported that overexpression
of collagens I and III induced by treatment with inhibitors of MMP
reduced the expression levels of miRNA-221, which in turn promoted
the progression of hypertrophy of ligamentum flavum in patients
with LSS.
As a member of miR-29 family, miR-29a was revealed
to be involved in the development of a variety diseases. In the
study of cerebellar alterations in mice, Papadopoulou et al
(20) reported that miR-29a
together with miR-29b-1 caused the ataxic features of this disease.
The expression levels of miR-29a were significantly upregulated in
serum of patients with acute graft-vs-host disease; increased
expression levels of miR-29a were suggested to activate dendritic
cells via Toll-like receptor (TLR) 8 and TLR 7 (21). However, to the best of the authors'
knowledge, expression profile and functionality of miR-29a in the
pathogenesis of LSS have yet to be reported. In the present study,
the expression levels miR-29a were observed to be significantly
lower in patients with LSS than in patients with LDH, as well as
healthy controls, indicating that the expression of miR-29a may be
particularly downregulated and correlated with the progression of
LSS. ADAMTSs and MMPs are main factors involved in the process of
disc degeneration and breakdown of the extracellular matrix
(22). miR-29a can negatively
regulate the expression of MMP9 and ADAMTS5 (11). In the present study, the expression
levels of MMP9 and ADAMTS5 were observed to be significantly higher
in patients with LSS than in patients with LDH, as well as healthy
controls. In addition, the expression levels of miR-29a were
reported to be negatively correlated with that of MMP9 and ADAMTS5.
The results of the present study suggest that reduced expression of
miR-29a may upregulate the expression of MMP9 and ADAMTS5, which in
turn may promote the progression of this LSS.
Due to the particular expression profile of miR-29a
in numerous pathological processes, miR-29a has been applied as a
biomarker for the diagnosis of a variety of human diseases. In the
study of colorectal cancer, Brunet Vega et al (23) reported that the expression levels
of miR-29a were significantly increased in patients with stage III
colorectal cancer, and expression levels of miR-29a may be used to
accurately predict this disease. In addition, the expression levels
of numerous miRNAs were detected to be upregulated in patients with
hypertrophic cardiomyopathy; only circulating miR-29a can be used
to sensitively and accurate diagnose both hypertrophy and fibrosis
(24). In the present study, the
expression levels of miR-29a in plasma reflected its expression
profile within intervertebral disc tissue. Therefore, plasma
expression levels of miR-29a may be employed to predict LSS; plasma
expression levels of miR-29a may be used to accurately predict LSS
but not LDH. In addition, miR-29a was also demonstrated to exhibit
low temperature sensitivity and high freeze-thaw stability. The
findings of the present study suggested that miR-29a may serve as a
reliable biomarker for the diagnosis of LSS.
In conclusion, the expression levels of miR-29a were
particularly reduced in patients with LSS, which in turn led to the
increased expression levels of MMP9 and ADAMTS5, resulting in the
progression of LSS. In addition, plasma expression levels of
miR-29a, which exhibited low temperature sensitivity and high
freeze-thaw stability, may be applied to accurately diagnose
LSS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GZ and LD designed the experiments. GZ, WZ and YH
performed the experiments. GZ, YC, JS and LD analyzed the data. LD
wrote the manuscript. All authors read the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Beijing Shi Ji Tan Hospital. All patients provided
written informed consent.
Consent for publication
All participants signed informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kreiner DS, Shaffer WO, Baisden JL,
Gilbert TJ, Summers JT, Toton JF, Hwang SW, Mendel RC and Reitman
CA: North American Spine Society: An evidence-based clinical
guideline for the diagnosis and treatment of degenerative lumbar
spinal stenosis (update). Spine J. 13:734–743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Backstrom KM, Whitman JM and Flynn TW:
Lumbar spinal stenosis-diagnosis and management of the aging spine.
Man Ther. 16:308–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lucio JC, VanConia RB, DeLuzio KJ, Lehmen
JA, Rodgers JA and Rodgers W: Economics of less invasive spinal
surgery: An analysis of hospital cost differences between open and
minimally invasive instrumented spinal fusion procedures during the
perioperative period. Risk Manag Healthc Policy. 5:65–74.
2012.PubMed/NCBI
|
|
4
|
Bressler HB, Keyes WJ, Rochon PA and
Elizabeth B: The prevalence of low back pain in the elderly: A
systematic review of the literature. Spine (Phila Pa 1976).
24:1813–1819. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Schepper EI, Overdevest GM, Suri P,
Peul WC, Oei EH, Koes BW, Bierma-Zeinstra SM and Luijsterburg PA:
Diagnosis of lumbar spinal stenosis: An updated systematic review
of the accuracy of diagnostic tests. Spine (Phila Pa 1976).
38:E469–E481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang X, Tsitsiou E, Herrick SE and
Lindsay MA: MicroRNAs and the regulation of fibrosis. FEBS J.
277:2015–2021. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shenoy A and Blelloch RH: Regulation of
microRNA function in somatic stem cell proliferation and
differentiation. Nat Rev Mol Cell Biol. 15:565–576. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baumjohann D and Ansel KM:
MicroRNA-mediated regulation of T helper cell differentiation and
plasticity. Nat Rev Immunol. 13:666–678. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen J, Liu Z, Zhong G, Qian L, Li Z, Qiao
Z, Chen B and Wang H: Hypertrophy of ligamentum flavum in lumbar
spine stenosis is associated with increased miR-155 level. Dis
Markers. 2014:7865432014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu YQ, Zhang ZH, Zheng YF and Feng SQ:
MicroRNA-221 regulates hypertrophy of ligamentum flavum in lumbar
spinal stenosis by targeting TIMP-2. Spine (Phila Pa 1976).
41:275–282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ko JY, Lee MS, Lian WS, Weng WT, Sun YC,
Chen YS and Wang FS: MicroRNA-29a counteracts synovitis in knee
osteoarthritis pathogenesis by targeting VEGF. Sci Rep. 7:35842017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han W, Han Y, Liu X and Shang X: Effect of
miR-29a inhibition on ventricular hypertrophy induced by pressure
overload. Cell Biochem Biophys. 71:821–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Q, Yin D, Zhang Y, Yu L, Li XD, Zhou
ZJ, Zhou SL, Gao DM, Hu J, Jin C, et al: MicroRNA-29a induces loss
of 5-hydroxymethylcytosine and promotes metastasis of
hepatocellular carcinoma through a TET-SOCS1-MMP9 signaling axis.
Cell Death Dis. 8:e29062017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Knutsson B, Sandén B, Sjödén G, Järvholm B
and Michaëlsson K: Body mass index and risk for clinical lumbar
spinal stenosis: A cohort study. Spine (Phila Pa 1976).
40:1451–1456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fujita N, Hosogane N, Hikata T, Iwanami A,
Watanabe K, Shiono Y, Okada E, Ishikawa M, Tsuji T, Shimoda M, et
al: Potential Involvement of obesity-associated chronic
inflammation in the pathogenesis of idiopathic spinal epidural
lipomatosis. Spine (Phila Pa 1976). 41:E1402–E1407. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakamura T, Okada T, Endo M, Nakamura T,
Oike Y and Mizuta H: Angiopoietin-like protein 2 promotes
inflammatory conditions in the ligamentum flavum in the
pathogenesis of lumbar spinal canal stenosis by activating
interleukin-6 expression. Eur Spine J. 24:2001–2009. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gu SX, Li X, Hamilton JL, Chee A, Kca R,
Chen D, An HS, Kim JS, Oh CD, Ma YZ, et al: MicroRNA-146a reduces
IL-1 dependent inflammatory responses in the intervertebral disc.
Gene. 555:80–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu B, Xu C, Tian Y, Shi C, Zhang Y, Deng
L, Zhou H, Cao P, Chen H and Yuan W: Inflammatory microRNA-194
and-515 attenuate the biosynthesis of chondroitin sulfate during
human intervertebral disc degeneration. Oncotarget. 8:49303–49317.
2017.PubMed/NCBI
|
|
20
|
Papadopoulou AS, Serneels L, Achsel T,
Mandemakers W, Callaerts-Vegh Z, Dooley J, Lau P, Ayoubi T,
Radaelli E, Spinazzi M, et al: Deficiency of the miR-29a/b-1
cluster leads to ataxic features and cerebellar alterations in
mice. Neurobiol Di. 73:275–288. 2015. View Article : Google Scholar
|
|
21
|
Ranganathan P, Ngankeu A, Zitzer NC,
Leoncini P, Yu X, Casadei L, Challagundla K, Reichenbach DK, Garman
S, Ruppert AS, et al: Serum miR-29a is upregulated in acute
graft-versus-host disease and activates dendritic cells through TLR
binding. J Immunol. 198:2500–2512. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang WJ, Yu XH, Wang C, Yang W, He WS,
Zhang SJ, Yan YG and Zhang J: MMPs and ADAMTSs in intervertebral
disc degeneration. Clin Chim Acta. 448:238–246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vega Brunet A, Pericay C, Moya I, Ferrer
A, Dotor E, Pisa A, Casalots À, Serra-Aracil X, Oliva JC, Ruiz A
and Saigí E: microRNA expression profile in stage III colorectal
cancer: Circulating miR-18a and miR-29a as promising biomarkers.
Oncol Rep. 30:320–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roncarati R, Anselmi Viviani C, Losi MA,
Papa L, Cavarretta E, Da Costa Martins P, Contaldi C, Jotti Saccani
G, Franzone A, Galastri L, et al: Circulating miR-29a, among other
up-regulated microRNAs, is the only biomarker for both hypertrophy
and fibrosis in patients with hypertrophic cardiomyopathy. J Am
Coll Cardiol. 63:920–927. 2014. View Article : Google Scholar : PubMed/NCBI
|