Introduction
Hepatocellular carcinoma (HCC) is the most common
type of primary liver cancer. The incidence of HCC keeps increasing
in recent years (1). Surgery,
chemotherapies and radiotherapy are the conventional treatments for
HCC. However, late diagnosis along with resistance to
chemotherapies relate to the limited clinical efficacy (2). Therefore, there is an urgent need to
develop effective agents for HCC treatment.
Vacquinol-1 (Vacq) is a quinolone derivative which
shows antitumor effects in glioblastomas (3). Vacq rapidly induced glioblastomas
cells (GCs) death and catastrophic vacuolization by membrane
ruffling, cell rounding, massive macropinocytic vacuole
accumulation, ATP depletion, and cytoplasmic membrane rupture of
GCs, which was morphologically distinct from apoptosis (3). Currently, whether Vacq could display
antitumor effects in other types of cancer remains to be examined.
In addition, whether Vacq could induce other types of cell death
besides catastrophic vacuolization in cancer cells needs to be
further investigated.
In the present study, we aimed to investigate the
effects of Vacq on human HCC cells. Distinct from observations in
previous study, we showed that Vacq triggered caspase dependent
apoptosis in HCC cells.
Materials and methods
Cell lines
HCC cell lines BEL7402 and Huh7 were provided by
Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
Huh7 was cultured in DMEM high glucose, and BEL7402 was maintained
in RPMI1640 (Hyclone; GE Healthcare, Logan UT, UTA). All the cell
culture medium was supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cells were
cultured in a humidified incubator containing 5% CO2 at
37°C.
Patients
This study was approved by the Medical Ethical
Committee of the Liaoning Cancer Hospital (China; Ethics Review
approval no. 20160213-3). Written informed consent was provided by
all of the participants. Ten patients who were pathologically
diagnosed as primary HCC were enrolled in this study. Patients who
received chemotherapy and radiotherapy or interventional therapy
were exclude from this study.
Compounds and antibodies
Vacq was purchased from Sigma Aldrich (St. Louis,
MO, USA) and dissolved in sterile dimethyl sulfoxide (DMSO).
Antibodies against caspase-3, caspase-8, caspase-9, BCL-xL, B-cell
lymphoma 2 (bcl-2)-associated X protein (Bax), Bim, and β-Actin
were purchased from Cell Signaling Technology Inc. (Danvers, MA,
USA). BCL-2 antibody was obtained from Thermo Fisher Scientific,
Inc. The following antibodies were purchased from Abcam (Cambridge
MA, USA): Anti-PARP, Goat anti-mouse IgG-HRP and Goat anti-Rabbit
IgG-HRP. Caspase inhibitor Z-VAD-FMK were purchased from Promega
(Madison, WI, USA). Hoechst 33342 was from Solarbio Science &
Technology (Beijing, China).
Cell viability assay
Cell viability was monitored by xCELLigence
Real-Time Cell Analyzer (RTCA)-MP system (Acea Biosciences, San
Diego, CA, USA). This device was put in 5% CO2 incubator
and could measure cellular growth status in real time. Briefly, 100
µl of culture medium was added in each well of E-Plate 96 (Acea
Biosciences) to obtain equilibrium. 2×104 cells for cell
line cells or 1×105 for human primary HCC cells in 100
µl of culture medium were seeded in E-Plate 96, which was coated by
biocompatible microelectrode. After 18 h, cells were treated with
25, 12.5, 6.26, 3.13 and 1.56 µM, respectively. DMSO-treated cells
were used as controls. Electrical impedance which reflects cell
growth status was presented as cell index and was read
automatically every 1 min. The half maximal inhibitory
concentration (IC50) values at 24, 48 and 72 h were calculated
automatically by RTCA software v. 2.0 (Acea Biosciences).
Colony formation
BEL7402 and Huh7 cells were seeded in 6-well culture
plates at the density of 2,000 cells/well. Cells were cultured for
18 h and then treated with different concentrations of Vacq for 24
h. Cells were then washed twice with PBS and cultured for 14 days.
Cells were fixed with formalin for 20 min and stained with crystal
violet for 15 min.
Apoptosis assay
Apoptosis was analyzed by flow cytometry (BD Accuri™
C6; BD Biosciences Franklin Lakes, NJ, USA). Briefly, cells were
harvested and washed twice with cold PBS. Collected cells were
stained with Annexin V-fluorescein isothiocyanate (FITC) and
propidium iodide (PI) and incubated at room temperature for 10
min.
Nuclear morphological observation
1×104 BEL7402 cells were seeded in 6-well
plates and treated with Vacq at 10 µM or Dox at 2 µM for 24 h,
respectively. Cells were stained with Hoechst 33342 for 30 min at
37°C. After staining, cells were washed with PBS for 3 times and
observed with Leica fluorescent microscope (DMi8; Leica
Microsystems, Inc., Buffalo Grove, IL, USA).
Western blot analysis
BEL7402 cells were seeded in 6-well culture plates
with the density at 3×105 cells/well. Cells were exposed
to 10 µM Vacq for 24 h and then processed for western blot analysis
as previously described (4). The
primary antibodies were used at 1:1,000 dilution and incubated at
4°C for overnight, and subsequently incubated with secondary
antibodies (Goat anti-mouse IgG-HRP, 1:10,000; and Goat anti-Rabbit
IgG-HRP, 1:15,000) for 2 h at room temperature. Immunodetection was
performed using Supersignal West Pico plus (Thermo Fisher
Scientific, Inc.) and detected by BIO-RAD GelDoc XR+ (Bio-Rad,
Berkeley, CA, USA).
Bax translocation
BEL7402 cells were exposed to 10 µM Vacq for
different durations (0, 1, 3, 6, 9, 12 h). Cytosolic and
mitochondrial proteins were extracted by using Cytoplasmic and
Mitochondrial Protein Extraction kit (Sangon Biotech, Shanghai,
China) according to the manufacturer's instructions. Mitochondrial
and cytosolic Bax expressions were detected by western blot
analysis. COX IV and β-Actin were used as mitochondrial and
cytosolic loading control, respectively.
Primary HCC cell isolation
Fresh liver cancer specimen obtained from surgery
was finely minced and digested with cell dispersion enzyme solution
(EZ; Nitta Gelatin Inc., Osaka, Japan) for 30 min. T-he dissociated
cancer cells were collected and filtered through an 80 µm nylon
mesh. Primary cells were cultured in a collagen gel coated flask
(CG-flask; Nitta Gelatin Inc.) in DMEM/F12 (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum at 37°C
5% CO2 for 24 h. Only the viable cells adhering to the
collagen gel were collected for the following use.
Primary HCC cell sensitivity test
One part of the primary HCC cells was used for cell
viability test monitored by xCELLigence RTCA. The rest part of the
cells was performed using collagen gel droplet-embedded CD-DST
followed by the standard procedure (5). Briefly, HCC cells were cultured in
the collagen droplet which provided a 3-D environment for HCC
cells. 15 µM Vacq was used for treatment. After 24 h, medium
containing Vacq was removed and changed to the prepared culture
media 2 (PCM-2; Kurabo, Oosaka, Japan) without FBS for 7 days.
Viable cells were stained with neutral red, fixed with 10% neutral
buffered formalin, washed in water, air dried, and quantified by
optical density image analysis using the Primage System (Solution
Systems, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed using one-way
analysis of variance and SPSS version 21 software (IBM Corp.,
Armonk, NY, USA). Multiple comparisons between treatment groups and
controls were evaluated using Dunnett's least significant
difference test. P<0.05 was considered to indicate a
statistically significant difference. All experiments were carried
out in triplicate in three independent experiments.
Results
Vacq suppresses human HCC cell
proliferation and colony formation
To determine the effect of Vacq on HCC cell
viability, BEL7402 and Huh7 cells were seeded in E-Plate 96, and
cell proliferation was dynamically monitored by RTCA system for 72
h. DMSO-treated HCC cells were used as controls. As shown in
Fig. 1A, HCC cell proliferation
was inhibited in a dose dependent manner. The IC50 of
Vacq for BEL7402 at 24, 48 and 72 h was 12.35, 11.48 and 10.21 µM
and for Huh7 cells was 6.21, 5.58 and 4.89 µM respectively. Next,
to investigate the inhibitory effect of Vacq on HCC cellular growth
potential, colony formation assay was performed. BEL7402 and Huh7
cells were exposed to Vacq at different concentrations for 24 h and
then cultured in complete medium for 14 days. As depicted in
Fig. 1B, the clonogenic abilities
of HCC cells were significantly inhibited by Vacq. Together, these
results indicated that Vacq suppresses human HCC cell viability
in vitro.
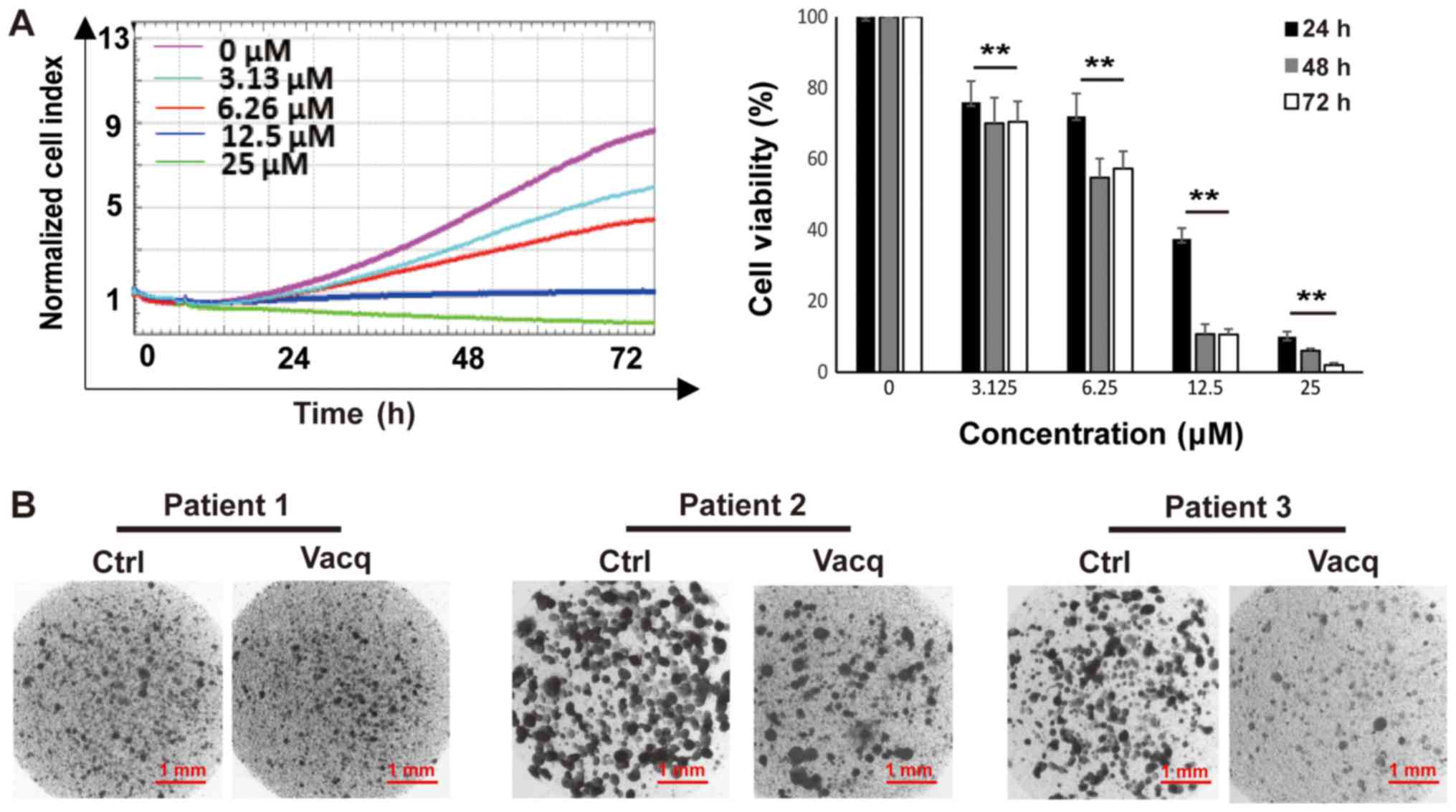
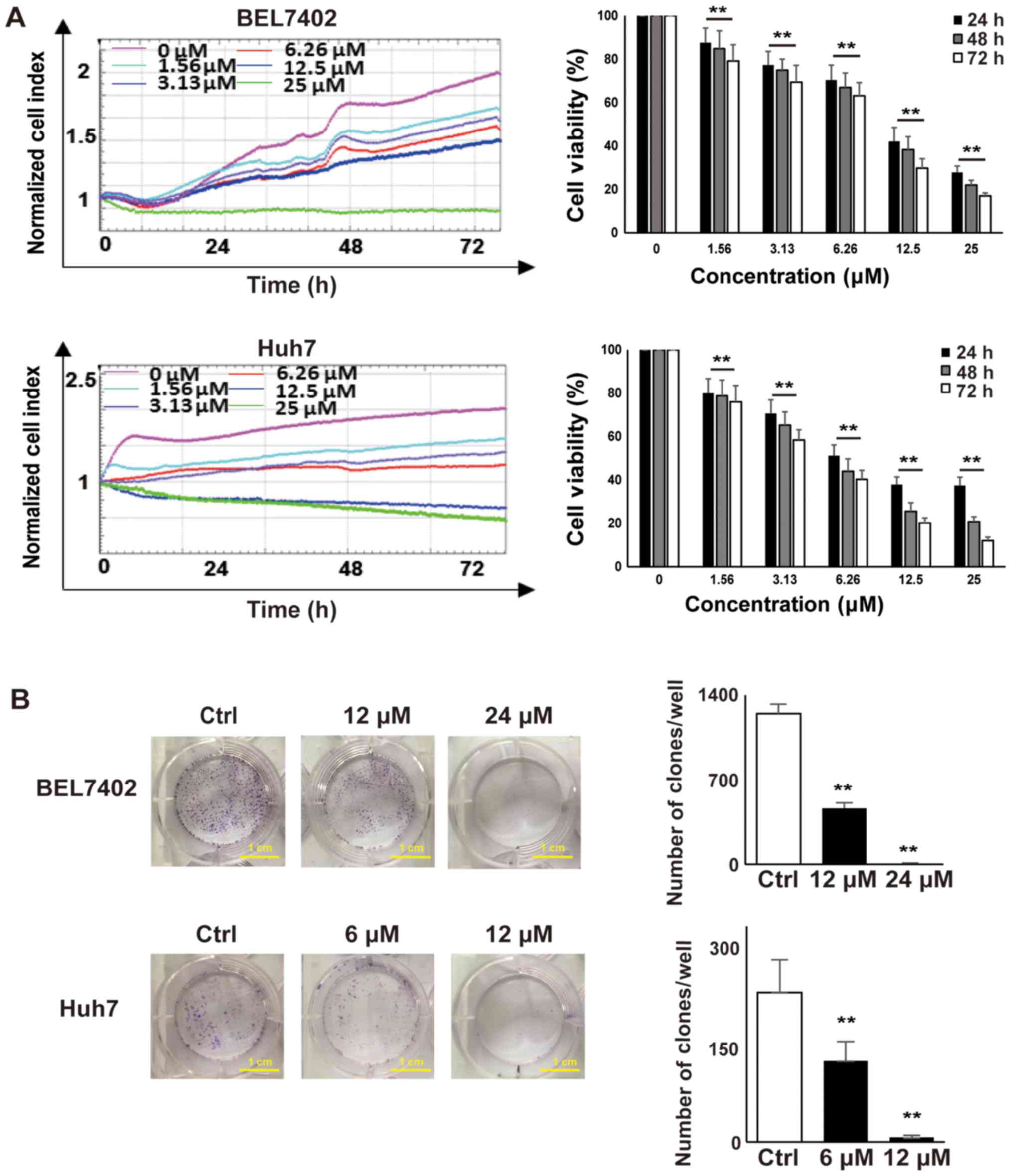 | Figure 1.Vacq suppressed human HCC cell
proliferation and colony formation. (A) 2×104 BEL7402
and Huh7 cells were seeded in an E-plate and treated with different
concentrations of Vacq (0, 1.56, 3.13, 6.25, 12.5 and 25 µM) for 72
h. Cell proliferation rates were monitored every 1 min and the
results were quantified by xCELLigence RTCA system. All of the
values were normalized to the cell indexes at the beginning of Vacq
treatment and were presented as the fraction relative to cell
indexes from the control group (set as 100%). (B) BEL7402 cells
were exposed to Vacq at 12 and 24 µM respectively and Huh7 cells
were at 6 and 12 µM for 24 h, and cultured in complete medium for
14 days. The number of the colonies was counted (scale bars, 1 cm).
Three independent experiments were performed. Data are presented as
mean ± standard error mean. **P<0.01 vs. control/as indicated.
HCC, hepatocellular carcinoma; Vacq, Vacquinol-1; RTCA, Real-Time
Cell Analyzer; Ctrl, control. |
Vacq induces human HCC cell
apoptosis
To determine whether the inhibitory effect by Vacq
on human HCC cells was due to apoptosis, BEL7402 cells were exposed
to Vacq for 24 h and then analyzed using an Annexin V/PI double
staining assay. DMSO-treated HCC cells were used as negative
controls. Doxorubicin (Dox) was reported to induce HCC cell
apoptosis (6). Therefore, 2 µM
Dox-treated cells were used as positive controls. As presented in
Fig. 2A, the number of cells
positive for either Annexin V+ PI− (early
apoptosis) or Annexin V+ PI+ (late apoptosis)
was increased in Vacq-treated cells compared to DMSO-treated cells,
suggesting that Vacq induces human HCC cell apoptosis. The
apoptotic effect induced by Vacq in HCC cells was further assessed
with Hoechst 33342 staining by fluorescence microscopy. As depicted
in Fig. 2B, nuclear fragmentations
and apoptotic bodies were observed in Vacq-treated HCC cells
whereas the control cells displayed round and normal nuclei. To
examine whether Vacq-initiated apoptosis is caspase-dependent, HCC
cells were pretreated with a broad specificity caspase inhibitor,
Z-VAD-FMK, and apoptosis was analyzed by flow cytometry.
Pretreatment with Z-VAD-FMK substantially attenuated the reduction
of cell viability compared to cells treated with Vacq alone
(Fig. 2C). Fig. 2D shows that apoptosis was inhibited
in Z-VAD-FMK pre-treatment group compared with the group exposure
to Vacq alone. Altogether, these results suggested that Vacq
induces apoptosis in HCC cells.
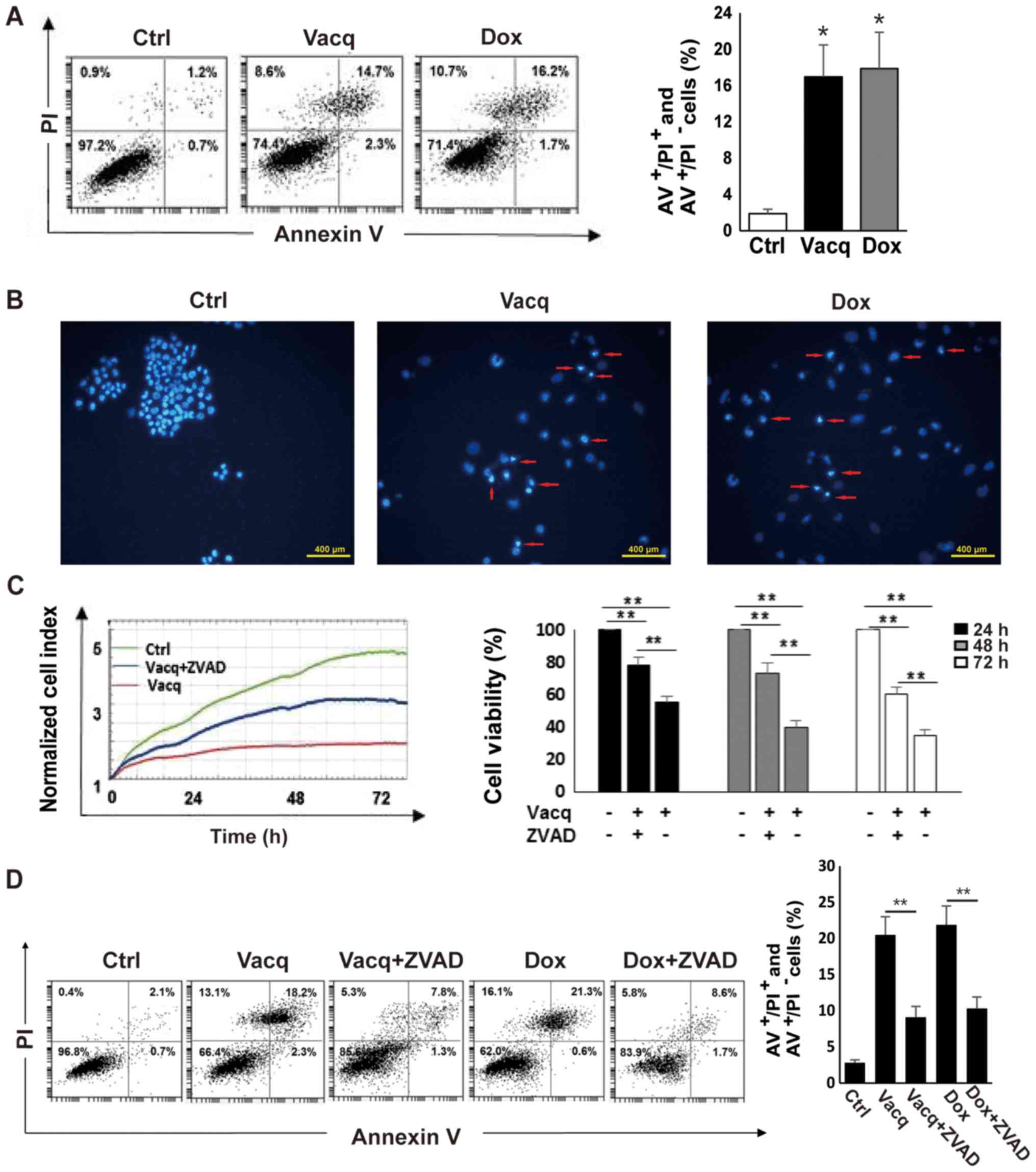 | Figure 2.Vacq induces human HCC cell apoptosis.
(A) BEL7402 cells were treated with Vacq at 10 µM. Following 24 h,
HCC cells were double stained with Annexin V and PI. A total of 2
µM Dox-treated cells were used as positive controls. Apoptotic
cells corresponding to Annexin+ PI−/+ were
quantified by flow cytometry. *P<0.05 vs. Ctrl. (B) A total of
10 µM Vacq was used to treat the BEL7402 cells for 24 h. HCC cells
were stained with Hoechst 33342 and observed by fluorescence
microscope. The red arrows indicate nuclear morphologies (scale
bars, 400 µm). BEL7402 cells were exposed to Vacq at 10 µM in the
presence or absence of 50 µM ZVAD. (C) Cell viabilities were
monitored by the xCELLigence RTCA system for 72 h. (D) Following 24
h treatment, apoptosis was detected by flow cytometry. Three
independent experiments were performed. Data are presented as the
mean ± standard error mean. **P<0.01, as indicated. Vacq,
Vacquinol-1; Dox, Doxorubicin; AV, Annexin V; ZVAD, Z-VAD-FMK;
Ctrl, Control; HCC, hepatocellular carcinoma; PI, propidium
iodide. |
Vacq induces intrinsic apoptosis in
HCC
Data from Fig. 2
suggested that Vacq might induce capase-depedent apoptosis in HCC
cells. We then analyzed whether Vacq triggered caspase activation.
Caspases, along with the poly(ADP-ribose) polymerase-1 (PARP-1)
cleavage are characteristic features of apoptosis (7). Compared with the control groups,
robust accumulations of cleaved caspase-3, caspase-9 and PARP-1 but
not caspase-8 were observed in HCC cells exposure to Vacq. We
further investigated the expression levels of the apoptosis related
proteins in Vacq-treated HCC cells. Bcl-2 family which consist of
both anti-apoptotic and pro-apoptotic proteins involves in the
regulation of apoptotic cell death (8). Vacq exposure upregulated the
pro-apoptotic proteins Bax and Bim while it downregulated the
pro-survival protein Bcl-2. The same protein expression patterns
were observed in Dox-treatment groups which were used as positive
control (Fig. 3A).
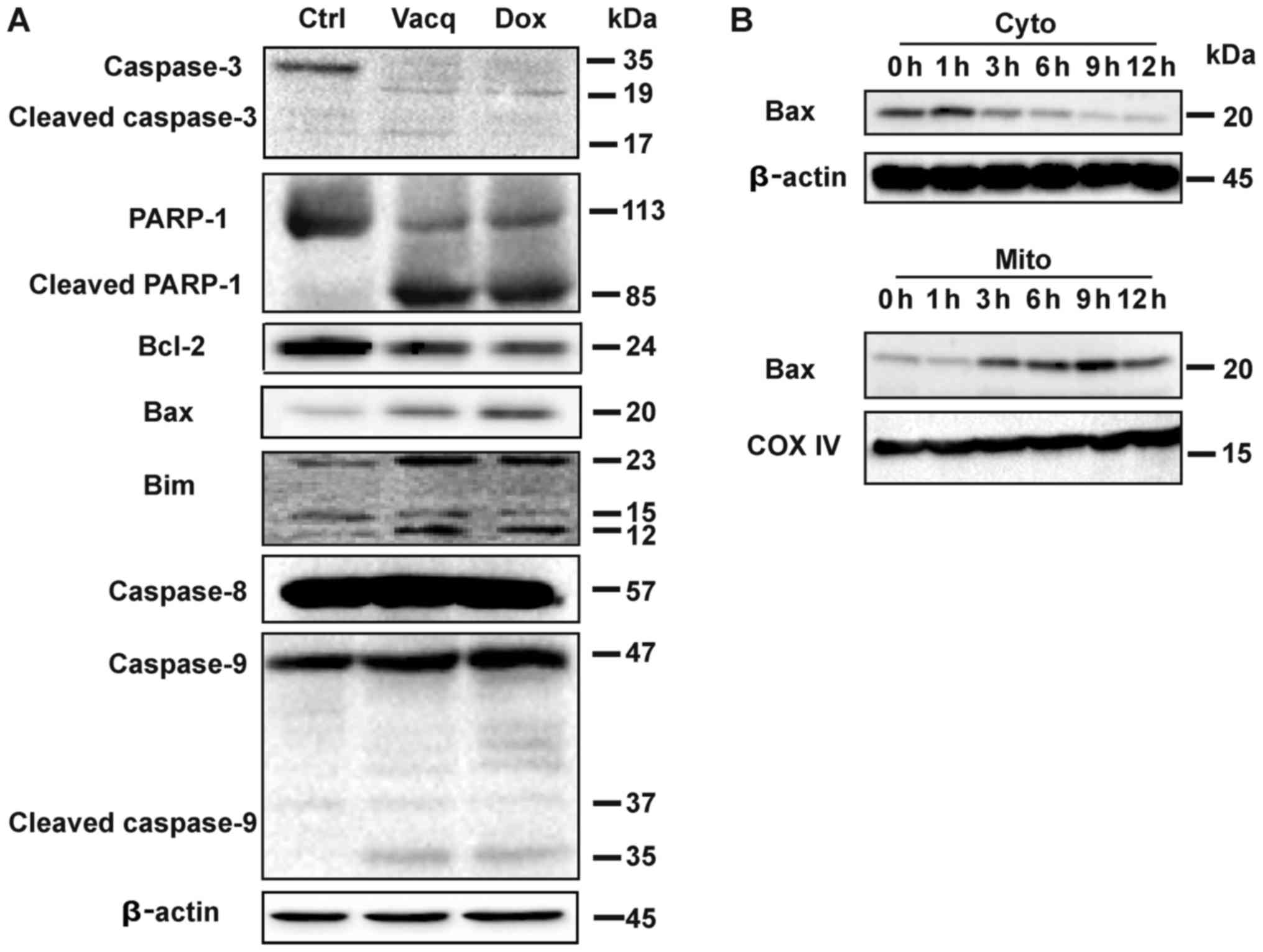 | Figure 3.Vacq induces intrinsic apoptosis in
HCC. (A) A total of 10 µM Vacq was used to treat the BEL7402 cells
for 24 h. Caspase-3, caspase-8, caspase-9, PARP-1, BCL-2, Bax and
Bim expression were detected by western blotting, using β-Actin as
the loading control. (B) BEL7402 cells were treated with Vacq for
the indicated time. The expression of cytosolic Bax and
mitochondrial Bax were detected by western blotting. COX-IV and
β-Actin were used as mitochondria and cytosol loading controls,
respectively. Three independent experiments were performed. Vacq,
Vacquinol-1; HCC, hepatocellular carcinoma; Dox, Doxorubicin; Ctrl,
Control; PARP-1, poly(adenosine diphosphate-ribose) polymerase-1;
BCL-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; Bim,
Bcl-2-like protein 11; Cyto, cytosol; Mito, mitochondria. |
Bax protein was reported to translocate from the
cell cytosol to the mitochondria during intrinsic apoptosis
(9). To investigate whether Vacq
induces Bax mitochondrial redistribution, Bel7402 cells were
treated with Vacq for different durations, and the expressions of
Bax proteins in cytosol and mitochondria fractions were assessed by
western blot, respectively. As depicted in Fig. 3B, Vacq induced the increase of
mitochondria Bax expression, combined with the significant
depletion of Bax in the cytosol compartment. Taken together, these
results indicated that Vacq induced intrinsic apoptosis in HCC.
Vacq inhibited patient-derived HCC
cells proliferation
To evaluate the clinical translational significance
of Vacq, we examined the antitumor effects of Vacq in
patient-derived HCC cells. Cells from 10 different individuals were
exposed to Vacq for 72 h at 3.13, 6.25, 12.5 and 25 µM,
respectively. DMSO was used as control. As shown in Fig. 4A, cell growth was inhibited by Vacq
in a dose-dependent manner. The average IC50 of these 10
individuals at 24, 48 and 72 h were 10.7, 8.9, 7.4 µM,
respectively. The sensitivities of these cells to Vacq were further
assessed by collagen gel droplet embedded CD-DST, an in
vitro anticancer drug sensitivity test in which the reported
clinical correlation was approximately 91% in several solid cancers
(10,11). Patient-derived HCC cells were
packaged into the collagen droplets to provide a 3-D culture
environment and treated with Vacq at 15 µM for 24 h. Viable cells
were deeply stained by neural red which presented in dark color in
Fig. 4B. Three results shown in
Fig. 4B. were represented to the
patients who were insensitive (patient 1) or sensitive (patient 2
and 3) to Vacq. In sensitive group, compared with DMSO treatment,
Vacq-treated cells were substantially stained, which related to the
inhibitory effect of Vacq in patient tumor-derived HCC cells.
Discussion
Vacq has been reported to induce the death of human
malignant glioma cells without affecting normal cells (3,12).
We here demonstrate that Vacq triggers apoptosis in both HCC cell
lines as well as in HCC patients-derived primary hepatoma cells. To
the best of our knowledge, this study has been the first to
identify that Vacq inhibits HCC cell growth. Thus, our data may
shed new lights into chemotherapy of HCC patients.
Previous study showed that Vacq induces GCs massive
macropinocytic vacuolization which was a novel form of cell death
termed as methuosis (13). Massive
macropinocytic vacuole accumulation leads to the rupture of GC
cytoplasmic membrane and cell death. Despite of caspase-3/7
activation, the GC death triggered by Vacq treatment was
morphologically distinct from apoptosis. Moreover, pretreatment
with caspase inhibitor zVAD-FMK only delayed the Vacq-induced GC
cell death and 100% cell death occurred later (12). These data suggest that Vacq induced
non-apoptotic cell death in GC. In addition, sensitivity to
macropinocytosis is a unique cellular property of GCs (14,15).
Therefore, the antitumor mechanism of vacq on other cancers needs
to be further explored.
In the present study, we show that Vacq induces
human HCC cell death which may be mediated through apoptosis. Flow
cytometry analysis revealed that Vacq increased both Annexin
V+ PI− and Annexin V+
PI+ population cells whereas the caspase inhibitor
Z-VAD-FMK efficiently decreased the apoptosis cells, suggesting
induction of apoptosis in Vacq-treated HCC cells. This notion was
further supported by the morphological observation of the nuclear
fragment formation in HCC cells exposure to Vacq. In addition, Vacq
treatment activated both caspase-3, caspase-9 and PARP-1 cleavage,
upregulated the expressions of Bim and downregulated pro-survival
protein Bcl-2 expression in HCC cells. Furthermore, Vacq treatment
induced Bax translocation to mitochondria. Taken together, Vacq
induces HCC cell death in an intrinsic apoptosis-dependent manner,
which is distinct from the massive macropinocytic vacuolization in
Vacq-treated glioma cells. Further studies necessary to elucidate
the exact underlying mechanism of the antitumor effects of Vacq on
HCC.
Notably, we also demonstrate that Vacq suppressed
patient-derived HCC cell growth in both two-dimensional (2D) and
three-dimensional (3D) cultures, suggesting of clinical
translational significance for the treatment of HCC. Further study
should include toxicity studies on a broad range of normal human
liver cells to confirm the selectivity of Vacq towards HCC cells.
In conclusion, the results of the present study provide preliminary
evidence that Vacq displays potent inhibitory effects on HCC cell
growth. Our data warrant further investigation of Vacq as a novel
antitumor compound for the treatment of HCC.
Acknowledgements
The authors would like to thank Dr Songshu Meng
(Institute of Cancer Stem Cell, Dalian Medical University Cancer
Center, Dalian, China) for the technical advice.
Funding
The present study was supported by Liaoning
Provincial Major Projects for Clinical Capacity-Building (grant no.
LNCCC-B04-2015) and Liaoning Province Ph.D. Research Start-up Fund
Project (grant no. 201501109).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SLS and HP conceived and designed the study. SLS was
a major contributor in writing the manuscript. XL analyzed the
data. NS and SC performed the experiments, including cell
viability, colony formation and nuclear morphological observation.
XG performed western blot analysis. GZ conducted the primary HCC
cell sensitivity test. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical
Ethical Committee of the Liaoning Cancer Hospital (China; Ethics
Review Approval no. 20160213-3). Written informed consent was
provided by all of the participants.
Consent for publication
All patients, or their parent, guardian or next of
kin, provided written informed consent for the publication of any
associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Vacq
|
Vacquinol-1
|
|
HCC
|
Hepatocellular carcinoma
|
|
GS
|
glioblastomas cell
|
|
DMSO
|
dimethyl sulfoxide
|
|
FITC
|
fluorescein isothiocyanate
|
|
PI
|
propidium iodide
|
|
CD-DST
|
collagen gel droplet-embedded culture
drug sensitivity test
|
|
Dox
|
Doxorubicin
|
|
PARP-1
|
poly(adenosine diphosphate-ribose)
polymerase-1
|
References
|
1
|
Karagonlar Firtina Z, Koc D, Iscan E,
Erdal E and Atabey N: Elevated hepatocyte growth factor expression
as an autocrine c-Met activation mechanism in acquired resistance
to sorafenib in hepatocellular carcinoma cells. Cancer Sci.
107:407–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Villanueva A, Hernandez-Gea V and Llovet
JM: Medical therapies for hepatocellular carcinoma: A critical view
of the evidence. Nat Rev Gastroenterol Hepatol. 10:34–42. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sander P, Mostafa H, Soboh A, Schneider
JM, Pala A, Baron AK, Moepps B, Wirtz CR, Georgieff M and Schneider
M: Vacquinol-1 inducible cell death in glioblastoma multiforme is
counter regulated by TRPM7 activity induced by exogenous ATP.
Oncotarget. 8:35124–35137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang X, Sun D, Tai J and Wang L: Ganoderic
acid A inhibits proliferation and invasion, and promotes apoptosis
in human hepatocellular carcinoma cells. Mol Med Rep. 16:3894–3900.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Naitoh H, Yamamoto H, Murata S, Kobayashi
H, Inoue K and Tani T: Stratified phase II trial to establish the
usefulness of the collagen gel droplet embedded culture-drug
sensitivity test (CD-DST) for advanced gastric cancer. Gastric
Cancer. 17:630–637. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Ma L, Tang X, Zhang X, Qiao Y, Shi
Y, Xu Y, Wang Z, Yu Y and Sun F: Doxorubicin induces apoptosis by
targeting Madcam1 and AKT and inhibiting protein translation
initiation in hepatocellular carcinoma cells. Oncotarget.
6:24075–24091. 2015.PubMed/NCBI
|
|
7
|
Los M, Mozoluk M, Ferrari D, Stepczynska
A, Stroh C, Renz A, Herceg Z, Wang ZQ and Schulze-Osthoff K:
Activation and caspase-mediated inhibition of PARP: A molecular
switch between fibroblast necrosis and apoptosis in death receptor
signaling. Mol Biol Cell. 13:978–988. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gillies LA and Kuwana T: Apoptosis
regulation at the mitochondrial outer membrane. J Cell Biochem.
115:632–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kobayashi H, Tanisaka K, Doi O, Kodama K,
Higashiyama M, Nakagawa H, Miyake M, Taki T, Hara S, Yasutomi M, et
al: An in vitro chemosensitivity test for solid human tumors
using collagen gel droplet embedded cultures. Int J Oncol.
11:449–455. 1997.PubMed/NCBI
|
|
11
|
Miyazaki R, Anayama T, Hirohashi K, Okada
H, Kume M and Orihashi K: In vitro drug sensitivity tests to
predict molecular target drug responses in surgically resected lung
cancer. PLoS One. 11:e01526652016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hammarström LG, Harmel RK, Granath M,
Ringom R, Gravenfors Y, Färnegårdh K, Svensson PH, Wennman D,
Lundin G, Roddis Y, et al: The oncolytic efficacy and in vivo
Pharmacokinetics of
[2-(4-Chlorophenyl)quinolin-4-yl](piperidine-2-yl)methanol
(Vacquinol-1) are governed by distinct stereochemical features. J
Med Chem. 59:8577–8592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Overmeyer JH, Young AM, Bhanot H and
Maltese WA: A chalcone-related small molecule that induces
methuosis, a novel form of non-apoptotic cell death, in
glioblastoma cells. Mol Cancer. 10:692011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi Y, Lim SK and Parada LF: The soft
underbelly of tumor cells. Cell Res. 24:910–911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lim JP and Gleeson PA: Macropinocytosis:
An endocytic pathway for internalising large gulps. Immunol Cell
Biol. 89:836–843. 2011. View Article : Google Scholar : PubMed/NCBI
|