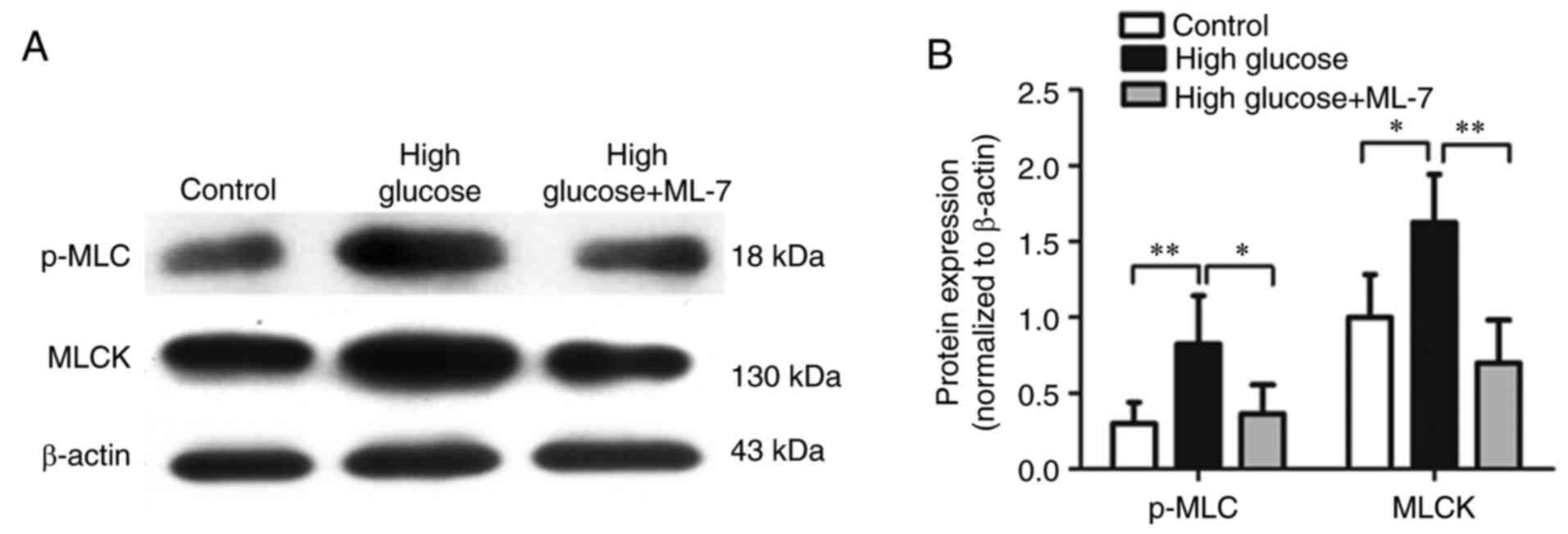
|
1
|
Duelli R, Maurer MH, Staudt R, Heiland S,
Duembgen L and Kuschinsky W: Increased cerebral glucose utilization
and decreased glucose transporter Glut1 during chronic
hyperglycemia in rat brain. Brain Res. 858:338–347. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malone JI, Hanna S, Saporta S, Mervis RF,
Park CR, Chong L and Diamond DM: Hyperglycemia not hypoglycemia
alters neuronal dendrites and impairs spatial memory. Pediatr
Diabetes. 9:531–539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ahtiluoto S, Polvikoski T, Peltonen M,
Solomon A, Tuomilehto J, Winblad B, Sulkava R and Kivipelto M:
Diabetes, Alzheimer disease, and vascular dementia: A
population-based neuropathologic study. Neurology. 75:1195–1202.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Summers WK: Alzheimer's disease, oxidative
injury, and cytokines. J Alzheimers Dis. 6:651–657; discussion
673–681. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pasquier F, Boulogne A, Leys D and
Fontaine P: Diabetes mellitus and dementia. Diabetes Metab.
32:403–414. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee A, Fischer RS and Fowler VM:
Stabilization and remodeling of the membrane skeleton during lens
fiber cell differentiation and maturation. Dev Dyn. 217:257–270.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Pan W, Yang GZ, Di YN, Zhao F, Zhu
LY and Jiang ZH: Proteome analysis of differential protein
expression in brain of rats with type 1 diabetes mellitus. Exp Clin
Endocrinol Diabetes. 119:265–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Tian Q, Zhang Q, Zhou X, Liu S
and Wang JZ: Hyperphosphorylation of microtubule-associated tau
protein plays dual role in neurodegeneration and neuroprotection.
Pathophysiology. 16:311–316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kruger L and Mandelkow EM: Tau
neurotoxicity and rescue in animal models of human tauopathies.
Curr Opin Neurobiol. 36:52–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Santacruz K, Lewis J, Spires T, Paulson J,
Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan
E, et al: Tau suppression in a neurodegenerative mouse model
improves memory function. Science. 309:476–481. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McLean WG, Pekiner C, Cullum NA and Casson
IF: Posttranslational modifications of nerve cytoskeletal proteins
in experimental diabetes. Mol Neurobiol. 6:225–237. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J and Dong XP: Dysfunction of
microtubule-associated proteins of MAP2/tau family in prion
disease. Prion. 6:334–338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Liang L, Zhan L, Zhou Y, Zheng L,
Sun X, Gong J, Sui H, Jiang R, Zhang F and Zhang L: ZiBuPiYin
recipe protects db/db mice from diabetes-associated cognitive
decline through improving multiple pathological changes. PLoS One.
9:e916802014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan Q, Zhan L, Zhou QY, Zhang LL, Chen
XM, Hu XM and Yuan XC: SIRT2 regulates microtubule stabilization in
diabetic cardiomyopathy. Eur J Pharmacol. 764:554–561. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stull JT, Kamm KE and Vandenboom R: Myosin
light chain kinase and the role of myosin light chain
phosphorylation in skeletal muscle. Arch Biochem Biophys.
510:120–128. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao N, Tsai MH, Chang AN, He W, Chen CP,
Zhu M, Kamm KE and Stull JT: Physiological vs. pharmacological
signalling to myosin phosphorylation in airway smooth muscle. J
Physiol. 595:6231–6247. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khapchaev AY and Shirinsky VP: Myosin
light chain kinase MYLK1: Anatomy, interactions, functions, and
regulation. Biochemistry (Mosc). 81:1676–1697. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martinsen A, Dessy C and Morel N:
Regulation of calcium channels in smooth muscle: New insights into
the role of myosin light chain kinase. Channels (Austin).
8:402–413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wilcox KS, Buchhalter J and Dichter MA:
Properties of inhibitory and excitatory synapses between
hippocampal neurons in very low density cultures. Synapse.
18:128–151. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gaspar JM, Castilho A, Baptista FI,
Liberal J and Ambrosio AF: Long-term exposure to high glucose
induces changes in the content and distribution of some exocytotic
proteins in cultured hippocampal neurons. Neuroscience.
171:981–992. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pardal R and Barneo Lopez J: Mature
neurons modulate neurogenesis through chemical signals acting on
neural stem cells. Dev Growth Differ. 58:456–462. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yarden-Rabinowitz Y and Yarom Y: In vivo
analysis of synaptic activity in cerebellar nuclei neurons unravels
the efficacy of excitatory inputs. J Physiol. 595:5945–5963. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fifkova E: Actin in the nervous system.
Brain Res. 356:187–215. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He Q and Roblodowski C: Functional
analysis of actin-binding proteins in the central nervous system of
drosophila. Methods Mol Biol. 1365:349–355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu L, Luo M, Yang B, Wu X, Zhu W, Guan Y,
Cai W, Troidl K, Schaper W and Schaper J: Actin-binding rho
activating protein is expressed in the central nervous system of
normal adult rats. Neural Regen Res. 7:965–970. 2012.PubMed/NCBI
|
|
26
|
Broschat KO, Stidwill RP and Burgess DR:
Phosphorylation controls brush border motility by regulating myosin
structure and association with the cytoskeleton. Cell. 35:561–571.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bresnick AR: Molecular mechanisms of
nonmuscle myosin-II regulation. Curr Opin Cell Biol. 11:26–33.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aromolaran AS, Albert AP and Large WA:
Evidence for myosin light chain kinase mediating
noradrenaline-evoked cation current in rabbit portal vein myocytes.
J Physiol. 3:853–863. 2000. View Article : Google Scholar
|
|
29
|
Yang X, Wang JG, Ma DB, Ma XF, Zhu GJ,
Zhou H, Yu CJ, Qian XY and Gao X: Myosin light chain kinase
regulates hearing in mice by influencing the F-actin cytoskeleton
of outer hair cells and cochleae. Int J Mol Med. 33:905–912. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mochida S, Kobayashi H, Matsuda Y, Yuda Y,
Muramoto K and Nonomura Y: Myosin II is involved in transmitter
release at synapses formed between rat sympathetic neurons in
culture. Neuron. 13:1131–1142. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee JS, Kim MH, Ho WK and Lee SH:
Presynaptic release probability and readily releasable pool size
are regulated by two independent mechanisms during posttetanic
potentiation at the calyx of Held synapse. J Neurosci.
28:7945–7953. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peng A, Rotman Z, Deng PY and Klyachko VA:
Differential motion dynamics of synaptic vesicles undergoing
spontaneous and activity-evoked endocytosis. Neuron. 73:1108–1115.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Garcia-Morales V, Montero F,
Gonzalez-Forero D, Rodriguez-Bey G, Gomez-Perez L,
Medialdea-Wandossell MJ, Dominguez-Vias G, Garcia-Verdugo JM and
Moreno-Lopez B: Membrane-derived phospholipids control synaptic
neurotransmission and plasticity. PLoS Biol. 13:e10021532015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Seabrooke S and Stewart BA: Synaptic
transmission and plasticity are modulated by nonmuscle myosin ii at
the neuromuscular junction of drosophila. J Neurophysiol.
105:1966–1976. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li L, Wu X, Yue HY, Zhu YC and Xu J:
Myosin light chain kinase facilitates endocytosis of synaptic
vesicles at hippocampal boutons. J Neurochem. 138:60–73. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Somlyo AP and Somlyo AV: Ca2+ sensitivity
of smooth muscle and nonmuscle myosin II: Modulated by G proteins,
kinases, and myosin phosphatase. Physiol Rev. 83:1325–1358. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Feng, He Yu, Liu Mi, Chang Xiao, Ren
Zhijing, Pan Wei and Li Xing: The establishment of the diabetic rat
model with cognitive dysfunction and the observation of cognitive
dysfunction in the diabetic rats. Chongqing Medicine. 16:2234–2236.
2015. View Article : Google Scholar
|
|
38
|
He Y, Wang F, Chen S, Liu M, Pan W and Li
X: The protective effect of radix polygoni multiflori on
diabetic encephalopathy via regulating myosin light chain kinase
expression. J Diabetes Res. 2015:4847212015. View Article : Google Scholar : PubMed/NCBI
|