Introduction
Nanoparticles (NPs) have been widely applied in the
field of healthcare owing to their unique physicochemical
characteristics including large surface-to-volume ratio and small
size (1,2). Superparamagnetic iron oxide
nanoparticles (SPIONs) have gained attention in medicine as a
result of their excellent magnetic properties and low cytotoxicity
(3). SPIONs are clinically
approved as magnetic resonance imaging contrast agents and have
also been used in hyperthermia treatment, chemotherapy and drug
delivery (4–7). The biomedical applications of NPs
require a complete understanding of their interaction with
biological systems. The cytotoxicity of SPIONs remains unclear. For
instance, carboxydextran-coated SPIONs did not affect the
proliferation and viability of murine macrophages, but
dextran-stabilized SPIONs could alter endothelial integrity and
function (8,9). Therefore, it is necessary to further
assess the biological toxicity of these NPs. The accumulation of
iron oxide NPs and release of free iron from the magnetite core may
damage iron homeostasis in cells, resulting in inflammation, DNA
damage or other toxin responses (10,11).
Furthermore, mitogen-activated protein kinases (MAPK) are induced
in cells following exposure to NPs (12).
It is well accepted that NPs can deliberately access
the vascular system via injection in the form of nano-medicine
(13). Monocytes are the portal of
NPs into the human body; they develop into macrophages or dendritic
cells and are also involved in various diseases and tissue
homeostasis (14). Although
certain studies have evaluated the toxic effects of iron oxide NPs
(15–17), the limited immune toxicity data and
its underlying signaling mechanism require further investigation.
It is preferable to select human primary cells for studying the
bio-safety of NPs, as they are closer to the in vivo
condition than non-human cells or immortalized cell lines (18). In the present study, therefore, the
effects of Dex-SPIONs on certain key activities of human primary
monocyte cells, including cell uptake, viability, pro-inflammatory
cytokines (IL-1β and TNF-α) and the potential signaling pathways
were focused on.
Materials and methods
Characterization of Dex-SPIONs
Dex-SPIONs were synthesized following a previously
described method (19). Briefly, a
mixture of 2.508 g dextran T70 (Mw: 70 kDa, Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) and 3.044 g iron (III) chloride
hexahydrate (Shanghai Aladdin Bio-Chem Technology Co., Ltd.,
Shanghai, China) was dissolved in 20 ml deionized water and placed
in a three-neck flask equipped with a mechanical stirrer. A freshly
prepared aqueous solution containing 1.27 g of ferrous chloride
tetrahydrate (Shanghai Aladdin Bio-Chem Technology Co., Ltd.)
dissolved in 2 ml deionized water was added to the mixture above.
While being rapidly stirred, 20 ml 7.5% ammonium hydroxide solution
was added into the mixture under argon protection. Subsequently,
the suspension was heated to 75°C and maintained at this
temperature for 30 min while stirring constantly. The black
suspension was cooled and centrifuged at a speed of 400 × g for 15
min at 4°C to separate large particles. The ammonium chloride along
with excess ammonia and dextran was removed by dialysis using a
citrate buffer (0.01 mol/l; Shanghai Aladdin Bio-Chem Technology
Co., Ltd.). Characterization of Dex-SPIONs was performed using TEM
(Jeol2100f; JEOL, Ltd., Tokyo, Japan). Dynamic light scattering was
used to analyze the ζ-potential and hydrodynamic size (Zeta Sizer,
Malven Nano ZS90; Malvern Panalytical Ltd., Malvern, UK). The
initial concentration of the NPs was determined by quantitative
prussian blue assay.
Isolation of human monocyte cells
The present study was approved by the Institutional
Review Board of the West China Second University Hospital of
Sichuan University (Chengdu, China). Peripheral blood (20 ml)
mononuclear cells were obtained from human buffy coat residues
(Chengdu Blood Center, Chengdu, China) using gradient
centrifugation (400 × g for 30 min at 16°C). Written informed
consent was obtained from all patients (n=15; 7 females and 8
males; age, 38±3 years) and samples were collected between October
and December 2016). Human monocyte cells [Cluster of
differentiation (CD)14+ cells] were purified using
magnetic beads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany)
according to the manufacturer's protocol. Cells with >98%
viability were obtained and the purity was 95–98% (data not shown),
verified respectively by cytospin preparations and flow cytometry
(BD Biosciences, Franklin Lakes, NJ, USA), as previously described
(20).
Cell culture
Monocytes were seeded in 24-well plates in RPMI 1640
culture medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% (v/v) heat-inactivated
fetal calf serum (FCS; Invitrogen; Thermo Fisher Scientific, Inc.)
and antibiotics (1% solution of penicillin 100 µg/ml and
streptomycin 100 µg/ml; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and incubated at 37°C, 5% (v/v) CO2,
and 95% relative humidity for 1 h (to allow enough time for
attachment of monocytes). The medium was discarded following
adhesion of the cells to the plate. Dex-SPIONs were mixed with RPMI
1640 medium #at a concentration of 13.2 mg/ml to form the stock
concentration from which the working concentration was derived.
Subsequently, the cells were incubated with Dex-SPIONs (20 or 100
µg/ml) in fresh pre-warmed medium for 24 h.
Transmission electron microscopy
(TEM)
Following 24 h of incubation, cells were collected
for TEM analysis as previously described (21). Briefly, monocyte cells were washed
thrice with PBS and fixed with 2.5% glutaraldehyde (Sigma-Aldrich;
Merck KGaA) in PBS at 4°C overnight. The fixed cells were
dehydrated and subsequently embedded with a layer of Spurr epoxy
resin at 60°C for 24 h (Polysciences Inc., Warrington, PA, USA).
Ultrathin sections (60–90 nm) were collected on 200 mesh copper
grids prior to staining with uranyl acetate and lead citrate
(Polysciences, Inc.) for 30 min at room temperature. Samples were
analyzed by TEM (Hitachi, Ltd., Tokyo, Japan; H600IV). Micrographs
were processed using Adobe Photoshop CS5 software (Adobe Systems,
Inc., San Jose, CA, USA).
Neutral red assays
Cells were seeded into 96-well plates
(1.5×105 cells/well) and incubated with Dex-SPIONs
(10–100 µg/ml) for 24 h at 37°C. Subsequently, the cells were
treated with Neutral Red Cell Proliferation and Cytotoxicology
Assay kit (Beyotime Institute of Biotechnology, Shanghai, China)
following the manufacturer's protocol and the absorbance was
measured at a wavelength of 540 nm using microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Flow cytometry
Cells were collected and washed with PBS.
Subsequently, cell suspensions (5×105 cells) were
stained with anti-CD14 (1:100) in 100 µl PBS with 5% FCS (cat no.
301829; Biolegend Inc., San Diego, CA, USA) for 30 min at room
temperature. An annexin V-fluorescein isothiocyanate/propidium
iodide apoptosis detection kit (Beijing Solarbio Science &
Technology, Co., Ltd., Beijing, China) was used according to the
manufacturer's protocol. Cells were subsequently analyzed within 1
h by flow cytometry (BD Biosciences). Flow cytometric data were
processed using FlowJo v.8.5.2 (Flowjo, LLC, Ashland, OR).
Quantification of cytokines
Cell supernatants were recovered following 24 h of
incubation. The levels of interleukin (IL)-1β (cat. no. P01584) and
tumor necrosis factor (TNF)-α (cat. no. P01375) were evaluated
using commercially available human ELISA kits (RayBiotech, Inc.,
Norcross, GA, USA) according to the manufacturer's protocol and the
absorbance was measured at 450 nm using a microplate reader
(Bio-Rad Laboratories, Inc.). Lipopolysaccharide (LPS; 1 µg/ml;
Merck KGaA) was used as a positive control.
To further assess the effects of Dex-SPIONs on the
MAPK pathway, cells were incubated with the MAPK inhibitors SB
203580 (for p38; Tocris Bioscience, Bristol, UK), SP 600125 (for
JNK; Absin Bioscience Inc., Shanghai, China) and PD98059 [for
extracellular regulated kinase (ERK)1/2; Cell Signaling Technology,
Inc., Danvers, MA, USA; 10 µM in all cases] for 1 h at 37°C prior
to the addition of Dex-SPIONs. The cells were cultured for 24 h at
37°C and cell supernatants were collected to analyze cytokine
concentrations.
Western blot analysis
Cells were seeded in 24-well plates
(1.2×106 cells/well) in the presence of Dex-SPIONs (100
µg/ml) for 24 h. Cells were harvested, pelleted (centrifugation at
300 × g for 5 min at room temperature) and lysed by adding
Western-IP lysis buffer (Beyotime Institute of Biotechnology).
Protein concentrations were determined with a bicinchoninic acid
protein assay. An equal amount of protein (35 µg) for each sample
was subjected to SDS-PAGE (10% and 12% separation gels) and
transferred onto polyvinylidene fluoride (PVDF) membranes.
Membranes were subsequently blocked with 5% non-fat dry milk for 1
h at room temperature and incubated with anti-GAPDH (1:1,000; cat.
no. 97166; CST Biological Reagents Co., Ltd., Shanghai, China),
anti-p38 (1:1,000; cat. no. 9212; CST Biological Reagents Co.,
Ltd.), anti-phosphorylated (p)-p38 (1:1,000; cat. no. 9211; CST
Biological Reagents Co., Ltd.), anti-JNK (1:1,000; cat. no. 9252;
CST Biological Reagents Co., Ltd.), anti-p-c-Jun N-terminal kinase
1 (JNK; 1:1,000; cat. no. 9251; CST Biological Reagents Co., Ltd.),
anti-ERK (1:1,000; cat. no. 4695S; CST Biological Reagents Co.,
Ltd.) and anti-p-ERK (1:1,000; cat. no. 9101S; CST Biological
Reagents Co., Ltd.) overnight at 4°C. The PVDF membranes were
incubated with an appropriate horseradish peroxidase-conjugated
secondary antibody (1:5,000; cat. no. AP132P; Merck KGaA) for 1 h
at room temperature, then protein bands were visualized with a
super signal chemiluminescence (ECL) kit (Beyotime Institute of
Biotechnology). The densitometric analysis of western blot was
performed using Chemi-Doc (Quantity One 4.6.8; Bio-Rad
Laboratories, Inc.).
Statistical analysis
Results are presented as the mean ± standard error
of mean and the data conformed to Gaussian distribution.
Statistical analyses were performed using SPSS 11.0 (SPSS Inc.,
Chicago, IL, USA). Significant differences were evaluated by
one-way analysis of variance followed by Dunnett's post-hoc test
(at least three experiments). P<0.05 was considered to indicate
a statistically significant difference.
Results
Characterization of Dex-SPIONs
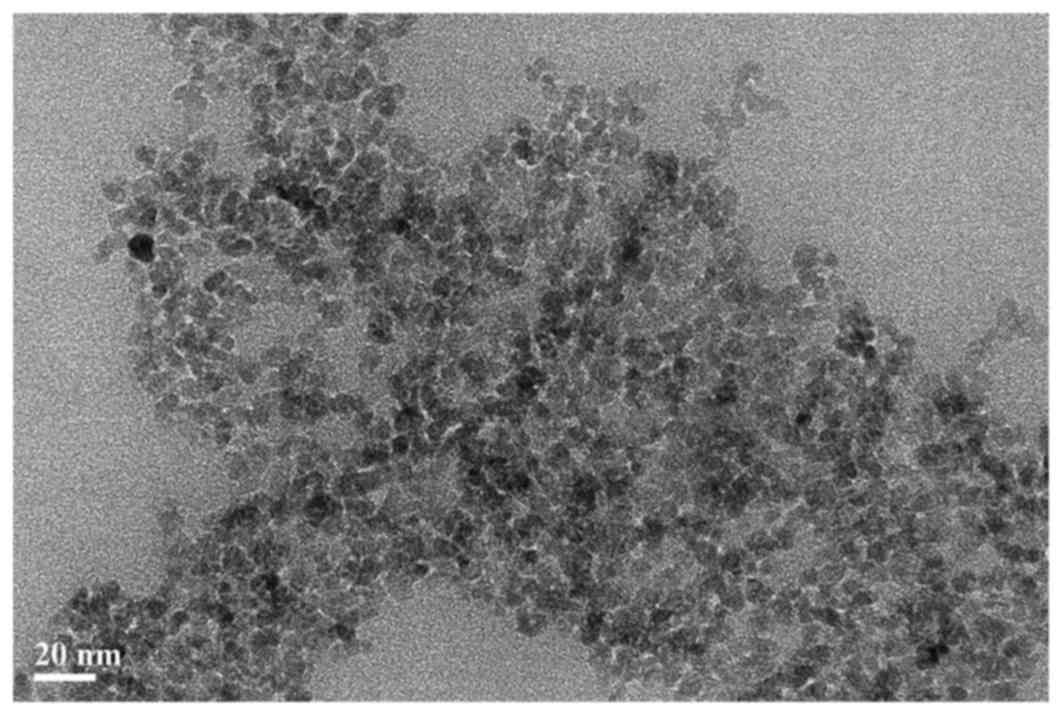
The size of the Dex-SPIONs were first characterized
prior to using them in the subsequent experiments. The mean sizes
of the NPs were calculated using Adobe Photoshop CS5 following
appropriate calibration. Dynamic light scattering was also used to
analyze the ζ-potential and hydrodynamic size. As demonstrated in
Fig. 1, Dex-SPIONs were observed
to be an average of 7 nm in diameter and demonstrated to be
spherical in shape. Furthermore, Dex-SPIONs exhibited a negative
potential (−11 mV) in cell culture medium and the hydrodynamic size
was ~63 nm (Table I). SPIONs are
divided into three main categories based on their hydrodynamic
diameter; this NP belongs to the standard size SPIONs, which have
an overall diameter of 50–150 nm (22).
 | Table I.Characterization of the particle
parameters of Dex-SPION. |
Table I.
Characterization of the particle
parameters of Dex-SPION.
| Nanoparticle | Hydrodynamic size
(nm) | ζ-potential
(mV) | Initial
concentration (mg/ml) |
|---|
| Dex-SPION | 62.8 | −11 | 13.2 |
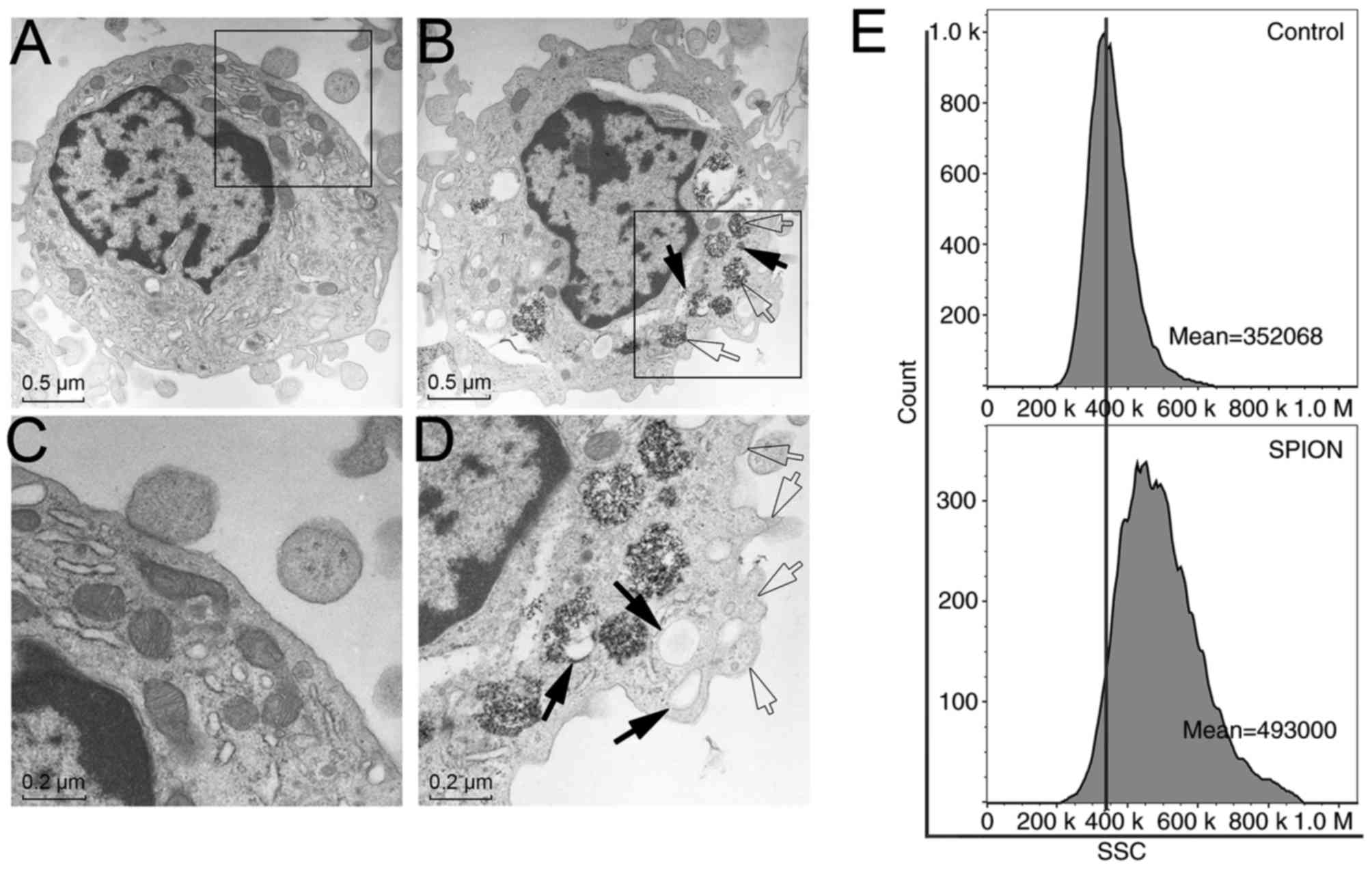
Cellular uptake and localization of
Dex-SPIONs
To follow the intracellular localization and
internalization of Dex-SPIONs, TEM was used to observe the cellular
response (Fig. 2). Monocytes
treated with Dex-SPIONs (100 µg/ml) exhibited vacuoles with highly
electron-dense Dex-SPIONs (Fig. 2B and
D) compared with the control group (Fig. 2A and C). Following careful
observation of the TEM images, Dex-SPIONs were demonstrated to
either be engulfed by monocytes in phagosomes or freed in the
cytoplasm (Fig. 2B). There were
bulky vacuoles present and a number of pseudopodia from the cell
membrane (Fig. 2D). Furthermore,
side-scatter light of flow cytometry representing cell granularity
was increased following Dex-SPIONs exposure and it also indicated
that Dex-SPIONs were phagocytosed by monocytes (Fig. 2E). These results definitively
demonstrated an interaction between these NPs and cells.
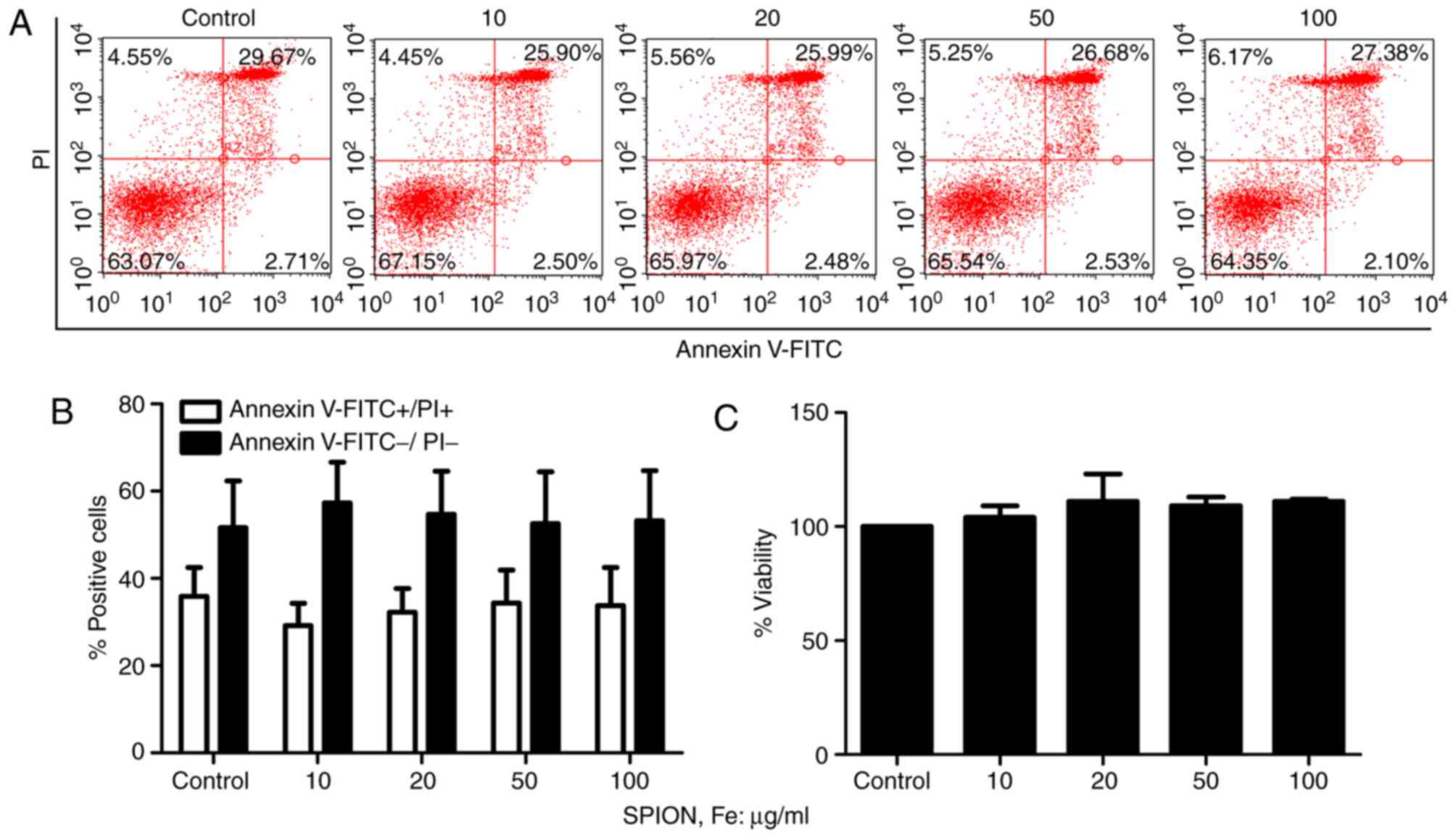
Effect of Dex-SPIONs on the cell
viability
In order to determine whether the Dex-SPIONs that
enter the cells evoke a cell viability response, the number of
Annexin V+/PI+ (apoptotic or dead) or Annexin
V−/PI− (live) cells following Dex-SPIONs
exposure at concentrations ranging from 10 to 100 µg/ml was
determined. It was demonstrated that Dex-SPIONs exhibited no
significant effects on cellular apoptosis and viability (Fig. 3A and B). In addition, cell
viability was further analyzed by neutral red assays. As
illustrated in Fig. 3C, Dex-SPIONs
did not influence the viability of cell even at 100 µg/ml compared
with the control group following 24 h of incubation.
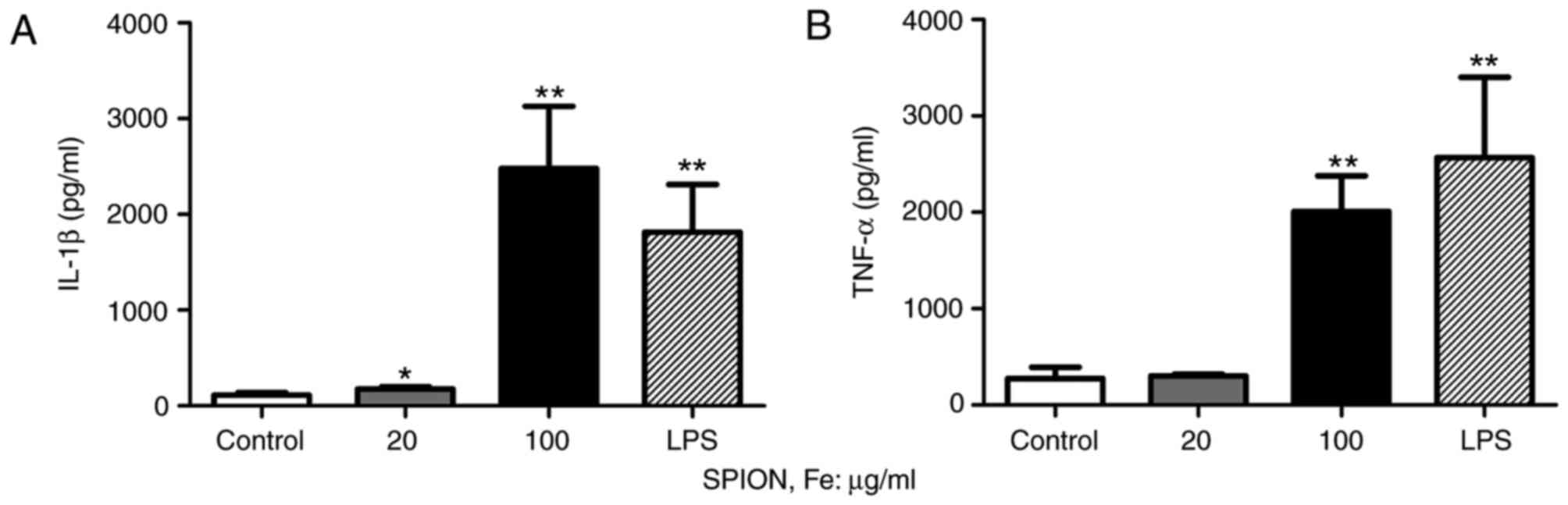
Effect of Dex-SPIONs on
pro-inflammatory cytokines production in human monocyte cells
To examine the activation of human monocyte cells,
an ELISA was used to analyze relevant parameters. The expression
level of IL-1β was significantly improved following Dex-SPIONs
treatment in a concentration-dependent manner (P<0.05 and
P<0.01; Fig. 4A). Dex-SPIONs
treatment significantly increased the production of TNF-α in the
group at 100 µg/ml (P<0.01; Fig.
4B). In addition, dextran did not affect the secretion of
cytokines in cells (data not shown). LPS (1 µg/ml) was used as a
positive control.
Effect of Dex-SPIONs on the MAPK
signaling pathway in human monocyte cells
To further investigate the underlying signaling
pathway of the activation of monocytes induced by Dex-SPIONs, MAPK
p38, JNK, ERK proteins and their respective inhibitors were used.
Dex-SPIONs treatment significantly increased the phosphorylation
levels of MAPK p38, JNK and ERK compared with the control group
(P<0.05; Fig. 5A-C). IL-1β
production only decreased significantly in the presence of SP
600125 (JNK inhibitor) or PD 98059 (ERK inhibitor; both P<0.01;
Fig. 5D). However, co-treatment of
Dex-SPIONs with inhibitors significantly reduced the expression
level of TNF-α (P<0.05; Fig.
5E). Additionally, the inhibitors themselves did not modify
cytokine expression in cells (data not shown).
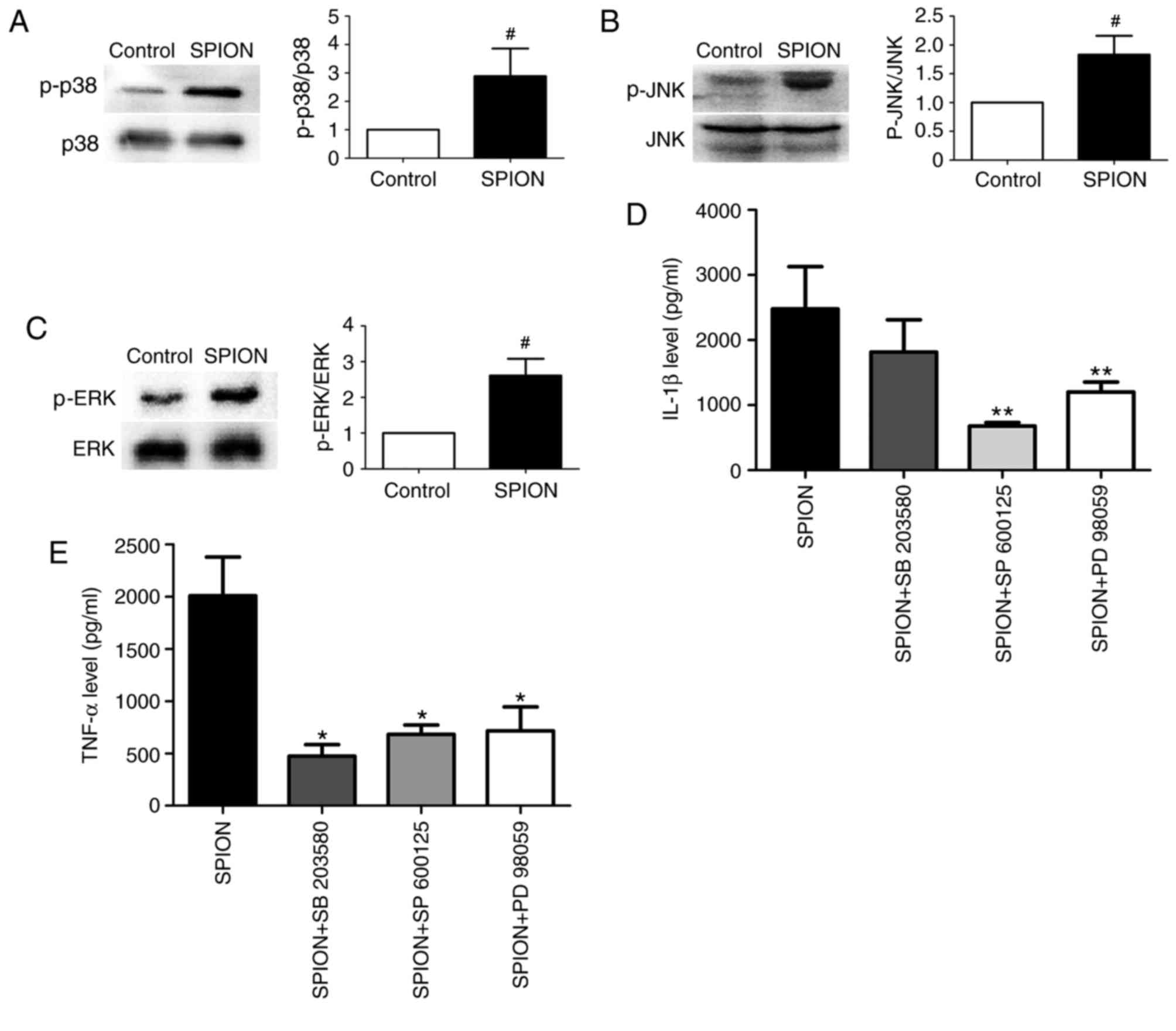 | Figure 5.Dex-SPION induced the activation of
MAPK p-p38, p-JNK and p-ERK in human monocyte cells. Monocytes were
incubated with Dex-SPION (100 µg/ml) for 24 h or cells were treated
with the MAPK inhibitors SB 203580 (for p38), SP 600125 (for JNK)
and PD98059 (for ERK1/2; 10 µM in all cases) for 1 h prior to the
addition of Dex-SPION. Cells were subsequently prepared for western
blotting and ELISA analyses. (A) p-p38/p38 expression. (B)
p-JNK/JNK expression. (C) p-ERK/ERK expression. (D) The expression
level of IL-1 β. (E) The expression level of TNF-α. Data were
presented as mean ± standard deviation, n=3. #P<0.05
vs. control; *P<0.05, **P<0.01 vs SPION. IL, interleukin;
TNF, tumor necrosis factor; ERK, extracellular signal regulated
kinase; p-JNK, phosphorylated c-Jun N-terminal kinase 1; MAPK,
mitogen-associated protein kinase; Dex-SPION, dextran
coated-superparamagnetic iron oxide nanoparticle. |
Discussion
SPIONs have enormous potential for biomedical
applications, but there remains a gap in the knowledge about their
reactions with the human immune system. In the present study,
Dex-SPIONs were demonstrated to be uptaken by human monocyte cells
and the cells were subsequently activated through the MAPK
signaling pathway. To a certain extent, the results of the present
study offered a better understanding and more convincing
experimental evidence for the issue of SPIONs safety.
Monocytes are crucial innate immune cells,
professional phagocytes whose main function is to recognize, ingest
and degrade foreign invaders including bacteria and NPs (23). The results of the present study
demonstrated that Dex-SPIONs were internalized by human monocyte
cells and located in phagosomes or freed in the cytoplasm, which is
similar to other studies (24,25).
Certain spherical vesicles resembling lysosomes fully packed with
Dex-SPIONs in the cytoplasm were observed. It has been demonstrated
that most endocytic routes of NPs are concentrated in the
lysosomes, which leads to lysosomes being the common site for
accumulation of NPs (26).
It is well documented that the cellular uptake of
NPs have a crucial role in influencing certain cellular functions,
including cell viability and inflammation (27). Whether SPIONs are cytotoxic or not
is controversial. Certain studies have demonstrated SPIONs to be
safe, while others have demonstrated SPIONs toxicity (18,28,29).
It may depend on the size of the NPs, any modifications or cell
types studied. Coating is vital to stabilize SPIONs as it can
decrease the toxicity of SPIONs by preventing aggregation and
leakage of free iron ions. Dextran has excellent biocompatibility
and has been used in the clinic for a long time, but it has been
reported to modify cell-cell interaction and cell viability
(30). However, the results of the
present study demonstrated that dextran had no impact on the
production of cytokines. Furthermore, Dex-SPIONs showed no
cytotoxicity and can't increase in apoptosis in human monocyte
cells. These results indicated that these NPs have a good
biocompatibility.
Bulky vacuoles and a number of pseudopodia of the
cell membrane were observed, indicating the potential activation of
human monocyte cells following Dex-SPIONs exposure. When undergoing
activation, monocytes can secrete various pro-inflammatory
cytokines, which have an important role in immune and inflammatory
responses. Of these cytokines, IL-1β and TNF-α are the commonly
used markers of monocytes activation (31). To further evaluate the activation
of cells, the expression levels of IL-1β and TNF-α were analyzed.
The production of IL-1β and TNF-α were enhanced following
Dex-SPIONs treatment. In one case, bare-iron oxide NPs increased
the secretion of TNF-α as well as the generation of reactive oxygen
species in RAW 264.7 cells (32).
It is noteworthy that cytokines, in particular pro-inflammatory
cytokines can be useful tools in evaluating immunotoxicity of NPs
(33). According to the literature
and the results of the present study, it was inferred that
Dex-SPIONs may induce immunotoxicity of monocytes by enhancing the
production of pro-inflammatory cytokines, but this conclusion
requires further testing in vivo.
p38, JNK and ERK, which are all the members of the
MAPK family, are involved in a number of functions, including
inflammation, cytokine production, transcriptional and apoptosis
(34). In order to prove the
potential signaling mechanism of cell activation, the
phosphorylation levels of p38, JNK and ERK were measured. It is
well documented that the MAPK signaling pathway can be induced in
response to a diverse range of extracellular stresses including
chemicals and cytokines (35,36).
Among these stresses, certain studies have reported that NPs can
induce MAPK pathway activation, such as ZnO and CuO (37,38).
Recently, Couto et al (39)
demonstrated that iron oxide NPs could cause cytokine secretion and
that the activation of MAPK p38 and JNK are involved in this
effect. In the present study, Dex-SPIONs were verified to increase
the expression levels of p-p38, p-JNK and p-ERK. Furthermore, MAPK
inhibitors also inhibited the production of pro-inflammatory
cytokines (IL-1β and TNF-α). Based on these results, it was
hypothesized that iron oxide NPs-caused inflammatory responses that
were associated with the MAPK signaling pathway. The data from the
present study demonstrated that there is an interaction between
human monocyte cells and Dex-SPIONs, and this should be considered
when deciding their various applications in the biomedical field.
Future studies in animal models should enhance understanding of the
health effects of these nanoparticles and may guide the risk
assessment of their applications.
In conclusion, it demonstrated that Dex-SPIONs were
engulfed by human monocyte cells and displayed the ability to
activate monocytes via the induction of the formation of
pseudopodia and the production of pro-inflammatory cytokines. In
addition, these inflammatory responses possibly depended on the
activation of MAPK signaling pathway.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the
National Natural Science Foundation of China (grant no. 81471848)
and the National Key Basic Research Program of Chain (grant no.
2013CB933903).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
QW designed the study and performed experiments. TM
and TF performed the western blot analysis. CY performed the flow
cytometry analysis. YG provided the nanoparticles, directed the
project and interpreted the data. HL supervised the project and
contributed to the study design. QW and YG wrote the paper. All
authors discussed the results and contributed to the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of the West China Second University Hospital of
Sichuan University (Chengdu, China). Written informed consent was
obtained.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gade A, Ingle A, Whiteley C and Rai M:
Mycogenic meta nanoparticles: Progress and application. Biothchnol
Lett. 32:593–600. 2010. View Article : Google Scholar
|
|
2
|
Jha RK, Jha PK, Chaudhury K, Rana SV and
Guha SK: An emerging interface between life science and
nanotechnology: Present status and prospects of reproductive
healthcare aide by nano-biotechnology. Nano Rev. 26:52014.
|
|
3
|
Jin R, Lin B, Li D and Ai H:
Superparamagnetic iron oxide nanoparticles for MR imaging and
therapy: Design considerations and clinical applications. Curr Opin
Pharmacol. 18:18–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hadjipanayis CG, Bonder MJ, Balakrishnan
S, Wang X, Mao H and Hadjipanayis GC: Metallic iron nanoparticles
for MRI contrast enhancement and local hyperthermia. Small.
4:1925–1929. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mahmoudi M, Hosseinkhani H, Hosseinkani M,
Boutry S, Simchi A, Journeay WS, Subramani K and Laurent S:
Magnetic resonance imaging tracking of stem cells in vivo using
iron oxide nanoparticles as a tool for the advancement of clinical
regenerative medicine. Chem Rev. 111:253–280. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mahmoudi M, Sant S, Wang B, Laurent S and
Sen T: Superparamagnetic iron oxide nanoparticles (SPIONs):
Development, surface modification and applications in chemotherapy.
Adv Drug Deliv Rev. 63:24–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun C, Lee JS and Zhang M: Magnetic
nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev.
60:1252–1265. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsiao JK, Chu HH, Wang YH, Lai CW, Chou
PT, Hsieh ST, Wang JL and Liu HM: Macrophage physiology function
after superparamagnetic iron oxide labeling. NMR Biomed.
21:820–829. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Astanina K, Simon Y, Cavelius C, Petry S,
Kraegeloh A and Kiemer AK: Superparamagnetic iron oxide
nanoparticles impair endothelial integrity and inhibit nitric oxide
production. Acta Biomater. 10:4896–4911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y and Wang J: Effects of DMSA-coated
Fe3O4 nanoparticles on the transcription of
gens related to iron and osmosis homeostasis. Toxicol Sci.
131:521–536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murray AR, Kisin E, Inman A, Young SH,
Muhammed M, Burks T, Uheida A, Tkach A, Waltz M, Castranova V, et
al: Oxidative stress and dermal toxicity of iron oxide
nanoparticles in vitro. Cell Biochem Biophys. 67:461–476. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Q, Chen B, Cao M, Sun J, Wu H, Zhao
P, Xing J, Yang Y, Zhang X, Ji M and Gu N: Response of MAPK pathway
to iron oxide nanoparticles in vitro treatment promotes osteogenic
differentiation of hBMSCs. Biomaterials. 86:11–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Donaldson K, Duffin R, Langrish JP, Miller
MR, Mills NL, Poland CA, Raftis J, Shah A, Shaw CA and Newby DE:
Nanoparticles and the cardiovascular system: A critical review.
Nanomedicine (Lond). 8:403–423. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Robbins CS and Swirski FK: The roles of
monocyte subsets in steady state and inflammation. Cell Mol Life
Sci. 67:2685–2693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Szalay B, Tátrai E, Nyírő G, Vezér T and
Dura G: Potential toxic effects of iron oxide nanoparticles in in
vivo and in vitro experiments. J Appl Toxicol. 32:446–453. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khan Ml, Mohammad A, Patil G, Nagvi SA,
Chauhan LK and Ahmad I: Induction ROS, mitochondrial damage and
autophagy in lung epithelial cancer cells by iron oxide
nanoparticles. Biomaterials. 33:1477–1488. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu X, Tan Y, Mao H and Zhang M: Toxic
effects of iron oxide nanoparticles on human umbilical vein
endothelial cells. Int J Nanomedicine. 5:385–399. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kunzmann A, Andersson B, Thurnherr T, Krug
H, Scheynius A and Fadeel B: Toxicology of engineered
nanomaterials: Focus on biocompatibility, biodistribution,
biodegradation. Biochim Biophys Acta. 1810:361–373. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hong RY, Feng B, Chen LL, Liu GH, Li HZ,
Zheng Y and Wei DG: Synthesis, characterization and MRI application
of dextran-coated Fe3O4 magnetic
nanoparticles. Bio Eng J. 42:290–300. 2008. View Article : Google Scholar
|
|
20
|
Xing Y and Hogguist KA: Isolation,
identification, and purification of murine thymic epithelial cells.
J Vis Exp. 90:e517802014.
|
|
21
|
Bachmann S, Schlichting U, Geist B, Mutig
K, Petsch T, Bacic D, Wagner CA, Kaissling B, Biber J, Murer H and
Willnow TE: Kidney-specific inactivation of the megalin gene
impairs trafficking of renal inorganic sodium phosphate
cotransporter (NaPi-lla). J Am Soc Nephrol. 15:892–900. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singh N, Jenkins GJ, Asadi R and Doak SH:
Potential toxicity of superparamagnetic iron oxide nanoparticles
(SPION). Nano Rev. 1:34022010. View Article : Google Scholar
|
|
23
|
Aderem A and Underhill DM: Mechanisms of
phagocytosis in macrophages. Annu Rev Immunol. 17:593–623. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Monteiro-Riviere NA, Inman AO and Zhang
LW: Limitations and relative utility of screening assays to assess
engineered nanoparticle toxicity in a human cell line. Toxicol Appl
Pharmacol. 234:222–235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Strehl C, Gaber T, Maurizi L, Hahne M,
Rauch R, Hoff P, Häupl T, Hofmann-Amtenbrink M, Poole AR, Hofmann H
and Buttgereit F: Effects of PVA coated nanoparticles on human
immune cells. Int J Nanomedicine. 10:3429–3445. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stern ST, Adiseshaiah PP and Crist RM:
Autophagy and lysosomal dysfunction as emerging mechanisms of
nanomaterial toxicity. Part Fibre Toxicol. 9:202012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oh WK, Kim S, Choi M, Kim C, Jeong YS, Cho
BR, Hahn JS and Jang J: Cellular uptake, cytotoxicity, and innate
immune response of silica-titania hollow nanoparticles based on
size and surface functionality. ACS Nano. 4:5301–5313. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thomsen LB, Linemann T, Pondman KM,
Lichota J, Kim KS, Pieters RJ, Visser GM and Moos T: Uptake and
transport of superparamagnetic iron oxide nanoparticles through
human brain capillary endothelial cells. ACS Chem Neurosci.
4:1352–1360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng M, Li H, Luo Z, Kong J, Wan Y, Zhang
Q, Niu H, Vermorken A, Ven de Ven W, Chen C, et al: Dectran-coated
superparamagnetic nanoparticles as potential cancer drug carriers
in vivo. Nanoscale. 7:11155–11162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rouleau L, Rossi J and Leask RL:
Concentration and time effects of dextran expouse on endothelial
cell viability, attachment, and inflammatory marker expression in
vitro. Ann Biomed Eng. 38:1451–1462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gordon S: Alternative activation of
macrophages. Nat Rev Immunol. 3:23–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park EJ, Umh HN, Kim SW, Cho MH, Kim JH
and Kim Y: ERK pathway is activated in bare-FeNPs-induced
autophagy. Arch Toxicol. 88:323–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Elsabahy M and Wooley KL: Cytokine as
biomarkers of nanoparticle immunotoxicity. Chem Soc Rev.
42:5552–5576. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dong C, Davis RJ and Flavell RA: MAP
kinases in immune response. Annu Rev Immunol. 20:55–72. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee IC, Ko JW, Lee SM, Kim SH, Shin IS,
Moon OS, Yoon WK, Kim HC and Kim JC: Time-course and molecular
mechanism of hepatotoxicity induced by 1,3-dichloro-2-propanol in
rats. Environ Toxicol Pharmacol. 40:191–198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zarubin T and Han J: Activation and
signaling of p38 MAP kinase pathway. Cell Res. 15:11–18. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ko JW, Park JW, Shin NR, Kim JH, Cho YK,
Shin DH, Kim JC, Lee IC, Oh SR, Ahn KS and Shin IS: Copper oxide
nanoparticle induces inflammatory response and mucus production via
MAPK signaling in human bronchial epithelial cells. Environ.
Toxicol Pharmacol. 43:21–26. 2016. View Article : Google Scholar
|
|
38
|
Yuan L, Wang Y, Wang J, Xiao H and Liu X:
Additive effects of zinc oxide nanoparticles and isoorientin on
apoptosis in human hepatoma cell line. Toxicol Lett. 225:294–304.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Couto D, Freitas M, Porto G,
Lopez-Quintela MA, Rivas J, Freitas P, Carvalho F and Fernandes E:
Polyacrylic acid-coated and non-coated iron oxide nanoparticles
induce cytokine activation in human blood cells through TAK1, p38
MAPK and JNK pro-inflammatory pathways. Arch Toxicol. 89:1759–1769.
2015. View Article : Google Scholar : PubMed/NCBI
|