Introduction
Osteoarthritis (OA) is the most prevalent form of
joint disease, with a high prevalence in the elderly, and an
increasing social and economic healthcare burden (1,2). The
etiology of OA is complex and has been associated with multiple
factors of mechanical stress, gender, age, environment and genes,
which may interact to cause its initiation and progression
(3–6). Its primary clinical symptoms are
joint pain and restricted movement, with the main pathologies of
synovitis, cartilage degeneration, subchondral bone sclerosis, and
osteophyte formation (7). The
pathogenesis of OA has been well reported and has been associated
with decreases in chondrocyte numbers and degradation of the
extracellular matrix (ECM) in cartilage (8). Matrix metalloproteinase-13 (MMP-13)
is one of the main enzymes responsible for ECM degradation
(9).
Vascular endothelial growth factor (VEGF) has
previously been reported to be expressed in osteoarthritic
articular cartilage (10–12); VEGF has the capacity to increase
expression levels of MMPs and to decrease expression levels of
their inhibitors (tissue inhibitors of metalloproteinases)
(13,14), VEGF appears to serve an important
role in the development of OA. In addition, a wide range of studies
suggest that increased VEGF expression levels are associated with
the progression of OA (15,16)
and the inhibition of VEGF expression may alleviate the development
of OA (17). Therefore,
downregulation of MMP-13 and VEGF expression levels may be
considered to be a promising therapy for OA.
Thalidomide, a drug first used to treat pregnant
women for nausea or vomiting, was retracted from administration due
to its teratogenic effects exerted on newborns (18). It was reported to be an angiogenic
inhibitor in 1994 (19) and its
first clinical success in treating multiple myeloma was reported in
1999 (20). Thalidomide was later
reported to decrease VEGF expression, at the mRNA and protein
levels (21); Mercurio et
al (22) proposed that
thalidomide may affect VEGF expression levels via
downregulation.
At present, thalidomide is used in erythema nodosum
leprosum, multiple myeloma, prostate cancer, glioblastoma, glioma,
renal cell carcinoma, colon cancer and advanced breast cancer,
diabetic retinopathy, rheumatoid arthritis and lupus, as well as in
other pathological conditions where inflammation and angiogenesis
co-exist (23–27).
To the best of our knowledge, the therapeutic
effects of thalidomide on OA in vivo have not been
investigated. Therefore, the present study utilized
surgically-induced OA mice to investigate the hypothesis that
thalidomide may attenuate the early development of OA by
suppressing VEGF expression.
Materials and methods
Reagents
Thalidomide, dimethylsulfoxide (DMSO), and a
Safranin-O/Fast Green Staining kit were obtained from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). Thalidomide was dissolved in DMSO
at 200 mg/ml as a 50 mM stock solution and then stored at 4°C; the
DMSO concentration was <0.1%. Primary rabbit polyclonal
antibodies against MMP-13 and VEGF were purchased from Abclonal
Biotech Co., Ltd. (Boston, MA, USA). Secondary anti-rabbit antibody
was purchased from Beijing Ray Antibody Biotechnology (Beijing,
China). Fluorescein isothiocyanate goat anti-rabbit immunoglobulin
G (IgG) antibody was purchased from OriGene Technologies, Inc.
(Beijing, China). A hematoxylin and eosin (HE) staining kit, a
diaminobenzidine tetrahydrochloride (DAB) kit, and a DAPI staining
kit were purchased from Beijing Solarbio Science & Technology
Co., Ltd. (Beijing, China). TRIzol total RNA reagent,
HiScript® II Q RT SuperMix for quantitative polymerase
chain reaction (qPCR; +gDNA wiper), and ChamQ™ SYBR®
qPCR Master Mix were purchased from Vazyme (Piscataway, NJ, USA).
An ELISA kit of VEGF (E-EL-M1292c) was purchased from Elabscience
Biotechnology Co., Ltd., Wuhan, China.
Mice model of OA
A total of 48 male C57BL/6 mice (10-weeks-old,
27.7±0.8 g) were purchased from the Laboratory Animal Center of Sun
Yat-Sen University (Guangzhou, China), were randomly divided into
experimental (Dmm+Th), control (Dmm), and Sham groups (n=16 in
each). The mice were housed under clean conditions with controlled
temperature (25±1°C), humidity (50±10%), and a 12 h light/dark
cycle, allowed free access to water and food, and received humane
care in the Laboratory Center of The Third Affiliated Hospital of
Southern Medical University (Guangzhou, China). In the Dmm+Th and
the Dmm groups, to induce experimental OA, each mouse was
anesthetized and then subjected to a destabilization of the medial
meniscus (DMM) procedure as previously described (28). In the Sham group, only the skin of
the right knee joint was resected. The skin of each mouse in the
three groups was then sutured in layers, and the mice were allowed
to move, eat and drink freely following surgery. All the surgical
procedures performed herein were performed under sterile conditions
and general anesthesia with 3% pentobarbital sodium (40 mg/kg) via
intraperitoneal injection. The Dmm+Th group was injected
intraperitoneally daily (8 mice for 2 weeks and another 8 mice for
4 weeks) with thalidomide (200 mg/kg body weight) the day following
surgery (29); the Dmm and Sham
groups were injected intraperitoneally daily with normal saline
according to the same standard. At 2 and 4 weeks following surgery,
8 mice of each group were first anesthetized to collect blood
samples from the heart vein via cardiac puncture approach and then
sacrificed to harvest the right knees for histological assessments
(n=4) and gene expression (n=4). All efforts were made to minimize
suffering. All animal experiments were approved by and conducted
according to the guidelines of The Third Affiliated Hospital of
Southern Medical University Animal Healthcare Ethics Committee
(Guangdong, China) (30).
Histological analysis
The specimens of the right knees were fixed in 4%
paraformaldehyde for 48 h at −4°C and then decalcified with 14.5%
EDTA at pH 7.3 for 3 weeks. Following embedding in paraffin, the
specimens were sectioned via the medial tibial plateau at a
thickness of 5 µm in the sagittal plane. Then, the sections were
dewaxed in xylene and hydrated with a graded ethanol series. To
assess cartilage destruction, staining with HE and Safranin O/Fast
Green staining was performed according to the manufacturer's
protocols. The hyaline cartilage/calcified cartilage (HC/CC) and
Osteoarthritis Research Society International (OARSI) cartilage OA
grading system (31) was employed
to determine the extent of cartilage degeneration. Image-Pro Plus
version 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA)
(32) was used to analyze the
HC/CC of the Safranin O/Fast Green-stained sections. A total of 3
different areas from the medial tibial plateau were randomly
selected to calculate and evaluate the OARSI score. The sections
were examined blindly by two musculoskeletal researchers
respectively, and the scores were averaged to minimize observer
bias. The OARSI score used for the present study was presented in
Table I.
 | Table I.Semi-quantitative analysis of the
extent of cartilage degeneration using the Osteoarthritis Research
Society International scoring system. |
Table I.
Semi-quantitative analysis of the
extent of cartilage degeneration using the Osteoarthritis Research
Society International scoring system.
| Grade | Osteoarthritic
damage |
|---|
| 0 | Normal |
| 0.5 | Loss of
proteoglycan with an intact surface |
| 1 | Superficial
fibrillation without loss of cartilage |
| 2 | Vertical clefts and
loss of surface lamina |
| 3 | Vertical
clefts/erosion to the calcified layer lesion for 1–25% of the
quadrant width |
| 4 | Lesion reaches the
calcified cartilage for 25–50% of the quadrant width |
| 5 | Lesion reaches the
calcified cartilage for 50–75% of the quadrant width |
| 6 | Lesion reaches the
calcified cartilage for >75% of the quadrant width |
Reverse transcription (RT)-qPCR
RT-qPCR was employed to quantify the mRNA expression
levels of MMP-13 and VEGF in the medial articular cartilage. At
weeks 2 and 4, a total of four mice in each group were sacrificed
to harvest the right knees, which were then stored at −80°C until
use. The frozen samples constituted collected articular cartilage
from the medial tibial plateau, as published previously (33) and crushed in a mortar under liquid
nitrogen. Subsequently total RNA extraction from articular
cartilage from the medial tibial plateau was performed using
TRIzol® (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's protocols to extract total
RNA. The concentration and purity of total RNA were determined with
a NanoDrop spectrophotometer (NanoDrop; Thermo Fisher Scientific,
Inc., Wilmington, DE, USA); total RNA was reversed transcribed into
cDNA using HiScript® II Q RT SuperMix for qPCR(+gDNA
wiper) according to the manufacturer's protocols. Subsequently,
qPCR was performed in triplicate using ChamQ™ SYBR qPCR Master Mix
according to the manufacturer's protocols. Relative gene expression
levels were calculated using the 2−ΔΔCq method (34). Target mRNA expression levels were
normalized to the reference gene GADPH, which served as an internal
control. The specific primer sequences (Sangon Biotech, Co., Ltd.,
Shanghai, China) employed in the present study were listed in
Table II.
 | Table II.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table II.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer | Reverse primer |
|---|
| MMP-13 |
5′-CTTCTTCTTGTTGAGCTGGACTC-3′ |
5′-CTGTGGAGGTCACTGTAGACT-3′ |
| VEGF |
5′-CTGCCGTCCGATTGAGACC-3′ |
5′-CCCCTCCTTGTACCACTGTC-3′ |
| GAPDH |
5′-AGGTCGGTGTGAACGGATTTG-3′ |
5′-TGTAGACCATGTAGTTGAGGTCA-3′ |
Immunohistochemistry (IHC) and
immunofluorescence (IF)
In the articular cartilage of the medial tibial
plateau, IHC was used to locate the protein expression of VEGF; IF
was employed to locate the protein expression of MMP-13, which was
conducted to detect cartilage lesions. For IHC, 5-µm
paraffin-embedded sections were dewaxed and hydrated using a graded
ethanol gradient, and incubated with rabbit polyclonal antibody
anti-VEGF (A12303) at dilution of 1:100 at 4°C overnight. Following
staining with Goat anti-Rabbit IgG(H+L)-HRP secondary antibody of
anti-VEGF (RM3002) at dilution of 1:100 for 1 h at room
temperature, DAB was used for 2 min at room temperature, followed
by counterstaining with hematoxylin for 30 sec at room temperature.
For IF, the sections were prepared and incubated with rabbit
polyclonal antibody anti-MMP-13 (A1606) at dilution of 1:100 at 4°C
overnight. Then, the sections were incubated with
fluorescein-conjugated goat anti-rabbit IgG antibody (TA130022) at
dilution of 1:100 for 1 h at room temperature and were mounted in
medium containing DAPI for 10 min at room temperature. The sections
were viewed with a FluoView FV1000 confocal laser scanning
microscope (magnification, ×400; Olympus Corporation, Tokyo,
Japan). The percentage of positive cells were calculated in
arbitrary three slices.
ELISA
The VEGF expression levels in serum were measured
via ELISA. Immediately after collection, blood samples were allowed
to coagulate at 4°C overnight and were then centrifuged at 1,000 ×
g at room temperature for 20 min. The supernatant was collected and
stored at −80°C until use. The concentration of VEGF in serum of
the different groups were analyzed via ELISA, according to the
manufacturer's protocols. In order to calculate the concentration
of VEGF in serum, the standard curve was constructed by plotting
the mean absorbance (Y) of standards against the known
concentration (X) of standards in logarithmic scale, using the
four-parameter algorithm.
Statistical analysis
All experiments were performed independently at
least three times. All quantitative data were expressed as the mean
± standard deviation. For three group comparisons, one-way analysis
of variance followed by Tukey's post-hoc test was performed. All
statistical data were analyzed using SPSS software (version 19.0,
IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Thalidomide treatment may attenuate
the progression of OA in a mice model
As presented in Fig. 1A
and B respectively, HE and Safranin O/Fast Green staining
demonstrated within the Sham group the articular cartilages had
smooth and intact surface and almost no fibrillation was observed.
Conversely, in the Dmm group, articular cartilage was easily
observed with superficial fibrillation at 2 weeks post-surgery,
notable loss of proteoglycan, as well as vertical clefts and
erosion to the calcified layer lesion at 4 weeks post-surgery.
Compared with in the Dmm group, articular cartilage in the Dmm+Th
group had rough surface at 2 weeks post-surgery, with less
proteoglycan loss, cartilage fibrillation, and loss of surface
lamina at 4 weeks post-surgery. Additionally, compared with in the
Sham group, the hyaline cartilage layer appeared thinner and
aberrant subchondral bone structure in the Dmm group was observed,
while the hyaline cartilage layer relatively restored its normal
thickness in the Dmm+Th group at 4 weeks following surgery.
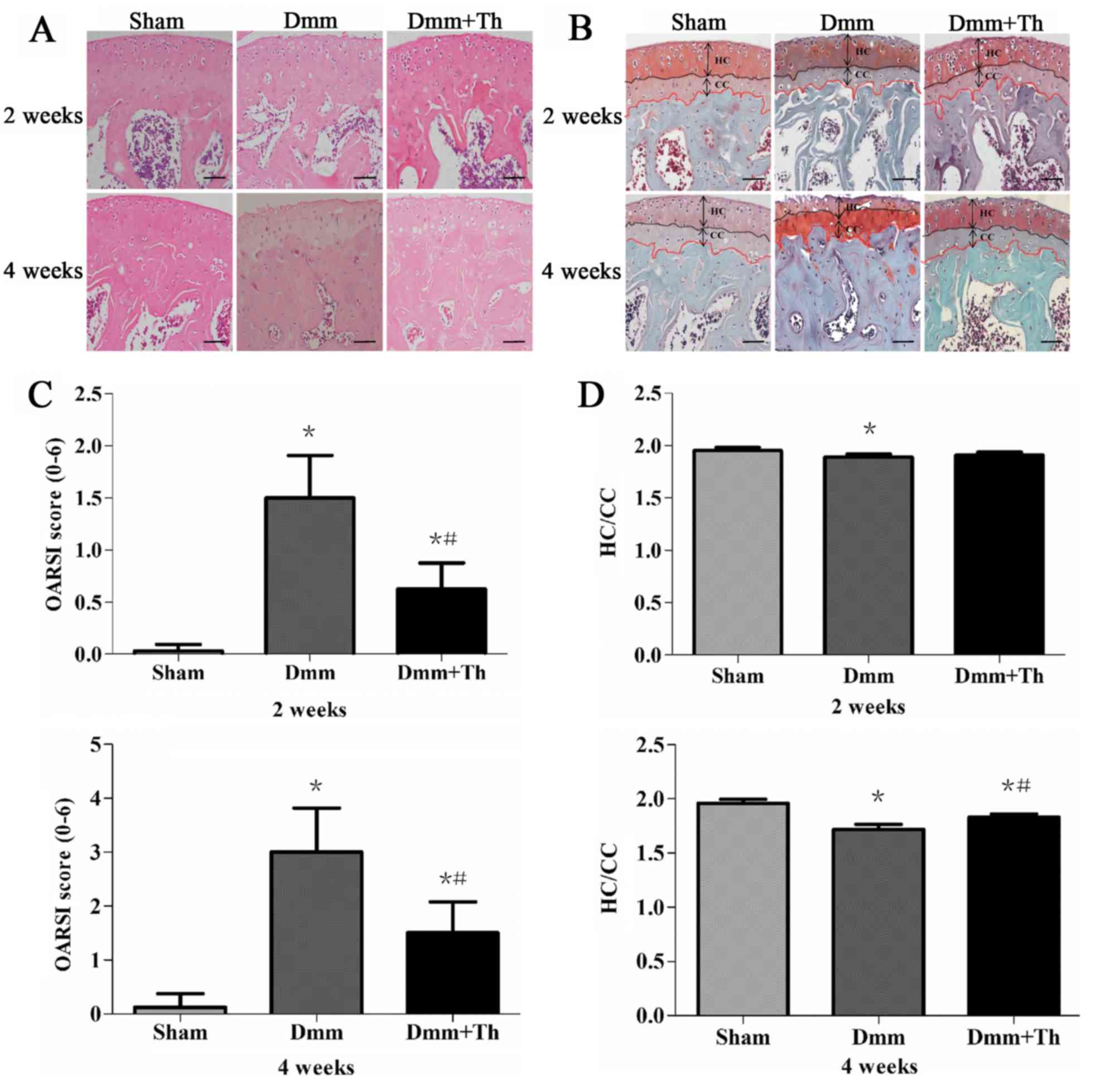 | Figure 1.Pathological alterations of knee
articular cartilage of mice among the Sham, Dmm and Dmm+Th groups
(n=4 in each group). (A) HE staining of the medial tibial plateau
(magnification, ×200, scale bar=50 µm). (B) Safranin O/Fast green
staining of the medial tibial plateau (magnification, ×200, scale
bar=50 µm). (C) Results of OARSI score of the medial tibial
plateau, based on the Safranin O/Fast green staining results. (D)
Results of HC/CC of the medial tibial plateau, based on the
Safranin O/Fast green staining results. The values are presented as
the mean ± standard deviation. *P<0.05 compared with the Sham
group; #P<0.05 compared with the Dmm group. CC,
calcified cartilage; Dmm, destabilization of the medial meniscus;
HC, hyaline cartilage; HE, hematoxylin and eosin; OARSI,
osteoarthritis research society international; Th, thalidomide. |
As presented in Fig.
1C, consistent with the results of Safranin O/Fast Green
staining, compared with the Sham group, the OARSI scores were
significantly higher in the Dmm group and the Dmm+Th group at 2 and
4 weeks post-surgery (P<0.05). However, the Dmm+Th group
revealed significantly lower OARSI scores than those of the Dmm
group at 2 and 4 weeks post-surgery (P<0.05).
As presented in Fig.
1D, HC/CC values in the Dmm group were slightly lower at 2
weeks post-surgery (P<0.05) and were significantly lower at 4
weeks post-surgery (P<0.05) compared with the Sham group. In
addition, the HC/CC value in the Dmm+Th group increased
significantly compared with the Dmm group at 4 weeks (P<0.05).
In addition, no significant difference between the Sham group and
the Dmm+Th group was observed at 2 weeks post-surgery
(P>0.05).
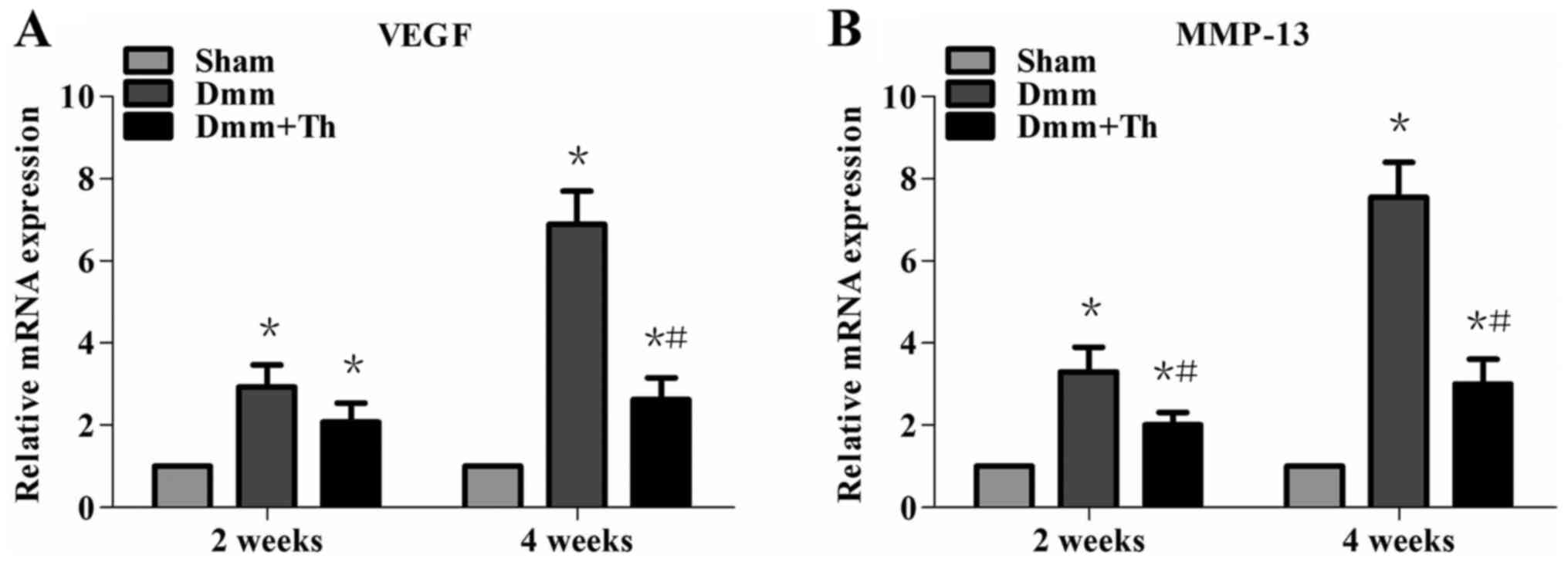
Thalidomide treatment downregulates
VEGF and MMP-13 mRNA expression levels in early OA mice
As presented in Fig. 2A
and B, the results obtained from RT-qPCR indicated that the
mRNA expression levels of VEGF and MMP-13 in the Dmm and Dmm+Th
groups significantly increased with the development of OA compared
with in the Sham group at 2 and 4 weeks post-surgery (P<0.05).
Compared with the Dmm group, the mRNA expression levels of VEGF and
MMP-13 were significantly downregulated by varying degrees in the
Dmm+Th group at 2 and 4 weeks post-surgery (P<0.05), with the
exception of VEGF mRNA expression levels between the Dmm+Th group
and the Dmm group at 2 weeks post-surgery (P>0.05).
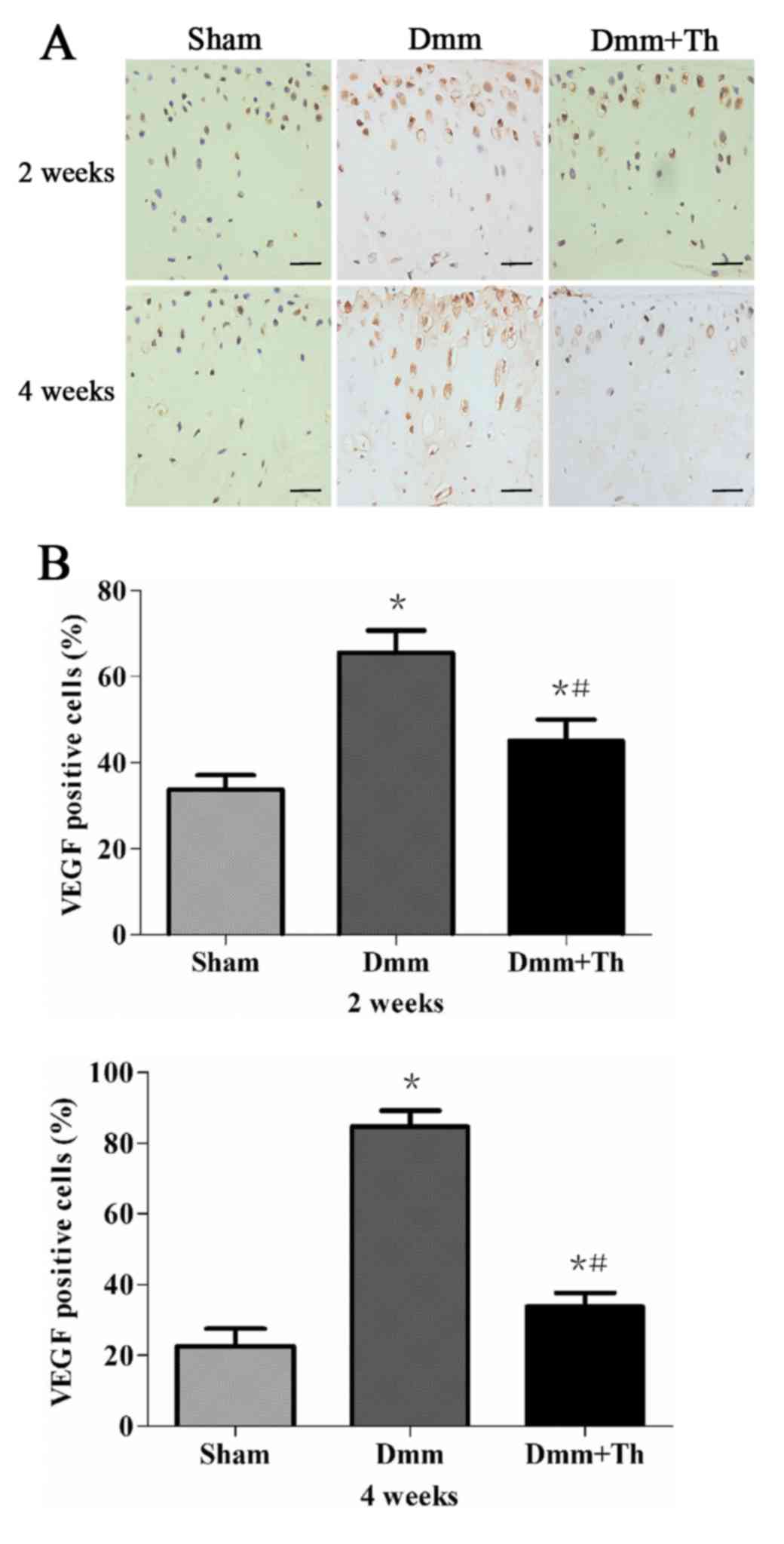
Thalidomide treatment inhibits VEGF
protein expression in early OA mice
As presented in Fig.
3A, immunohistochemical staining revealed that the
VEGF-positive cells were detected mainly in the superficial layers
of the articular cartilage among the three groups. Compared with in
the Sham group, the percentage of VEGF-positive cells increased as
OA progressed and were significantly higher in the Dmm group and
the Dmm+Th group at 2 and 4 weeks post-surgery (P<0.05; Fig. 3B). However, the Dmm+Th group
demonstrated significantly lower percentages of VEGF positive cells
than those of the Dmm group at 2 and 4 weeks post-surgery
(P<0.05).
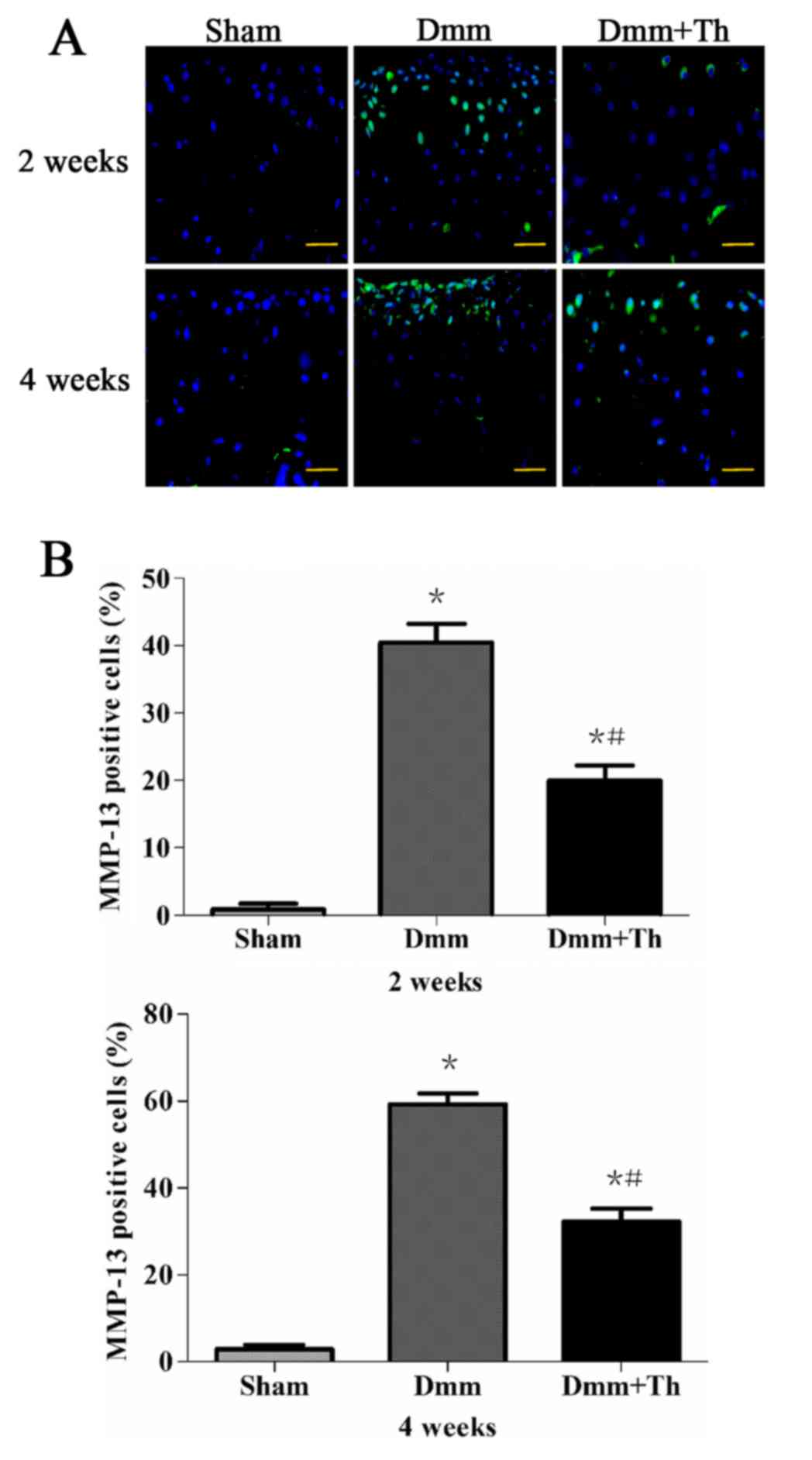
Thalidomide treatment reduces MMP-13
protein expression in early OA mice
As presented in Fig.
4A, immuno-positive cells were detected in the areas of
cartilage breakdown. In the Dmm and Dmm+Th groups, the majority of
these cells were observed in the superficial layers and very few in
the middle layers of the articular cartilage; however, in the Sham
group, where the articular cartilage had a smooth and intact
surface, almost no such staining was observed. The percentages of
MMP-13-positive cells in the articular cartilage were significantly
higher in the Dmm group and the Dmm+Th group as compared with the
Sham group at 2 and 4 weeks post-surgery (P<0.05; Fig. 4B). The Dmm+Th group demonstrated a
significant decrease in the number of VEGF-positive cells in the
articular cartilage compared with the Dmm group at 2 and 4 weeks
post-surgery (P<0.05).
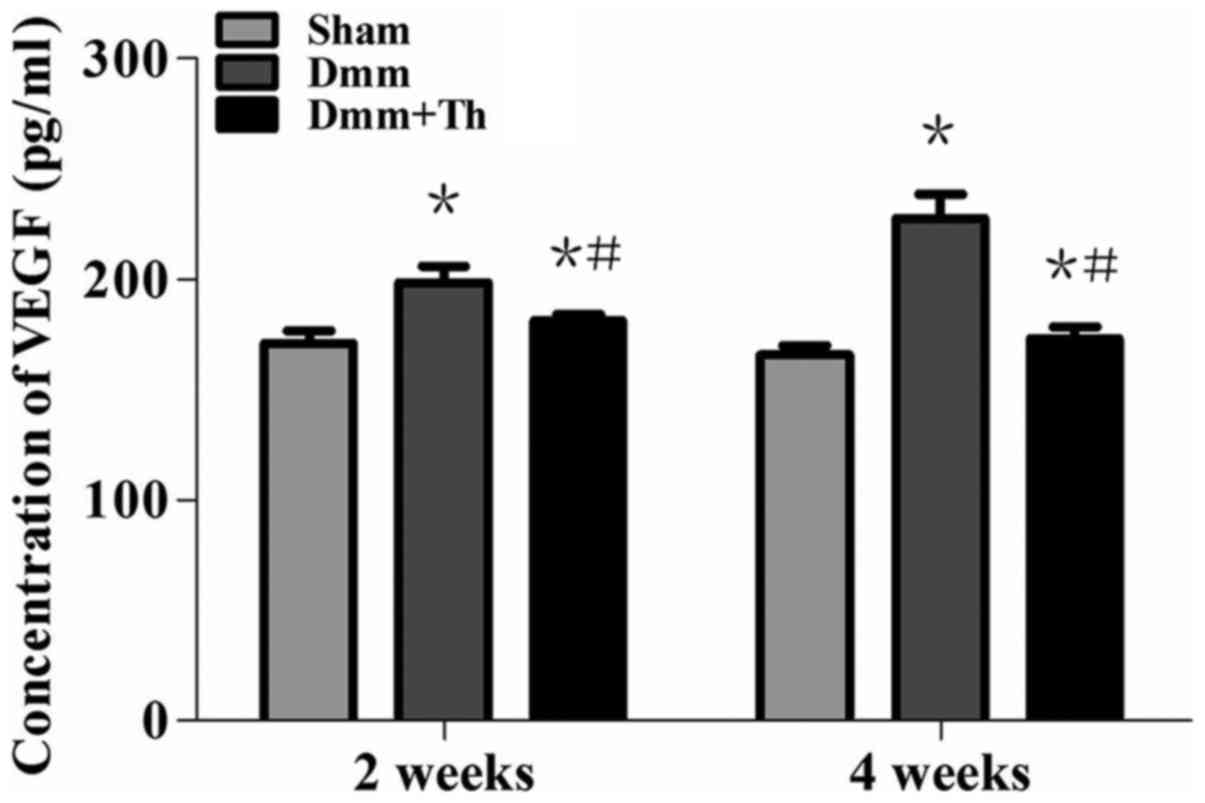
Thalidomide treatment reduces serum
VEGF concentration in early OA mice
As presented in Fig.
5, compared with in the Sham group, serum VEGF concentration in
the Dmm and the Dmm+Th groups were significantly increased by
varying degrees; however, compared with in the Dmm group, the serum
VEGF concentration was significantly lower within the Dmm+Th group
at 2 and 4 weeks following surgery (P<0.05).
Discussion
Thalidomide was first developed as a sedative in the
1950s in European countries, and was later reported to be useful in
the treatment of morning sickness in pregnant women, but was
withdrawn from the market globally due to its teratogenic effects
(35). Then thalidomide, which was
researched by scientists for numerous years, was reported to
exhibit antiangiogenic properties and suppress VEGF expression
(19,23). In addition, in the present study,
thalidomide was observed to inhibit the expression of VEGF at the
mRNA and protein levels, detected via RT-qPCR and IHC.
Additionally, the concentration of VEGF in serum was also
significantly reduced in the Dmm+Th group compared with in the Dmm
group. The results of the present study revealed that thalidomide
may be effective in reducing the production of VEGF. Furthermore,
Alberto et al (36)
revealed that thalidomide may decrease serum levels of VEGF in
patients with severe intestinal bleeding.
In the present study, the method of intraperitoneal
injection of thalidomide was employed. Thalidomide may not be
suitable for intra-articular injection as the administration of
dosing is greater than the volume of the articular cavity of the
C57BL/6 mouse. In addition, intravenous administration has been
reported to have some relevant complications, including delayed
wound healing, hemorrhage, thromboembolism, proteinuria (37). The administration of
intraperitoneal injection of thalidomide is rather mature and has
been used in previous studies for numerous years (38,39),
and has been reported to be more effective in inhibiting VEGF than
oral administration of thalidomide (29,40).
OA is a widely prevalent and degenerative disease of
the joints and occurs mainly in elderly people. The pathology is
mainly characterized by the degeneration of cartilage.
Chondrocytes, unique cells in articular cartilage, may synthesize
ECM components, which mainly comprises aggrecans and type II
collagen (Collagen II), and serve a vital role in maintaining the
dynamic balance of ECM metabolism (8). Destruction of articular cartilage,
involving the destruction of aggrecans and Collagen II, is mainly
caused by proteolytic enzymes of aggrecanase families and MMPs
(9). Xia et al (41) published a review suggesting that
disruptions of the ECM dynamic equilibrium are pivotal events in
the pathogenesis of OA. MMPs constitute a family of proteolytic
enzymes, which regulate a variety of functions in tissue
remodeling, such as ECM degradation (42). Among the MMPs, MMP-13 has been
reported to serve an important role in the progression of OA in
that it irreversibly and efficiently breaks down Collagen II
(43), which has made it an
attractive therapeutic target for OA. In addition, the expression
levels of several MMPs have been observed to be raised within
cartilage tissues of patients with OA (44). Studies have revealed that selective
inhibitors of MMP-13, as a latent therapy, may attenuate the
progression of OA (45,46).
Based on the findings of the present study, compared
with the Sham group, the mRNA and protein expression levels of
MMP-13 in the Dmm group had significantly increased, but exhibited
declined levels in the Dmm+Th group. In addition, HE and Safranin
O/Fast green staining qualitatively demonstrated that the Dmm group
suffered severe damage compared with the Dmm+Th group. Furthermore,
the OARSI scores quantitatively presented that the Dmm+Th group
exhibited markedly less severe pathological alterations compared
with the Dmm group. Collectively, the results of the present study
indicated that the Dmm+Th group exhibited reduced OA progression
compared with the Dmm group; thalidomide may downregulate the
expression of MMP-13 which is associated with the progression of
OA. The results of the present study also indicated that the
superficial layers of cartilage in surgically-induced OA mice,
which were characterized by various degrees of fibrillations and
cartilage erosion, contained a high proportion of cells positive
for MMP-13 as observed via immunostaining; however, almost no
immuno-positive cells of MMP-13 were detected in the Sham group.
These observations were consistent with previous findings (44). Baragi et al (45) and Janusz et al (47) reported that MMPs or MMP-13
inhibitors may protect cartilage from degeneration (48).
VEGF, which was discovered and isolated in 1989, is
a protein that stimulates blood vessel growth (49). Kim et al (50) demonstrated the significant
inhibition of VEGF-induced angiogenesis, as well as the progression
of diverse forms of tumors by a means of specific monoclonal
antibodies. Yuan et al (16) reported a meta-analysis of the
association between OA and VEGF expression levels, and indicated
that higher VEGF expression levels were strongly correlated with
the pathogenesis of OA.
From the results of IHC analysis of VEGF expression,
VEGF was also expressed in normally growing mice and decreased
progressively over time in the Sham group. This phenomenon was
similar to the study of Tibesku et al (15) who reported an initial VEGF-increase
in sham-operated New Zealand rabbits which decreased after 6 weeks.
In the present study VEGF expression levels increased along with
the progression of early OA, and may be inhibited by the
intraperitoneal injection of thalidomide in the Dmm+Th group. Nagai
et al (17) suggested that
intravenous administration of an antibody against VEGF may
contribute to articular cartilage repair in an osteochondral defect
model. Furthermore, the findings of the present study indicated
that the expression of VEGF at the mRNA and protein levels were
downregulated in the Dmm+Th group and that the degree of cartilage
damage in OA mice model, which would lead to cartilage degeneration
and loss of proteoglycans, was significantly improved by
suppression of VEGF expression within the Dmm+Th group, compared
with the Dmm group. The results of present study supported the
hypothesis that intraperitoneal administration of thalidomide may
alleviate early OA development by suppressing VEGF expression in
mice; to the best of our knowledge, this has not been previously
reported.
There were some limitations to the present study: i)
The OA model was surgically-induced via the Dmm method. This is a
classical and effective method to establish an animal OA model for
study in vivo (51);
however, the method differs from the processes by which OA develops
clinically. The etiology of OA is multifactorial, as the disease is
caused by aging, as well as trauma (52). ii) The present study focused mainly
on the effects of thalidomide on chondrocytes and cartilage, but
did not investigate its effects on other types of cell (meniscus
cells, osteoblasts/osteoclasts, and fibroblasts) or tissues
(meniscus, subchondral bone, and synovium). iii) Only a single dose
of thalidomide (200 mg/kg/day) for the treatment of early-stage OA.
Thus, the effects of various doses and longer treatment courses on
the different stages of OA requires further investigation.
Additionally, the findings of the present study indicated that
treatment with thalidomide may alleviate early OA development by
suppressing VEGF expression; however, the exact molecular
mechanisms associated with the interaction between thalidomide,
VEGF, MMP-13 and chondrocytes requires further investigation.
In conclusion, the results of the present study
suggested that intraperitoneal injection of thalidomide may
alleviate the development of early OA by suppressing VEGF
expression in mice. Thalidomide may be a promising treatment for
OA; however, additional comprehensive studies to elucidate the
exact molecular mechanisms and the clinically potential treatments
for OA are required.
Acknowledgements
The authors are grateful to the staff in the
Laboratory Center of the Third Affiliated Hospital of Southern
Medical University, Guangzhou, China.
Funding
The present study was supported by the Projects of
National Natural Science Foundation of China (grant no. 81371990),
and Specialized Research Fund for the Doctoral Program of Higher
Education of China (grant no. 20124433110021).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
JLS, HF and DZC conceived and designed the study.
JLS and DLL performed the experiments. JLS, HF and DLL collected,
analyzed and interpreted the data. All authors drafted and edited
the manuscript, and gave final approval of the article.
Ethics approval and consent to
participate
The present study was approved by and conducted
according to the guidelines of The Third Affiliated Hospital of
Southern Medical University Animal Healthcare Ethics Committee
(Guangdong, China).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
OA
|
osteoarthritis
|
|
ECM
|
extracellular matrix
|
|
MMP-13
|
matrix metalloproteinase-13
|
|
VEGF
|
vascular endothelial growth factor
|
|
MMPs
|
matrix metalloproteinases
|
|
DMSO
|
dimethyl sulfoxide
|
|
DAB
|
diaminobenzidine
tetrahydrochloride
|
|
HE
|
hematoxylin and eosin
|
|
DMM
|
destabilization of the medial
meniscus
|
|
OARSI
|
Osteoarthritis Research Society
International
|
|
RT-qPCR
|
reverse transcription quantitative
polymerase chain reaction
|
|
IHC
|
immunohistochemistry
|
|
IF
|
immunofluorescence
|
|
ELISA
|
enzyme linked immunosorbent assay
|
|
Collagen II
|
type II collagen
|
References
|
1
|
Bitton R: The economic burden of
osteoarthritis. Am J Manag Care. 15 Suppl 8:S230–S235.
2009.PubMed/NCBI
|
|
2
|
Ackerman IN, Pratt C, Gorelik A and Liew
D: Projected burden of osteoarthritis and rheumatoid arthritis in
Australia: A population-level analysis. Arthritis Care Res
(Hoboken). 2017.
|
|
3
|
Cicuttini FM and Wluka AE: Osteoarthritis:
Is OA a mechanical or systemic disease? Nat Rev Rheumatol.
10:515–516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Musumeci G, Szychlinska MA and Mobasheri
A: Age-related degeneration of articular cartilage in the
pathogenesis of osteoarthritis: Molecular markers of senescent
chondrocytes. Histol Histopathol. 30:1–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Srikanth VK, Fryer JL, Zhai G, Winzenberg
TM, Hosmer D and Jones G: A meta-analysis of sex differences
prevalence, incidence and severity of osteoarthritis.
Osteoarthritis Cartilage. 13:769–781. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Felson DT: An update on the pathogenesis
and epidemiology of osteoarthritis. Radiol Clin North Am. 42:1–9.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei Y and Bai L: Recent advances in the
understanding of molecular mechanisms of cartilage degeneration,
synovitis and subchondral bone changes in osteoarthritis. Connect
Tissue Res. 57:245–261. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van der Kraan PM and van den Berg WB:
Chondrocyte hypertrophy and osteoarthritis: Role in initiation and
progression of cartilage degeneration? Osteoarthritis Cartilage.
20:223–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fosang AJ, Last K, Knauper V, Murphy G and
Neame PJ: Degradation of cartilage aggrecan by collagenase-3
(MMP-13). FEBS Lett. 380:17–20. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pufe T, Petersen W, Tillmann B and
Mentlein R: The splice variants VEGF121 and VEGF189 of the
angiogenic peptide vascular endothelial growth factor are expressed
in osteoarthritic cartilage. Arthritis Rheum. 44:1082–1088. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Enomoto H, Inoki I, Komiya K, Shiomi T,
Ikeda E, Obata K, Matsumoto H, Toyama Y and Okada Y: Vascular
endothelial growth factor isoforms and their receptors are
expressed in human osteoarthritic cartilage. Am J Pathol.
162:171–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pfander D, Körtje D, Zimmermann R, Weseloh
G, Kirsch T, Gesslein M, Cramer T and Swoboda B: Vascular
endothelial growth factor in articular cartilage of healthy and
osteoarthritic human knee joints. Ann Rheum Dis. 60:1070–1073.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pufe T, Harde V, Petersen W, Goldring MB,
Tillmann B and Mentlein R: Vascular endothelial growth factor
(VEGF) induces matrix metalloproteinase expression in immortalized
chondrocytes. J Pathol. 202:367–374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen P, Jiao Z, Zheng JS, Xu WF, Zhang SY,
Qin A and Yang C: Injecting vascular endothelial growth factor into
the temporomandibular joint induces osteoarthritis in mice. Sci
Rep. 5:162442015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tibesku CO, Daniilidis K, Skwara A,
Paletta J, Szuwart T and Fuchs-Winkelmann S: Expression of vascular
endothelial growth factor on chondrocytes increases with
osteoarthritis-an animal experimental investigation. Open Orthop J.
5:177–180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan Q, Sun L, Li JJ and An CH: Elevated
VEGF levels contribute to the pathogenesis of osteoarthritis. BMC
Musculoskelet Disord. 15:4372014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagai T, Sato M, Kutsuna T, Kokubo M,
Ebihara G, Ohta N and Mochida J: Intravenous administration of
anti-vascular endothelial growth factor humanized monoclonal
antibody bevacizumab improves articular cartilage repair. Arthritis
Res Ther. 12:R1782010. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Therapontos C, Erskine L, Gardner ER, Figg
WD and Vargesson N: Thalidomide induces limb defects by preventing
angiogenic outgrowth during early limb formation. Proc Natl Acad
Sci USA. 106:8573–8578. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
D'Amato RJ, Loughnan MS, Flynn E and
Folkman J: Thalidomide is an inhibitor of angiogenesis. Proc Natl
Acad Sci USA. 91:4082–4085. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singhal S, Mehta J, Desikan R, Ayers D,
Roberson P, Eddlemon P, Munshi N, Anaissie E, Wilson C, Dhodapkar
M, et al: Antitumor activity of thalidomide in refractory multiple
myeloma. N Engl J Med. 341:1565–1571. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tan H, Chen H, Xu C, Ge Z, Gao Y, Fang J,
Liu W and Xiao S: Role of vascular endothelial growth factor in
angiodysplasia: An interventional study with thalidomide. J
Gastroenterol Hepatol. 27:1094–1101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mercurio A, Adriani G, Catalano A, Carocci
A, Rao L, Lentini G, Cavalluzzi MM, Franchini C, Vacca A and Corbo
F: A mini-review on thalidomide: Chemistry, mechanisms of action,
therapeutic potential and anti-angiogenic properties in multiple
myeloma. Curr Med Chem. 24:2736–2744. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Andersen NF, Vogel U, Klausen TW, Gimsing
P, Gregersen H, Abildgaard N and Vangsted AJ: Vascular endothelial
growth factor (VEGF) gene polymorphisms may influence the efficacy
of thalidomide in multiple myeloma. Int J Cancer. 131:E636–E642.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Behl T, Kaur I, Goel H and Kotwani A:
Significance of the antiangiogenic mechanisms of thalidomide in the
therapy of diabetic retinopathy. Vascul Pharmacol. 92:6–15. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Israyelyan A, Shannon EJ, Baghian A,
Kearney MT and Kousoulas KG: Thalidomide suppressed the growth of
4T1 cells into solid tumors in Balb/c mice in a combination therapy
with the oncolytic fusogenic HSV-1 OncdSyn. Cancer Chemother
Pharmacol. 64:1201–1210. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lainer-Carr D and Brahn E: Angiogenesis
inhibition as a therapeutic approach for inflammatory synovitis.
Nat Clin Pract Rheumatol. 3:434–442. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gordon JN and Goggin PM: Thalidomide and
its derivatives: Emerging from the wilderness. Postgrad Med J.
79:127–132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vasheghani F, Zhang Y, Li YH, Blati M,
Fahmi H, Lussier B, Roughley P, Lagares D, Endisha H, Saffar B, et
al: PPARγ deficiency results in severe, accelerated osteoarthritis
associated with aberrant mTOR signalling in the articular
cartilage. Ann Rheum Dis. 74:569–578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kotoh T, Dhar DK, Masunaga R, Tabara H,
Tachibana M, Kubota H, Kohno H and Nagasue N: Antiangiogenic
therapy of human esophageal cancers with thalidomide in nude mice.
Surgery. 125:536–544. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu CY, Jiang XX, Zhu YH and Wei DN:
Metabotropic glutamate receptor 5 antagonist
2-methyl-6-(phenylethynyl)pyridine produces antidepressant effects
in rats: Role of brain-derived neurotrophic factor. Neuroscience.
223:219–224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Glasson SS, Chambers MG, Van Den Berg WB
and Little CB: The OARSI histopathology initiative-recommendations
for histological assessments of osteoarthritis in the mouse.
Osteoarthritis Cartilage. 18 Suppl 3:S17–S23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang MJ, Wang L, Jin DD, Zhang ZM, Chen
TY, Jia CH, Wang Y, Zhen XC, Huang B, Yan B, et al: Enhancement of
the synthesis of n-3 PUFAs in fat-1 transgenic mice inhibits mTORC1
signalling and delays surgically induced osteoarthritis in
comparison with wild-type mice. Ann Rheum Dis. 73:1719–1727. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jonason JH, Hoak D and O'Keefe RJ: Primary
murine growth plate and articular chondrocyte isolation and cell
culture. Methods Mol Biol. 1226:11–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vargesson N: Thalidomide-induced
teratogenesis: History and mechanisms. Birth Defects Res C Embryo
Today. 105:140–156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alberto SF, Felix J and de Deus J:
Thalidomide for the treatment of severe intestinal bleeding.
Endoscopy. 40:788–789. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gu X, Zheng Y, Ren B, Zhang R, Mei F,
Zhang J and Ma Z: Intraperitoneal injection of thalidomide
attenuates bone cancer pain and decreases spinal tumor necrosis
factor-alpha expression in a mouse model. Mol Pain. 6:642010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Daruwalla J, Nikfarjam M,
Malcontenti-Wilson C, Muralidharan V and Christophi C: Effect of
thalidomide on colorectal cancer liver metastases in CBA mice. J
Surg Oncol. 91:134–140. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kenyon BM, Browne F and D'Amato RJ:
Effects of thalidomide and related metabolites in a mouse corneal
model of neovascularization. Exp Eye Res. 64:971–978. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xia B, Di Chen, Zhang J, Hu S, Jin H and
Tong P: Osteoarthritis pathogenesis: A review of molecular
mechanisms. Calcif Tissue Int. 95:495–505. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brinckerhoff CE and Matrisian LM: Matrix
metalloproteinases: A tail of a frog that became a prince. Nat Rev
Mol Cell Biol. 3:207–214. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Klein T and Bischoff R: Physiology and
pathophysiology of matrix metalloproteases. Amino Acids.
41:271–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tetlow LC, Adlam DJ and Woolley DE: Matrix
metalloproteinase and proinflammatory cytokine production by
chondrocytes of human osteoarthritic cartilage: Associations with
degenerative changes. Arthritis Rheum. 44:585–594. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Baragi VM, Becher G, Bendele AM, Biesinger
R, Bluhm H, Boer J, Deng H, Dodd R, Essers M, Feuerstein T, et al:
A new class of potent matrix metalloproteinase 13 inhibitors for
potential treatment of osteoarthritis: Evidence of histologic and
clinical efficacy without musculoskeletal toxicity in rat models.
Arthritis Rheum. 60:2008–2018. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hu Y, Xiang JS, DiGrandi MJ, Du X, Ipek M,
Laakso LM, Li J, Li W, Rush TS, Schmid J, et al: Potent, selective,
and orally bioavailable matrix metalloproteinase-13 inhibitors for
the treatment of osteoarthritis. Bioorg Med Chem. 13:6629–6644.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Janusz MJ, Bendele AM, Brown KK, Taiwo YO,
Hsieh L and Heitmeyer SA: Induction of osteoarthritis in the rat by
surgical tear of the meniscus: Inhibition of joint damage by a
matrix metalloproteinase inhibitor. Osteoarthritis Cartilage.
10:785–791. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Janusz MJ, Hookfin EB, Heitmeyer SA,
Woessner JF, Freemont AJ, Hoyland JA, Brown KK, Hsieh LC, Almstead
NG, De B, et al: Moderation of iodoacetate-induced experimental
osteoarthritis in rats by matrix metalloproteinase inhibitors.
Osteoarthritis Cartilage. 9:751–760. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ferrara N and Henzel WJ: Pituitary
follicular cells secrete a novel heparin-binding growth factor
specific for vascular endothelial cells. Biochem Biophys Res
Commun. 161:851–858. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim KJ, Li B, Winer J, Armanini M, Gillett
N, Phillips HS and Ferrara N: Inhibition of vascular endothelial
growth factor-induced angiogenesis suppresses tumour growth in
vivo. Nature. 362:841–844. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Thysen S, Luyten FP and Lories RJ:
Targets, models and challenges in osteoarthritis research. Dis
Model Mech. 8:17–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis. Lancet.
386:376–387. 2015. View Article : Google Scholar : PubMed/NCBI
|