Introduction
Urethral defects, which are caused by urethral
trauma, congenital malformations and tumors, are common urological
diseases. At present, it is still rather difficult to repair a long
urethral defect. When the length exceeds 4 cm, additional tissue
transplantation is in need (1,2).
Researchers have endeavored to repair urethral defects by using
various tissue materials, such as the foreskin, bladder mucosa,
small intestinal submucosal tissue and tunica vaginalis. However,
there is still no ideal substitute material for urethral tissue
hitherto, because of high incidence rates of metabolic
abnormalities, infection, urethral fistula, urethral stricture and
other complications. Besides, repairing tissues with defects by
using autologous tissues prolongs the time of urethral surgery. By
using tissue-engineered technology, Atala et al (3) reconstructed the bladder by autologous
bladder tissues and constructed the urethra.
In traditional urethral tissue engineering, seed
cells are mainly derived from autologous urethral tissues. The
number of passages of urethral cells is limited, which can be
solved by stem cells with strong differentiation and proliferation
abilities. For the first time, Zuk et al (4) isolated stem cells with multipotential
differentiation from a suspension of adipose tissues that were
obtained by suction lipectomy, and referred to them as processed
lipoaspirate cells. Afterwards, Li et al (5) reported that bone marrow mesenchymal
stem cells (MSCs) had osteogenic and chondrogenic differentiation
potentials and secreted proteins (stem cell-derived factor-1 and
hepatocyte growth factor). These biological advantages should be
considered in the selection of an MSC source for specific clinical
application (6).
During the repair of urethral defects, it is also
important to find an appropriate biological scaffold material which
can relieve inflammation, and benefit the adhesion, growth and
proliferation of seed cells, with high tissue compatibility
(7–9). As a natural biological material
derived from silk, silk fibroin (SF) barely shows immune response
after purification that removes silk sericin. In addition, it can
promote cell adhesion, growth and proliferation (10–12).
SF carries great promises in biomedical fields and can be used as
surgical sutures, wound protection materials, artificial blood
vessels, artificial tendons, contact lenses, sustained release
carriers and anticoagulant substances. Up to now, however, it has
seldom been employed as a scaffold material for urethral defect
repair. Meanwhile, SF has never been composited with adipose
mesenchymal stem cells (ADMSCs) to repair urethral defects. Thereby
motivated, we herein aimed to investigate whether it was possible
to repair urethral defects with an ADMSCs-porous SF material.
Materials and methods
Ethics
All animal experiments were performed in accordance
with the National Institutes of Health Guide for the Care and Use
of Laboratory Animals, with the approval of the Animal Care and Use
Committee of Shanghai Jiao Tong University Affiliated Sixth
People's Hospital (Shanghai, China).
Materials
Porous SF material was prepared through
freeze-drying by Professor Mingzhong Li from College of Chemistry,
Chemical Engineering and Material Science, Soochow University
(Suzhou, China). This material had a dense lower surface, a lowly
porous upper surface and a porous inner structure. The SF membrane
consisted of filamentous core protein, SF and sericin. A total of
39 adult New Zealand white rabbits weighing about 3.0–4.5 kg
(preoperative urethrography disclosed normal urethrae) were
randomly divided into a control group, an SF group and a
BrdU-labeled ADMSCs-SF group (SSF group) (n=13).
Establishment of urethral defect
model
The rabbits were anesthetized by intravenous
injection with 30 mg/kg pentobarbital sodium in the ear. The
surgical area was routinely sterilized three times and covered by a
drape. Then an F6 catheter was inserted into the urethra. A
longitudinal incision of about 1.5 cm was made on the anterior wall
in the middle of the ventral urethra. Finally, the segmental
urethral defect model was established after cutting open
subcutaneous tissues and the urethra, and longitudinally resecting
the posterior urethral wall of about 2.5×1.0 cm.
Culture of ADMSCs
A healthy New Zealand white rabbit (about 2.0 kg)
was anesthetized by intravenous injection with 30 mg/kg
pentobarbital sodium, and fat near the epididymis was collected
under sterile conditions. Discernible small vessels and fascia were
resected, and tissues were washed by PBS, cut into pieces, added a
double volume of 0.15% type I collagen, digested by shaking at 37°C
for 45 min, added an equal amount of DMEM-F12 containing 10% fetal
bovine serum (FBS) to stop digestion, filtered through a 200-mesh
screen, lysed with 160 mM ammonium chloride at 37°C for 10 min,
centrifuged at 2,600 × g for 15 min, washed once with PBS, and
centrifuged at 2,600 × g for 15 min. The precipitated ADMSCs were
inoculated into 6-wells plate with 3 ml of DMEM-F12 containing 10%
FBS in each well, and cultured in a 37°C incubator with 5%
CO2 and saturated humidity.
Preparation of BrdU-labeled ADMSCs
suspension
After almost complete confluence was reached, ADMSCs
were digested by 0.25% trypsin and centrifuged to discard the
culture medium. Then ADMSCs were incubated in DMEM containing 10 µM
BrdU and 10% FBS at 37°C in 5% CO2 and saturated
humidity for 3 days. Afterwards, they were diluted by PBS into a
final density of 1×106/ml and stored in sterile tubes at
4°C prior to use.
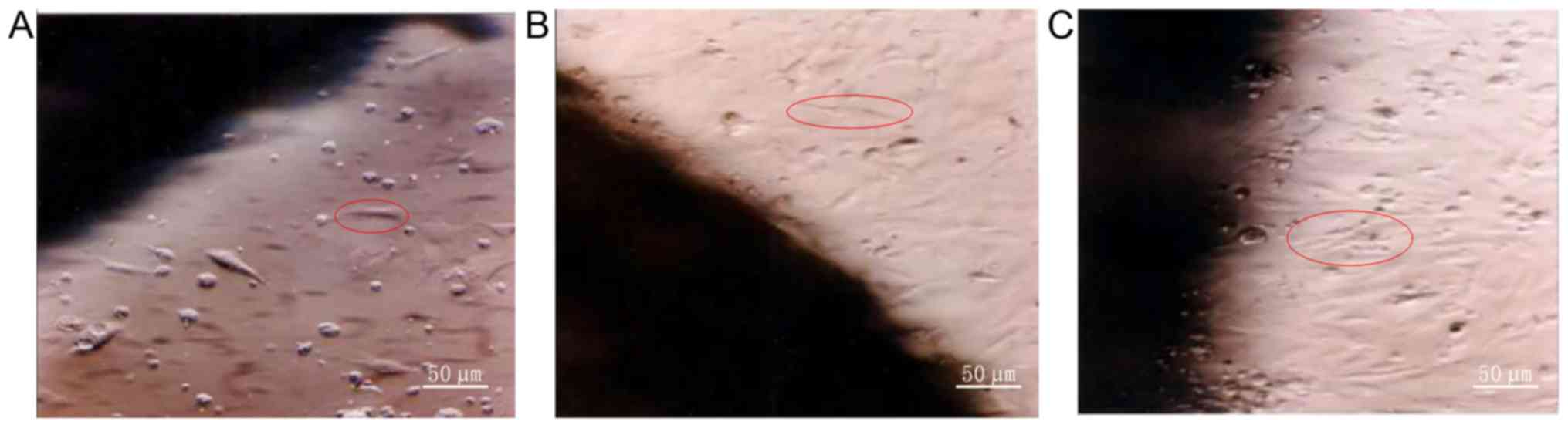
Observation of BrdU-labeled ADMSCs on
SF material by inverted microscope
SF material was prewetted with culture medium and
placed in a 24-well plate. Then BrdU-labeled ADMSCs were inoculated
onto the material at a density of 1×106/ml, incubated
for 1 h and further cultured after adding 1.5 ml of culture medium.
The growth of these cells was observed by inverted microscope
(Fig. 1).
Surgical procedure and postoperative
treatment of urethral defects
For the control group, the same width of the
urethral mucosa was cut off, and four corners were sutured with 5-0
nylon thread (Cheng-He Microsurgical Instruments Factory, Ningbo,
China), as a postoperative marker for tissue collection. An F10
disposable catheter was retained, and the glans was fixed, with
about 5 cm retained in vitro. A 6-0 PGA absorbable thread
(Suzhou Medical Appliance Factory, Suzhou, China) was used to
suture the anterior wall of the urethra continuously as well as the
subcutaneous fat and skin interruptedly. The head was fixed with a
cervical gear.
For the SF group, the urethral wall defect was
repaired by SF material through Inlay surgery, and the edge was
sutured interruptedly with 6-0 PGA absorbable thread. Afterwards,
the material was longitudinally sutured using 6-0 absorbable thread
in an interrupted way, with the penis in the middle, and four
corners were sutured with 5-0 nylon thread, as a postoperative
marker for tissue collection. The remaining procedures were the
same as those of the control group.
For the SSF group, after a suspension of
BrdU-labeled ADMSCs at the density of 1×106 was dropped
on the posterior wall defect of the urethra into a layer, the
defect was covered and repaired with SF material through Inlay
surgery, and then the material surface was evenly dropped with the
ADMSC suspension. The remaining procedures were the same as those
of the SF group.
The urethral catheter, which was washed with
nitrofurazone once per day, was retained for about 3 weeks after
surgery. The collected urine was diluted by intravenous infusion
with 250 ml of glucose-sodium chloride (once per day) for about 3
weeks. To prevent removal of the catheter by the rabbit itself, the
cervical gear was retained for about 3 weeks (or 2 weeks for those
sacrificed in the postoperative second week), and 800,000 U of
penicillin sodium was injected intravenously for about 2 weeks
(once per day).
Postoperative observation
All rabbits were sacrificed by air embolization 2
(n=2), 4 (n=9) and 6 weeks (n=2) after surgery respectively.
Urethrography was performed after surgery to observe the urethral
patency. The repaired urethral segment was taken, fixed in 4%
neutral paraformaldehyde, embedded in paraffin and subjected to
H&E staining for histological examination. Cells with positive
expressions of factor VIII related antigen (FVIII-RAg), α-smooth
muscle actin (α-SMA) and AE1/AE3 as well as macrophages were
detected by immunohistochemical assay. Two microscopists
independently observed sections without knowing the experimental
design. Ten visual fields were randomly selected for each section.
The positive cells in each field were counted, and the results were
averaged.
Statistical analysis
All experimental data were analyzed by GraphPad
software (GraphPad Software, Inc., San Diego, CA, USA). All
experiments were performed in triplicate, and the results were
expressed as mean ± standard deviation. The categorical data were
subjected to the t test, and the numerical data were subjected to
the χ2 test. P<0.05 was considered statistically
significant.
Results
Urethrography results
All rabbits had normal urethral morphologies before
surgery. Urethral stricture and fistula were observed by retrograde
urethrography before the end of the experiment after surgery. The
incidence rates of postoperative complications in control, SF and
SSF groups were 76.92 (7/13), 23.07 (3/13) and 15.38% (2/13)
respectively, with significant differences (P<0.05). The
incidence rates of postoperative complications in SSF and SF groups
were similar (P>0.05) (Table
I).
 | Table I.Incidence rates of urethral fistula
and stricture. |
Table I.
Incidence rates of urethral fistula
and stricture.
|
| Urethral fistula and
stricture (n) |
|
|
|---|
|
|
|
|
|
|---|
| Group | + | − | Total (n) | Incidence rate
(%) |
|---|
| Control | 10 | 3 | 13 | 76.92 |
| SF | 3 | 10 | 13 | 23.07a |
| SSF | 2 | 11 | 13 | 15.38a,b |
Histological observation results
In the control group, the urethral defect did not
form mucous epithelium 2 weeks after surgery, and many lymphocytes
infiltrated, with few blood vessels. A discontinuous urethral
mucosa formed 4 weeks after surgery, and the surface was uneven,
accompanied by infiltration of lymphocytes and fibrous tissue
hyperplasia. The number of layers of mucous epithelial cells in the
urethral defect increased 6 weeks after surgery, and the cells were
arranged irregularly, with disordered polarity. Many submucosal
lymphocytes infiltrated, with fibrous tissue hyperplasia and
disordered tissue structures. They were scattered within a small
amount of smooth muscle fibers (Fig.
2A and D).
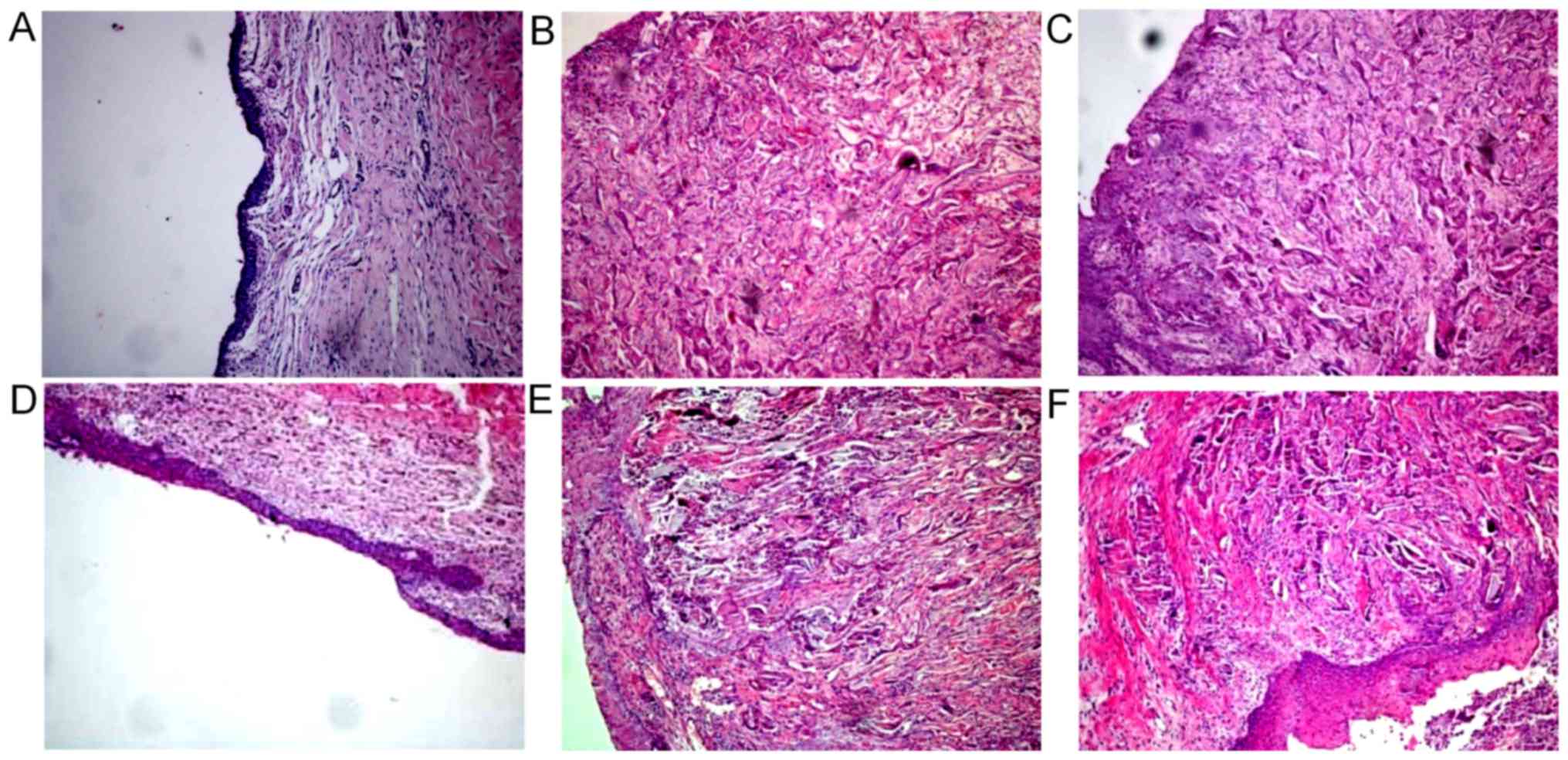 | Figure 2.Histological observation results of
(A) control group, (B) SF group and (C) SSF group 4 weeks following
surgery, and those of (D) control group, (E) SF group and (F) SSF
group 6 weeks following surgery. (A) Urethral epithelial defects
and many infiltrated lymphocytes were observed. The number of
microvessels was (10.11±1.54)/HP. (B) Urethral epithelial cells
formed a multilayer structure and tissue growth was incomplete. The
number of microvessels was (20.77±2.14)/HP. (C) Urethral epithelial
cells formed a multilayer structure, and a considerable number of
blood vessels, smooth muscle cells and fibrous tissues under the
mucous membrane grew toward the SF pores. The number of
microvessels was (25.18±2.51)/HP. (D) Many lymphocytes infiltrated
and tissue structure was disordered. The number of microvessels was
(15.32±1.67)/HP. (E) Urethral epithelial cells formed a multilayer
structure, and many blood vessels, smooth muscle cells and fibrous
tissues under the mucous membrane grew toward SF pores. The number
of microvessels was (23.18±2.21)/HP. (F) SF material decomposed
into large pieces, and a considerable number of blood vessels,
smooth muscle cells and fibrous tissues under the mucous membrane
grew toward SF pores. Urethral epithelial cells were arranged
regularly. The number of microvessels was (28.67±2.32)/HP
(magnification, ×100). SF, silk fibroin; SSF,
bromodeoxyuridine-labeled adipose mesenchymal stem cells-SF group;
HP, high power field. |
In the SF group, the surface of SF material did not
form epithelium 2 weeks after surgery. There were a few blood
vessels and collagen tissues at the bottom of SF material, with
infiltration of a small number of lymphocytes. In addition, the
structure of SF was intact, without tissue growth on the top. A
stratified epithelial structure formed on the surface of SF
material 4 weeks after surgery, and blood vessels, smooth muscle
and fibrous tissue grew along SF pores. A part of SF tissues were
incomplete and only a few lymphocytes infiltrated. Six weeks after
surgery, 3–4 layers of epithelial cells formed on the surface of SF
material. Many submucous blood vessels, smooth muscle and fibrous
tissue grew along SF pores, with infiltration of a few lymphocytes
and complete SF structure. Meanwhile, decomposition hardly occurred
(Fig. 2B and E).
In the SSF group, no epithelial cells formed on the
surface of SF material 2 weeks after surgery. Many blood vessels
and collagen tissues grew at the bottom of SF material, with
infiltration of a few lymphocytes and complete SF structure.
Tissues did not grow on the top of SF material. Three to four
layers of epithelial cells formed on the SF material surface 4
weeks after surgery, which were irregularly arranged. Many
submucosal blood vessels, smooth muscles and fibrous tissues grew
along SF pores, also with infiltration of a small number of
lymphocytes (Fig. 2C). Six weeks
after surgery, 6–7 layers of epithelial cells formed on the SF
material surface. The cells were arranged in a regular pattern.
Considerable blood vessels, smooth muscles and fibrous tissues grew
along SF pores. A small number of lymphocytes infiltrated, and SF
material decomposed into small pieces (Fig. 2F).
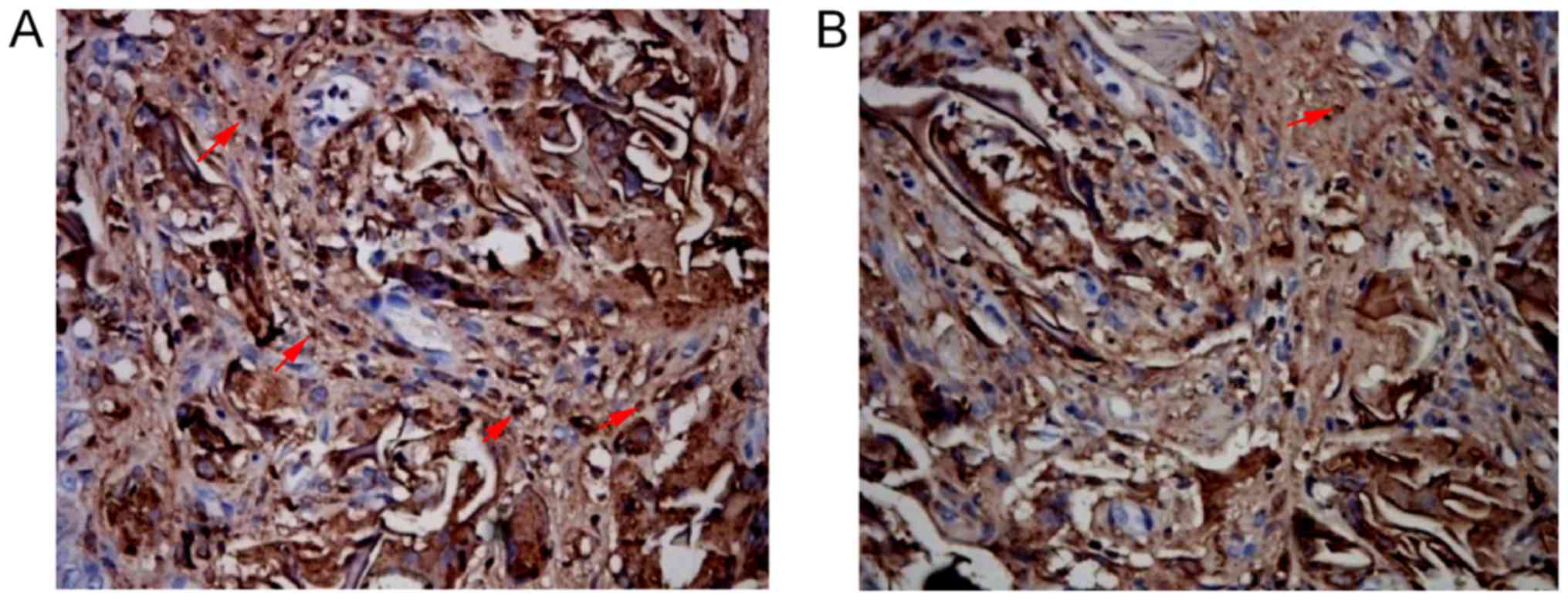
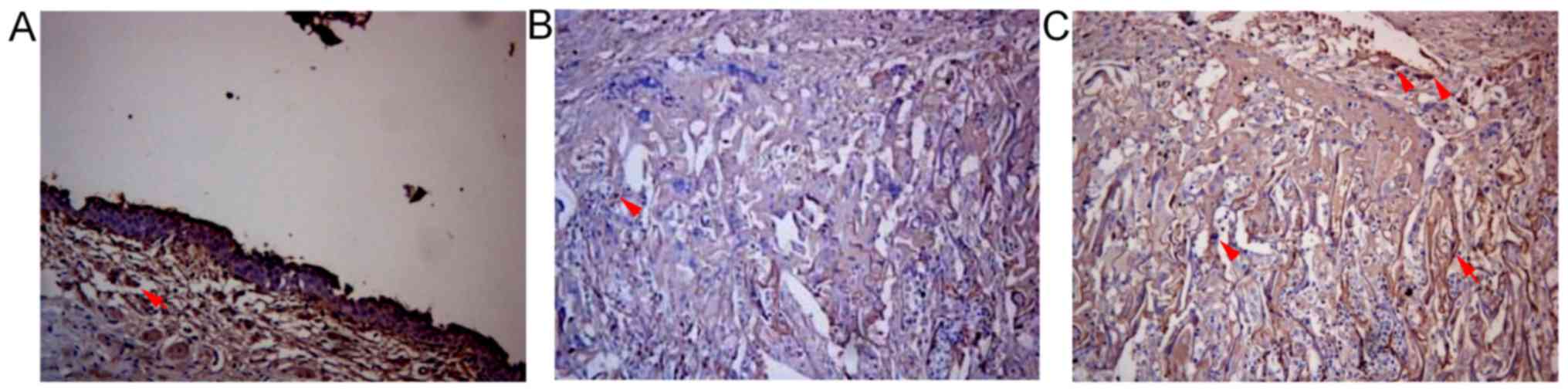
Distribution of macrophages, BrdU,
FVIII-RAg, α-SMA and AE1/AE3 positive cells
There were macrophages at the gap and edge of SF
material in both SSF and SF groups 2, 4 and 6 weeks after surgery
respectively. The number of macrophages with positive expression in
the SSF group was (11.66±1.58)/HP, which was significantly lower
than that of the SF group ((13.88±2.08)/HP) at the same time point
(P<0.05) (Fig. 3; Table II).
 | Table II.Number of FVIII-Rag, α-SMA and
macrophage positive cells 4 weeks following surgery. |
Table II.
Number of FVIII-Rag, α-SMA and
macrophage positive cells 4 weeks following surgery.
| Group | FVIII-RAg | α-SMA | Macrophage |
|---|
| SSF |
23.44±2.40a,b |
33.00±3.27a,b |
11.66±1.58b |
| SF |
20.77±2.38a |
29.00±3.20a | 13.88±2.08 |
| Control | 15.11±1.61 | 16.11±1.53 | 0.00 |
In the SSF group, BrdU positive cells were scattered
within SF material in the urethral defect 2, 4 and 6 weeks after
surgery, which were more obvious at the intersection between this
material and the urethra (Fig. 4).
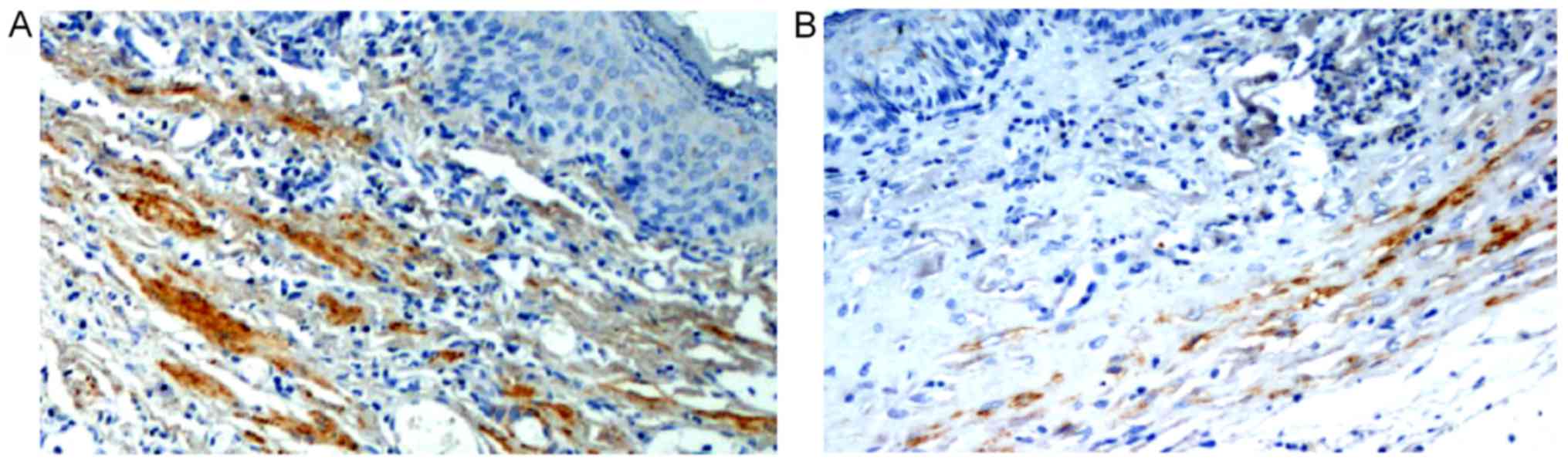
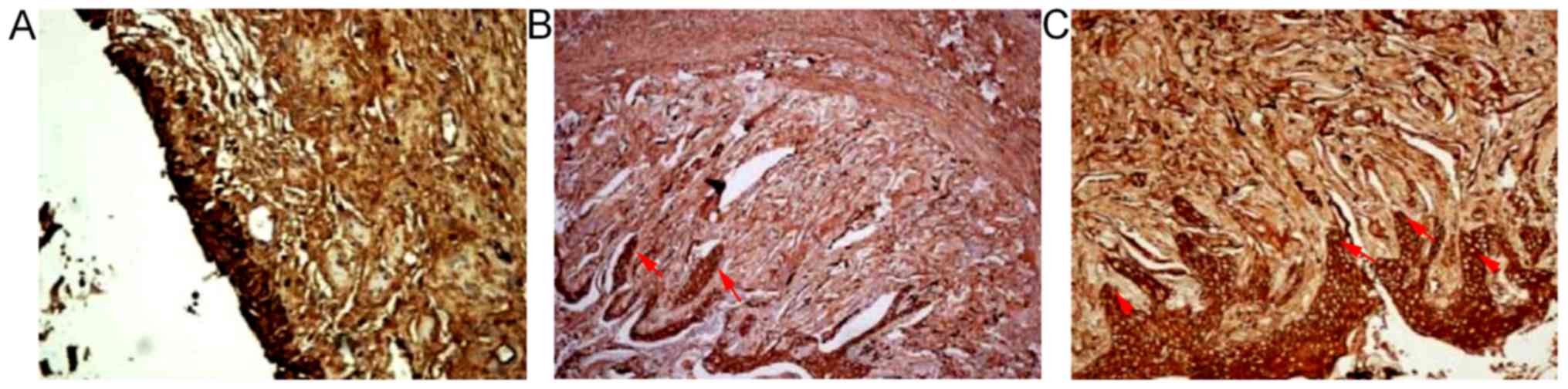
There were FVIII-RAg positive cells in the urethral defects of the
three groups 2, 4 and 6 weeks after surgery. There were
considerable FVIII-RAg positive cells under the mucosae of both SSF
and SF groups 4 and 6 weeks after surgery. The numbers of positive
cells in SSF and SF groups were (23.44±2.40)/HP and (20.77±2.38)/HP
respectively 4 weeks after surgery, both of which were
significantly higher than that of the control group
((15.11±1.61)/HP) (P<0.01). The number of the SSF group
significantly exceeded that of the SF group (P<0.05) (Fig. 5; Table II).
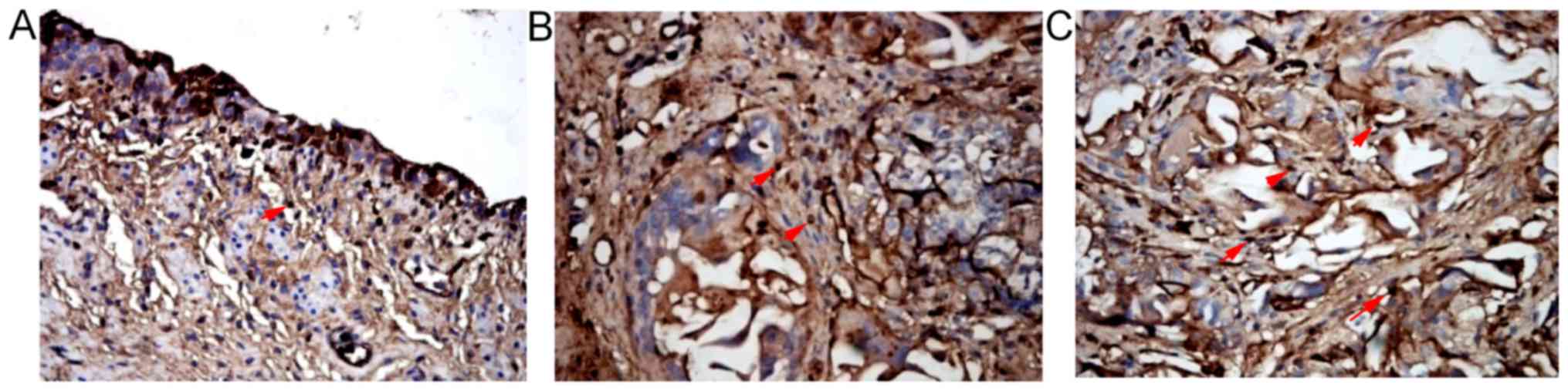
There were α-SMA positive cells in the urethral
defects of the three groups 2, 4 and 6 weeks after surgery. A large
number of α-SMA positive cells were observed at the intersections
of SF materials with the urethra in SSF and SF groups 4 and 6 weeks
after surgery. The numbers of α-SMA positive cells in SSF and SF
groups were (33.00±3.27)/HP and (29.00±3.20)/HP respectively 4
weeks after surgery, which were significantly higher than that of
the control group at the same time point ((16.11±1.53)/HP)
(P<0.01). The number of the SSF group was significantly higher
than that of the SF group (P<0.05) (Fig. 6; Table II). Pan-cytokeratin (AE1/AE3) in
both SSF and SF groups was stained positive. The cytoplasm, which
was stained brown uniformly, was reticular under the
high-magnification microscope, like normal urethral mucosa. It also
contained many papillary structures. However, compared with normal
urethral mucosa, the urethral mucosa of the control group had
yellow cytoplasm. The epithelial cells composed a single layer,
lacking a multilayer columnar epithelium or a papillary mucosa. In
contrast, normal urethral mucosa had positive pan-cytokeratin
staining, and the cytoplasm was brown (Fig. 7).
Discussion
Autologous substitutes, such as acellular matrix,
tunica vaginalis, oral mucosa and small intestinal submucosal
tissue, have commonly been used to repair urethral defect. However,
they all have disadvantages, such as prolonged surgical time and
hospital stay, aggravated trauma, urethral stricture and fistula
(13–15).
Shokeir et al (16) repaired a 3-cm defect in the canine
urethra by using an acellular matrix material, and found urethral
stricture in experimental group by urethrography. Subsequently, Hu
et al (17) repaired a
rabbit urethral defect of 1.5×1.0 cm by employing a urethral
extracellular matrix, and found that the urethral TNF-α level of
experimental group exceeded that of control group. Bhargava et
al (7) evaluated the clinical
outcomes of repair using oral mucosa, and concluded that it was
unsuitable for repairing large urethral defect. El-Assmy et
al (18) treated a rabbit
model with a commercially available small intestinal submucosal
tissue. As a result, the experimental group suffered from urethral
fistula and obvious hyperplasia of fibrous tissues. In addition,
there were no smooth muscle bundles in tissues, so the clinical
application of small intestinal submucosal tissue was
controversial. The development of tissue engineering has shed a new
light on addressing the problems mentioned above (19,20).
Dal Pra et al (21)
implanted SF material in subcutaneous tissue, and found a large
number of vascular reticular connective tissues therein on the
180th day, with a small number of macrophages. There was no
lymphocyte infiltration or formation of fibrous capsule. Herein, we
found that significantly more new blood vessels formed in SF
material of the SSF group than in the SF group. Additionally, Fuchs
et al (22) found that
after peripheral vascular endothelial progenitor cells and SF
material were cultured for 4 weeks in vitro, the vascular
structure began to form 1 week later, and the vessel-derived matrix
(collagen) was evidently deposited on the surface of SF material.
With extended culture time, the vascular area and length as well as
the number of blood vessels all increased. SF material was
infiltrated with only a few lymphocytes in the urethra, with mild
inflammatory reaction. In both SSF and SF groups, a small number of
lymphocytes infiltrated in vivo after repair of the urethra,
but the control group had considerable lymphocyte infiltration.
Panilaitis et al (23)
found that SF induced minimal inflammation, but SF particles with
diameters of 10–200 µm significantly stimulated macrophages to
release TNF-α. Thus, the role of SF material in macrophages was
limited by its size, shape and interaction with other molecules.
Until now, the inflammatory response to SF materials implanted with
stem cells has seldom been studied. In this study, the number of
macrophages in the SSF group was significantly lower than that of
the SF group. The inflammatory response of the SSF group was
significantly milder than that of the SF group, indicating that the
response was alleviated due to the interaction between ADMSCs and
SF material, the surrounding microenvironment as well as the
material surface characteristics, size and chemical composition.
Moreover, SF material composited with ADMSCs further mitigated the
inflammatory response of tissues.
Large amounts of smooth muscle tissues were observed
in the SF material, and the number of urethral smooth muscle cells
in the SSF group exceeded that of the SF group. Besides, basic
urethral structures formed in both groups. There were many smooth
muscles at the periphery of SF material. The interaction between
the urethral epithelium and mesenchymal tissue may be conducive to
the formation of urethral smooth muscle. Meanwhile, the labeled
ADMSCs existed for a long time, which may also play an important
role in smooth muscle regeneration. In a specific microenvironment,
stem cells may differentiate into smooth muscle cells or promote
their proliferation through the paracrine effect. In the SF group,
smooth muscles and blood vessels grew markedly on SF material in
the urethral defect, without obvious decomposition. Contrarily, in
the SSF group, SF material decomposed into large pieces 6 weeks
after surgery, possibly owing to enzymatic reaction. Accordingly,
we postulated that stem cells may promote the secretion of
proteolytic enzymes in vivo, accelerating the decomposition
and segmentation of SF material. In both groups, SF material was
mostly exposed on the urethra luminal surface 2 weeks after
surgery, lacking epithelial coverage. New tissues only grew at the
bottom, and SF material was not fixed tightly. Four and six weeks
after surgery, a small amount of SF material shed off, with the
formation of many new tissues. Urethral epithelial cells were not
observed 2 weeks after repair, and SF material was exposed in the
urethra. In the fourth week, urethral epithelium formed in both SF
and SSF groups, and SF scaffolds were sufficiently immobilized,
indicating that tissues began to grow into the gap between SF
materials from the second to the fourth week. Therefore, it was
necessary to extend the cathetering time to enhance the binding of
SF material to the urethra. The urine of normal rabbits contained
many phosphate crystals that easily blocked the catheter, so
nitrofurazone was used to wash the bladder, with intravenous
infusion with an appropriate amount of glucose-sodium chloride
solution. As a result, the urine was effectively diluted,
preventing possible infection and urethral obstruction. Stratified
columnar urethral epithelium structures formed in the urethral
defects of both SSF and SF groups 6 weeks after surgery, like
normal urethral mucosa. However, the epithelial cells in the SSF
group were arranged more orderly, probably because ADMSCs promoted
the growth of adjacent urethral mucosal cells along the SF material
surface.
In summary, SF material had high biocompatibility
with ADMSCs. Repairing urethral defect by ADMSCs-composited SF
material induced the formation of a large number of blood vessels,
and promoted the growth of smooth muscle tissues, with mild
inflammatory reaction. Furthermore, it restored the basic functions
of the urethra by benefiting the formation of urethral epithelial
cells. Hence, it is feasible to apply the composite ADMSCs-SF
material to repair urethral defect safely.
Acknowledgements
Not applicable.
Funding
The present study was supported by Shanghai
Municipal Commission of Health and Family Planning Research
Projects (grant no. 20144310).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
BT, LS, TL, ZL, XY and QF performed the study and
analyzed experimental data; BT and YL designed the study and
prepared the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Care and Use Committee of Shanghai Jiao Tong University Affiliated
Sixth People's Hospital (Shanghai, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li C, Xu YM, Liu ZS and Li HB: Urethral
reconstruction with tissue engineering and RNA interference
techniques in rabbits. Urology. 81:1075–1080. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang F, Liu T, Yang L, Zhang G, Liu H, Yi
X, Yang X, Lin TY, Qin W and Yuan J: Urethral reconstruction with
tissue-engineered human amniotic scaffold in rabbit urethral injury
models. Med Sci Monit. 20:2430–2438. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Atala A, Bauer SB, Soker S, Yoo JJ and
Retik AB: Tissue-engineered autologous bladders for patients
needing cystoplasty. Lancet. 367:1241–1246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: Implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li CY, Wu XY, Tong JB, Yang XX, Zhao JL,
Zheng QF, Zhao GB and Ma ZJ: Comparative analysis of human
mesenchymal stem cells from bone marrow and adipose tissue under
xeno-free conditions for cell therapy. Stem Cell Res Ther.
6:552015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lotfy A, Salama M, Zahran F, Jones E,
Badawy A and Sobh M: Characterization of mesenchymal stem cells
derived from rat bone marrow and adipose tissue: A comparative
study. Int J Stem Cells. 7:135–142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhargava S, Patterson JM, Inman RD,
MacNeil S and Chapple CR: Tissue-engineered buccal mucosa
urethroplasty-clinical outcomes. Eur Urol. 53:1263–1269. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lv X, Li Z, Chen S, Xie M, Huang J, Peng
X, Yang R, Wang H, Xu Y and Feng C: Structural and functional
evaluation of oxygenating keratin/silk fibroin scaffold and initial
assessment of their potential for urethral tissue engineering.
Biomaterials. 84:99–110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Filippo RE, Kornitzer BS, Yoo JJ and
Atala A: Penile urethra replacement with autologous cell-seeded
tubularized collagen matrices. J Tissue Eng Regen Med. 9:257–264.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murphy AR, John PS and Kaplan DL:
Modification of silk fibroin using diazonium coupling chemistry and
the effects on hMSC proliferation and differentiation.
Biomaterials. 29:2829–2838. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kundu B, Rajkhowa R, Kundu SC and Wang X:
Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv
Rev. 65:457–470. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koh LD, Cheng Y, Teng CP, Khin YW, Loh XJ,
Tee SY, Low M, Ye E, Yu HD, Zhang YW and Han MY: Structures,
mechanical properties and applications of silk fibroin materials.
Prog Polym Sci. 46:86–110. 2015. View Article : Google Scholar
|
|
13
|
Raya-Rivera A, Esquiliano DR, Yoo JJ,
Lopez-Bayghen E, Soker S and Atala A: Tissue-engineered autologous
urethras for patients who need reconstruction: An observational
study. Lancet. 377:1175–1182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chun SY, Kim BS, Kwon SY, Park SI, Song
PH, Yoo ES, Kim BW, Kwon TG and Kim HT: Urethroplasty using
autologous urethral tissue-embedded acellular porcine bladder
submucosa matrix grafts for the management of long-segment urethral
stricture in a rabbit model. J Korean Med Sci. 30:301–307. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim BS, Kim HT, Kwon SY, Chun SY, Choi KH,
Park M, Kim DH, Song PH and Kwon TG: Nontransected ventral
onlay-augmented urethroplasty using autologous saphenous vein graft
in a rabbit model of urethral stricture. Urology. 83:225–231. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shokeir A, Osman Y, El-Sherbiny M, Gabr M,
Mohsen T and El-Baz M: Comparison of partial urethral replacement
with acellular matrix versus spontaneous urethral regeneration in a
canine model. Eur Urol. 44:603–609. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu YF, Yang SX, Wang LL and Jin HM:
Curative effect and histocompatibility evaluation of reconstruction
of traumatic defect of rabbit urethra using extracellular matrix.
Chin J Traumatol. 11:274–278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
El-Assmy A, El-Hamid MA and Hafez AT:
Urethral replacement: A comparison between small intestinal
submucosa grafts and spontaneous regeneration. BJU Int.
94:1132–1135. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sartoneva R, Nordback PH, Haimi S, Grijpma
DW, Lehto K, Niall R, Seppänen-Kaijansinkko R, Miettinen S and
Lahdes-Vasama T: Comparison of poly (L-lactide-co-ε-caprolactone)
and poly (trimethylene carbonate) membranes for urethral
regeneration: An in vitro and in vivo study. Tissue Eng Part A.
24:117–127. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Osman NI, Hillary C, Bullock AJ, MacNeil S
and Chapple CR: Tissue engineered buccal mucosa for urethroplasty:
Progress and future directions. Adv Drug Deliv Rev. 82–83:69–76.
2015. View Article : Google Scholar
|
|
21
|
Dal Pra I, Freddi G, Minic J, Chiarini A
and Armato U: De novo engineering of reticular connective tissue in
vivo by silk fibroin nonwoven materials. Biomaterials.
26:1987–1999. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fuchs S, Jiang X, Schmidt H, Dohle E,
Ghanaati S, Orth C, Hofmann A, Motta A, Migliaresi C and
Kirkpatrick CJ: Dynamic processes involved in the
pre-vascularization of silk fibroin constructs for bone
regeneration using outgrowth endothelial cells. Biomaterials.
30:1329–1338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Panilaitis B, Altman GH, Chen J, Jin HJ,
Karageorgiou V and Kaplan DL: Macrophage responses to silk.
Biomaterials. 24:3079–3085. 2003. View Article : Google Scholar : PubMed/NCBI
|