Introduction
Glioblastoma multiforme (GBM) is the most common
malignant tumour of central nervous system (CNS), and it currently
remains incurable. Despite drastic treatment methods, including
maximal safe resection followed by radiotherapy in combination with
temozolomide, the patient survival rate has not effectively
improved, with a 5-year survival rate of only 5.5% being observed
(1,2). Therefore, more refined studies are
vital to elucidate the potential mechanism of therapeutic
tumourigenesis and resistance of this malignancy.
In the previous classification scheme of gliomas,
GBM were commonly diagnosed as astrocytomas or oligodendrogliomas
based on their morphological resemblance and further distinguished
by malignant grades (I to IV) according to cellular features
(proliferation, angiogenesis and necrosis) (3). However, this partition method is
highly subjective and inconsistent in its designation in glioma
subtypes and grades (4). In
addition, the pathological criteria diagnosis of gliomas especially
GBMs, which has caused artificial heterogeneity and complexities in
investigations, did not bring valid effects to our clinical
management (5). Recent studies
focused on the molecule-based GBM classification which benefited
from the availability of the datasets generated by The Cancer
Genome Atlas (TCGA) workgroup have established the groundwork for a
better understanding of the etiology and improved personalized
therapy for subgroup patients (6–9). In
2016, the new WHO classification of diffuse gliomas was refined,
and formally brought the histomolecular conception into our sight
for the first time, which incorporated 1p/19q codeletion, IDH1/2
mutation, and histone H3-K27M mutation into previous diagnostic
criteria (10–12).
Because of robust gene expression, TCGA classified
GBMs into four subtypes: proneural, neural, classical and
mesenchymal. Each subtype differs greatly in terms of its cellular
features, genetic contexts and signalling pathways involved
(7). Due to a more aggressive
biological nature and higher overall fraction of necrosis evident
in these tumours, the mesenchymal group is usually placed in a more
malignant base (7,13). It is proposed that not a single
molecule but the alterations of a small regulatory module can
induce and maintain a specific phenotypic state in glioma cells
(14). For instance, the
activation of a small group of hub genes may facilitate mesenchymal
transformation, which is characterized by extensive necrosis,
angiogenesis, and an enhanced inflammatory/immune response
(15,16).
CXCR4, a cell surface chemokine receptor, is
involved in many cell fate decisions, such as growth, invasion,
angiogenesis and metastasis in a wide range of malignant cancers,
including leukaemia, breast cancer and, recently, in glioma
(17–19). In this study, we clarified the
prognosis and clinical significance of CXCR4 in glioma and observed
that CXCR4 has shown to be selectively overexpressed in the
mesenchymal subtype compared with other phenotypes of GBMs, which
indicates that CXCR4 may participate in phenotype transformation of
the mesenchymal GBMs.
Methods to classify tumours according to key
molecular events that manage growth of their most aggressive
cellular component and to search for the genetic alterations that
accompany disease recurrence might greatly facilitate development
of targeted therapies (13,20).
In the present study, we aimed to investigate the associations and
uncover the critical pathways that CXCR4 mediated in mesenchymal
glioblastoma using computational methods to analyse the
relationship between a wide range of expression patterns and the
activation of specific hub genes or pathways.
Materials and methods
Glioma specimen and brain tissue
collection
Glioma surgical specimens were collected in Tianjin
Medical University General Hospital between October 2011 and
November 2017 in accordance with institution-approved protocols.
All patients signed and approved consent forms prior to the surgery
and the study was approved by the Ethics Committee of Tianjin
Medical University Hospital (Tianjin, China). These tissue samples
were analyzed retrospectively in the present study. Collected
specimens were split into two parts for 4% paraformaldehyde (PFA)
fixation/cryo-sectioning and primary tissues culture establishment
respectively. Specimens were examined by pathologists to verify
tumour types and grades.
Analysis of glioma patients' survival
and expression data
Tumour gene expression and clinical data of glioma
patients were retrieved from The Cancer Genome Atlas (TCGA) and
French dataset which were available on R2 analysis and
visualization platform (http://hgserver1.amc.nl/cgi-bin/r2/main.cgi). Patients
were classified into CXCR4low and
CXCR4high expression groups by the mean
expression levels.
Differential expression genes analysis
of TCGA microarray data
The gene expression profiles of TCGA were downloaded
from UCSC Xena Browser (https://xenabrowser.net/heatmap/), which contains 539
GBM samples including 145 classical subtype, 158 mesenchymal
subtype, 87 neural subtype and 139 proneural subtype samples.
Morpheus (https://software.broadinstitute.org/morpheus/) online
software was used to perform heat maps, and differential expression
genes (DEGs) were determined using a threshold P-value of 0.05.
Immunohistochemistry staining
Paraffin embedded tumour tissues were sectioned at 6
µm, for immunohistochemistry After quenching the endogenous
peroxidase activity and blocking with normal goat serum, sections
were incubated sequentially with the primary antibodies at 4°C
overnight, the next day, after rewarming for 1 h, sections were
incubated with secondary antibodies (ZSGB-Bio, Beijing, China) for
1 h at 37°C. Immunostaining was performed using DAB kit (ZSGB-Bio),
which resulted in a brown precipitate at the antigen site.
Subsequently, sections were counterstained with Mayer Haematoxylin
solution (ZSGB-Bio) and mounted in mounting medium. After
dehydration, sections were examined using a light microscope.
Protein expression levels were quantified on the basis of a
multiplicative index of staining extent (0–3) and the average
staining intensity (0–3). The staining score is the product of
staining extent and the staining intensity.
Gene ontology and pathway enrichment
analysis
DAVID database (https://david.ncifcrf.gov/) is an essential foundation
for the success of any high-throughput gene functional analysis. To
understand the significance of genes, we performed the Gene
Ontology (GO) classification, making use of the following
categories: BP_Fat (biological process), CC_Fat (cellular
component), and MF_Fat (molecular function). We also performed the
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment
analysis to detect the potential pathway of target genes and had
the hypergeometric test with P-value <0.05. GO enrichment and
KEGG pathway analysis were performed using the DAVID online tool.
P<0.05 was considered to indicate a statistically significant
difference.
Statistical analysis
The Kaplan-Meier survival analysis was used to
estimate the survival distributions, and the log-rank test was used
to assess the statistical significance between stratified survival
groups using the mean value as the cut-off. The Pearson correlation
array was performed to determine significant differences. One-way
ANOVA was used to test for differences among at least 3 groups. The
Newman-Keuls multiple comparisons test was performed after ANOVA.
The t-test was used to determine differences in each 2-group
comparison. All data are presented as mean ± standard error.
Results
CXCR4 is a strong predictor of poor
prognosis in GBM patients
To testify whether alterations at the genetic locus
of CXCR4 could be implicated as a predictor in GBM patients
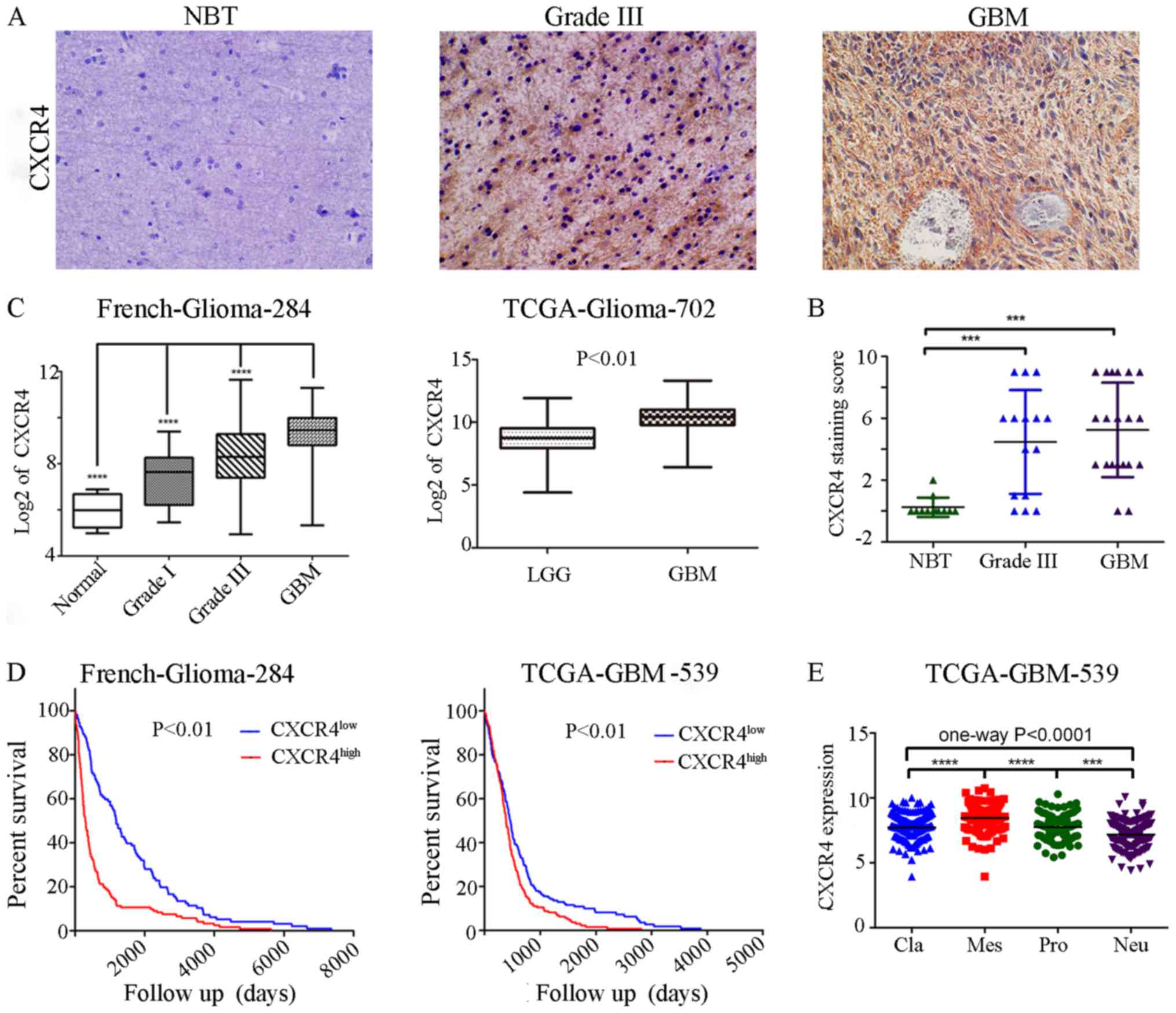
prognosis. We checked CXCR4 expressions in various glioma specimens
(12 normal brain tissues (NBT), 15 grade III and 20 GBMs) on a
tissue array using immunohistochemistry followed by quantitation.
As a result, in contrast to negligible CXCR4 expressions in NBT,
profound CXCR4 expression can be seen in high grade glioma (HGG,
WHO Grade III–IV) samples, especially in GBMs (WHO Grade IV)
(Fig. 1A). At the same time, CXCR4
owns a distinctly 85.7% (30/35) to 16.7% (2/12) positive expression
samples between HGG and NBT (Fig.
1B). TCGA and French datasets retrieved from the R2 genomics
analysis and visualization platform also showed that compared to
lower grade glioma or normal brain tissue, the highest expression
levels were detected in GBMs (Fig.
1C). TCGA and French datasets was employed to evaluate the
effects of CXCR4 on overall patient survival using the KM curve.
The mRNA expression levels of CXCR4 from the two databases were
used to classify patients into upregulation and downregulation
groups according to their mean expression values. Compared to the
downregulation CXCR4 cases, patients with high levels of CXCR4 were
associated with a significantly shorter overall survival time
(Fig. 1D). These data indicated
that CXCR4 expression correlates with glioma grade at the protein
and transcriptional level and is a strong predictor of poor
prognosis in GBM patients.
CXCR4 is a clinical prognostic factor
in glioma patients
In the French glioma dataset (n=284), a high
expression of CXCR4 was remarkably associated with an older age at
diagnosis, shorter overall survival years, non IDH1 mutation, lower
expression of PTEN, and a high expression of KI67 and EGFR
amplification (Table I). While in
the TCGAs 539 GBM samples, a high expression of CXCR4 was
selectively associated with a shorter overall survival and days to
recurrence (Table II). Consistent
with the theory that recurrence of glioblastoma after
radio-chemotherapy is associated with an angiogenic switch to the
CXCL12-CXCR4 pathway (21),
CXCR4high patients experience a shorter days to
recurrence. These results demonstrate that CXCR4 may be involved in
the recurrence of the glioma and could be conferred as a new
clinical prognostic factor of GBM.
 | Table I.Clinical and molecular pathology
features of French glioma samples in association with CXCR4
expression. |
Table I.
Clinical and molecular pathology
features of French glioma samples in association with CXCR4
expression.
| Variables | Low | High | P-value |
|---|
| Age | 46.1±13.4 | 53.4±14.9 |
<0.0001a |
| Gender,
female/male | 43/72 | 47/106 | 0.2283b |
| KPS ≥80/<80 | 82/28 | 100/54 | 0.1229b |
| OS, years ± SD | 4.1±4.0 | 1.7±2.7 |
<0.0001c |
| Resection
complete/partial | 40/57 | 46/89 | 0.3643b |
| IDH1 mutation/no
mutation | 50/49 | 33/94 | 0.0001b |
| 1p mutation/no
mutation | 39/39 | 11/59 | 0.5433b |
| 19q mutation/no
mutation | 39/38 | 13/57 | 0.6312b |
| KI67 low/high | 66/57 | 52/107 | 0.0009b |
| PTEN low/high | 42/81 | 93/68 |
<0.0001b |
| EGFR
amplification/wild | 12/63 | 31/45 | 0.0004b |
| EGFR low/high | 55/68 | 83/78 | 0.8005b |
 | Table II.Clinical and molecular pathology
features of TCGA GBM samples in association with CXCR4
expression. |
Table II.
Clinical and molecular pathology
features of TCGA GBM samples in association with CXCR4
expression.
| Variables | Low | High | P-value |
|---|
| Age (years ±
SD) | 56.4±13.1 | 58.6±13.1 | 0.1496a |
| Gender,
female/male | 75/136 | 122/178 | 0.2424b |
| OS (days ± SD) | 521.8±391.9 | 413.5±610.2 | 0.0129c |
| KPS ≥80/<80 | 117/80 | 146/124 | 0.2536b |
|
Treated/untreated | 10/201 | 10/289 | 0.4251b |
| Radiation therapy,
yes/no | 20/170 | 33/247 | 0.6726b |
| Chemo therapy,
yes/no | 58/112 | 105/157 | 0.4703b |
| Days to progression
± SD | 311.8 to pro | 242.3 to pro | 0.1140a |
| Days to recurrence
± SD | 515.6 to 1.0 | 257.3 to 1.0 | 0.0036a |
| MGMT promoter
methylation (unmethylation/methylation) | 9/17 | 13/46 | 0.0573b |
CXCR4 is preferentially expressed in
mesenchymal subtype of glioma
The Cancer Genome Atlas (TCGA) network described a
robust gene expression based molecular classification. We retrieved
539 GBM samples from TCGA, including 145 classical subtype, 158
mesenchymal subtype, 87 neural subtype and 139 proneural subtype
samples. One-way ANOVA indicated a markedly significant difference
in CXCR4 expression between the four glioma subtypes in the TCGA
datasets. In particular, CXCR4 is preferentially expressed in the
mesenchymal subtype of glioma with a notable statistical
significance (P<0.0001; Fig.
1E). Similarly, to confirm the CXCR4 as a biomarker of
mesenchymal subtype of glioma, we further analysed the association
between CXCR4 and the mesenchymal markers such as CHI3L1 (also
known as YKL40), MET, CD44, MERTK and NF-κB pathway genes (TRADD,
RELB, TNFRSF1A) using a Pearson correlation array (7). Likewise, CXCR4 was positively
correlated with MES markers (CHI3L1, R=0.3959; MET, R=0.1267; CD44,
R=0.5432; MERTK, R=0.4662; TLR2, R=0.6143; TLR4, R=0.4136; TRADD,
R=0.4343; RELB, R=0.2583; TNFRSF1A, R=0.4676) (Fig. 2). These results foreshadowed that
CXCR4 acts as a marker for the glioma molecular subtype and may
regulate gene expression patterns in glioma mesenchymal
transition.
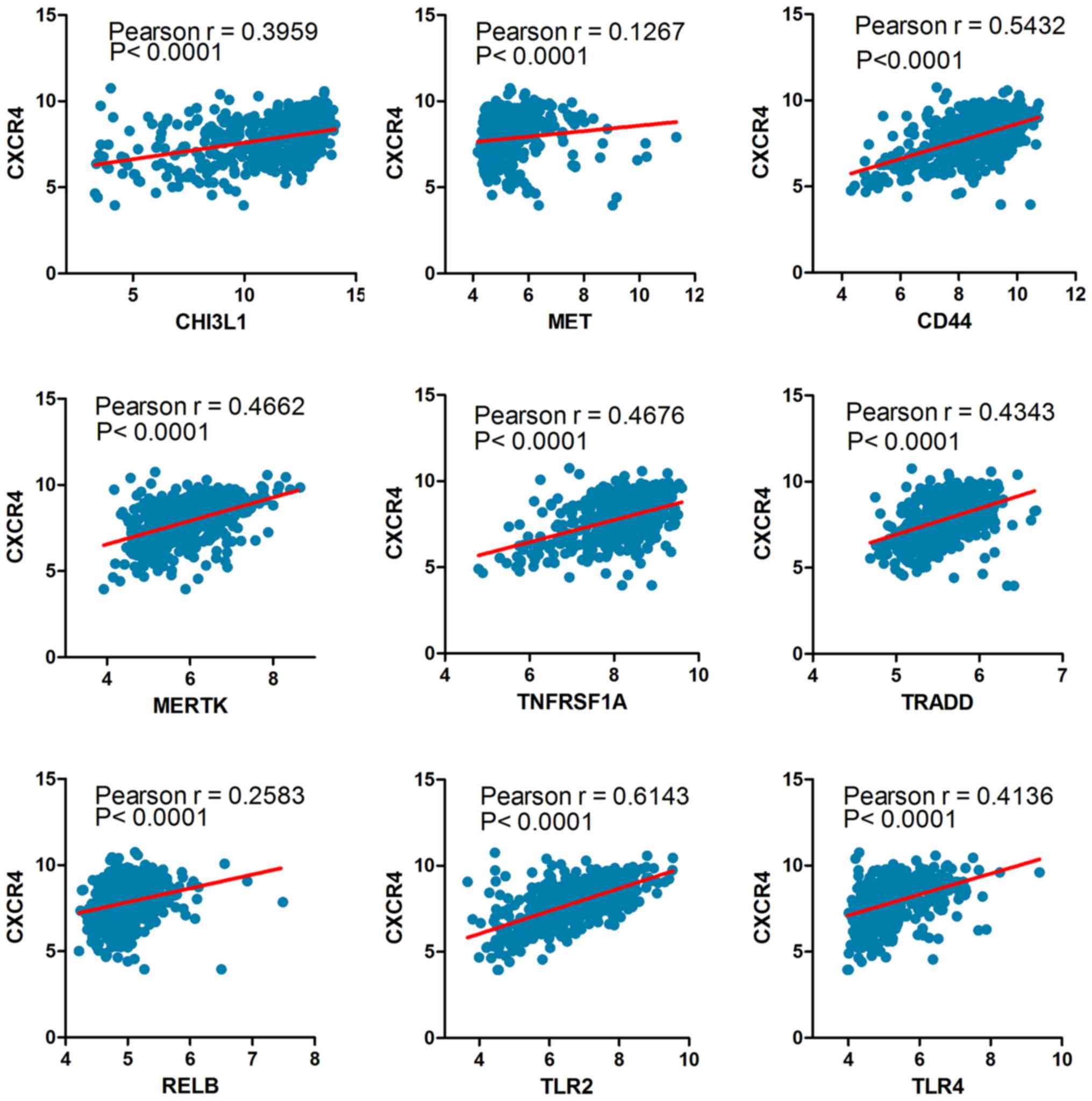 | Figure 2.The Pearson correlation coefficient
between CXCR4 and mesenchymal markers. TLR, toll-like receptor;
CHI3L1, Chitinase 3 Like 1; MET, MET proto-oncogene, receptor
tyrosine kinase; CD, cluster of differentiation; MERTK, MER
proto-oncogene, tyrosine kinase; TRADD, TNFRSF1A associated via
death domain; RELB, RELB proto-oncogene; TNFRSF1A, TNF receptor
superfamily member 1A. |
Selection of genes associated with
CXCR4 signalling in mesenchymal subtype of GBM
To study the underlying gene and pathway pattern
that CXCR4 regulated in mesenchymal glioma. First, the students
t-test was performed on the DEGs to test the difference between the
mesenchymal subtype and other subtypes of glioblastoma. The
differential expression was determined using a threshold P-value of
0.05, and the top 100 genes were shown in the heatmaps (Fig. 3A-C). Next, we selected 3198 genes
(Fig. 3D) that were highly related
in CXCR4high patients (classified by the mean value of
CXCR4 mRNA expression) of mesenchymal GBM, namely, CXCR4 correlated
genes in mesenchymal glioblastoma (CCGIM). Therefore, we found that
4004 genes that were differentially expressed between the classical
subtype and the mesenchymal subtype, 3455 genes that were
differentially expressed between the neural subtype and the
mesenchymal subtype and 3289 genes were differentially expressed
between the proneural subtype and the mesenchymal subtype. Then, we
compared the genes that were differentially expressed in
mesenchymal CXCR4high patients with the genes that were
differentially expressed between mesenchymal and three other
subtypes (classical, neural, proneural), which comprised 838, 1397,
1187 overlapping genes respectively (Fig. 3E-G). Finally, we compared the
abovementioned 838, 1397 and 1187 genes that overlapped between
groups to identify DEGs that were specific to the mesenchymal
CXCR4high subgroup, yielding a total of 34 genes
(Fig. 3H).
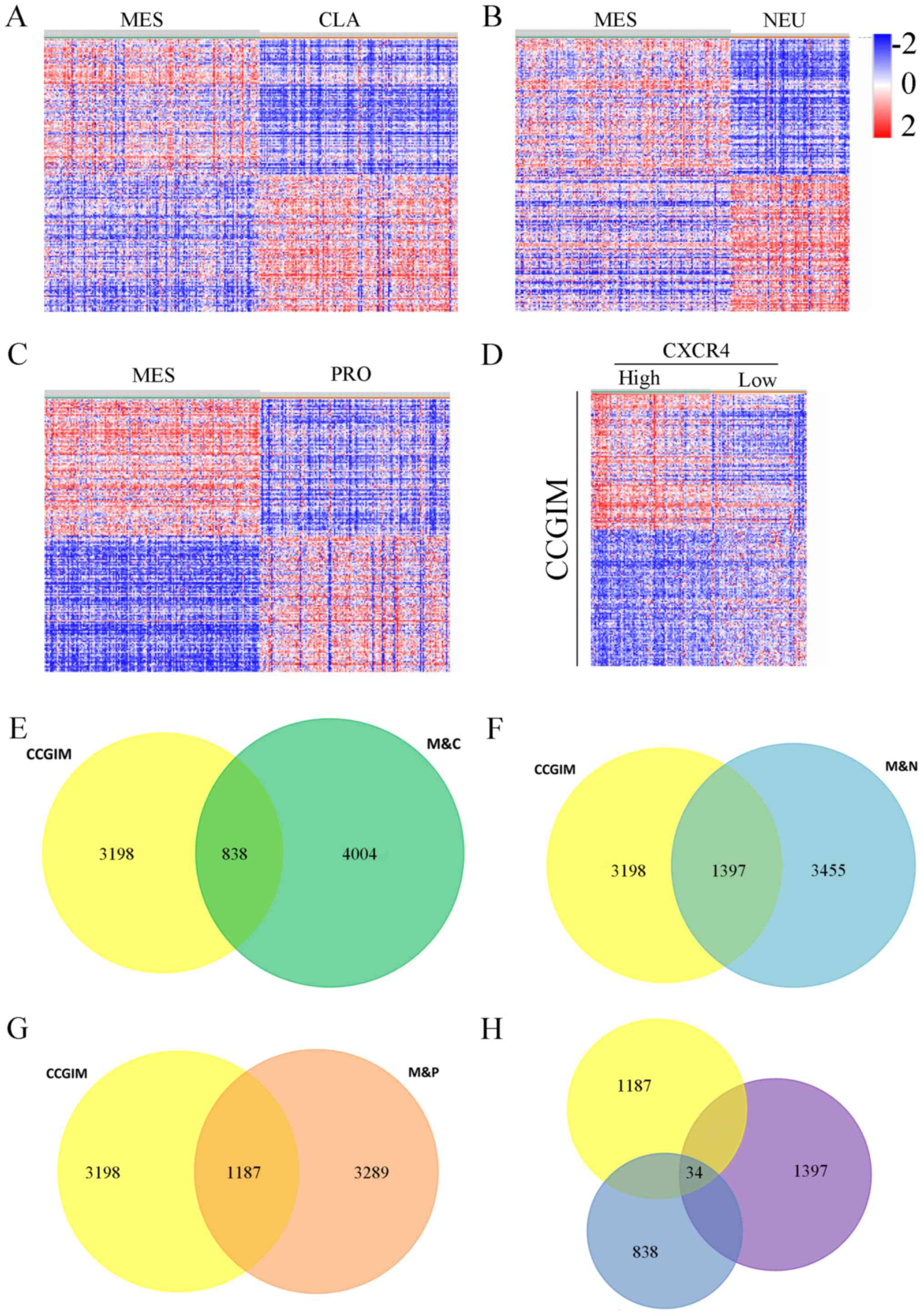 | Figure 3.The heat maps displaying the DEGs
between MES and (A) CLA, (B) NEU and (C) PRO subtypes of GBM. (D)
Heat map displaying the DEGs between the CXCR4high and
CXCR4low group in MES GBM. (E) Total of 3198 CCGIM
subtype were compared with 4004 genes DEGs between the mesenchymal
and classical groups, yielding a set of 838 overlapping genes. (F)
3455 DEGs expressed between the mesenchymal and neural groups,
yielding a set of 1397 overlapping genes. (G) 3289 DEGs between the
mesenchymal and proneural groups, yielding a set of 1187
overlapping genes. (H) A comparison of the 838, 1397 and 1187 genes
revealed 34 common genes specific to the CXCR4 correlated
mesenchymal subgroup. M&C, the overlap between mesenchymal and
classical subtype; M&N, the overlap between mesenchymal and
neural subtype; M&P, the overlap between mesenchymal and
proneural subtype; CCGIM, CXCR4 correlated genes in the mesenchymal
GBM; GBM, glioblastoma; DEG, different expressed genes. |
Gene ontology and KEGG pathway
analysis of CXCR4 associated genes in mesenchymal GBM
We uploaded all 34 DEGs to the online software DAVID
to identify overrepresented Gene Ontology (GO) categories and KEGG
pathways. The GO categories and KEGG pathways were ranked by
P-value, and the top 5 items were displayed in the table. GO cell
component analysis displayed that CXCR4 associated genes were
significantly enriched in the golgi apparatus, cytoplasmtic
membrane-bounded vesicle, vacuole, golgi apparatus part and
endoplasmic reticulum with a notable statistical significance of
P<0.05. For biological processes (BP) CXCR4 associated genes
were significantly enriched in regulation of MAP kinase activity,
regulation of protein serine/threonine kinase activity, regulation
of protein kinase activity, regulation of kinase activity and
mitogen-activated protein kinase (MAPK) cascade, while in the GO
molecular function (MF) analysis no item was involved with a
threshold of P<0.05 (Table
III). Table III also
contains the most significantly enriched pathways of the CXCR4
associated genes in mesenchymal GBM analyzed by KEGG analysis. The
34 DEGs were enriched in melanoma, prostate cancer, pathways in
cancer, protein processing in endoplasmic reticulum and regulation
of actin cytoskeleton. In collection, Gene ontology and KEGG
pathway analyses demonstrated that MAPK signalling pathway is
associated with CXCR4 activation in the mesenchymal subtype.
Meanwhile, the activation of CXCR4 is also enriched in melanoma and
prostate cancer, which demonstrated there might be a co-therapeutic
target or strategies in the management of melanoma, prostate cancer
and mesenchymal GBM.
 | Table III.Gene ontology and KEGG pathway
analysis of CXCR4 correlated genes in mesenchymal glioblastoma. |
Table III.
Gene ontology and KEGG pathway
analysis of CXCR4 correlated genes in mesenchymal glioblastoma.
| Category | Term, function | Count | Percentage | P-value | Genes |
|---|
| GOTERM_CC_FAT | GO:0005794, golgi
apparatus | 13 | 23.6 |
7.78×10−6 | NCSTN, NUCB1,
SPRY1, GANAB, ARFRP1, PDGFC, ADAM19, TM9SF4, TRIP10, KDELR1, SPRY4,
HS2ST1, AP3B1 |
| GOTERM_CC_FAT | GO:0016023,
cytoplasmic, membrane-bounded vesicle | 7 | 12.7 |
2.30×10−2 | NCSTN, NUCB1,
FGFR1, GANAB, SPRED2, KDELR1, AP3B1 |
| GOTERM_CC_FAT | GO:0005773,
vacuole | 7 | 12.7 |
2.55×10−2 | NCSTN, NUCB1,
TM9SF1, TRAF6, TM9SF4, TRIP10, AP3B1 |
| GOTERM_CC_FAT | GO:0044431, golgi
apparatus part | 6 | 10.9 |
2.81×10−2 | NUCB1, ARFRP1,
PDGFC, TRIP10, KDELR1, HS2ST1 |
| GOTERM_CC_FAT | GO:0005783,
endoplasmic reticulum | 8 | 14.5 |
3.44×10−2 | NCSTN, NUCB1,
RAB3GAP2, GANAB, BAX, PDGFC, EDEM3, KDELR1 |
| GOTERM_BP_FAT | GO:0043405,
regulation of MAP kinase activity | 6 | 10.9 |
2.70×10−4 | FGFR1, SPRY1,
SPRED2, PDGFC, TRAF6, SPRY4 |
| GOTERM_BP_FAT | GO:0071900,
regulation of protein serine/threonine kinase activity | 6 | 10.9 |
1.48×10−3 | FGFR1, SPRY1,
SPRED2, PDGFC, TRAF6, SPRY4 |
| GOTERM_BP_FAT | GO:0045859,
regulation of protein kinase activity | 7 | 12.7 |
1.68×10−3 | FGFR1, SPRY1, BAX,
SPRED2, PDGFC, TRAF6, SPRY4 |
| GOTERM_BP_FAT | GO:0043549,
regulation of kinase activity | 7 | 12.7 |
2.47×10−3 | FGFR1, SPRY1, BAX,
SPRED2, PDGFC, TRAF6, SPRY4 |
| GOTERM_BP_FAT | GO:0000165, MAPK
cascade | 7 | 12.7 |
3.42×10−3 | FGFR1, SPRY1, ARAF,
SPRED2, PDGFC, TRAF6, SPRY4 |
| GOTERM_MF_FAT | GO:0042802,
identical protein binding | 6 | 10.9 |
8.25×10−2 | BCAT1, FGFR1, BAX,
PDGFC, TRAF6, TRIP10 |
| KEGG_PATHWAY | hsa05218,
melanoma | 3 | 5.4 |
8.75×10−3 | FGFR1, ARAF,
PDGFC |
| KEGG_PATHWAY | hsa05215, prostate
cancer | 3 | 5.4 |
1.32×10−2 | FGFR1, ARAF,
PDGFC |
| KEGG_PATHWAY | hsa05200, pathways
in cancer | 4 | 7.3 |
4.16×10−2 | FGFR1, BAX, ARAF,
TRAF6 |
| KEGG_PATHWAY | hsa04141, protein
processing in endoplasmic reticulum | 3 | 5.4 |
4.46×10−2 | GANAB, BAX,
EDEM3 |
| KEGG_PATHWAY | hsa04810,
regulation of actin cytoskeleton | 3 | 5 |
6.64×10−2 | FGFR1, ARAF,
PDGFC |
Discussion
Glioblastoma is the most malignant primary tumour of
the CNS with a devastating outcome. This tumour often invades
healthy brain tissue leading to tumour progression or recurrence
despite drastic treatments such as chemotherapy and radiotherapy
(22,23). The classification of GBMs into
classical, mesenchymal, neural and proneural subtypes based on gene
expression profiles bring a new insight into understanding of the
molecular mechanisms of GBM. This phenomenon is reminiscent of a
newly emerging concept that differential activation of critical
signalling pathways induces and maintains each subtype in cancer
biology. Extensive reports suggested that CXCR4 is required for
tumour proliferation, invasion, angiogenesis, modulation of the
immune response and recently enriched in neural stem cells
(24,25). In this study, we demonstrated that
CXCR4 could serve as a prognostic factor in characterizing subsets
of GBM, as patients with high expression of CXCR4 gliomas seem to
have poorer prognosis. Additionally, CXCR4 is preferentially
expressed in the MES subtype of GBM and highly consistent with MES
makers such as CHI3L1 (also known as YKL40) and MET. However,
little has been reported regarding about the role of activated
CXCR4 in mesenchymal GBM.
In the present study, gene expression data of 539
GBM patients were retrieved from the TCGA dataset. We identified
3198 DEGs associated with CXCR4 in mesenchymal GBM, 4004 DEGs
between mesenchymal and classical GBM, 3455 DEGs between
mesenchymal and neural GBM, and 3289 DEGs between mesenchymal and
proneural GBM. Subsequently, we intersected of the gene sets
mentioned above to determine key pathways and genes in the CXCR4
mediated mesenchymal subtype of glioblastoma. As a result, 34
overlapped genes were found to be distinctively specific to the
CXCR4high group of mesenchymal patients.
In addition, Gene ontology and KEGG pathway analyses
were performed to compare functional annotations associated with
CXCR4 signalling across the mesenchymal GBM subtype. In accordance
with expectations, CXCR4 was found to be enriched in the cell
component of golgi apparatus, cytoplasmic membrane, vacuole and
endoplasmic reticulum. Interestingly, CXCR4 was demonstrated to be
involved in the regulation of the MAPK pathway, which is reported
to be associated with the many cell fate decision including
proliferation, differentiation, migration, senescence and apoptosis
(26,27). Numerous studies investigated the
linkage between CXCR4 and MAPK in a wide range of malignant
tumours, covering small-cell lung cancer, breast cancer and,
recently, osteosarcoma (28–30).
These studies indicated that the CXCL12/CXCR4 axis could act as a
mediator to promote tumour biology through the activation of the
MAPK pathway. Sun et al (31), reported that the expression of
β-arrestin2 strengthened the CXCR4-mediated activation of both p38
MAPK and ERK in HeLa cells. Rhodes et al (28), showed that enhanced CXCR4
signalling is sufficient to drive ER-positive breast cancers to a
metastatic and endocrine therapy-resistant phenotype via increased
MAPK signalling. However, fewer literatures are written about the
potential association between CXCR4 and MAPK pathway in the
mesenchymal subtype of GBM. Our study demonstrated that the MAPK
pathway may play a pivotal role in the progression of
CXCR4-mediated mesenchymal GBM or the mesenchymal phenotype
transition. Given the poor prognosis and aggressiveness of
mesenchymal GBM, it is anticipated that these patients would
benefit most from gene based chemotherapeutic strategies through
targeting CXCR4/MAPK related factors.
In conclusion, our data provide a comprehensive
bioinformatics analysis of CXCR4 and its DEGs, which may play a
functional role in the development of GBM and the maintenance of
the mesenchymal phenotype. The study also provides preliminary
evidence that the CXCR4 mediated MAPK pathway was identified
specifically in patients with mesenchymal GBM and CXCR4 be a
co-therapeutic target in the management of melanoma, prostate
cancer and mesenchymal GBM. Therefore, targeting CXCR4 and its
related cooperative pathways and genes could be a promising
approach for the efficient suppression or elimination of this
devastating cancer.
Acknowledgements
Not applicable.
Funding
The research was funded by the National Natural
Science Foundation of China (grant no. 81472352) and the Natural
Science Foundation of Tianjin City (grant no. 15JCZDJC36200).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the R2 analysis and visualization
platform (hgserver1.amc.nl/cgi-bin/r2/main.cgi) and UCSC Xena
(xenabrowser.net/datapages/).
Authors' contributions
LY drafted the manuscript. LY and LT performed the
experiments. PL and IRA participated in the design of the study. TL
and LH downloaded and interpreted the raw data. ZT and HM analyzed
the data. YX, SY, JL and FY contributed to the data collection and
processing. XY helped design the study and revised and approved the
final version of manuscript.
Ethics approval and consent to
participate
All participants provided written informed consent
prior to their inclusion within the study and the study was
approved by the Ethics Committee of Tianjin Medical University
Hospital.
Consent for publication
All participants provided written informed consent
for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ostrom QT, Gittleman H, Xu J, Kromer C,
Wolinsky Y, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: Primary brain and other central nervous system tumors
diagnosed in the United States in 2009–2013. Neuro Oncol. 18
Suppl_5:v1–v75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chinot OL, Wick W, Mason W, Henriksson R,
Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea
D, et al: Bevacizumab plus radiotherapy-temozolomide for newly
diagnosed glioblastoma. N Engl J Med. 370:709–722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coons SW, Johnson PC, Scheithauer BW,
Yates AJ and Pearl DK: Improving diagnostic accuracy and
interobserver concordance in the classification and grading of
primary gliomas. Cancer. 79:1381–1393. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun Y, Zhang W, Chen D, Lv Y, Zheng J,
Lilljebjörn H, Ran L, Bao Z, Soneson C, Sjögren HO, et al: A glioma
classification scheme based on coexpression modules of EGFR and
PDGFRA. Proc Natl Acad Sci USA. 111:3538–3543. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brennan CW, Verhaak RG, McKenna A, Campos
B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ,
Berman SH, et al: The somatic genomic landscape of glioblastoma.
Cell. 155:462–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Verhaak RG, Hoadley KA, Purdom E, Wang V,
Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al:
Integrated genomic analysis identifies clinically relevant subtypes
of glioblastoma characterized by abnormalities in PDGFRA, IDH1,
EGFR, and NF1. Cancer Cell. 17:98–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brennan CW, Verhaak RG, McKenna A, Campos
B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ,
Berman SH, et al: The somatic genomic landscape of glioblastoma.
Cell. 155:462–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eckel-Passow JE, Lachance DH, Molinaro AM,
Walsh KM, Decker PA, Sicotte H, Pekmezci M, Rice T, Kosel ML,
Smirnov IV, et al: glioma groups based on 1p/19q, IDH and TERT
promoter mutations in tumors. N Engl J Med. 372:2499–2508. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tabouret E, Nguyen AT, Dehais C,
Carpentier C, Ducray F, Idbaih A, Mokhtari K, Jouvet A, Uro-Coste
E, Colin C, et al: Prognostic impact of the 2016 WHO classification
of diffuse gliomas in the French POLA cohort. Acta Neuropathol.
132:625–634. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dunn GP, Rinne ML, Wykosky J, Genovese G,
Quayle SN, Dunn IF, Agarwalla PK, Chheda MG, Campos B, Wang A, et
al: Emerging insights into the molecular and cellular basis of
glioblastoma. Genes Dev. 26:756–784. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Phillips HS, Kharbanda S, Chen R, Forrest
WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, et
al: Molecular subclasses of high-grade glioma predict prognosis,
delineate a pattern of disease progression, and resemble stages in
neurogenesis. Cancer Cell. 9:157–173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng W, Zhang C, Ren X, Jiang Y, Han S,
Liu Y, Cai J, Li M, Wang K, Liu Y, et al: Bioinformatic analyses
reveal a distinct Notch activation induced by STAT3 phosphorylation
in the mesenchymal subtype of glioblastoma. J Neurosurg.
126:249–259. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cooper LA, Gutman DA, Chisolm C, Appin C,
Kong J, Rong Y, Kurc T, Van Meir EG, Saltz JH, Moreno CS and Brat
DJ: The tumor microenvironment strongly impacts master
transcriptional regulators and gene expression class of
glioblastoma. Am J Pathol. 180:2108–2119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krock BL, Skuli N and Simon MC:
Hypoxia-induced angiogenesis: Good and evil. Genes Cancer.
2:1117–1133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gagliardi F, Narayanan A, Reni M, Franzin
A, Mazza E, Boari N, Bailo M, Zordan P and Mortini P: The role of
CXCR4 in highly malignant human gliomas biology: Current knowledge
and future directions. Glia. 62:1015–1023. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Passaro D, Irigoyen M, Catherinet C,
Gachet S, Da Costa De Jesus C, Lasgi C, Quang Tran C and Ghysdael
J: CXCR4 Is required for leukemia-initiating cell activity in t
cell acute lymphoblastic leukemia. Cancer Cell. 27:769–779. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luker KE, Lewin SA, Mihalko LA, Schmidt
BT, Winkler JS, Coggins NL, Thomas DG and Luker GD: Scavenging of
CXCL12 by CXCR7 promotes tumor growth and metastasis of
CXCR4-positive breast cancer cells. Oncogene. 31:4750–4758. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seo CH, Kim JR, Kim MS and Cho KH: Hub
genes with positive feedbacks function as master switches in
developmental gene regulatory networks. Bioinformatics.
25:1898–1904. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tabouret E, Tchoghandjian A, Denicolai E,
Delfino C, Metellus P, Graillon T, Boucard C, Nanni I, Padovani L,
Ouafik L, et al: Recurrence of glioblastoma after
radio-chemotherapy is associated with an angiogenic switch to the
CXCL12-CXCR4 pathway. Oncotarget. 6:11664–11675. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huse JT and Holland EC: Targeting brain
cancer: Advances in the molecular pathology of malignant glioma and
medulloblastoma. Nat Rev Cancer. 10:319–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen J, McKay RM and Parada LF: Malignant
glioma: Lessons from genomics, mouse models, and stem cells. Cell.
149:36–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li M, Chang CJ, Lathia JD, Wang L, Pacenta
HL, Cotleur A and Ransohoff RM: Chemokine receptor CXCR4 signaling
modulates the growth factor-induced cell cycle of self-renewing and
multipotent neural progenitor cells. Glia. 59:108–118. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ho SY, Ling TY, Lin HY, Liou JT, Liu FC,
Chen IC, Lee SW, Hsu Y, Lai DM and Liou HH: SDF-1/CXCR4 signaling
maintains stemness signature in mouse neural stem/progenitor cells.
Stem Cells Int. 2017:24937522017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rhodes LV, Short SP, Neel NF, Salvo VA,
Zhu Y, Elliott S, Wei Y, Yu D, Sun M, Muir SE, et al: Cytokine
receptor CXCR4 mediates estrogen-independent tumorigenesis,
metastasis, and resistance to endocrine therapy in human breast
cancer. Cancer Res. 71:603–613. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Burger M, Glodek A, Hartmann T,
Schmitt-Gräff A, Silberstein LE, Fujii N, Kipps TJ and Burger JA:
Functional expression of CXCR4 (CD184) on small-cell lung cancer
cells mediates migration, integrin activation, and adhesion to
stromal cells. Oncogene. 22:8093–8101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liao YX, Fu ZZ, Zhou CH, Shan LC, Wang ZY,
Yin F, Zheng LP, Hua YQ and Cai ZD: AMD3100 reduces CXCR4-mediated
survival and metastasis of osteosarcoma by inhibiting JNK and Akt,
but not p38 or Erk1/2, pathways in in vitro and mouse experiments.
Oncol Rep. 34:33–42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun Y, Cheng Z, Ma L and Pei G:
Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis,
and this is mediated by its enhancement of p38 MAPK activation. J
Biol Chem. 277:49212–49219. 2002. View Article : Google Scholar : PubMed/NCBI
|