Introduction
Atherosclerosis is a chronic pathophysiological
process involving large and medium-sized vessels (1). It is considered to be the result of
cholesterol accumulation in the artery over a long period of time.
However, a series of landmark studies have allowed an increasingly
clear understanding of the key role of inflammation in the genesis
and development of atherosclerosis (2). Various types of immunocytes are the
major components of early atherosclerotic plaques and effector
molecules released by these immunocytes accelerate the plaque
progression (3). Acute coronary
syndrome is the manifestation of intra-plaque inflammation
activation (3). Therefore,
atherosclerosis is considered to be an inflammatory disease
(3) and is the consequence of a
combination of immune factors and metabolic risk factors (4). Atherosclerosis manifests as the
genesis and development of atherosclerotic plaques within the
vascular wall (4).
Interleukin (IL)-6 is a multifunctional circulating
cytokine. Its major biological activities include inducing B cells
to produce antibody and promoting cytotoxic T cell formation
(5). Elevated levels of IL-6 are
observed following cardiac surgery, which peak at 4–6 h (5). IL-6 has proinflammatory and
anti-inflammatory effects and is an important acute phase reaction
factor during the wound and repair process (6). IL-6 is able to activate neutrophils
and also delay the phagocytosis of aging and non-functional
neutrophils by phagocyte. Thus, it promotes an inflammatory
environment (7). IL-6 has also
been reported to promote the release of soluble tumor necrosis
factor (TNF) receptors and IL-1 receptor (8), while another study demonstrated that
IL-6 weakened the effect of TNF-α and IL-1, thus exerting an
anti-inflammatory effect. Excessive IL-6 release is a risk factor
for patients (9).
The heart incessantly contracts and relaxes
throughout the lifetime of an individual to drive the systemic
blood circulation. In order to allow its function, the maintenance
of sufficient blood, oxygen and nutrients is required (10). Energy is supplied to myocardial
cells primarily through β-oxidation of fatty acids under aerobic
conditions (11) and myocardial
cells almost completely depend on aerobic metabolism to supply
energy. Therefore, myocardial cells have a high dependency on
oxygen. Myocardial cells have a high sensitivity to ischemia and
anoxia; once myocardial cells suffer ischemia and anoxia, cell
dysfunction, paramorphia and potentially death may occur (11).
Sirtuin 1 (Sirt1), also termed silent mating type
information regulation 2 homolog 1, is a NAD2-dependent
multifunctional transcription regulatory factor that is involved in
the regulation of various signaling pathways that are involved in
mammalian cell lifespan, glucose metabolism and insulin secretion
(12). Previous studies have
demonstrated that knock-out of Sirt1 aggravated myocardial
ischemia-reperfusion injury in mice, indicating that SIRT1 may have
a role in the protection against myocardial injury (12,13).
The nuclear factor erythroid 2-related factor 2
(Nrf2)-antioxidant response element (ARE) pathway is the most
important endogenous antioxidative stress pathway that has been
identified at present. It is widely distributed in the
cardiovascular system and upregulates the endogenous antioxidative
system once activated, thereby reducing oxidative damage to the
myocardium (14). In addition, as
a receptor of oxidative stress, Nrf2 has important roles in the
regulation of cellular oxidative stress and functions as a
transcription factor of antioxidative stress (15). ARE has a unique induction mechanism
to prevent against oxidative stress, and as a cis-acting element,
is activated by various factors, including hydrogen peroxide,
reactive oxygen species, electrophilic species and other
xenobiotics, and induces the expression of antioxdative genes
(16).
p38 mitogen-activated protein kinase (MAPK) is a
type of tyrosine/threonine protein kinase with a molecular weight
of ~40–60 kDa; all members of the MAPK family are activated by dual
phosphorylation of tyrosine and threonine (17). Tyrosine kinase receptors,
G-protein-coupled receptors and ion channel-coupled receptors may
initiate the phosphorylation of tyrosine and threonine sites on
MAPKs through various intermediary links to activate p38 MAPK, and
activated p38 MAPK subsequently translocates to its corresponding
transcription factors and activates gene transcription, resulting
in effects on cell proliferation, growth or apoptosis (17,18).
p38 MAPK is considered to be the focal point or common pathway of
the transmission of various extracellular signals that lead to cell
proliferation, hypertrophy and apoptosis, and participates in
myocardial hypertrophy, proliferation and apoptosis induced by a
various stimuli (19).
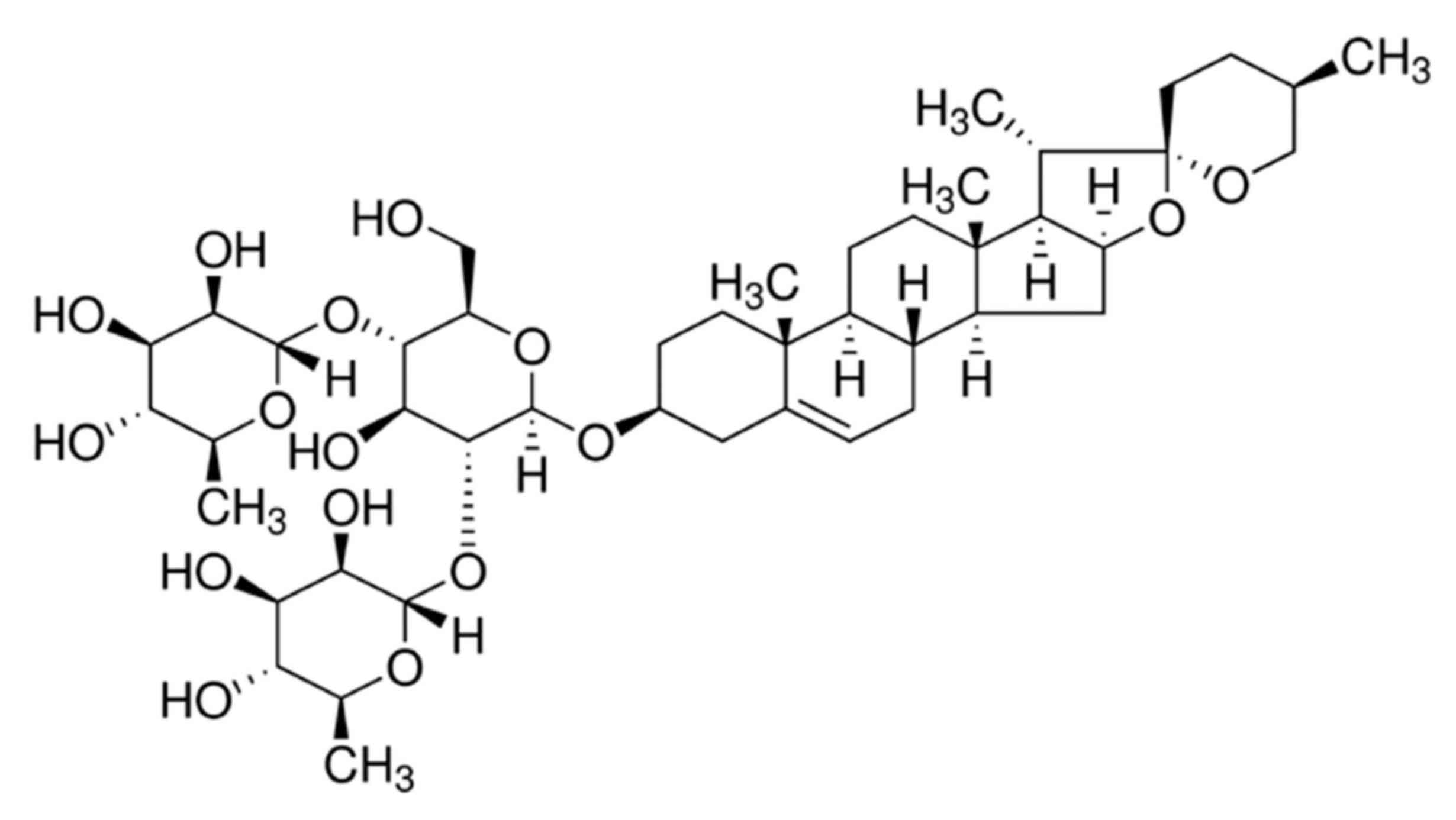
Dioscin is a type of steroid sapogenin synthesized
naturally by plants and belongs to the group of spirostanols
(20). As an important raw
material for the synthesis of steroid hormones and steroidal
contraceptives, dioscin has generally been used in the production
of pregnenolone, progesterone, cortisol and other drugs (21). In the last several decades, the
pharmacological effects of dioscin have been investigated
extensively (20,21). Dioscin exhibits antineoplastic
function and regulates blood lipids, prevents platelets from
aggregation and promotes bile secretion; therefore, it is primarily
employed to treat cardiovascular disease, encephalitis, skin
diseases and tumors (22). The
purpose of the current study was to investigate the potential
protective effects of dioscin against coronary heart disease
(CHD)-induced inflammation in a pig model and the underlying
mechanisms.
Materials and methods
Experimental animals and groups
Chinese miniature pigs (male, 20–30 kg, 1–2 month)
were acquired from the Institute of Laboratory Animal Science,
Jining Medical University (Jining, China) and housed in 22–24°C,
55–60% humidity, 0.038% CO2, fed a standard laboratory
diet and water ad libitum with a 12 h light/dark cycle. A
total of 26 pigs were randomly assigned into control (sham
treatment, n=6), vehicle (CHD model, n=10) and dioscin-treated (CHD
model + dioscin, n=10) groups. Ethical approval for the present
study was provided by the Chinese PLA General Hospital (Beijing,
China). The chemical structure of dioscin is presented in Fig. 1.
CHD model induction and dioscin
treatment
In the CHD model and dioscin-treated groups, pigs
were fed with a high-fat diet for 4 weeks. Subsequently, pigs were
intravenously administered with 30 mg/kg sodium pentobarbital
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) into the ear vein
and the common carotid artery was separated and a 6F arterial
sheath tube was advanced and then the ligature was checked to
ensure it was tight. The left anterior descending branch was
accessed by a guide wire and the sacculus was merged into the left
anterior descending branch and air pressure was maintained at 10–12
ATM for 30 sec and this process was repeated 3 times with the
pressure being maintained in between at 1–1.5 ATM. Subsequently, in
CHD model and dioscin treated groups, pigs were fed with a high-fat
diet for 4 weeks. In the dioscin-treated group, pigs
intraperitoneal received with 80 mg/kg/ every 3 days dioscin
(Sigma-Aldrich; Merck KGaA) for 4 weeks. In control group, pigs
were intravenously administered with 30 mg/kg sodium pentobarbital
(Sigma-Aldrich; Merck KGaA) into ear vein without induction of
CHD.
Hematoxylin and eosin staining
methods
After treatment with dioscin, tissue samples were
washed with PBS and fixed with 4% paraformaldehyde for 24 h at room
temperature. Tissue samples were embedded in paraffin and sectioned
at 10 µM. Tissue samples were stained with hematoxylin and eosin
staining for 15 min at room temperature. Tissue samples were
observed using a LSM 780 NLO confocal microscope (Carl Zeiss, AG,
Oberkochen, Germany).
Left ventricular ejection fraction
(LVEF) and systolic internal diameter (LVIDs)
Following treatment with dioscin, LVEF and LVIDs
were analyzed by a S5-1 linear probe (iE33 xMatrix Ultrasound;
Philips Healthcare, Andover, MA, USA).
Determination of serum levels of heart
injury and inflammatory markers using ELISA kits
Following treatment with dioscin, serum samples from
each group were obtained from whole blood samples by centrifugation
at 2,000 × g for 10 min at 4°C. The levels of creatine kinase (CK;
cat. no. A032), CK-MB (cat. no. H197), lactate dehydrogenase (LDH;
cat. no. A020-2), cardiac troponin T (cTnT; cat. no. H149-4), TNF-α
(cat. no. H052), IL-1β (cat. no. H002), IL-6 (cat. no. H007) and
IL-18 (cat. no. H015) were measured using respective ELISA kits
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China). SOD
(cat. no. A001-1-1), MDA (cat. no. A003-1), CAT (cat. no. A007-1-1)
and GSH (cat. no. A006-2) levels were measured using commercial
kit.
Western blot analysis
Following treatment with dioscin for 4 weeks,
proteins were extracted from coronary tissue samples using
radioimmunoprecipitation assay lysis buffer and protein
concentration was measured using a BCA protein assay kit (both
Beyotime Institute of Biotechnology, Haimen, China) following
centrifugation at 12,000 × g for 10 min at 4°C. Total protein (50
µg) was separated on 8–12% SDS-PAGE gels and transferred to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were subsequently blocked with 5% non-fat milk
for 1 h at 37°C followed by incubation with Bax (1:1,000; cat. no.
sc-6236), Caspase-3 (1:1,000; cat. no. sc-98785), PARP (1:1,000;
cat. no. sc-7150), p53 (1:1,000; cat. no. sc-6243), Sirt1 (1:1,000,
cat. no. sc-15404), Nrf2 (1:1,000; cat. no. sc-722), p38 (1:1,000;
sc-728) and p-p38 (1:1,000; sc-17852-R) and GAPDH (cat. no.
sc-25778; 1:500; all Santa Cruz Biotechnology, Inc.) at 4°C
overnight. The membrane was washed with TBS-0.1% Tween-20 and
incubated with horseradish peroxidase-conjugated secondary antibody
(cat. no. sc-2004; 1:5,000; Santa Cruz Biotechnology, Inc.) for 1 h
at 37°C. Subsequently, the membrane was stained with ECL Plus
(Beyotime Institute of Biotechnology) and analyzed using
Image_Lab_3.0 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± standard error of
the mean (n=6). Data were analyzed by SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA) and were analyzed by one-way analysis of
variance and the post-hoc Bonferroni test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Dioscin protects against CHD in a pig
model
The present study investigated whether dioscin may
exert protective effects against CHD in a pig model. The results of
ELISA demonstrated that the serum levels of heart injury markers
CK, CK-MB, LDH and cTnT in the CHD model group were significantly
higher compared with the control group (Fig. 2). Following treatment with 80 mg/kg
dioscin, the serum levels of CK, CK-MB, LDH and cTnT were
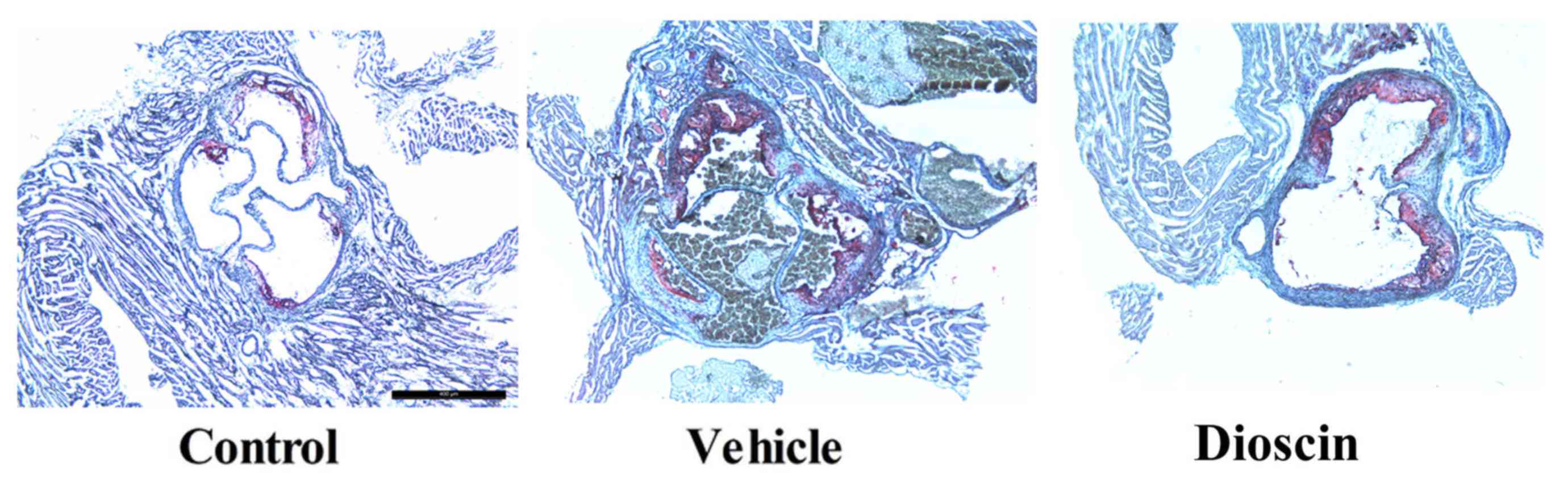
significantly lower compared with the CHD model group (Fig. 2). Furthermore, hematoxylin and
eosin staining demonstrated that there was a higher number of
arterial plaques in the CHD model group compared with the control
group. However, dioscin (80 mg/kg) markedly prevented the formation
of arterial plaques in CHD pigs, compared with the CHD model group
(Fig. 3).
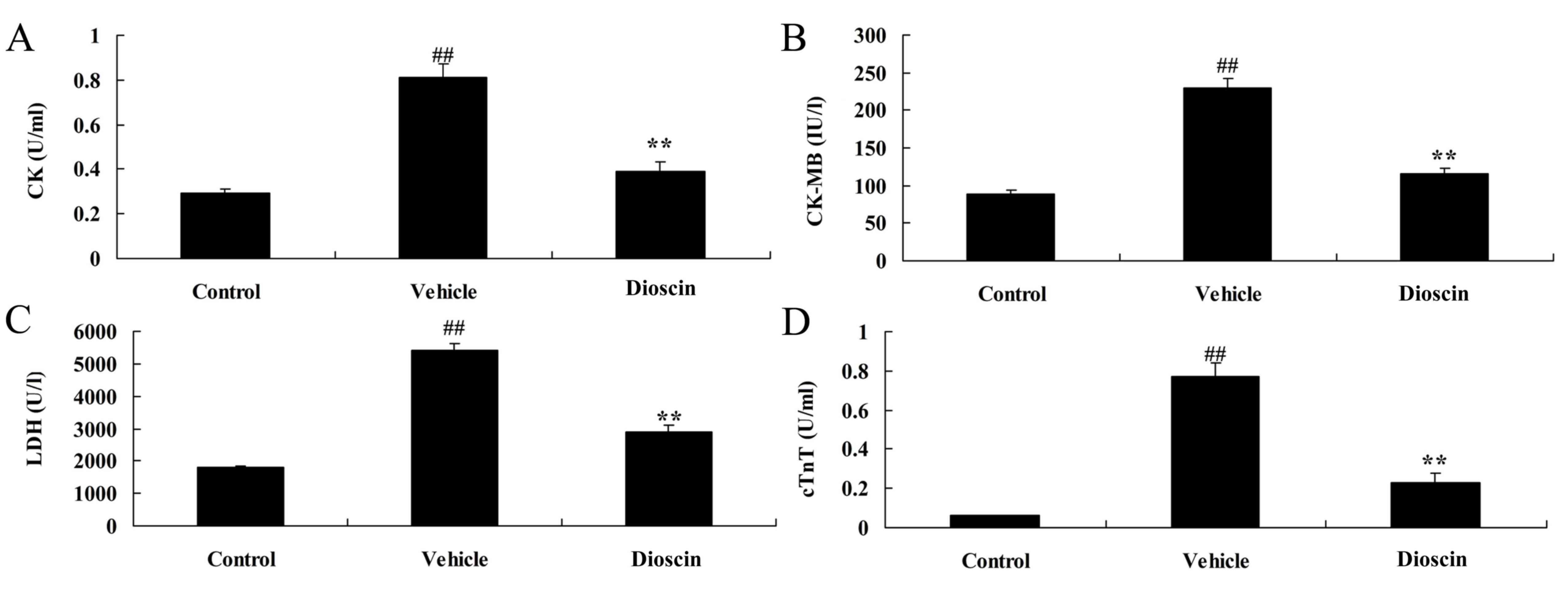 | Figure 2.Dioscin reduces the levels of CK,
CK-MB, LDH and cTnT in CHD model pigs. ELISA was performed
following treatments to determine the levels of (A) CK, (B) CK-MB,
(C) LDH and (D) cTnT in the serum of pigs. ##P<0.01
vs. control group; **P<0.01 vs. vehicle group. CK, creatine
kinase; LDH, lactate dehydrogenase; cTnT, cardiac troponin T; CHD,
coronary heart disease; control, sham group; vehicle, CHD model
group; dioscin, CHD model + dioscin group. |
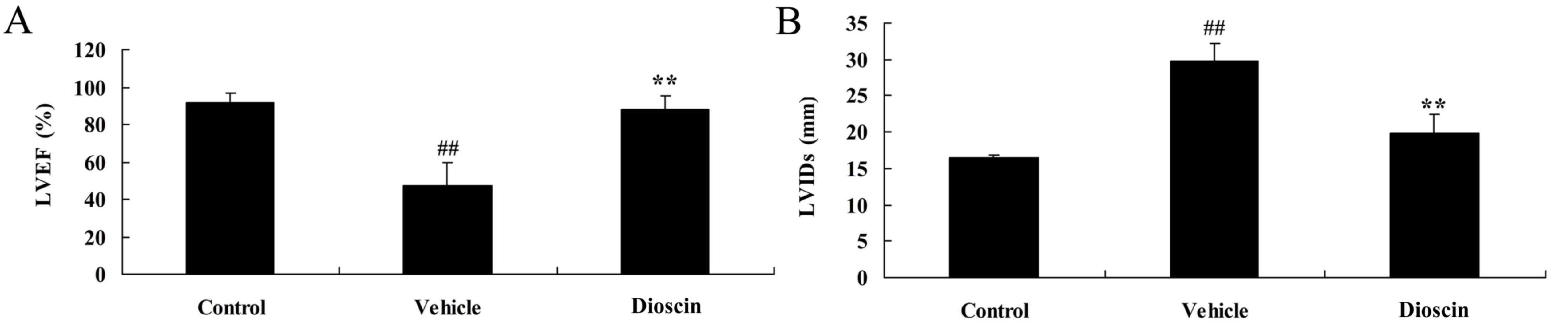
Dioscin improves heart function in CHD
model pigs
To determine whether dioscin protects heart function
in CHD model pigs, LVEF and LVIDs were measured in each group. In
the CHD model group, the LVEF was lower compared with the control
group (Fig. 4A). However,
CHD-induced reduction of LVEF was significantly reversed by 80
mg/kg dioscin (Fig. 4A). By
contrast, LVIDs was higher in the CHD model group compared with the
control group and dioscin (80 mg/kg) significantly reduced the
LVIDs in CHD pigs, compared with the CHD model group (Fig. 4B).
Dioscin reduces oxidative stress and
the levels of inflammation in a CHD pig model
The results demonstrated that the levels of
superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH)
were significantly decreased, while malondialdehyde (MDA) levels
were significantly increased, in the CHD model group compared with
the control group (Fig. 5).
Treatment with dioscin (80 mg/kg) significantly increased SOD, CAT
and GSH levels, and inhibited MDA levels, in CHD pigs, compared
with the CHD model group (Fig. 5).
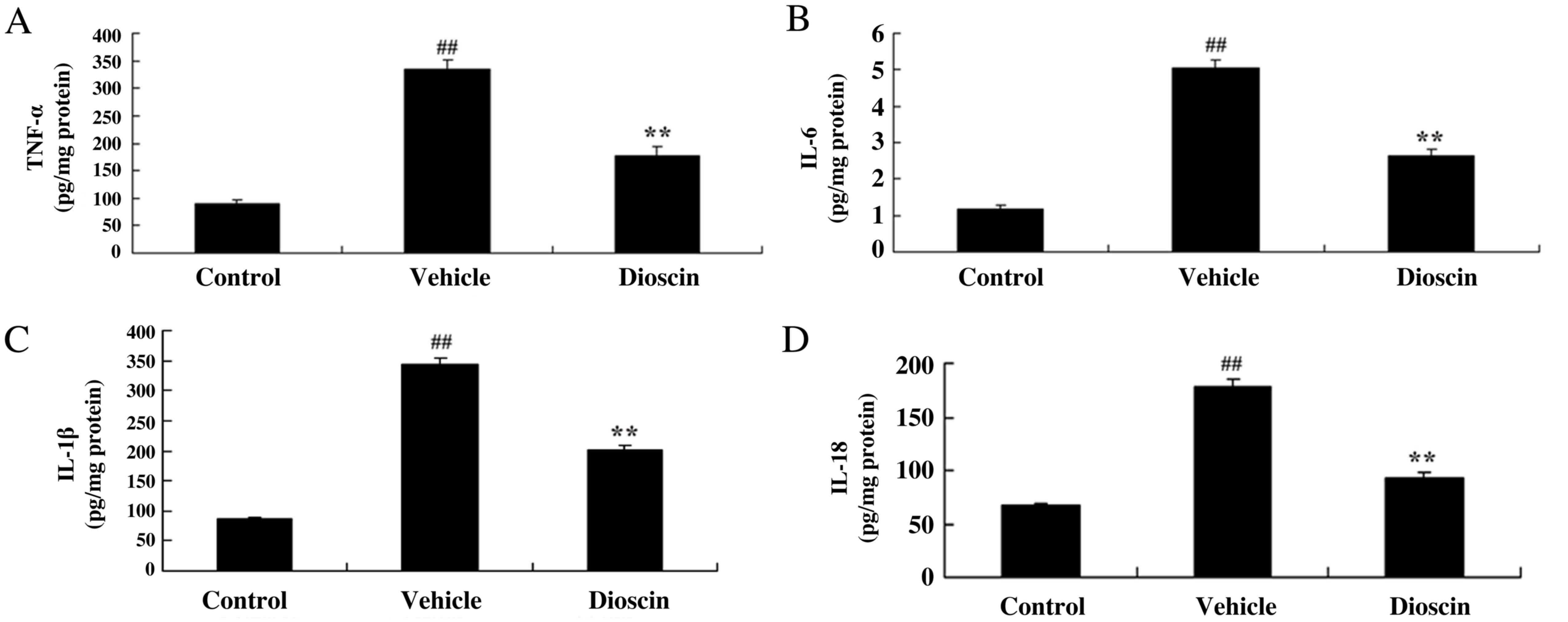
Furthermore, TNF-α, IL-1β, IL-6 and IL-18 levels were significantly
increased in the CHD model group compared with the control group,
and these CHD-induced increases in TNF-α, IL-1β, IL-6 and IL-18
were significantly reduced by treatment with 80 mg/kg dioscin
(Fig. 6).
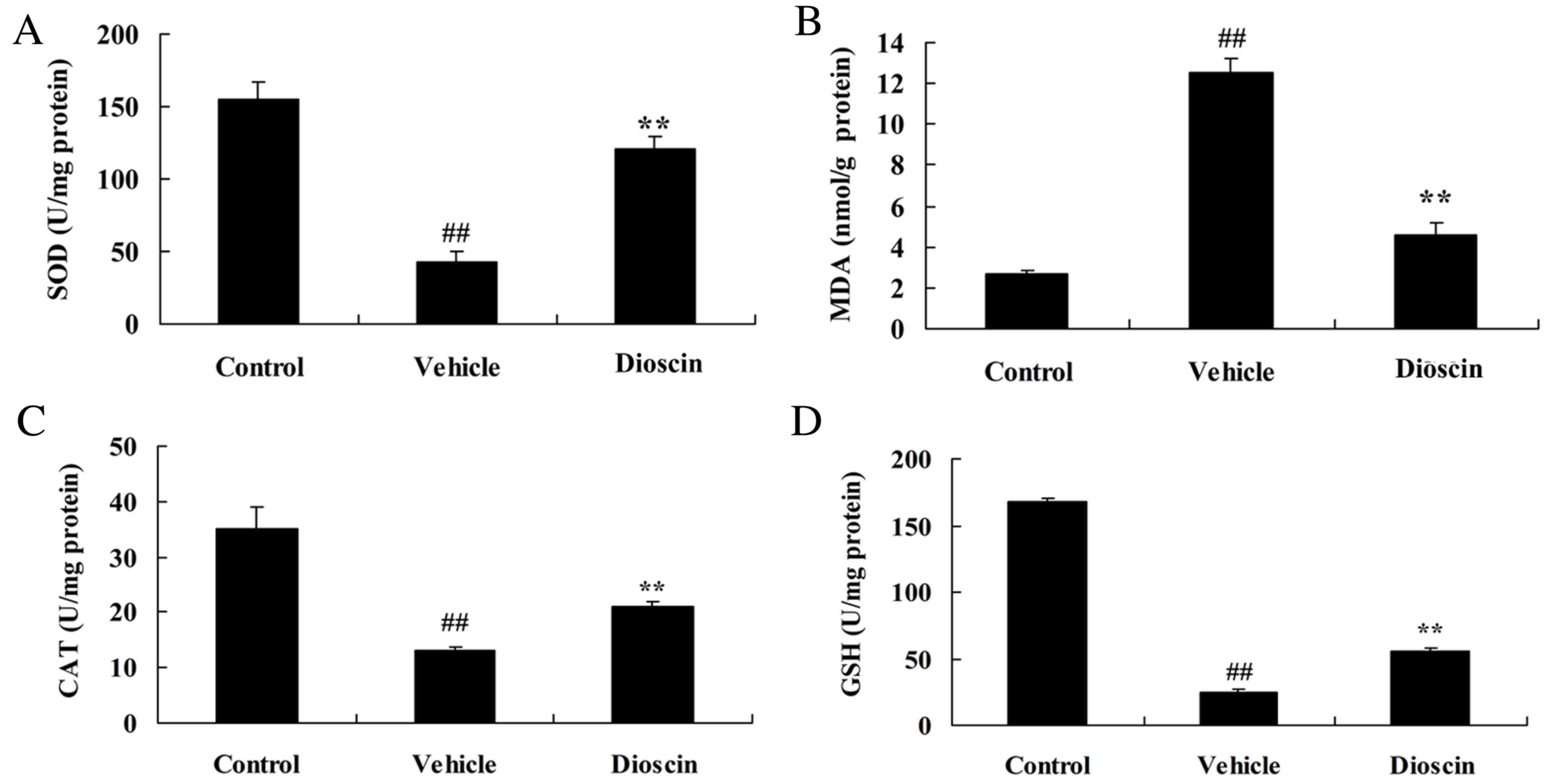 | Figure 5.Dioscin reduces oxidative stress in
CHD model pigs. The effect of dioscin on the serum levels of (A)
SOD, (B) MDA, (C) CAT and (D) GSH in CHD model pigs was determined
by ELISA. ##P<0.01 vs. control group; **P<0.01 vs.
vehicle group. CHD, coronary heart disease; SOD, superoxide
dismutase; MDA, malondialdehyde; CAT, catalase; GSH, glutathione;
control, sham group; vehicle, CHD model group; dioscin, CHD model +
dioscin group. |
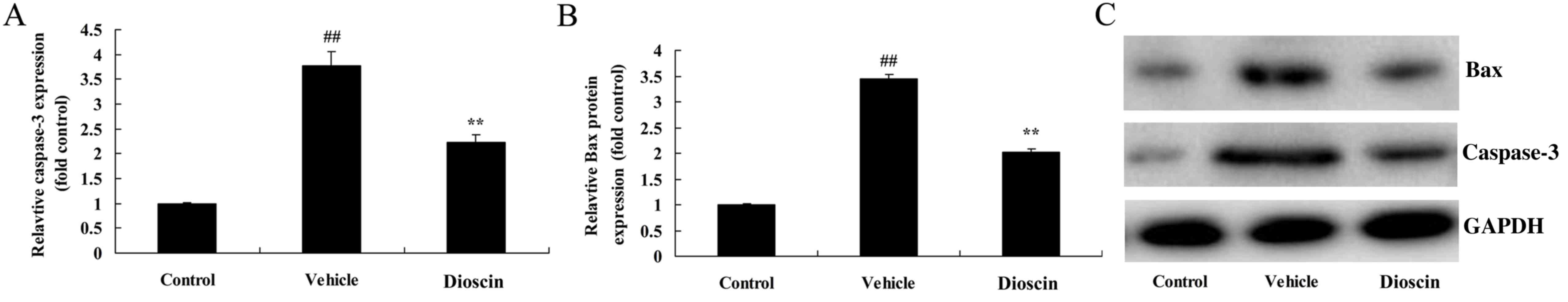
Dioscin inhibits apoptosis in the
heart of CHD model pigs
Compared with the control group, the protein
expression of caspase-3 and Bcl-2-associated X (Bax) in coronary
tissues was significantly increased in the CHD model group
(Fig. 7). However, 80 mg/kg
dioscin significantly suppressed the CHD-induced increases in the
expression of caspase-3 and Bax (Fig.
7).
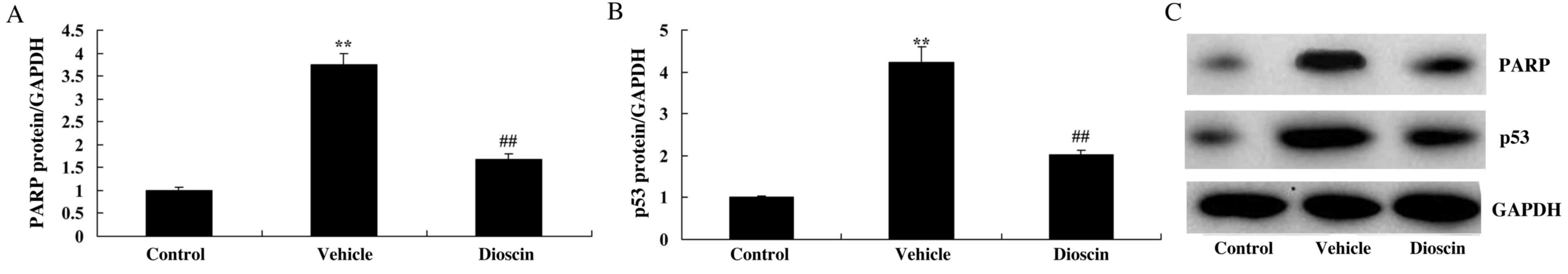
Dioscin suppresses poly (ADP-ribose)
polymerase 1 (PARP) and p53 protein expression in CHD model
pigs
To investigate the signal transduction mechanisms of
dioscin-mediated protection against heart cell apoptosis in CHD
observed in the present study, the alterations in PARP and p53
protein expression were also investigated. In the CHD model group,
PARP and p53 protein expression were significantly increased
compared with the control group (Fig.
8). However, dioscin significantly suppressed PARP and p53
protein expression compared with the CHD model group (Fig. 8). These results indicated that the
protective effects of dioscin on heart cell apoptosis in CHD may be
mediated via PARP and p53 proteins.
Dioscin induces Sirt1/Nrf2 and
suppresses p38 MAPK pathways in a CHD pig model
To investigate the roles of Sirt1/Nrf2 and p38 MAPK
pathways in dioscin-mediated protection against oxidative stress
and inflammation in CHD, the protein expression of components of
Sirt1/Nrf2 and p38 MAPK pathways was measured. As demonstrated in
Fig. 9, Sirt1 and Nrf2 protein
expression was significantly suppressed, while phosphorylated
(p)-p38 MAPK protein expression was significantly induced, in the
CHD model group compared with the control group. However, 80 mg/kg
dioscin significantly induced Sirt1 and Nrf2 protein expression and
suppressed p-p38 MAPK protein expression in CHD model pigs,
compared with the CHD model group (Fig. 9). These results indicated that
dioscin may reduce oxidative stress and inflammation in CHD through
activation of Sirt1/Nrf2 and suppression of p38 MAPK pathways.
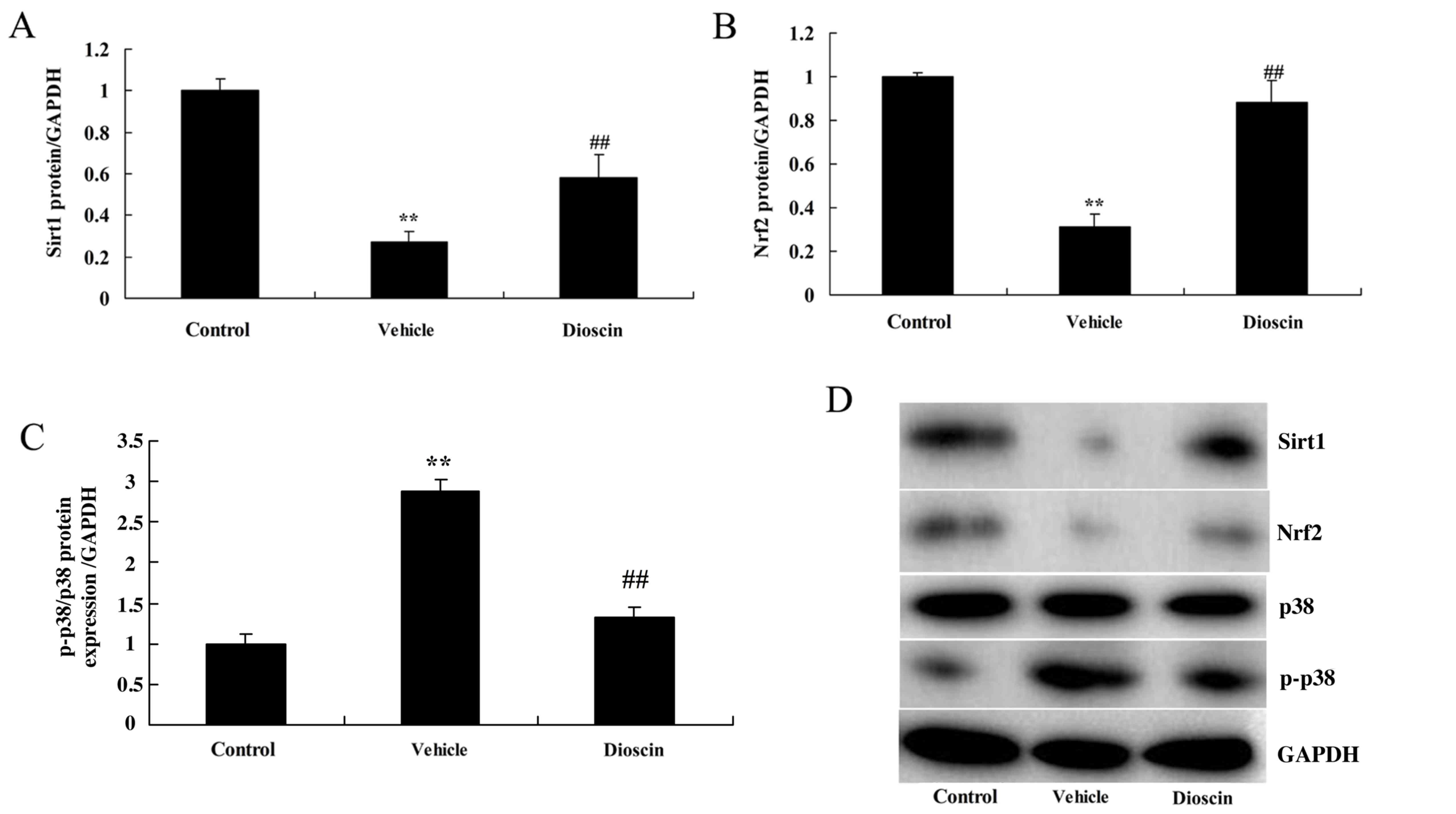 | Figure 9.Dioscin activates Sirt1/Nrf2 and
inhibits p38 MAPK pathways. Densitometric analysis of western
blotting results was performed to determine the effect of dioscin
on the protein levels of (A) Sirt1, (B) Nrf2 and (C) p-p38 MAPK in
coronary heart disease model pigs. (D) Representative western blot
bands for Sirt1, Nrf2, p38 and p-p38 MAPK, and GAPDH in each of the
three groups. ##P<0.01 vs. control group; **P<0.01
vs. vehicle group. Sirt1, sirtuin 1; Nrf2, nuclear factor erythroid
2-related factor 2; MAPK, mitogen-activated protein kinase; p-,
phosphorylated-; control, sham group; vehicle, CHD model group;
dioscin, CHD model + dioscin group. |
Discussion
Research concerning atherosclerosis has been
increasingly intensive. Thus, the importance of inflammation in the
genesis and development of atherosclerosis has become increasingly
clear. Inflammation was reported to have an important role in acute
coronary syndrome (23) and is
also a factor associated with the genesis and development of
atherosclerotic plaques (24). At
present, the concept that atherosclerosis is an inflammatory
disease has been widely accepted. Atherosclerosis is the result of
a combination of immune factors and metabolic risk factors and
manifests as the genesis and development of atherosclerotic plaques
within the vascular wall (25).
The results of the present study demonstrated that dioscin reduced
the serum levels of CK, CK-MB, LDH and cTnT, increased the LVEF and
reduced the LVIDs in CHD model pigs. In addition, Qin et al
(26) demonstrated that Dioscin
prevents the mitochondrial apoptosis and attenuates oxidative
stress in cardiac H9c2 cells.
ROS is a type of metabolic substance that is
generated from oxygen (27). There
are various types of active oxygen and oxygen radicals are the
major type, which include hydroxyl radicals, superoxide anion,
nitric oxide and certain non-free radicals. A certain level of
active oxygen is required for the maintenance of normal life and
usually exists in a dynamic equilibrium in the body at a normal
level (10). The physiological
functions of active oxygen include participation in electron
transfer and metabolic processes within the body (28). When stress is induced in the body
by various conditions, including ischemia, anoxia, ion radiation
and chemical reagents, the generation of active oxygen forms may be
induced in body, which may lead to an imbalance between the levels
of oxidative and antioxidative factors, and subsequent cell damage
(28). In the current study,
dioscin significantly increased SOD, CAT and GSH levels and
decreased MDA levels in CHD model pigs. In addition, Zhao et
al (29) suggested that
dioscin alleviated doxorubicin-induced cardiotoxicity through
modulating miR-140-5p-mediated myocardial oxidative stress.
Sirt1 was reported to alleviate oxidative stress and
inflammatory reactions, activate the autophagy of myocardial cells
and inhibit the apoptosis of myocardial cells induced by
ischemia-reperfusion to protect the myocardium (30). Furthermore, in a study concerning
the effect of Sirt1 on the expression of endoplasmic reticulum
stress-associated proteins during myocardial ischemia-reperfusion,
it was demonstrated that the mRNA and protein expression levels of
endoplasmic reticulum stress-associated genes (glucose-regulated
protein 78 kDa, caspase-3 and DNA damage-inducible transcript 3)
decreased with the activation of Sirt1 and increased with the
inhibition of Sirt1 (31).
Therefore, Sirt1 was indicated to protect the myocardium from
ischemia-reperfusion by inhibiting the expression of endoplasmic
reticulum stress-associated proteins (31). The results of the present study
indicated that dioscin significantly increased the expression of
Sirt1 protein in CHD model pigs.
Nrf2 and the downstream antioxidant genes that it
regulates participate in the protective mechanism against
myocardial ischemia-reperfusion injury (32). Previous studies demonstrated that
the basal expression of antioxidants and two-phase enzyme in Nrf2
knockout mice was markedly lower compared with wild-type mice, with
sensitivity to ROS-induced cytotoxicity increased, indicating that
antioxidant genes were vital to protecting against myocardial
injury and the expression of Nrf2 was involved in the expression
and induction of antioxidants. In addition, knockout of Nrf2
inhibited the ability of fibroblasts to fight against injury
induced by ROS (33,34). Importantly, the present study
demonstrated that dioscin significantly increased Nrf2 protein
expression in the heart of CHD model pigs, and the results
indicated that the effects of dioscin may be mediated by Sirt1/Nrf2
to protect against CHD in pigs. Additionally, Gu et al
(22) demonstrated that dioscin
alleviated hepatic fibrosis through the Sirt1/Nrf2 and p38 MAPK
pathways.
As an important member of the MAPK family, p38 MAPK
has been reported to be involved in the ischemia, reperfusion and
apoptosis of myocardial cells in a previous study (35). Numerous stimuli may lead to the
activation of p38 MAPK and activated p38 MAPK has been implicated
in the regulation of myocardial cell apoptosis (35). Myocardial ischemia-reperfusion has
been reported to initiate the stress-activated protein kinase
pathway and MAPK pathway in cells, and these signal pathways were
demonstrated to be closely associated with calcineurin (36). The activity of calcineurin was
reported to be enhanced during myocardial ischemia-reperfusion and
participated in myocardial apoptosis induced by
ischemia-reperfusion; p38 MAPK are important signal pathways that
mediate myocardial apoptosis (17). The results of the present study
demonstrated that dioscin significantly suppressed p-p38 MAPK
protein expression in CHD model pigs, indicating that p38 MAPK
signaling pathways may also be involved in the effects of dioscin
in CHD model pigs. Wang et al (37) demonstrated that dioscin induced the
apoptosis of HL-60 cells through activation of p38 MAPK and c-Jun
N-terminal kinase pathways.
In conclusion, the results of the current study
demonstrated that dioscin reduced the levels of CK, CK-MB, LDH and
cTnT, increased the LVEF and inhibited the LVIDs CHD model pigs
through anti-inflammatory and antioxidative effects. Furthermore,
the results of further experiments indicated that the protective
effects of dioscin on CHD in pigs may be associated with the
Sirt1/Nrf2 and p38 MAPK pathways, and dioscin maybe a novel
possible drug for CHD in future research.
Acknowledgements
Not applicable.
Funding
The present study was partially supported by the
National Natural Science Foundation of China (grant no. 81570272,
to Dr Bo Yang), the Beijing Natural Science Foundation (grant no.
7132227, to Dr Bo Yang), National Science Foundation of China
(grant no. 61471064, GX Kang and B Yang), the Nova Programme from
Beijing Municipal Science and Technology Commission (grant no.
Z141107001814113-XXHZ201401, to Dr Bo Yang) and the Discovery
Foundation from The Chinese Medical Doctor Association (grant no.
DFCMDA201311, to Dr Bo Yang).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BY designed the study; BX, HZ, YBW, JZ, CWL, QW,
YKC, YL and FC performed the experiments; BY and FC analyzed the
data; BY wrote the manuscript.
Ethics approval and consent to
participate
Ethical approval for the present study was provided
by the Chinese PLA General Hospital (Beijing, China).
Consent for publication
Not applicable.
Competing interests
All authors declared that they have no competing of
interests.
References
|
1
|
Andersson C, Shilane D, Go AS, Chang TI,
Kazi D, Solomon MD, Boothroyd DB and Hlatky MA: Beta-blocker
therapy and cardiac events among patients with newly diagnosed
coronary heart disease. J Am Coll Cardiol. 64:247–252. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Strissel KJ, Denis GV and Nikolajczyk BS:
Immune regulators of inflammation in obesity-associated type 2
diabetes and coronary artery disease. Curr Opin Endocrinol Diabetes
Obes. 21:330–338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hakeem A, Bhatti S and Chang SM: Screening
and risk stratification of coronary artery disease in end-stage
renal disease. JACC Cardiovasc Imaging. 7:715–728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alizade E, Avci A, Acar G, Açar G, Fidan
S, Öcal L, Bulut M, Tellice M, Akçakoyun M, Pala S and Esen AM: The
relationship between rheumatoid factor levels and coronary artery
lesion complexity and severity in patients with stable coronary
artery disease. Postepy Kardiol Interwencyjnej. 11:26–31.
2015.PubMed/NCBI
|
|
5
|
Tan F, Chen Y, Yuan D, Gong C, Li X and
Zhou S: Dexmedetomidine protects against acute kidney injury
through downregulating inflammatory reactions in endotoxemia rats.
Biomed Rep. 3:365–370. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meyer S, Neeff H, Thomusch O, Strate T,
Tittelbach-Helmrich D, Hopt UT and von Dobschuetz E: Everolimus
improves microcirculatory derangements in experimental postischemic
pancreatitis modulating the expression of vascular endothelial
growth factor, Interleukin 6, and toll-like receptor 4. Pancreas.
44:1245–1251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Correia GD, Ng Wooi K, Wijeyesekera A,
Gala-Peralta S, Williams R, MacCarthy-Morrogh S, Jiménez B, Inwald
D, Macrae D, Frost G, et al: Metabolic profiling of children
undergoing surgery for congenital heart disease. Crit Care Med.
43:1467–1476. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yin YW, Li JC, Zhang M, Wang JZ, Li BH,
Liu Y, Liao SQ, Zhang MJ, Gao CY and Zhang LL: Influence of
interleukin-6 gene −174G>C polymorphism on development of
atherosclerosis: A meta-analysis of 50 studies involving 33,514
subjects. Gene. 529:94–103. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sakthivel KM and Guruvayoorappan C: Acacia
ferruginea inhibits tumor progression by regulating inflammatory
mediators-(TNF-a, iNOS, COX-2, IL-1β, IL-6, IFN-γ, IL-2, GM-CSF)
and pro-angiogenic growth factor-VEGF. Asian Pac J Cancer Prev.
14:3909–3919. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Somacal S, Figueiredo CG, Quatrin A,
Ruviaro AR, Conte L, Augusti PR, Roehrs M, Denardin IT, Kasten J,
da Veiga ML, et al: The antiatherogenic effect of bixin in
hypercholesterolemic rabbits is associated to the improvement of
lipid profile and to its antioxidant and anti-inflammatory effects.
Mol Cell Biochem. 403:243–253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Badalzadeh R, Mohammadi M, Yousefi B,
Farajnia S, Najafi M and Mohammadi S: Involvement of glycogen
synthase kinase-3β and oxidation status in the loss of
cardioprotection by postconditioning in chronic diabetic male rats.
Adv Pharm Bull. 5:321–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fleming DS and Miller LC: Identification
of small non-coding RNA classes expressed in swine whole blood
during HP-PRRSV infection. Virology. 517:56–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saito S, Thuc LC, Teshima Y, Nakada C,
Nishio S, Kondo H, Fukui A, Abe I, Ebata Y, Saikawa T, et al:
Glucose fluctuations aggravate cardiac susceptibility to
ischemia/reperfusion injury by modulating microRNAs expression.
Circ J. 80:186–195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, Hu X and Jiang H: ERS-PERK
signaling pathway-mediated Nrf2/ARE-HO-1 axis: A novel therapeutic
target for attenuating myocardial ischemia and reperfusion injury.
Int J Cardiol. 203:779–780. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mleczko AM and Bąkowska-Żywicka K: When
small RNAs become smaller: Emerging functions of snoRNAs and their
derivatives. Acta Biochim Pol. 63:601–607. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li W, Wu M, Tang L, Pan Y, Liu Z, Zeng C,
Wang J, Wei T and Liang G: Novel curcumin analogue 14p protects
against myocardial ischemia reperfusion injury through
Nrf2-activating anti-oxidative activity. Toxicol Appl Pharmacol.
282:175–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liao J, Yu L, Mei Y, Guarnera M, Shen J,
Li R, Liu Z and Jiang F: Small nucleolar RNA signatures as
biomarkers for non-small-cell lung cancer. Mol Cancer. 9:1982010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ravo M, Cordella A, Rinaldi A, Bruno G,
Alexandrova E, Saggese P, Nassa G, Giurato G, Tarallo R, Marchese
G, et al: Small non-coding RNA deregulation in endometrial
carcinogenesis. Oncotarget. 6:4677–4691. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Langhendries JL, Nicolas E, Doumont G,
Goldman S and Lafontaine DL: The human box C/D snoRNAs U3 and U8
are required for pre-rRNA processing and tumorigenesis. Oncotarget.
7:59519–59534. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang W, Yin L, Tao X, Xu L, Zheng L, Han
X, Xu Y, Wang C and Peng J: Dioscin alleviates
dimethylnitrosamine-induced acute liver injury through regulating
apoptosis, oxidative stress and inflammation. Environ Toxicol
Pharmacol. 45:193–201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu B, Xu Y, Xu L, Cong X, Yin L, Li H and
Peng J: Mechanism investigation of dioscin against CCl4-induced
acute liver damage in mice. Environ Toxicol Pharmacol. 34:127–135.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gu L, Tao X, Xu Y, Han X, Qi Y, Xu L, Yin
L and Peng J: Dioscin alleviates BDL- and DMN-induced hepatic
fibrosis via Sirt1/Nrf2-mediated inhibition of p38 MAPK pathway.
Toxicol Appl Pharmacol. 292:19–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Konstanty-Kalandyk J, Piatek J,
Miszalski-Jamka T, Rudziński P, Walter Z, Bartuś K,
Urbańczyk-Zawadzka M and Sadowski J: The combined use of
transmyocardial laser revascularisation and intramyocardial
injection of bone-marrow derived stem cells in patients with
end-stage coronary artery disease: One year follow-up. Kardiol Pol.
71:485–492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tahara N, Tahara A, Narula J and Imaizumi
T: Statin therapy resolves coronary artery inflammation. JACC
Cardiovasc Imaging. 6:1119–1120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gupta GK, Agrawal T, DelCore MG, Mohiuddin
SM and Agrawal DK: Vitamin D deficiency induces cardiac hypertrophy
and inflammation in epicardial adipose tissue in
hypercholesterolemic swine. Exp Mol Pathol. 93:82–90. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qin J, Kang Y, Xu Z, Zang C, Fang B and
Liu X: Dioscin prevents the mitochondrial apoptosis and attenuates
oxidative stress in cardiac H9c2 cells. Drug Res (Stuttg).
64:47–52. 2014.PubMed/NCBI
|
|
27
|
Sartore G, Seraglia R, Burlina S, Bolis A,
Marin R, Manzato E, Ragazzi E, Traldi P and Lapolla A: High-density
lipoprotein oxidation in type 2 diabetic patients and young
patients with premature myocardial infarction. Nutr Metab
Cardiovasc Dis. 25:418–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mentese U, Dogan OV, Turan I, Usta S,
Dogan E, Mentese SO, Demir S, Ozer T, Aykan AC and Alver A:
Oxidant-antioxidant balance during on-pump coronary artery bypass
grafting. ScientificWorldJournal. 2014:2630582014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao L, Tao X, Qi Y, Xu L, Yin L and Peng
J: Protective effect of dioscin against doxorubicin-induced
cardiotoxicity via adjusting microRNA-140-5p-mediated myocardial
oxidative stress. Redox Biol. 16:189–198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ge J, Wu XM, Yang XT, Gao JM, Wang F and
Ye KF: Role of long non-coding RNA SNHG1 in occurrence and
progression of ovarian carcinoma. Eur Rev Med Pharmacol Sci.
22:329–335. 2018.PubMed/NCBI
|
|
31
|
Ren J, Yang Y, Xue J, Xi Z, Hu L, Pan SJ
and Sun Q: Long noncoding RNA SNHG7 promotes the progression and
growth of glioblastoma via inhibition of miR-5095. Biochem Biophys
Res Commun. 496:712–718. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bachellerie JP, Nicoloso M, Qu LH, Michot
B, Caizergues-Ferrer M, Cavaille J and Renalier MH: Novel
intron-encoded small nucleolar RNAs with long sequence
complementarities to mature rRNAs involved in ribosome biogenesis.
Biochem Cell Biol. 73:835–843. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J, Cao L, Wu J and Wang Q: Long
non-coding RNA SNHG1 regulates NOB1 expression by sponging miR-326
and promotes tumorigenesis in osteosarcoma. Int J Oncol. 52:77–88.
2018.PubMed/NCBI
|
|
34
|
Mei YP, Liao JP, Shen J, Yu L, Liu BL, Liu
L, Li RY, Ji L, Dorsey SG, Jiang ZR, et al: Small nucleolar RNA 42
acts as an oncogene in lung tumorigenesis. Oncogene. 31:2794–2804.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Koduru SV, Tiwari AK, Leberfinger A,
Hazard SW, Kawasawa YI, Mahajan M and Ravnic DJ: A comprehensive
NGS data analysis of differentially regulated miRNAs, piRNAs,
lncRNAs and sn/snoRNAs in triple negative breast cancer. J Cancer.
8:578–596. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao L, Ma J, Mannoor K, Guarnera MA,
Shetty A, Zhan M, Xing L, Stass SA and Jiang F: Genome-wide small
nucleolar RNA expression analysis of lung cancer by next-generation
deep sequencing. Int J Cancer. 136:E623–E629. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, He QY and Chiu JF: Dioscin induced
activation of p38 MAPK and JNK via mitochondrial pathway in HL-60
cell line. Eur J Pharmacol. 735:52–58. 2014. View Article : Google Scholar : PubMed/NCBI
|