Introduction
Osteosarcoma (OS) is regarded as the most common
primary malignant bone tumor. It occurs chiefly in children and
adolescents who account for ~70% of all cases (1). Like the vast majority of malignant
tumors, OS is highly invasive and has distant metastatic potential,
especially in the lung (2).
Despite surgery in combination with chemotherapy and/or
radiotherapy has greatly improved the prognosis of OS patients,
five-year survival rate is still lower. Moreover, chemotherapy can
lead to drug-resistance and produce life-threatening side effects,
such as cardiotoxicity and nephrotoxicity. Thus, there is an urgent
need to develop safer and more effective anti-OS drugs.
The biologically active compounds extracted from
plants and other natural sources have become an attractive strategy
for treating cancers, including OS. Scutellaria baicalensis
(SB) Georgi, a traditional Asian herb, is widely applied in the
clearance of heat dampness and purgation of fire detoxification
(3). Baicalein
(5,6,7-trihydroxyflavone), one of the primary active elements of
this plant, exerts a variety of pharmacological functions, such as
anti-oxidation, anti-proliferation, and apoptosis induction
(4,5). There is growing evidence that
administration of baicalein inhibits the development of many
tumors, including cervical cancer, colorectal cancer, bladder
cancer and breast cancer (6–9).
Notably, baicalein has also a potent anti-OS effect. However, the
potential molecular mechanisms of baicalein on human OS are still
vague.
MicroRNAs (miRNAs) are a class of small and
non-coding RNA molecules, which can regulate gene expression at the
post-transcriptional level by binding to the 3′-untranslated region
(UTR) of target mRNAs. Dysregulation of miRNAs is involved in the
maligantly biological behaviors of tumors (10,11).
As an evolutionarily conserved miRNA, miR-183 has been showed to
serve as either a tumor suppressor or oncogene in various human
tumors (12). Ezrin gene is
located in 6q25 and composed of 585 amino acids and can mediate the
tumor invasion and metastasis (13,14).
Research has revealed that miR-183 level was dramatically decreased
in OS cells and tissues, enhancing the migration and invasion of OS
by targeting Ezrin (15).
Moreover, our previous study also confirmed that Ezrin aggravated
the aggressiveness of OS, and was a direct target of miR-183
(16). Therefore, it could be
ascertained that the miR-183/Ezrin pathway plays vital roles in the
development and progression of OS. However, there have not yet been
any investigations concerning whether the antitumor effects of
baicalein on OS were triggered by miR-183/Ezrin pathway until
now.
In the present study, we found that baicalein
inhibited the proliferation, migration and invasion and induced
apoptosis in two human OS cell lines, MG-63 and Saos-2.
Importantly, activation of the miR-183/Ezrin pathway was required
for these effects. These results provide a novel insight into the
mechanisms by which baicalein protects against OS.
Materials and methods
Drugs, reagents and antibodies
Baicalein was obtained from Sigma-Aldrich Chemical
Co. (St. Louis, MO, USA). Dulbecco's modified Eagle's medium
(DMEM), penicillin/streptomycin, 0.25% trypsin-EDTA, and dimethyl
sulphoxide (DMSO) were purchased from Gibco (Grand Island, NY,
USA). Anti-Ezrin (1:500) and anti-phosphor-Ezrin (1:500) antibodies
were acquired from Abcam (Cambridge, MA, USA). In addition,
anti-GAPDH (1:1,000) and secondary antibodies (1:3,000) were
obtained from the Cell Signaling Technology (CST; Beverly, MA,
USA).
Cell culture
Human OS cell lines MG-63 and Saos-2 were purchased
from China Center for Type Culture Collection (Wuhan, China), and
human osteoblast cell line hFOB1.19 was obtained from the Chinese
Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
MG-63 and Saos-2 cells were maintained in complete medium
containing DMEM, 10% fetal bovine serum (FBS; Biolnd, Israel), 50
U/ml penicillin/streptomycin in a humidified incubator with 5%
CO2 at 37°C. hFOB1.19 cells were cultured in complete
medium consisting of DMEM/F12 (1:1) medium, 10% FBS (Biolnd,
Israel), and G418 (0.3 mg/ml) with 5% CO2 at 34°C.
Sebsequently, these cells were digested by 0.25% trypsin-EDTA
solution for all in vitro experiments and for cell pass and
27age unless stated otherwise after cells reached at least 80%
confluence.
Cell proliferation assay
Baicalein was dissolved in DMSO solution to a final
concentration of 4 mM. MG-63, Saos-2 and hFOB cells were seeded at
density 5,000 cells/well in 96-well plates and cultured for 24 h.
Cells were treated with baicalein at different concentrations (0,
25, 50, 75 and 100 µM) at 0 h, and then were cultured for 24, 48 or
72 h. Subsequently, the proliferation assay was performed using a
Cell Counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) according to the manufacturer's instructions. A
microplate reader (Thermolex Molecular Device Co.) was utilized to
detect the absorbance at 450 nm.
Cell apoptosis analysis
Following maintenance in culture by different
treatments, cell apoptosis was assessed using an Annexin
V/fluorescein isothiocyanate (FITC) Apoptosis Detection kit I (BD
Biosciences, San Jose, CA, USA). The untreated or treated MG-63 and
Saos-2 cells were harvested after indicated treatments with 0.25%
trypsin-EDTA, and a single cell suspension was prepared. Cells were
washed twice with cold PBS, centrifugated at 1,500 rpm for 5 min
and resuspended in 1X Binding Buffer so that cell density reached
1×106 cells/ml. 5 µl Annexin V/FITC was added to 300 µl
the above resuspended solution and incubated for 10 min, followed
by adding 5 µl propidium iodide (PI) for a 10 min incubation at
room temperature in the dark. Cell apoptosis were then detected
using flow cytometry (BD Biosciences). The results were analyzed by
cell quest software (BD Biosciences) to determine the rate of
apoptosis in the lower right quadrant.
Cell invasion and migration assay
Cell invasion assay was carried out using Millicell
Hanging Cell Culture Insert (24-well, pore size 5 µm; Merck
Millipore, Billerica, MA, USA). After treatment, MG-63 and Saos-2
cells resuspended to a density of 5×104 cells/ml in
serum-free DMEM medium were transferred into upper chamber of the
inserts coated with 30 mg/cm2 of Matrigel (BD
Biosciences), DMEM medium with 20% FBS were added to the well out
of the insert and then incubation for different concentration or
time based on experimental program. Ultimately, cells on the upper
membrane surface were removed through careful wiped off a cotton
swab, and the cells on lower surface were fixed with 95% ethanol
for 30 min and stained with 0.2% Crystal Violet solution for 30
min. Five vision fields per chamber were randomly selected and then
counted the number of invasion cells under an inverted microscope.
The general procedure of the migration assay was performed the same
as that of the invasion assay described above, except that Matrigel
was not applied.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNAs were extracted from either MG-63 or
Saos-2 cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturers'
protocol. The purity and concentration of RNAs were detected by a
NanoDrop ND-1,000 spectrophotometer (Thermo Fisher Scientific,
Inc., Wilmington, DE, USA). The reverse transcription reactions
were conducted by a miRcute miRNA Reverse Transcription kit
(TianGen Biotech Co., Ltd., Beijing, China) and PrimeScript RT
Master Mix (Perfect Real Time; Takara Biotechnology Co., Ltd.,
Dalian, China). All reactions were performed using a 7500 Real-Time
PCR System (Thermo Fisher Scientific, Inc.) in a reaction volume of
20 µl. The levels of miR-183 and Ezrin were normalized to U6 small
nuclear RNA and GAPDH, respectively. The relative quantification
was calculated by applying the 2−∆∆Cq method (17). The PCR primers were designed and
synthesized by Shanghai Sangon Biological Engineering Technology
Co., Ltd., (Shanghai, China) (Table
I).
 | Table I.Primer sequences used for RT-qPCR
analyses. |
Table I.
Primer sequences used for RT-qPCR
analyses.
| Gene | Sequence |
|---|
| miR-183 |
|
|
Forward |
5′-TATGGCACTGGTAGAATTCACT-3′ |
|
Reverse |
5′-GCGAGCACAGAATTAATACGAC-3′ |
| U6 |
|
|
Forward |
5′-CGCTTCGGCAGCACATATAC-3′ |
|
Reverse |
5′-AAATATGGAACGCTTCACGA-3′ |
| Ezrin |
|
|
Forward |
5′-TGGGATGCTCAAAGATAATGC-3′ |
|
Reverse |
5′-ACTCCAAGCCAAAGGTCTGTT-3′ |
| GAPDH |
|
|
Forward |
5′-GGCACAGTCAAGGCTGAGAATG-3′ |
|
Reverse |
5′-ATGGTGGTGAAGACGCCAGTA-3′ |
Western blot analysis
Total proteins were extracted from MG-63 or Saos-2
cells using RIPA Lysis Buffer (Beyotime, Shanghai, China). The
protein concentrations were measured via Enhanced BCA Protein Assay
kit (Bio-Rad, Richmond, CA, USA). Lysates of Total protein were
separated by a 10% sodium dodecyl sulfate-polyacrylamide (SDS-PAGE)
gel and transferred to a polyvinylidene fluoride (PVDF) membrane
(Merck Millipore). The membrane was blocked by 5% (v/v) skim milk
in Tris-buffered saline Tween-20 (TBST) at room temperature for 2
h, followed by incubation with the primary antibodies at 4°C
overnight. After washing, the membrane was incubated with
HRP-conjugated secondary antibody at room temperature for 1 h. The
proteins were visualized using a chemiluminescence method
(electrochemiluminescence and western blot detection system;
Amersham Biosciences, Foster City, CA, USA). The results were
normalized using GAPDH.
Cell transfection
MiR-183 mimics/negative controls and
inhibitors/negative controls were supplied by RiboBio (Guangzhou,
China). The whole DNA fragment of Ezrin was amplified from the OS
cells. The obtained products were cloned into recombinant plasmid
pcDNA3.1(+)-EGFP to overexpress Ezrin (pcDNA3.1-Ezrin; Invitrogen;
Thermo Fisher Scientific, Inc.), with the empty vector as a
negative control (pcDNA3.1-Mock). The constructs were identified by
DNA sequencing. Both MG-63 and Saos-2 cells were seeded into a
12-well plate at a 1×105/ml density, and then
transfected with 20 nM miR-183 mimic/negative controls or 40 nM
miR-183 inhibitors/negative controls or pcDNA3.1-Ezrin using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions.
Dual luciferase reporter assay
A fragment of 3′UTR of Ezrin containing the putative
miR-183 binding site was amplified by RT-PCR. The amplified product
was then subcloned into a pMIR-REPORT luciferase reporter vector
applying restriction sites XhoI (Promega, Madison, WI, USA). The
mutated sequences of predicred miR-183 binding located in the 3′UTR
of Ezrin mRNA were introduced using the QuikChange kit (Stratagene,
La Jolla, CA, USA). All plasmid constructs were verified by DNA
sequencing (Biology Engineering Corp., Shanghai, China).
Successively, the 20 nM miR-183 mimics/negative controls or 40 nM
miR-183 inhibitor/negative controls and 0.1 µg pMIR-REPORT-Ezrin
(WT) or pMIR-REPORT-Ezrin (MUT) constructs were co-transfected into
the cells using Lipofectamine 2000. After transfection for 48 h,
the Dual-Luciferase Reporter Assay System (Promega) was used to
detect the luciferase activity. The Renilla luciferase values were
regarded as a correction factor, and the firefly/Renilla ratio was
obtained. Luciferase activity was averaged from three replicates
for each transfection.
Statistical analysis
All data were presented as the means ± standard
deviation (SD), and all of graphs were produced by GraphPad Prism
5.0 Software (Graph Pad Software, Inc., La Jolla, CA, USA). SPSS
22.0 software (IBM Corp., Armonk, NY, USA) was used for all
statistical analyses. Results were analyzed using Student's
t-test for comparison between two groups or one-way ANOVA
followed by the Tukey's test for comparison of multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baicalein inhibits proliferation and
promotes apoptosis in MG-63 and Saos-2 cells
To investigate the effect of baicalein on OS cell
proliferation, cells were treated with various concentrations of
baicalein for the indicated time, followed by the CCK-8 assay. Our
results showed that baicalein inhibited the proliferation of MG-63
and Saos-2 cells in a concentration- and time-dependent manner
(Fig. 1A and B), but did not
affect hFOB cell growth (Fig. 1C).
Given that 100 µM baicalein had the optimal efficacy, we selected
this dose for subsequent experiments.
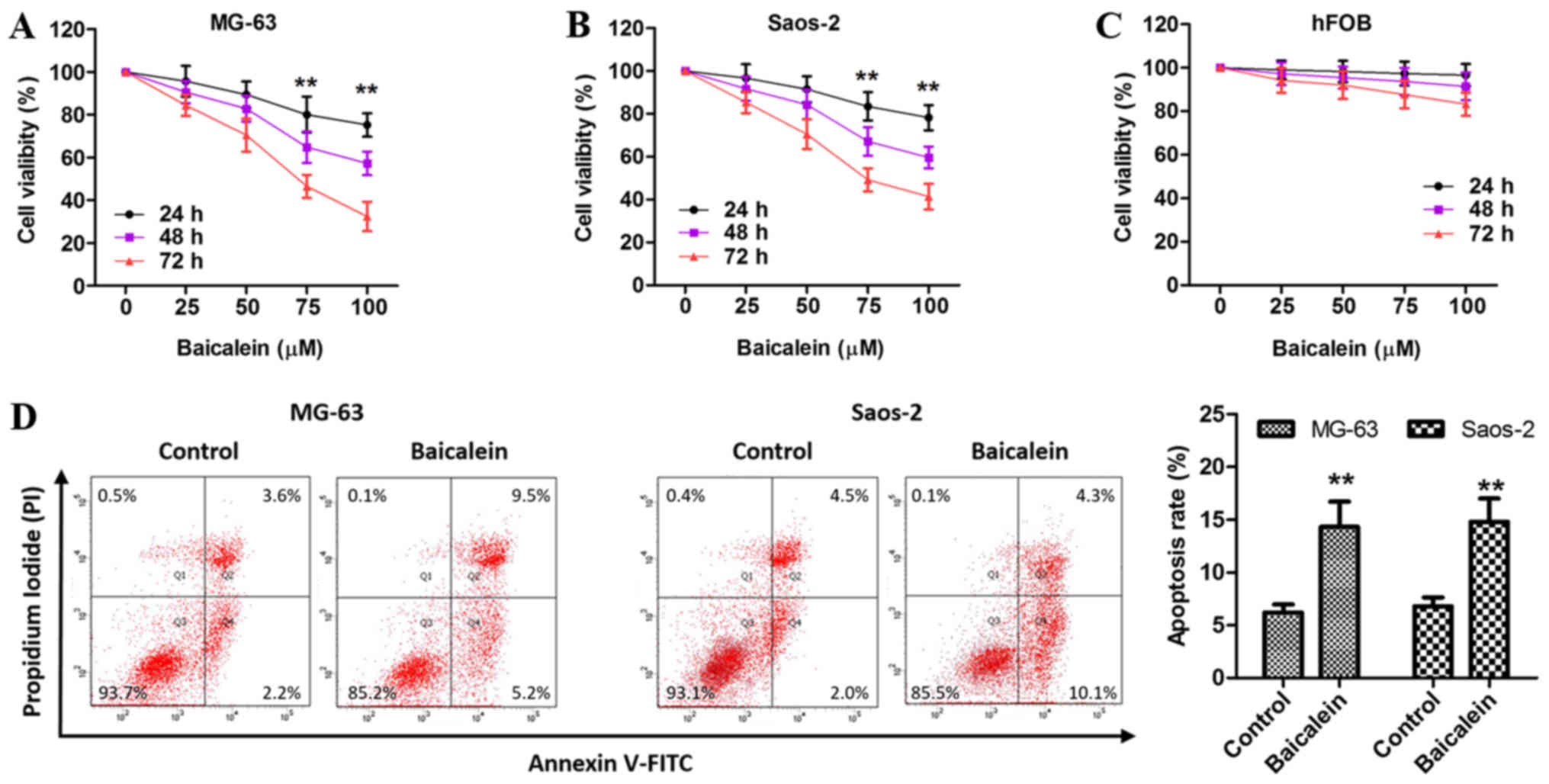 | Figure 1.Baicalein regulates the proliferation
and apoptosis of OS cells. (A-C) after exposure to various
concentrations (0, 25, 50, 75, 100 µM) of baicalein for 24, 48, 72
h, the viability of MG-63, Saos-2 and hFOB cells was performed
using the CCK-8 assay kit. DMSO was regarded as control. (D) MG-63
and Saos-2 cells were treated with 100 µM baicalein for 48 h, and
then subjected to double staining with Annexin V-FITC and PI for
flow cytometry detection. Q2+Q4 represents apoptotic cells (%).
Data is presented as the means ± SD from three independent
experiments. **P<0.01 vs. control. OS, osteosarcoma; CCK-8, Cell
Counting kit-8; DMSO, dimethyl sulphoxide; FITC, fluorescein
isothiocyanate; SD, standard deviation. |
Given the fact that the inhibition of apoptosis
contributed to cell proliferation in various type of tumor cells,
next we employed fluorescence activated cell sorter (FACS) analysis
via Annexin V-FITC and PI double-staining to evaluate the apoptosis
rate of MG-63 and Saos-2 cells exposed to 100 µM baicalein for 48
h. As shown in Fig. 1D, the
apoptosis rate of MG-63 and Saos-2 cells was low in control group
but significantly elevated in baicalein-treated group, suggesting
an inductive effect of baicalein on MG-63 and Saos-2 cell
apoptosis.
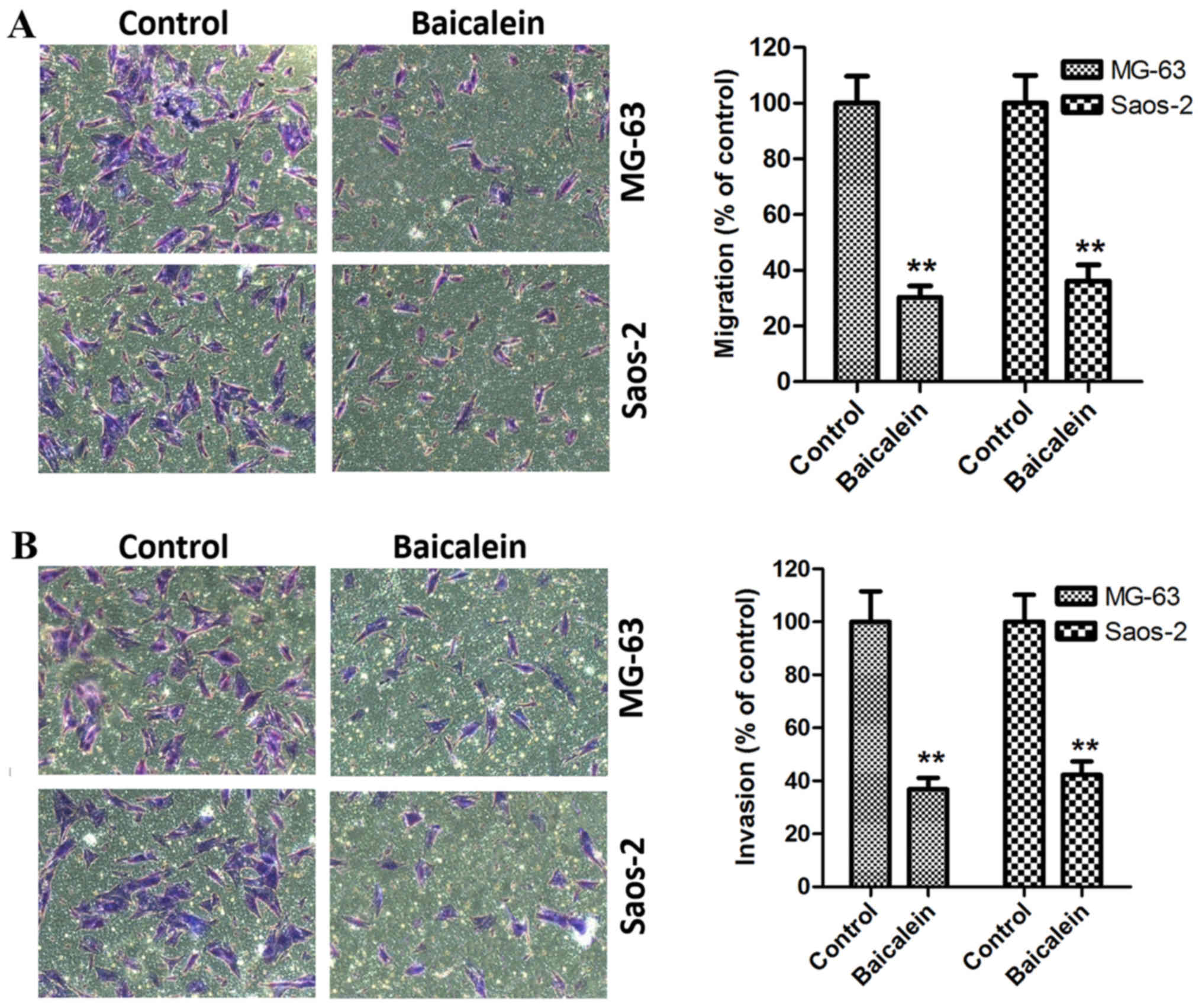
Baicalein represses the migration and
invasion of OS cells
It is well known that migration and invasion are the
two pivotal stages in malignant progression and metastasis for
tumors. Thus, the present study further evaluated the effects of
baicalein on the aggressive potential of MG-63 and Saos-2 cells
using cell migration and invasion assays. After stimulation of
MG-63 and Saos-2 cells with 100 µM baicalein for 48 h, the
migration ability of cells was measured. In Fig. 2A, we found that the migration
ability of MG-63 and Saos-2 cells was obviously inhibited. Aside
from migration test, we also employed the cell invasion assay to
measure the invasion ability of MG-63 and Saos-2 cells. As shown in
Fig. 2B, the similar effect on
invasive ability was also observed in parallel invasive test,
compared with the control group. Altogether, the above results
reveal that baicalein can suppress the migration and invasion
ability of MG-63 and Saos-2 cells.
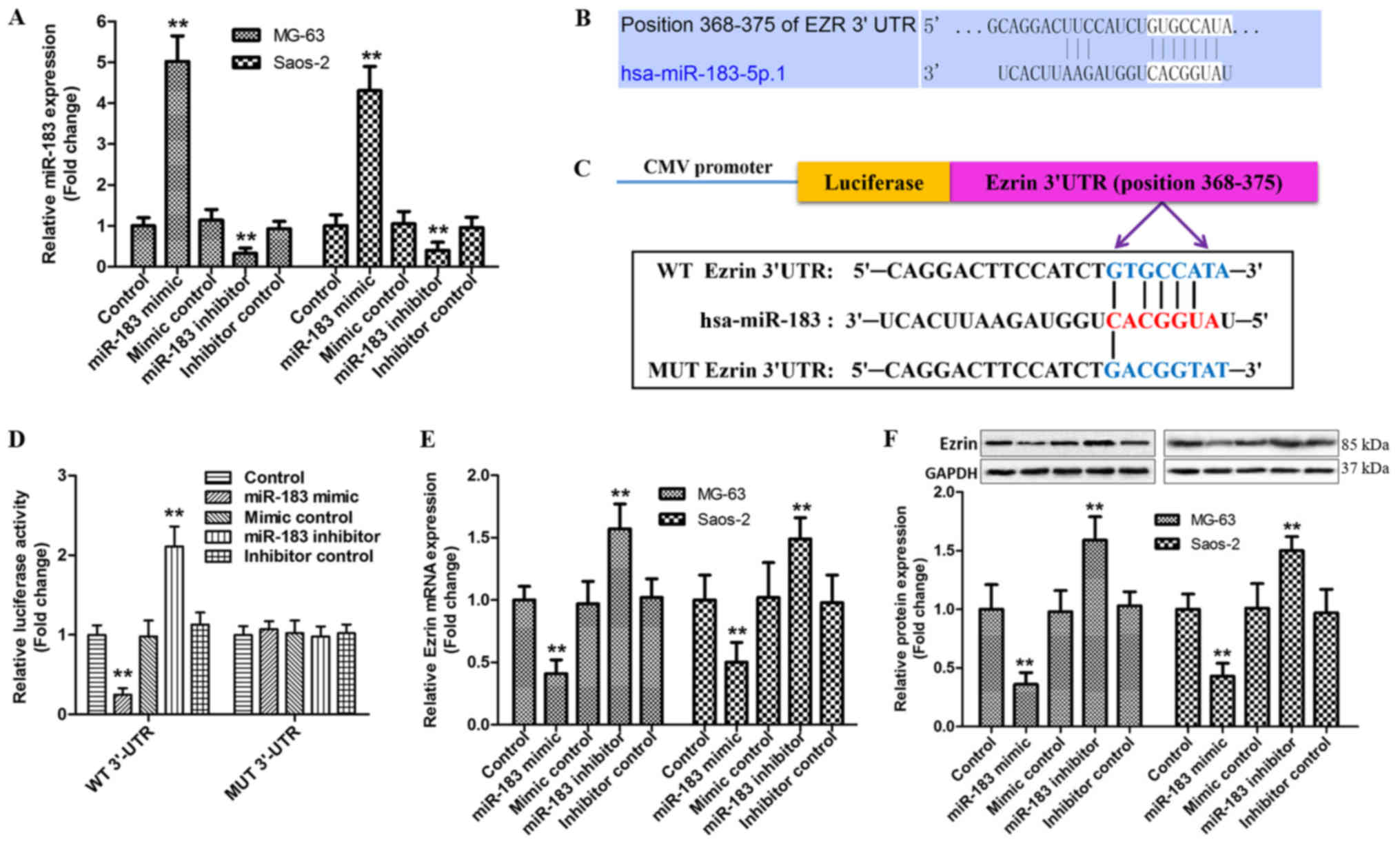
Identification of Ezrin as a direct
target of miR-183
It has been reported that miRNAs are important
regulators in the aggressive behavior of tumor, including OS
(18). Our previous study has been
proposed that Ezrin as a direct target of miR-183 promotes the
migration and invasion of OS MG-63 cells through increased
N-cadherin and activating ERK signaling (16). However, whether baicalein regulates
the activation of miR-183/Ezrin pathway in MG-63 and Saos-2 cells
remains unclear. In order to explore this hypothesis, we validated
again the relationship between Ezrin and miR-183 in the current
research. Treatment of MG-63 and Saos-2 cells with miR-183
mimic/negative control or miR-183 inhibitors/negative control both
exerted a higher transfection efficiency (Fig. 3A). The pairing site of Ezrin 3′-UTR
with miR-183 were predicted by TargetScan (an open sourced
software) (Fig. 3B). A luciferase
reporter gene with 3′-UTR of Ezrin downstream (WT-Ezrin), its
mutant version (MUT-Ezrin) via the binding site mutagenesis were
also constructed (Fig. 3C). As
shown in Fig. 3D, the luciferase
activity of cells transfected with miR-183 mimic was dramatically
decreased in wild type, but not mutant. Furthermore, the RT-qPCR
and western blot analyses showed that overexpression of miR-183
noteworthily reduced the levels of Ezrin mRNA and protein compared
with control group, whereas an opposite effect appeared in response
to miR-183 inhibitor (Fig. 3E and
F). Overall, these results demonstrate that miR-183 directly
targets the 3′-UTR of Ezrin and negatively regulates its
expression.
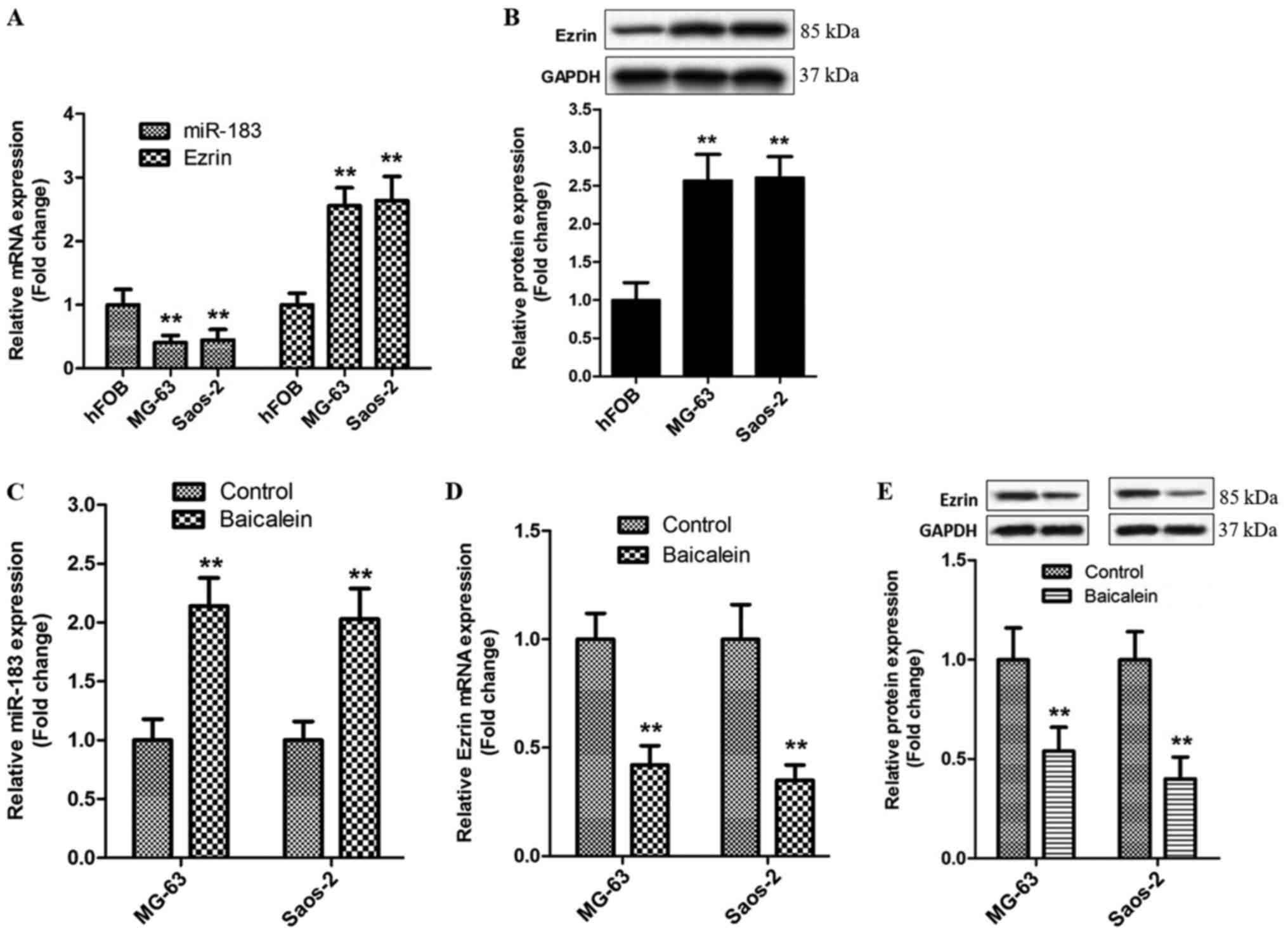
Baicalein increases miR-183 levels and
represses Ezrin expression in OS cells
We firstly detected the contents of miR-183 and
Ezrin in MG-63, Saos-2 and hFOB cells, respectively. RT-qPCR showed
that miR-183 levels in MG-63 and Saos-2 cells were lower than those
in hFOB cells (Fig. 4A). However,
the expression of Ezrin mRNA and protein displayed an opposed
tendency in above cells (Fig. 4A and
B). These data indicate that abnormal expressions of miR-183
and Ezrin might be correlated highly with the pathogenesis and
metastasis of OS.
Next, the underlying effects of baicalein on
miR-183/Ezrin pathway were investigated using RT-qPCR and western
blot in MG-63 and Saos-2 cells treated by 100 µM baicalein for 48
h. As demonstrated in Fig. 4C,
baicalein-stimulated MG-63 and Saos-2 cells displayed a significant
increase in miR-183 level in comparison with control group.
However, the alterations in the mRNA and protein levels of Ezrin
presented an opposite tendency in above parallel groups (Fig. 4D and E). In conclusion, these data
corroborate that stimulation of baicalein is able to promote the
generation of miR-183 and then retard the expression of Ezrin in
MG-63 and Saos-2 cells.
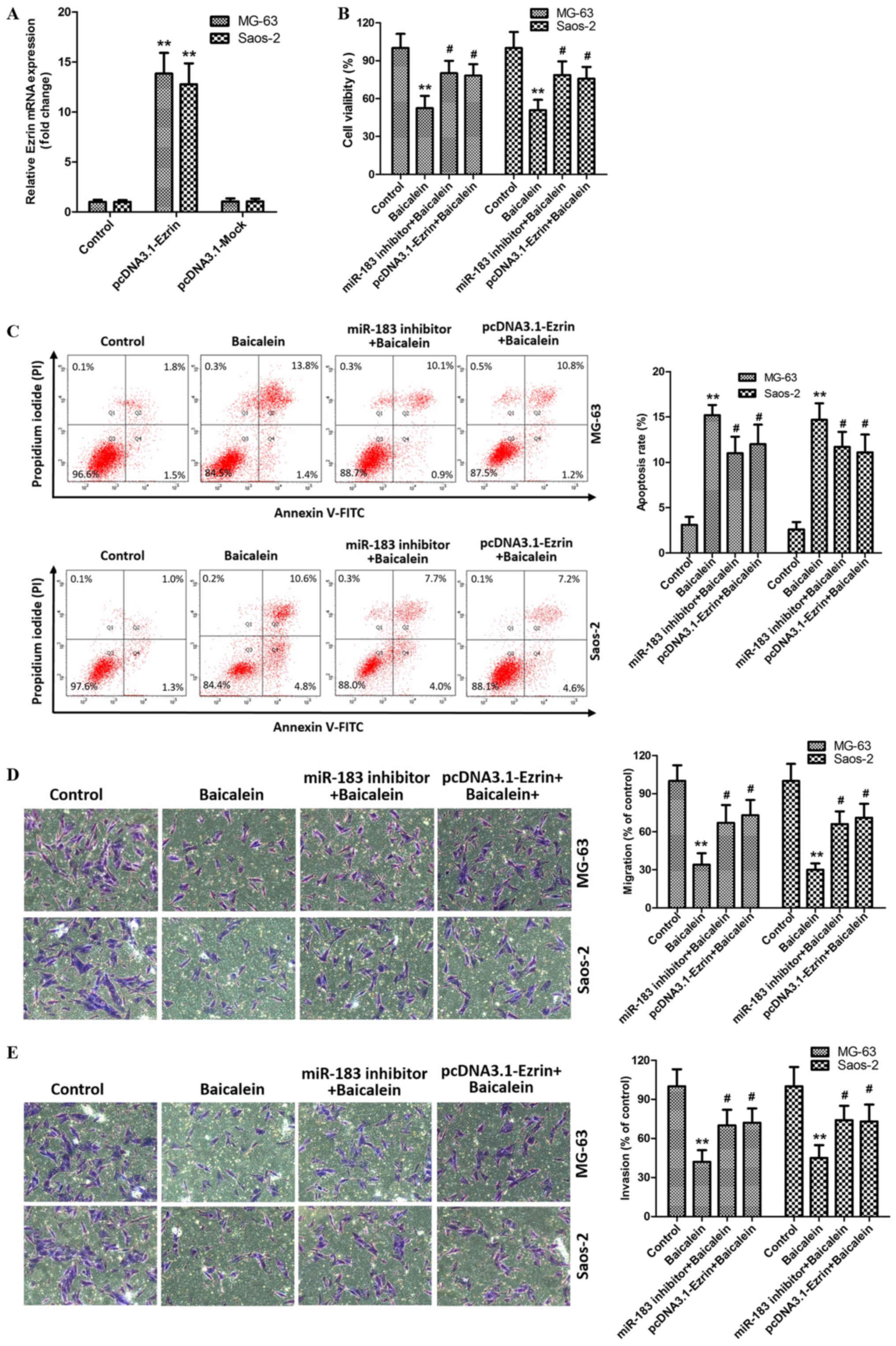
miR-183/Ezrin pathway is required for
the effects of baicalein on OS cells
As evidenced above experiments, baicalein represses
the proliferation and migration and invision abilitiess, and
participates in the activation of apoptosis in MG-63 and Saos-2
cells. Meanwhile, it as well negatively regulates the Ezrin
expression via upregulating miR-183 level in MG-63 and Saos-2
cells. Therefore, we hypothesized that the miR-183/Ezrin pathway
might be responsible for the antitumor effects of baicalein on OS
cells. To verify the assumption, MG-63 and Saos-2 cells were
co-cultured with baicalein and miR-183 inhibitors or
pcDNA3.1-Ezrin, which showed a higher transfection efficiency
(Fig. 5A). As expected, miR-183
inhibitor significantly reversed baicalein-induced high apoptosis
rate and low proliferation, migration and invasive ability in MG-63
and Saos-2 cells, compared with baicalein group (Fig. 5B-E). Besides, we also found that
overexpression of Ezrin by pcDNA3.1-Ezrin exerts similary effect in
stumilation of baicalein with MG-63 and Saos-2 cells (Fig. 5B-E). Taken together, these
observations indicate that baicalein induces apoptosis and
attenuates proliferation and migration and invasion abilities of
MG-63 and Saos-2 cells via activating miR-183/Ezrin pathway.
Discussion
OS is originated from mesenchymal stem cells
producing osteoid or immature bone, and mainly characterized by
high aggressiveness and metastasis. Multi-drug chemotherapy
approaches for OS patients have obviously improved the survival
rate subsequent to surgical resection (19). However, it is undesirable to the
drug-resistance and life-threatening side effects induced by
chemotherapy, leading to treatment failure or shorter survival.
Thus, there is an urgent need for newer effective and low-toxic
agents for patients with OS, especially for patients suffered from
metastatic disease or disease recurrence. Enhancing the
understanding of basic biology of therapeutic agents to OS has been
a high priority in recent years.
Baicalein, a bioactive flavone purified from SB, is
widely used in nutraceuticals and pharmaceuticals. It has been
shown that baicalein has considerable potential in the treatment
and prevention of cancers by inducing apoptosis, inhibiting the
colony formation, migration and invasion (3,20).
Moreover, baicalein only suppresses the growth of cancer cells
without the effects on normal cells (21,22).
In addition, researches have shown that baicalein can inhibit the
progression of OS cells by a variety of biological pathways.
Baicalein suppresses progression of OS cells via inactivating the
Wnt/β-catenin signaling pathway (23). Zhang and his colleagues reported
that baicalein inhibitors reduces the viability of MG-63 cells by
repressing c-MYC expression through the Wnt signaling pathway
(24). Baicalein induces MG-63
cells apoptosis via ROS-induced BNIP3 expression (25). In the present study, we found that
baicalein markedly promoted apoptosis and inhibited cell
proliferation, migration and invasion of MG-63 and Saos-2 cells,
but not hFOB cells. Taken together, these studies suggest that
baicalein may be a promising and potential candidate for
chemotherapeutic agents to treat OS. However, the precision
mechanism by which baicalein mediates the development and
progression of OS cells remains unclear.
Ectopic expression of miRNAs has been shown to be
implicated in regulating proliferation, apoptosis, migration and
invasion in multifarious cancer cells, including OS cells (26–28).
These small molecules have emerged as potential targets for the
diagnosis, therapy and prognosis of OS. MiR-183 has been identified
as a promising biomarker, which was attributed to its early cancer
detection and exact prognosis as well as high-efficiency treatment
(29). Previous reports have shown
that downexpression of miR-183 was observed in OS tissues or
various cell lines (15,30,31).
Similarly, our previous research and this current study also
revealed a similar tendency for miR-183 expression in OS MG-63 and
Saos-2 cells (16). These data
suggest that downregulation of miR-183 plays a pivotal role in
promoting aggressiveness of OS.
It is generally known that the effect of miRNAs on
the target gene is achieved by binding to the 3′-UTR of target
gene. Ezrin, a target gene of miR-183, is a widely explored
oncogene in various cancers, and as well involved in the
tumorgenesis of OS. Our previous data and present research have
demonstrated that Ezrin expression was markedly increased in MG-63
and Saos-2 cells, promoting invasion and migration of cells; by
contrast, these effects were abolished by knockdown of Ezrin
(16). Furthermore, positive Ezrin
expression is closely correlated with clinical grade,
free-metastasis or metastasis, and adverse prognosis of patients
with OS (32,33). Thereby, Ezrin may serve as a
contributor for the invasiveness and carcinogenesis of OS.
Increasing evidence has illustrated that the
miR-183/Ezrin pathway is involved in the progression of OS. It has
been shown that downexpression of miR-183 promotes migration and
invasion of OS by targeting Ezrin (15). MiR-183 inhibits the metastasis of
OS via downregulation of Ezrin expression in F5M2 cells (31). Interestingly, research has shown
that the aberrant expression of miR-183/Ezrin axis may be related
to the prediction of aggressiveness and poor prognosis for patients
with OS (30). In the current
study, we validated that Ezrin was a target gene of miR-183,
suggesting that miR-183 negatively regulated Ezrin expression.
Given the roles of baicalein or miR-183/Ezrin pathway in OS, we
rationally hypothesize that the antitumor effects of baicalein on
OS were presented by activating the miR-183/Ezrin pathway. In this
study, we found that baicalein significantly increased the miR-183
levels and decreased Ezrin expression in human OS MG-63 and Saos-2
cells. In addition, our findings showed that transfection of
miR-183 inhibitor or overexpression of Ezrin abolished
baicalein-induced antitumor effects in MG-63 and Saos-2 cells.
Thus, the miR-183/Ezrin pathway is involved in the suppressive
effects of baicalein on MG-63 and Saos-2 cells.
In summary, our study clearly demonstrates that
baicalein enhances apoptosis and inhibits cell proliferation,
migration and invasion by activation of miR-183/Ezrin pathway in
human OS MG-63 and Saos-2 cells. These findings reveal a molecular
mechanism by which baicalein inhibits progression of OS, providing
in vitro evidence to support baicalein as an efficient agent
for the treatment of OS.
Acknowledgements
The authors would like to thank all of the
volunteers who took part in this study.
Funding
Financial support of this study was obtained from
the Natural Science Foundation in Hunan Province, China (grant no.
2015JJ4043), the Key Project of Health and Family Planning
Commission in Hunan Province (grant no. A2017016), Clinical
Research Center For Spinal Minimally Invasive Techniques in Hunan
Province (grant no. 2017SK4004) and the Key Research and
Development Plan in Hunan Province (grant no. 2017SK2104).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ performed the molecular experiments and drafted
the manuscript. WY, Y-BZ, Y-XX, L-SW and W-KH performed the
statistical analysis. W-JW conceived and designed the current study
and revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
OS
|
Osteosarcoma
|
|
SB
|
Scutellaria baicalensis
|
|
miRNAs
|
microRNAs
|
|
3′-UTR
|
3′-untranslated region
|
References
|
1
|
Li J, Yang Z, Li Y, Xia J, Li D, Li H, Ren
M, Liao Y, Yu S, Chen Y, et al: Cell apoptosis, autophagy and
necroptosis in osteosarcoma treatment. Oncotarget. 7:44763–44778.
2016.PubMed/NCBI
|
|
2
|
Zhang J, Yan YG, Wang C, Zhang SJ, Yu XH
and Wang WJ: MicroRNAs in osteosarcoma. Clin Chim Acta. 444:9–17.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou RT, He M, Yu Z, Liang Y, Nie Y, Tai S
and Teng CB: Baicalein inhibits pancreatic cancer cell
proliferation and invasion via suppression of NEDD9 expression and
its downstream Akt and ERK signaling pathways. Oncotarget.
8:56351–56363. 2017.PubMed/NCBI
|
|
4
|
Liu X and Liu S, Chen J, He L, Meng X and
Liu S: Baicalein suppresses the proliferation of acute
T-lymphoblastic leukemia Jurkat cells by inhibiting the
Wnt/β-catenin signaling. Ann Hematol. 95:1787–1793. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wei N, Wei Y, Li B and Pang L: Baicalein
promotes neuronal and behavioral recovery after intracerebral
hemorrhage via suppressing apoptosis, Oxidative stress and
neuroinflammation. Neurochem Res. 42:1345–1353. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ye H, Zhang Y, Wang Y, Xia J, Mao X and Yu
X: The restraining effect of baicalein and U0126 on human cervical
cancer cell line HeLa. Mol Med Rep. 16:957–963. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chai Y, Xu J and Yan B: The
anti-metastatic effect of baicalein on colorectal cancer. Oncol
Rep. 37:2317–2323. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi EO, Park C, Hwang HJ, Hong SH, Kim
GY, Cho EJ, Kim WJ and Choi YH: Baicalein induces apoptosis via
ROS-dependent activation of caspases in human bladder cancer 5637
cells. Int J Oncol. 49:1009–1018. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma X, Yan W, Dai Z, Gao X, Ma Y, Xu Q,
Jiang J and Zhang S: Baicalein suppresses metastasis of breast
cancer cells by inhibiting EMT via downregulation of SATB1 and
Wnt/β-catenin pathway. Drug Des Devel Ther. 10:1419–1441. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Palmini G, Marini F and Brandi ML: What is
new in the miRNA world regarding osteosarcoma and chondrosarcoma?
Molecules. 22:pii: E417. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zoni E and van der Pluijm G: The role of
microRNAs in bone metastasis. J Bone Oncol. 5:104–108. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao LL, Xie JW, Lin Y, Zheng CH, Li P,
Wang JB, Lin JX, Lu J, Chen QY and Huang CM: miR-183 inhibits
invasion of gastric cancer by targeting Ezrin. Int J Clin Exp
Pathol. 7:5582–5594. 2014.PubMed/NCBI
|
|
13
|
Li M, Feng YM and Fang SQ: Overexpression
of ezrin and galectin-3 as predictors of poor prognosis of cervical
cancer. Braz J Med Biol Res. 50:e53562017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kong J, Li Y, Liu S, Jin H, Shang Y, Quan
C, Li Y and Lin Z: High expression of ezrin predicts poor prognosis
in uterine cervical cancer. BMC Cancer. 13:5202013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu J, Feng Y, Ke Z, Yang Z, Zhou J, Huang
X and Wang L: Down-regulation of miR-183 promotes migration and
invasion of osteosarcoma by targeting Ezrin. Am J Pathol.
180:2440–2451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Zuo J, Lei M, Wu S, Zang X and
Zhang C: Ezrin promotes invasion and migration of the MG63
osteosarcoma cell. Chin Med J (Engl). 127:1954–1959.
2014.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kushlinskii NE, Fridman MV and Braga EA:
Molecular mechanisms and microRNAs in osteosarcoma pathogenesis.
Biochemistry (Mosc). 81:315–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anderson ME: Update on survival in
osteosarcoma. Orthop Clin North Am. 47:283–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao Y, Snyder SA, Smith JN and Chen YC:
Anticancer properties of baicalein: A review. Med Chem Res.
25:1515–1523. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dinda B, Dinda S, DasSharma S, Banik R,
Chakraborty A and Dinda M: Therapeutic potentials of baicalin and
its aglycone, baicalein against inflammatory disorders. Eur J Med
Chem. 131:68–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chung H, Choi HS, Seo EK, Kang DH and Oh
ES: Baicalin and baicalein inhibit transforming growth
factor-β1-mediated epithelial-mesenchymal transition in human
breast epithelial cells. Biochem Biophys Res Commun. 458:707–713.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dai G, Zheng D, Wang Q, Yang J, Liu G,
Song Q, Sun X, Tao C, Hu Q, Gao T, et al: Baicalein inhibits
progression of osteosarcoma cells through inactivation of the
Wnt/β-catenin signaling pathway. Oncotarget. 8:86098–86116. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He N and Zhang Z: Baicalein suppresses the
viability of MG-63 osteosarcoma cells through inhibiting c-MYC
expression via Wnt signaling pathway. Mol Cell Biochem.
405:187–196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ye F, Wang H, Zhang L, Zou Y, Han H and
Huang J: Baicalein induces human osteosarcoma cell line MG-63
apoptosis via ROS-induced BNIP3 expression. Tumour Biol.
36:4731–4740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pal MK, Jaiswar SP, Dwivedi VN, Tripathi
AK, Dwivedi A and Sankhwar P: MicroRNA: A new and promising
potential biomarker for diagnosis and prognosis of ovarian cancer.
Cancer Biol Med. 12:328–341. 2015.PubMed/NCBI
|
|
27
|
Taylor MA and Schiemann WP: Therapeutic
opportunities for targeting microRNAs in cancer. Mol Cell Ther.
2:1–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nugent M: microRNA and bone cancer. Adv
Exp Med Biol. 889:201–230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang QH, Sun HM, Zheng RZ, Li YC, Zhang
Q, Cheng P, Tang ZH and Huang F: Meta-analysis of microRNA-183
family expression in human cancer studies comparing cancer tissues
with noncancerous tissues. Gene. 527:26–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mu Y, Zhang H, Che L and Li K: Clinical
significance of microRNA-183/Ezrin axis in judging the prognosis of
patients with osteosarcoma. Med Oncol. 31:8212014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao H, Guo M, Zhao G, Ma Q, Ma B, Qiu X
and Fan Q: miR-183 inhibits the metastasis of osteosarcoma via
downregulation of the expression of Ezrin in F5M2 cells. Int J Mol
Med. 30:1013–1020. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abdou AG, Kandil M, Asaad NY, Dawoud MM,
Shahin AA and Eldayem Abd AF: The prognostic role of Ezrin and
HER2/neu expression in osteosarcoma. Appl Immunohistochem Mol
Morphol. 24:355–363. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao DH, Zhu J, Wang WB, Dong F, Zhang Q,
Fan HW, Zhang JZ and Wang YM: Correlations of ezrin expression with
pathological characteristics and prognosis of osteosarcoma: A
meta-analysis. ScientificWorldJournal. 2014:8375432014. View Article : Google Scholar : PubMed/NCBI
|