Introduction
Mesenchymal stem cells (MSCs) are a class of
pluripotent stem cells derived from the mesoderm that support the
in vitro growth of long-term culture-initiating cells and
promote in vivo hematopoietic embedding and reconstruction,
thus having important roles in tissue repair, anti-inflammation,
and the prevention and treatment of graft versus host disease
(GVHD) (1,2). However, harvesting bone marrow
mesenchymal stem cells (BMSCs) is invasive and their ability to
differentiate decreases with age, which restricts their utility in
clinical and scientific research (3). As MSCs originating from the amniotic
membrane, termed amniotic MSCs (AMSCs), could be accessed
relatively easily compared with BMSCs and without ethical barriers,
there are numerous potential applications for AMSCs. The biological
characteristics of BMSC and AMSC were previously reported to be
similar, including hematopoiesis multipotency properties with low
immunogenicity as well as possessing the ability to inhibit the
proliferation of allogeneic T cells (4,5). The
combined transplantation of BMSCs with hematopoietic stem cells has
been reported to be an effective method for increasing
hematopoietic reconstitution and reducing the occurrence of GVHD
(2,6). One study demonstrated that direct
injection of MSCs into the bone marrow cavity promoted
hematopoietic recovery and reduced GVHD symptoms (7), indicating that improving MSC homing
and implantation methods may lead to improved therapeutic effects
of MSC transplantation. Previous studies have also demonstrated
that stimulation with a cytokine cocktail [fms-related tyrosine
kinase-3 ligand, recombinant human stem cell factor, interleukin
(IL)-6, hepatocyte growth factor and IL-3 increased the expression
of C-X-C motif chemokine receptor 4 (CXCR4) on the surface of BMSCs
(8) and that the stromal
cell-derived factor-1 (SDF-1)/CXCR4 axis facilitated BMSC homing
and accelerated hematopoietic recovery in a rat pancreatic
transplant recipient (9). However,
not all of the aforementioned cytokines are suitable for
therapeutic use in humans, and the cytokine cocktail may induce
severe adverse side effects due to their pleiotropic properties
(10). Therefore, although this
method is effective, it cannot be applied clinically. Determining
whether there is a simpler, safer and more effective way to promote
MSC homing clinically requires further investigation.
Amniotic epithelial cells (AECs) are derived from
the embryonic ectoderm. These cells are able to synthetize and
secrete a variety of cytokines, and have the ability to grow and
proliferate in serum-free conditions (11,12).
Therefore, we hypothesized that co-culture of AMSCs with AECs may
maintain AMSC activity and also stimulate the expression of CXCR4
on AMSC surfaces to enhance AMSC migration and homing ability.
In the current study, the effects of co-culture with
AECs on the biological characteristics of AMSCs, including their
viability, CXCR4 expression and migration ability, as well as the
roles of the SDF-1/CXCR4 axis in the migration and homing of AMSCs,
were investigated.
Materials and methods
Samples and approval
Samples of human amniotic membrane were obtained
from 43 healthy women aged 22–30 years that had undergone a
caesarean delivery (negative in hepatitis B virus, human
immunodeficiency virus and syphilis tests) from the First
Affiliated Hospital of Kunming Medical University (Kunming, China).
All the samples were collected between October 2012 and March 2014.
The study was approved by Ethics Committee of the First Affiliated
Hospital of Kunming Medical University. Written informed consent
was obtained from donors for the use of amniotic membranes in this
study.
Isolation, culture and identification
of AMSCs
The amniotic membrane was isolated and repeatedly
rinsed under aseptic conditions. Following the removal of blood
clots, the amniotic membrane was cut into sections (~0.5–1.0
mm2) and seeded onto the bottom of culture flasks.
Complete Dulbecco's modified Eagle's medium (DMEM)/F12, containing
10% fetal bovine serum (FBS) and 1% cyan-streptomycin, all of which
were purchased from HyClone (GE Healthcare Life Sciences, Logan,
UT, USA), was added and the flasks were cultured at 37°C and in 5%
CO2 and saturated humidity. When the cell density
reached 80–90%, the cells were passaged. P3-6 generation AMSCs were
used in the experiments.
MSCs were identified as described previously
(8,13). Briefly, indirect immunofluorescence
was performed using MSCs (1×106). Cells were blocked
with 0.5 % bovine serum albumin (BSA) and 2% normal FBS in 1X PBS
at 4°C for 30 min; both serums of which were purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Following this,
cells were incubated at 4°C for 30 min with primary mouse
anti-human monoclonal antibodies against CD11a (cat. no. 301202;
1:100), CD11b (cat. no. 301302; 1:100), CD29 (cat. no. 303002;
1:100), CD31 (cat. no. 303102; 1:50), CD34 (cat. no. 343502; 1:25),
CD44 (cat. no. 338802; 1:100), CD45 (cat. no. 368502; 1:100), CD90
(cat. no. 328102; 1:100), CD105 (cat. no. 323202; 1:50), human
leukocyte antigen D-related (HLA-DR; cat. no. 307602; 1:100) and
pan-cytokeratins (Pan-CK; cat. no. 628602; 1:250); which were all
purchased from BioLegend, Inc. (San Diego, CA, USA). Cells were
then incubated with fluorescein isothiocyanate-labeled goat
anti-mouse secondary antibody (cat. no. 1015-02; 1:200; Southern
Biotech, Birmingham, IL, USA) at 4°C for 30 min. Isotype antibodies
were used as the control. MSCs were subsequently detected by flow
cytometry and the results were analyzed using WinMDI 2.9 software
(Scripps Research Institute, La Jolla, CA, USA).
To induce differentiation, AMSCs were inoculated
into culture flasks at a density of 2–3×104/cm at 37°C
for 3 weeks in adipocyte differentiation medium [Iscove's modified
Dulbecco's medium (IMDM) + 10−6 mol/l dexamethasone +
0.5 mol/l 1-methyl-3-isobutyl-xanthine + 0.1 mol/l vitamin C + 100
U/ml penicillin + 100 µg/ml streptomycin + 10% FBS)], all reagents
of which were purchased from Sigma Aldrich; Merck KGaA. Following
this, AMSCs were fixed in ice cold 10% formalin for 10 min and
stained with oil red O for 5 min at room temperature. Osteogenic
induction was performed in Iscove's modified Dulbecco's medium
containing 10% FBS, 10−7 mol/l dexamethasone, 10 mol/l
β-glycerophosphate, 0.05 mol/l vitamin C, 100 U/ml penicillin and
100 µg/ml streptomycin (all from Sigma Aldrich, St Louis, MO, USA).
A total of 3 weeks post-induction, cells were fixed with 10%
formalin for 10 min at room temperature and then incubated in 5%
silver nitrate (American Master Tech Scientific, Inc., Lodi, CA,
USA) at room temperature for 1 h. Observation was subsequently
performed using a light microscope (magnification, ×400). Negative
controls refer to AMSCs stained with Oil Red O or Von Kossa that
had been cultured in DMEM/F12 medium without adipogenic and
osteogenetic induction.
Isolation, culture and identification
of AECs
The amnion tissue was digested with 0.125% trypsin
(Biological Industries, Kibbutz Beit Haemek, Israel) at 37°C for
30–40 min. The digested liquid was collected and filtered through a
200-mesh screen to collect the cells following centrifugation at
200 × g for 5 min at room temperature. The collected cells were
then cultured at 37°C with 5% CO2 in complete medium
(DMEM/F12 containing 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin), the reagents of which were purchased from HyClone
(GE Healthcare Life Sciences). Cells were then passaged when they
reached 80–90% confluence.
The AECs were prepared for the cell climbing slice
assay (14), followed by fixation
in 4% neutral formaldehyde, staining with hematoxylin,
differentiation with 1% hydrochloric acid for 30–60 sec,
re-staining with 1% aqueous ammonia for 1 min and eosin for 30 min,
alcohol dehydration, 5–10 min of hyalinization and mounting on a
film (14). All steps were
performed at room temperature. Observation was subsequently
performed using a light microscope (magnification, ×100).
For immunohistochemical analysis, cell climbing
slices were immersed in DMEM/F12 medium and then fixed in 4%
neutral formaldehyde for 15 min at room temperature, subsequently
inactivated via incubation with 3% H2O2 for
10 min at room temperature and then blocked with 5% goat serum
(cat. no. 0060-01; Southern Biotech, Birmingham, AL, USA) for 30
min at room temperature. Following blocking, incubation was
performed for 45 min at room temperature with a primary antibody
against Pan-CK (cat. no. sc-8018; 1:100; Santa Cruz Biotechnology,
Inc., Dallas, Texas, USA). As a negative control, cells were
treated with PBS in the absence of primary antibodies. The cells
were then incubated with goat anti-mouse IgG-Biotin secondary
antibodies for 30 min at room temperature, which were included in
the SABC kit purchased from Beijing Solarbio Science &
Technology Co., Ltd. (Beijing, China; cat. no. SA0011), according
to the manufacturer's protocol. Then cells were observed under a
light microscope (magnification, ×200).
A direct immunofluorescence assay was performed
after rupturing cell membranes and fixing the cells (107
cells/ml) using a Fixation/Permeabilization Solution kit (cat. no.
554714; BD Biosciences, Franklin Lakes, NJ, USA). Cells were then
incubated at room temperature for 40 min with phycoerythrin
(PE)-labeled Pan-CK antibodies (cat. no. ab52460; 1:100; Abcam,
Cambridge, UK). Cells were blocked using 10% normal human serum
(cat. no. 31876; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at room temperature for 30 min. As a negative
control, cells were treated with isotype IgG in the absence of the
antibodies. The cells were subsequently resuspended and identified
by flow cytometry. The results were analyzed using WinMDI 2.9
software.
Co-culture groups and comparison of
adipogenic and osteogenic abilities
AMSCs were digested with 0.125% trypsin at 37°C,
resuspended in serum-free DMEM/F12, seeded into 6-well plates at a
density of 1×105 cells/well and placed into a Millicell
chamber (0.4 µm; EMD Millipore, Billerica, MA, USA). The AECs were
inoculated into the small chamber at a density of 1×104
cells/well. Together, this co-culture was labeled the co-culture
group. The same batch of AMSCs were digested, resuspended in
serum-free DMEM/F12 medium or complete medium (DMEM/F12 with the
addition of 10% FBS) and seeded into 6-well plates using the
above-mentioned methods and concentrations. These AEC-free
cultures, which were termed the serum-free and serum groups,
respectively, were used as controls. The AMSCs were detached using
0.125% trypsin at 37°C and subsequently collected for use following
incubation at 37°C for 24, 48 or 72 h time intervals.
In order to compare the adipogenic and osteogenic
abilities between the three culture groups, cells were inoculated
into culture flasks at a density of 2–3×104
cells/cm2 in adipocyte differentiation medium (IMDM +
10−6 mol/l dexamethasone + 0.5 mol/l
1-methyl-3-isobutyl-xanthine + 0.1 mol/l vitamin C + 100 U/ml
penicillin + 100 µg/ml streptomycin + 10% FBS) at 37°C for 2 weeks.
Osteogenic induction was performed in IMDM containing 10% FBS,
10−7 mol/l dexamethasone, 10 mol/l β-glycerophosphate,
0.05 mol/l vitamin C, 100 U/ml penicillin and 100 g/ml streptomycin
at 37°C for 2 weeks. Following 2 weeks of adipogenic and osteogenic
differentiation, total RNA from the AMSCs in the three groups was
extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and reverse-transcribed into cDNA using a
PrimeScript™ RT-PCR kit (Takara Bio, Inc., Otsu, Japan) at 42°C for
50 min. SYBR® Premix Ex Taq™ (Takara Bio, Inc.) was used
for quantitative polymerase chain reaction (qPCR). qPCR was
performed using a 7500 Fast Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Alkaline phosphatase
(ALP) and osteopontin (OPN) were measured as osteogenic indexes,
while peroxisome proliferator-activated receptor γ (PPARγ) and
CCAAT/enhancer-binding protein α (C/EBPα) were measured as the
adipogenic indexes and GAPDH was used as the internal reference.
The primer sequences used were as follows: ALP (162 bp) forward,
5′-ACCATTCCCACGTCTTCACATTTG-3′ and reverse,
5′-AGACATTCTCTCGTTCACCGCC-3′; OPN (416 bp) forward,
5′-AGCCAGGACTCCATTGACTCGAAC-3′ and reverse,
5′-GTTTCAGCACTCTGGTCATCCAGC-3′; C/EBPα (171 bp) forward,
5′-GAAGTTGGTGGAGCTGTCGG-3′ and reverse,
5′-TGAGGTATGGGTCGTTGCTGA-3′; PPARγ (89 bp) forward,
5′-AGCCTCATGAAGAGCCTTCCA-3′ and reverse,
5′-ACCCTTGCATCCTTCACAAGC-3′; and GAPDH (393 bp) forward,
5′-GTCTTCACCACCATGGAGAAGGCT-3′ and reverse,
5′-CATGCCAGTGAGCTTCCCGTTCA-3′. The reaction conditions were
pre-denaturation at 90°C for 10 sec, followed by degeneration at
95°C for 5 sec, annealing and extension at 60°C for 60 sec, for a
total of 40 cycles. The experiment was repeated three times. The
results were analyzed using the 2−ΔΔCq method (15), and the expression levels of
adipogenic and osteogenic indexes were compared among the three
groups after 24, 48 and 72 h of culture. The results were expressed
in terms of 2−ΔΔCq using the following formula: ΔΔCq=ΔCq
(co-culture group or serum-free group)-ΔCq (serum group). The
difference between the co-culture (or serum-free) group and the
serum group was 2−ΔΔCq times.
Comparison of AMSC viability
For the Cell Counting kit-8 (CCK-8) assay, AMSC
suspensions from each group at each time point were inoculated into
96-well plates (104 cells/well) and cultured overnight
at 37°C. 10% CCK-8 solution (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was added to each well and plates were incubated
for an additional 3–4 h at 37°C. The optical density (OD) value of
each well was determined by a microplate reader.
For the trypan blue assay, AMSC suspensions from
each group at each time point (105 cells/well) were
stained with 0.4% trypan blue dye for 30–60 sec at room
temperature. Following this, cells were delivered to a
hemocytometer by capillary action. The number of blue-stained cells
was determined under a light microscope (magnification, ×40). The
following formula was used for cell counting: Survival rate
(%)=[(total number of cells-number of blue-stained cells)/total
number of cells] ×100.
Comparison of CXCR4 expression
levels
CXCR4 expression was detected using a direct
immunofluorescence assay. Cells (2×105) were blocked via
incubation with 0.5% BSA (Beijing Solarbio Science & Technology
Co., Ltd.) at 4°C for 30 min. Following this, CXCR4 cell surface
expression was investigated via incubation of cells with a
PE-labeled mouse anti-human CXCR4 monoclonal antibody (cat. no.
12-9999-41; 1:20; eBioscience; Thermo Fisher Scientific, Inc.) at
room temperature for 30 min. To detect intracellular CXCR4, cells
(2×105) were blocked via incubation with 0.5% BSA
(Beijing Solarbio Science & Technology Co., Ltd.) at 4°C for 30
min and then incubated with unlabeled CXCR4 monoclonal antibodies
(cat. no. 14-9999-80; 1:20; eBioscience; Thermo Fisher Scientific,
Inc.) at room temperature for 1 h. Following the rupturing cell
membranes and fixing of the lysates using the
Fixation/Permeabilization Solution kit (BD Biosciences), cells were
then incubated with PE-labeled CXCR4 monoclonal antibodies for 30
min at room temperature for staining (cat. no. 12-9999-41; 1:20;
eBioscience; Thermo Fisher Scientific, Inc.). The results obtained
by flow cytometry were analyzed by WinMDI 2.9 software.
The mRNA expression of CXCR4 was also investigated
in cells after 24, 48 and 72 h of culture using reverse
transcription (RT)-qPCR. Total RNA was extracted and reverse
transcribed into cDNA according to the aforementioned protocol,
followed by amplification with SYBR Green dye and plotting of
amplification curves using a qPCR instrument. The sequences of
primers targeting the CXCR4 gene were forward,
5′-ACTTCAGTTTGTTGGCTGCGGC-3′ and reverse,
5′-ACCGCTGGTTCTCCAGATGCG-3′. The sequences of primers targeting the
internal reference (GAPDH) were forward, 5′-GAAGGTGAAGGTCGGAGTC-3′
and reverse, 5′-GAAGATGGGATGGGATTTC-3′. The following reaction
conditions were used: Pre-denaturation at 95°C for 10 sec, followed
by 40 cycles of denaturation at 95°C for 5 sec and annealing and
elongation at 60°C for 40 sec. The experiment was repeated three
times and the results were expressed as the 2−ΔΔCq.
In vitro migration assay
The assay was performed in a Millicell chamber (EMD
Millipore), with the upper chamber membrane (pore size, 12 µm)
coated with fibronectin (EMD Millipore) and the lower chamber
filled with different concentrations of SDF-1 (100, 200 and 300
ng/ml; PeproTech, Inc., Rocky Hill, NJ, USA) as well as DMEM/F12
medium and 0.5% BSA (Beijing Solarbio Science & Technology Co.,
Ltd.). AMSCs (3×105 cells/ml) in DMEM/F12 medium were
added to the upper chamber. The antibody blocking group represents
cells that have been incubated with PE-labeled CXR4 monoclonal
antibodies as aforementioned, which blocked cell surface CXCR4.
After 24 h of culture at 37°C, the filter was removed and stained
with 0.1% crystal violet for 15–30 min at room temperature, and the
number of cells that migrated to the outer surface of the membrane
was counted under a light microscope (magnification, ×200). The
number of cells in five random fields of view of the filter was
counted and the experiment was repeated three times.
Statistical analysis
SPSS package version 17.0 for Windows (SPSS, Inc.,
Chicago, IL, USA) was used for the statistical analysis.
Experimental data are presented as the mean ± standard deviation.
Comparisons among groups were performed using one-way analysis of
variance and pairwise comparisons were performed using Fisher's
least significant difference test. All experiments were performed
in triplicate. P<0.05 was considered to indicate a statistically
significant difference.
Results
Isolation, culture and identification
of AMSCs
Consistent with our previous studies (13,16),
the isolated and cultured AMSCs were spindle-shaped or polygonal,
homogeneous, and transparent. Flow cytometry demonstrated that
CD29, CD44, CD90 and CD105 expression was observed in AMSCs, but
there was no or limited expression of CD11a, CD11b, CD31, CD34,
CD45, HLA-DR and Pan-CK (data not shown). Additionally, the cells
successfully differentiated into adipocytes and osteoblasts
following induction in vitro (data not shown), which is
consistent with other recent reports (17–19).
Isolation, culture and identification
of AECs
The AECs were polygonal or oval, with a clear
outline, rich cytoplasm and pavement-like appearance following
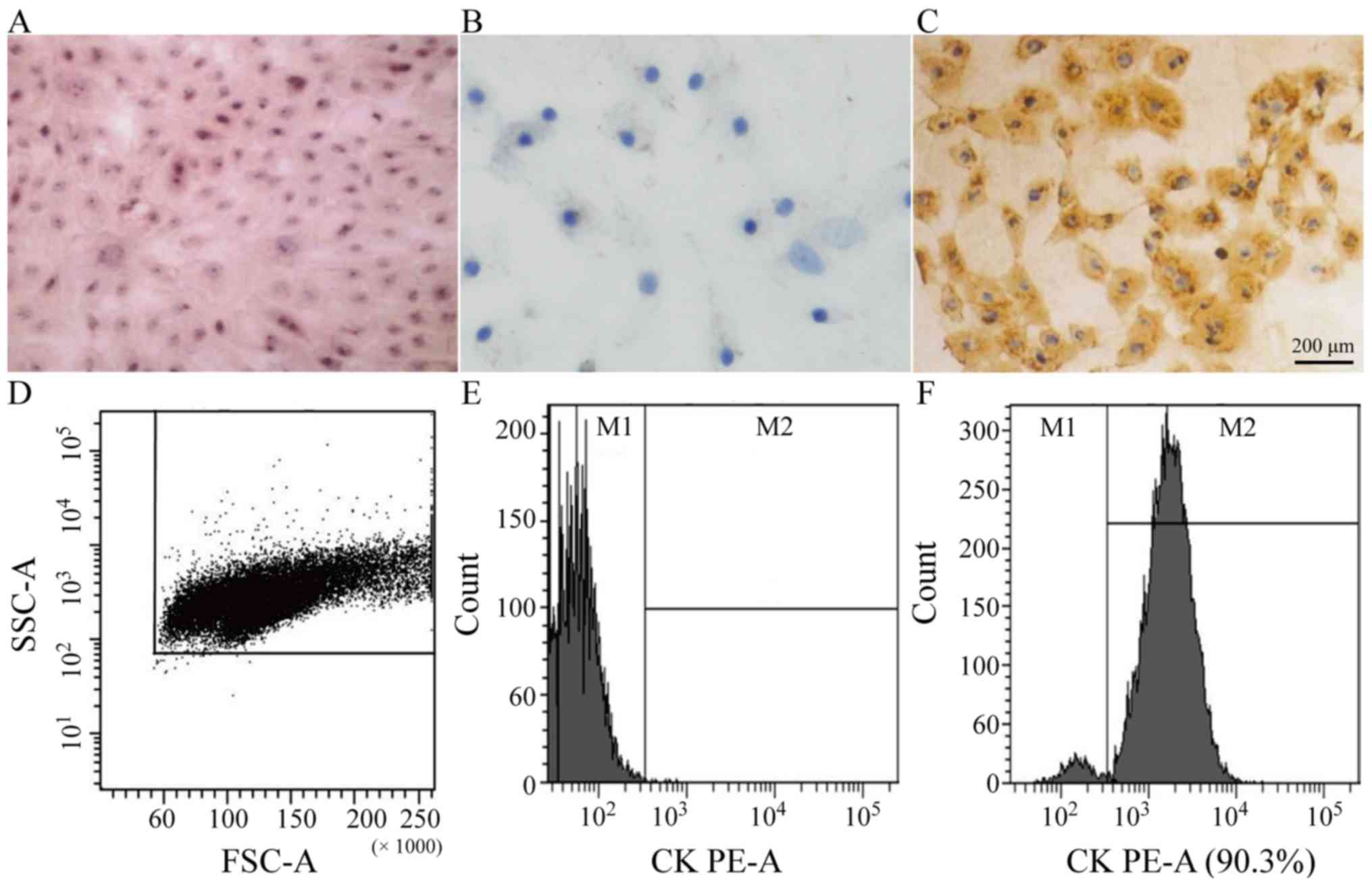
growing in flakes or clusters. Hematoxylin and eosin staining
demonstrated abundant cytoplasm and blue-stained nuclei (Fig. 1A). The expression of Pan-CK in AECs
was observed by immunohistochemistry (Fig. 1B and C) and flow cytometry
(Fig. 1D-F), both of which
indicated a high degree of positive Pan-CK expression in AECs.
Pan-CK represents the main structural protein and differentiation
marker of the epithelium (12).
The immunofluorescence staining results revealed a high expression
of Pan-CK in AEC, as well as positive Pan-CK revealed by
immunohistochemistry. Considering this as well as the morphological
characteristics revealed, it was confirmed that the cultured cells
were epithelial cells.
Basic biological characteristics of
AMSCs
The AMSCs in the three groups exhibited no marked
alterations in morphology after 24, 48 or 72 h of culture; the
morphology of AMSCs in the co-cultured group, serum-free cultured
group and serum cultured group at 72 h are presented in Fig. 2A-C, respectively. Following
co-culture with AECs for 72 h, AMSCs also maintained stable
immunophenotypic features, including a highly expressed matrix and
stromal cell antigen (CD29, CD44, CD105 and CD90), and no
exhibition of hematopoietic cell markers (CD11a, CD11b, CD34 and
CD45), major histocompatibility antigen complex class II molecules
(HLA-DR), or epithelial Pan-CK and endothelial markers (CD31)
(Fig. 2D-N). M1 indicates cells
with negative expression and M2 indicates cells with positive
expression. Furthermore, oil red O and Von Kossa staining confirmed
that AMSCs co-cultured with AECs for 72 h were able to
differentiate into adipocytes and osteoblasts in vitro
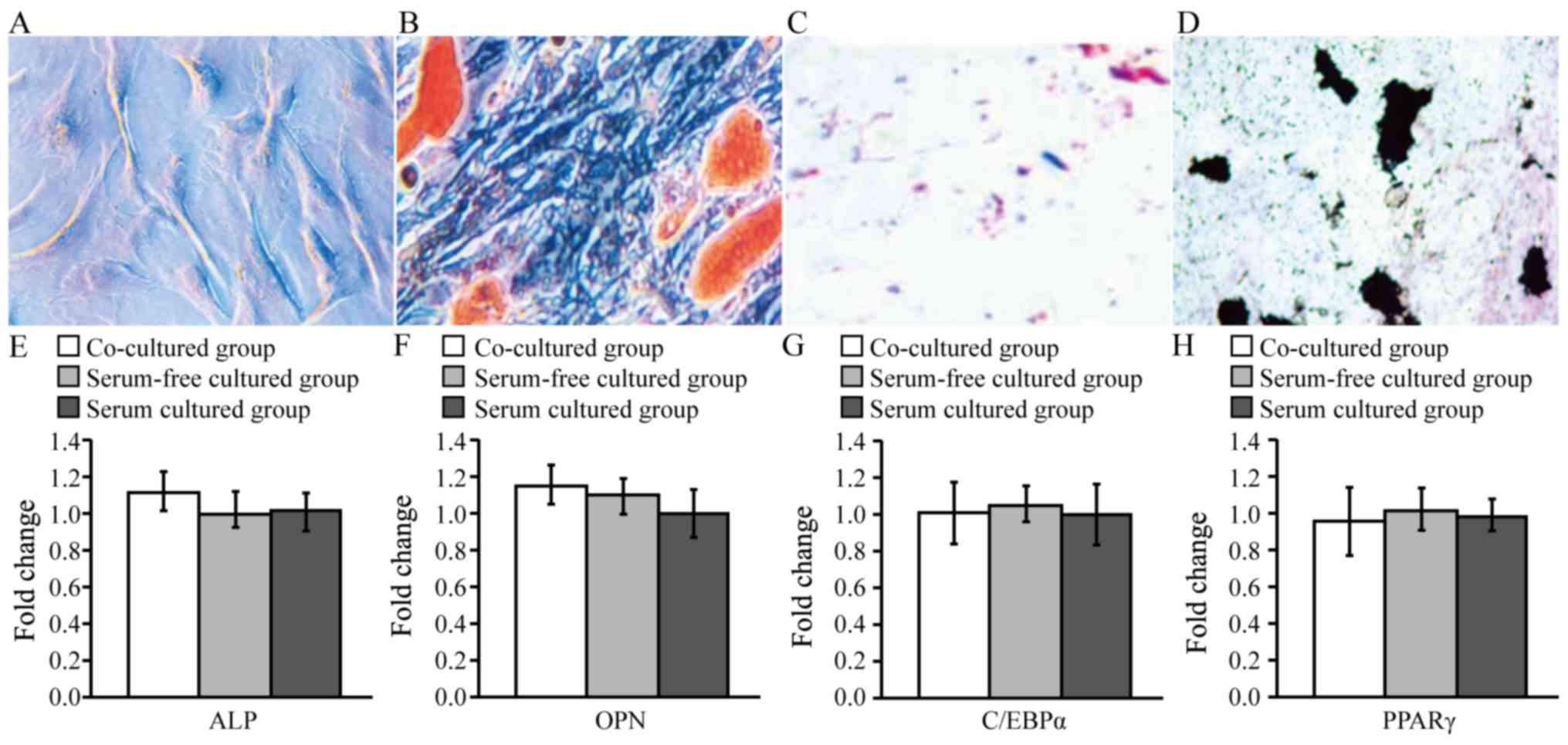
(Fig. 3A-D). Additionally, RT-qPCR
was performed to measure the mRNA expression of osteogenic (ALP and
OPN) and adipogenic (C/EBPα and PPARγ) markers in AMSCs following
co-culture with AECs, and the results presented in Fig. 3E-H confirmed that the osteogenic
and adipogenic differentiation potential of AMSCs co-cultured with
AECs for 72 h was not altered compared with serum-free and serum
cultured control groups at the same time-point.
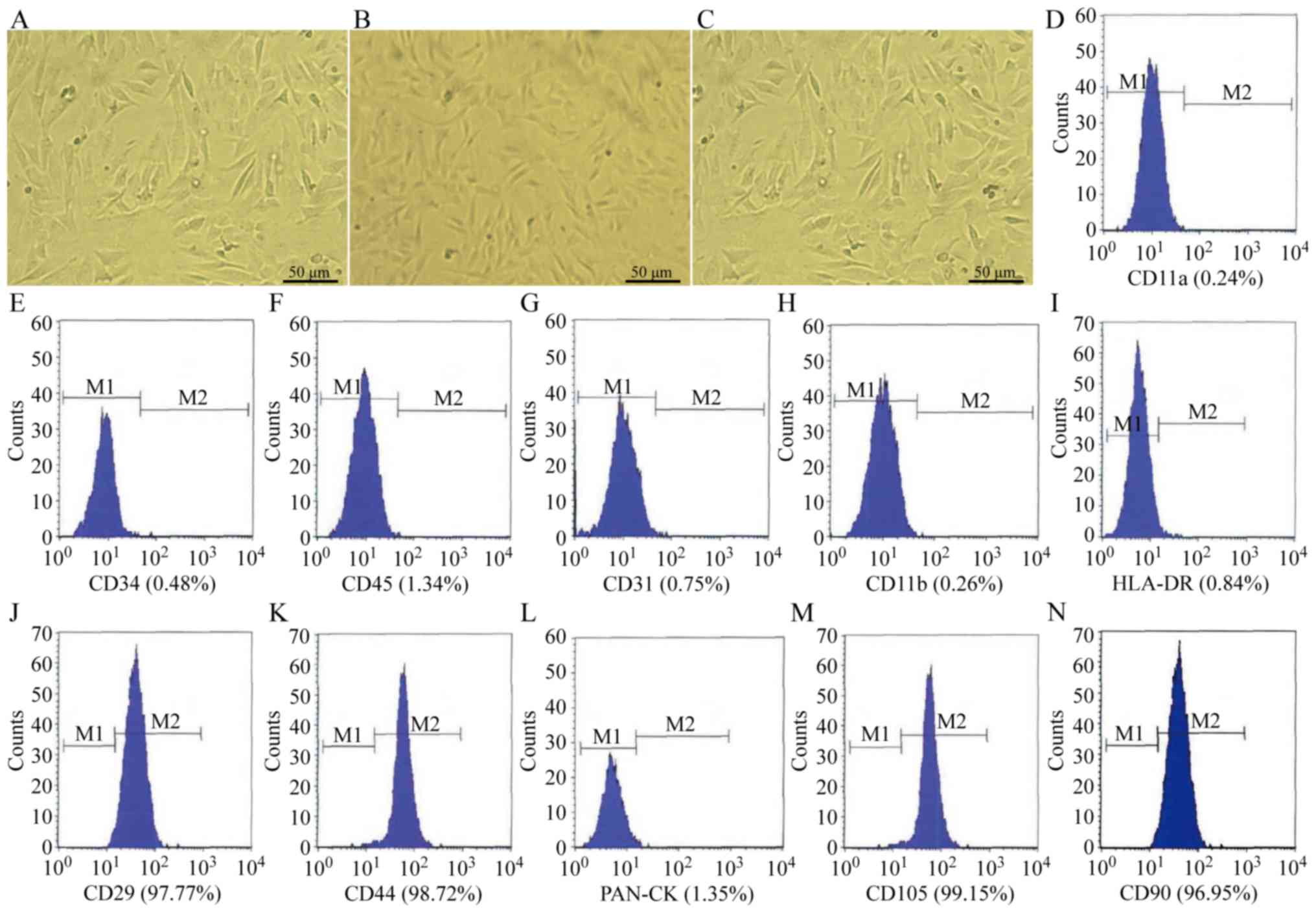 | Figure 2.Biological characteristics of AMSCs
following co-culture with AECs. The morphology of AMSCs at 72 h in
the (A) co-cultured group, (B) serum-free cultured group and (C)
serum cultured group. Magnification, ×40. Phenotype analysis of
culture-expanded AMSCs following co-culture with AECs for 72 h. The
expression of (D) CD11a, (E) CD34, (F) CD45, (G) CD31, (H) CD11b,
(I) HLA-DR, (J) CD29, (K) CD44, (L) Pan-CK, (M) CD105 and (N) CD90
was measured using fluorescence-labeled antibody staining and flow
cytometry. The graph outlined the region of fluorescent intensity
for cells fluorescently labeled with primary antibodies for
different markers. M1 indicates negative cells and M2 indicates
positive cells. AMSCs, amniotic mesenchymal stem cells; AECs,
amniotic epithelial cells; HLA-DR, human leukocyte antigen
D-related; Pan-CK, pan cytokeratins. |
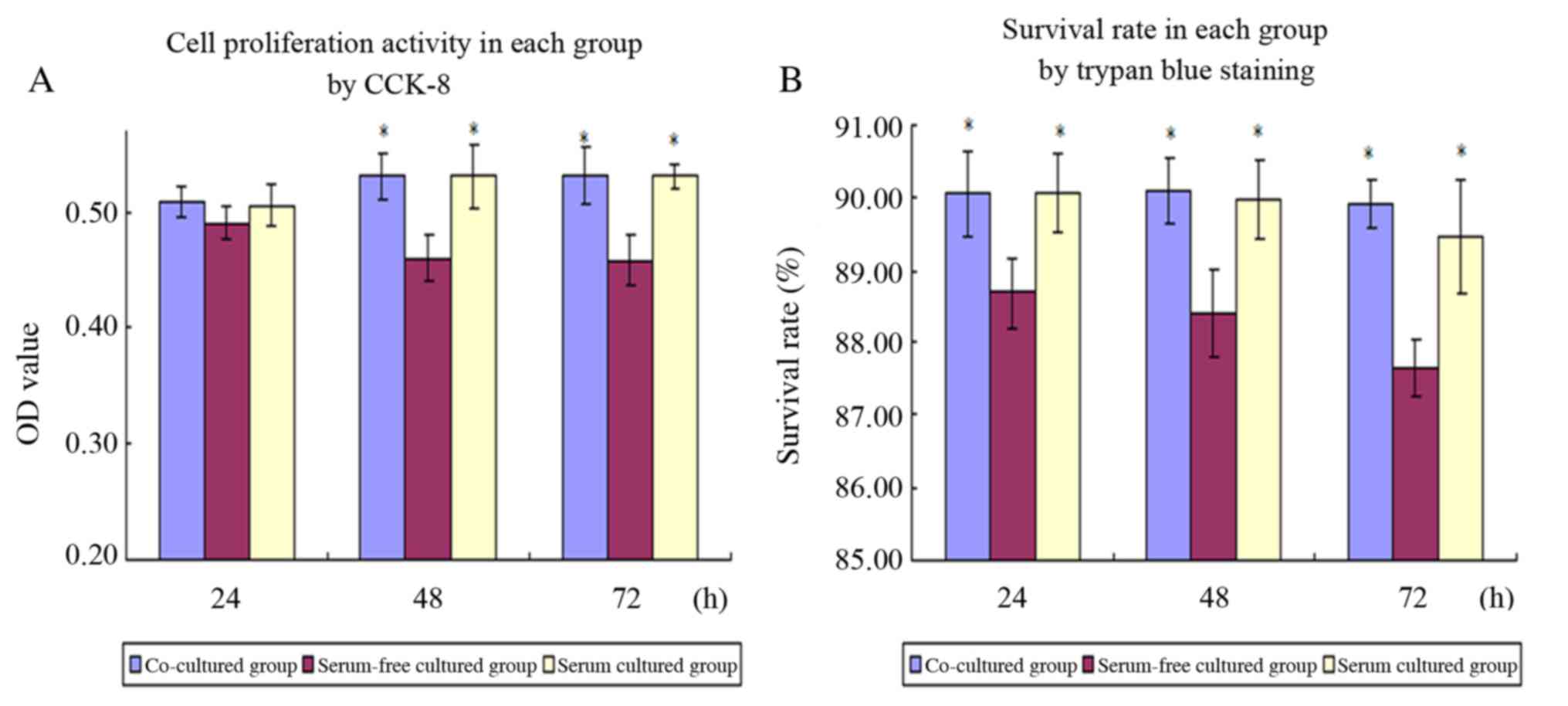
Comparison of AMSC viability
The results of the CCK-8 assay demonstrated that no
significant differences were observed in the viability among the
three groups after 24 h of culture. However, after 48 and 72 h of
culture, the absorbance of the co-culture and serum groups was
significantly higher compared with the serum-free cultured group
(P<0.05), indicating that the viability of AMSCs was higher in
the co-culture and serum cultured groups (Fig. 4A).
Furthermore, trypan blue staining demonstrated that
the survival rates of AMSCs in the co-culture and serum groups at
all three time-points were significantly higher compared with AMSCs
in the serum-free group (P<0.05; Fig. 4B).
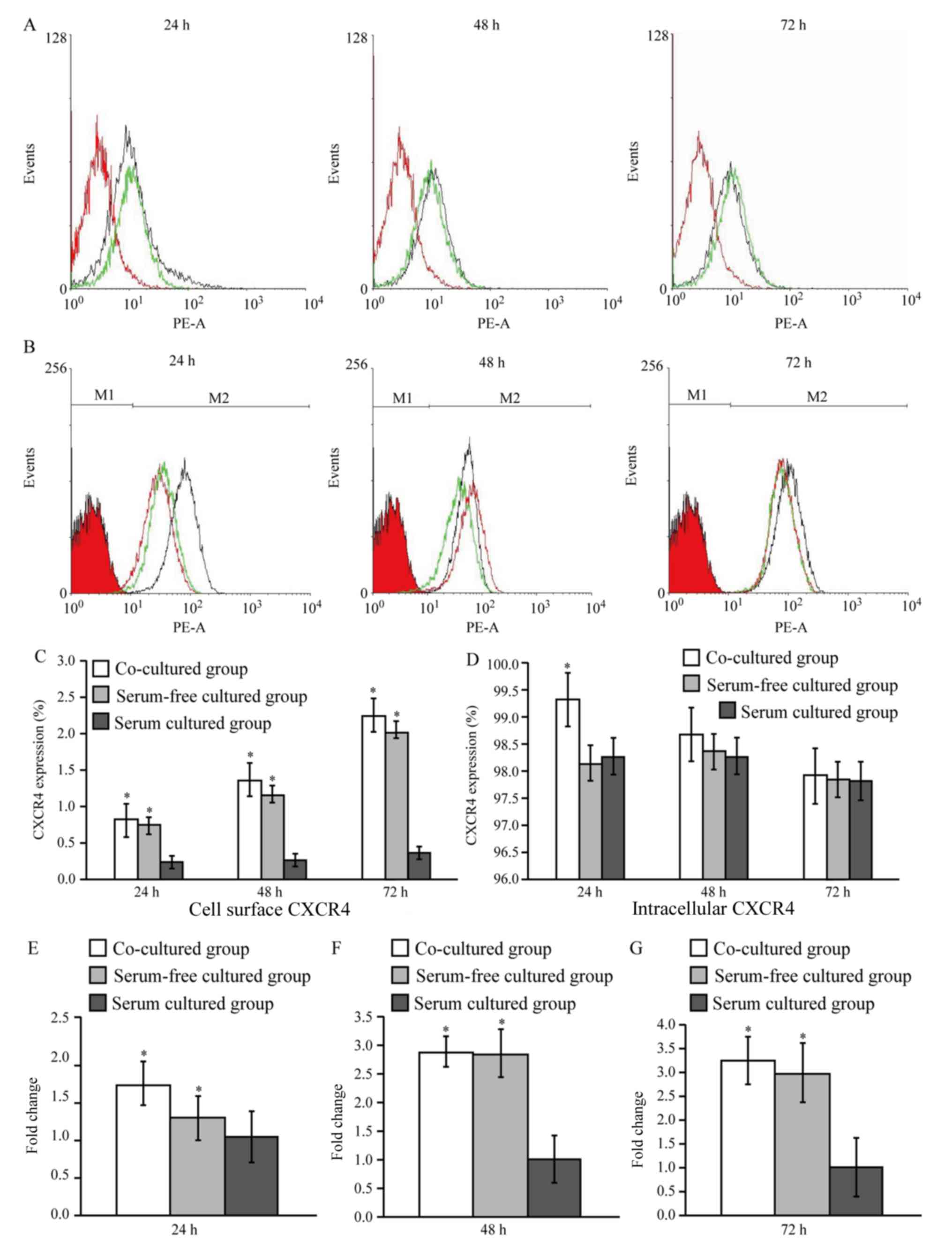
CXCR4 expression
CXCR4 expression was initially measured using a
direct immunofluorescence and flow cytometry assay. The results
demonstrated that CXCR4 expression on cell surfaces in the
co-culture and serum-free groups was higher compared with the serum
cultured group at each of the three time points (P<0.05;
Fig. 5A-D). Intracellular CXCR4
expression in the co-culture group was significantly higher
compared with the other two groups at 24 h (P<0.05), but the
expression of CXCR4 among the three groups at 48 and 72 h was not
significantly different (Fig. 5B and
D).
CXCR4 mRNA expression was also
measured in AMSCs by RT-qPCR
The results indicated that the mRNA expression of
CXCR4 at 24 h was 1.664±0.288 and 1.227±0.289 times higher in the
co-culture and serum-free groups, respectively, compared with the
serum group. At 48 h, the levels of CXCR4 expression were
2.875±0.260 and 2.842±0.413 times greater, respectively, and at 72
h, these levels were 3.241±0.511 and 2.998±0.632 times greater,
respectively (P<0.05; Fig.
5E-G).
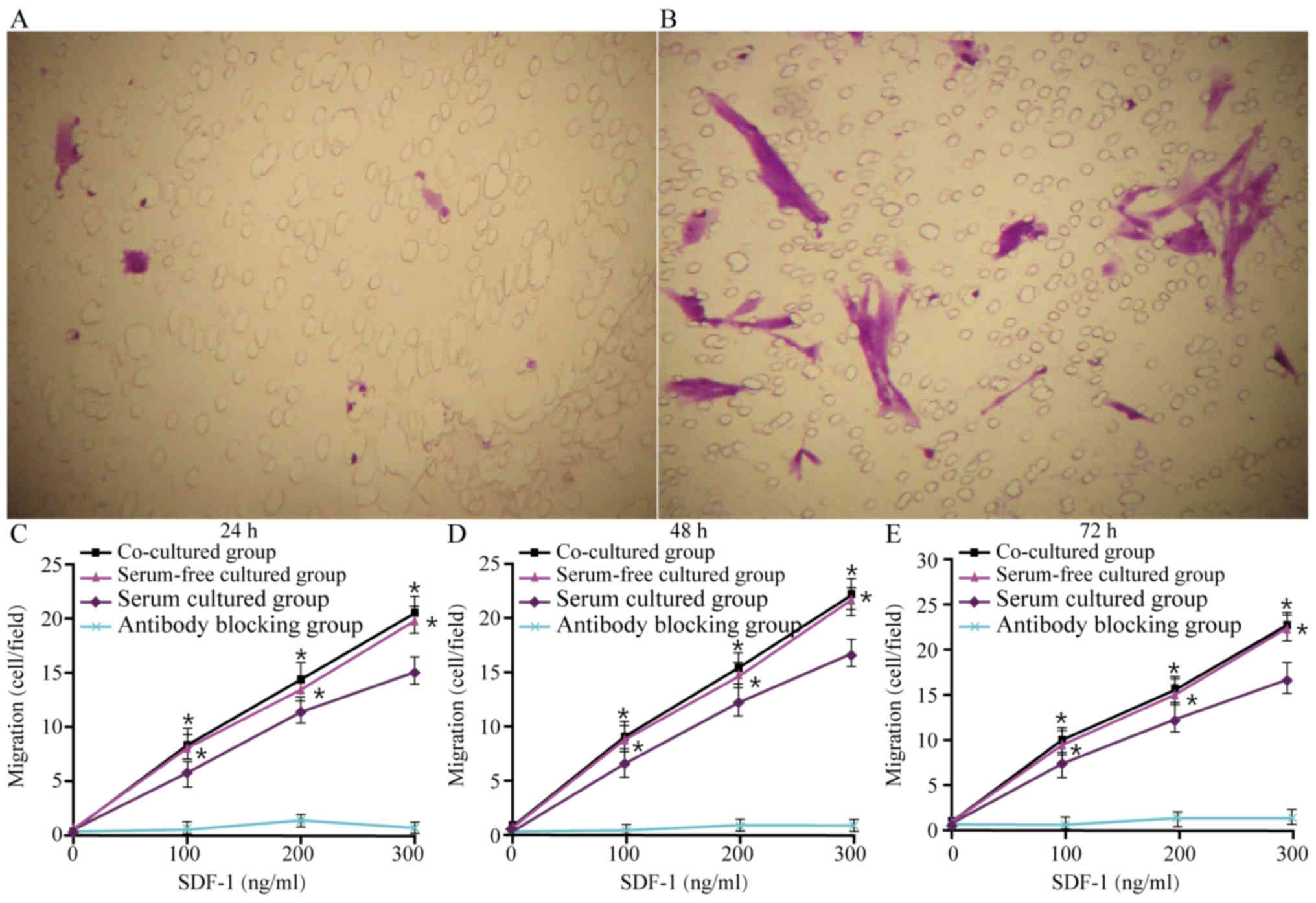
Migration assay
The results presented in Fig. 6A and B indicate that after 48 h of
culture in the co-culture group, the number of migrating AMSCs was
increased when 200 ng/ml SDF-1 was added compared with the addition
of 0 ng/ml SDF-1. The results of the migration assays also
demonstrated that, in all three groups, cells migrated towards
SDF-1 in a dose-dependent manner. Compared with AMSCs in the serum
group, the migration of AMSCs in the co-culture and serum-free
groups was significantly higher at concentrations of 100, 200 and
300 ng/ml at all time-points (P<0.05), but there were no
significant differences between the co-culture and serum-free
groups (Fig. 6C-E). Pre-incubation
with AMSC-neutralizing antibodies prevented migration, which
confirmed the specificity of this migration (Fig. 6C-E).
Discussion
MSCs have been used in the field of stem cell
transplantation due to their multi-directional differentiation
potential and ability to regulate immune responses (20). The number of homing MSCs is
reported to be closely associated with treatment outcomes, whereas
outcomes are not positively associated with the number of MSCs
transplanted (21). Therefore, the
efficiency of MSC homing and target tissue implantation is key in
effective treatment. The SDF-1/CXCR4 axis has been reported to have
an important role in MSC homing (22). Increasing the expression of CXCR4
contributed to the migration of MSCs toward target organs and, if
receptors were blocked, this ability was reduced (23).
The clinical application of BMSCs has been limited
by few donors and an invasive method of obtaining them, while AMSCs
are abundantly available as a by-product of childbirth and exhibit
similar biological characteristics to BMSCs (24), indicating that they may have
potential for numerous applications. Due to the ability of AECs to
secrete various cytokines, the present study co-cultured AECs with
AMSCs under serum-free conditions, aiming to maintain the growth
activity of AMSCs and upregulate CXCR4, thus improving the homing
and migration abilities of AMSCs. AMSCs were incubated with AECs in
serum-free medium through a Millicell chamber, in which only active
substances secreted by the cells were available to meet the
nutritional requirements of the cells and the effects of cell
contact were excluded. The results demonstrated that the
co-cultured AMSCs were not different in morphology, immunophenotype
or multidirectional differentiation ability when compared with
those without co-culture. Furthermore, the co-cultured cells
exhibited a similar growth ability compared with the serum cultured
group, and this ability was superior to that in the serum-free
cultured group, indicating that the cytokines produced by the AECs
were sufficient to maintain the biological activity of AMSCs for at
least 72 h.
CXCR4 is primarily expressed in the cytoplasm, and
cells regulate the expression of CXCR4 on cell membrane surfaces
through endocytosis (25). Under
normal circumstances, only a very small proportion of CXCR4 is
expressed on the surface of MSCs (1–3.9%); however, following the
rupture of membranes and exposure of intracellular antigens, CXCR4
expression is reported to increase (26). Li et al (27) demonstrated that CXCR4 expression on
the surface of MSCs was minimal, consistent with the results of the
immunofluorescence assay in the present study. Li et al
(27) also revealed that the
expression of CXCR4 within cells is always higher (>95%) than
the cell surface expression (<5%), regardless of the method of
detection used. Studies have indicated that the expression of CXCR4
on the cell surface maybe regulated by externalization and
endocytosis (25,28,29).
Therefore, it maybe hypothesized that co-culture of
AECs and AMSCs promotes the expression of CXCR4 in AMSCs through
autocrine or paracrine secretion in a serum-free environment. These
cytokines may even promote the migration of intracellular CXCR4 to
the cell surface. Notably, it was observed in the present study
that in serum-free conditions, CXCR4 was upregulated on cell
surfaces, which may explain why, even in the absence of nutritional
supplements, AMSCs are able to secrete certain cytokines in an
autocrine manner to maintain their growth requirements, and these
cytokines may also increase the surface expression of CXCR4.
However, these limited cytokines cannot meet the requirements for
cell growth and proliferation, which may explain why, despite CXCR4
expression in the serum-free group being higher compared with the
co-culture and serum groups, cell viability was lower in the
serum-free group compared with other groups. Conversely, although
the serum cultured group did not appear to have impaired cell
viability, its CXCR4 expression was significantly lower compared
with the other two groups. Therefore, the AMSCs co-cultured with
AECs had the advantages of both upregulated CXCR4 and increased
cell viability, while the other two groups had only one of these
advantages. Although the serum-free cultured cells exhibited a
similar migratory ability to the co-cultured group, the
proliferation activity and survival rate of the cells were
suppressed. However, further studies are required in order to
confirm the above findings.
The effectiveness of chemokine receptors depends on
their expression on cell surfaces. In our previous study (8), BMSCs were treated with five cytokines
that upregulated the expression of CXCR4 on and within the cells,
which enhanced their ability to migrate towards SDF-1, and promoted
their ability to home towards bone marrow and be successfully
implanted in radiated NOD/SCID mice. CXCR4-expressing MSCs have
also be reported to migrate towards target organs or tissues along
an SDF-1 concentration gradient and to participate in tissue repair
(27,30). The migration assay performed in the
current study demonstrated that the in vitro migration
ability of the co-culture group along the SDF-1 concentration
gradient was increased, potentially in response to the increased
expression of CXCR4 on cell surfaces. Pre-incubation with
AMSC-neutralizing antibodies to block surface CXCR4 prevented
migration and thus confirmed that the expression of chemotactic
receptors was on cell surfaces rather than intracellular, and that
surface CXCR4 represents the main factor affecting migration.
There were a number of limitations associated with
the presents study. Although the current study has discussed the
biological characteristics of AMSCs co-cultured with AECs; however,
further studies are required to investigate the biological
characteristics of BMSCs co-cultured with AECs. Numerous studies
have revealed that the CXCR4 expression on cell surface can be
regulated by externalization and endocytosis (28,29).
In the present study, the results of the intracellular expression
levels of CXCR4 were not entirely consistent with the results
demonstrating the expression levels of CXCR4 on the cell surface.
Therefore, it can be hypothesized that AECs and AMSCs upregulate
the expression of CXCR4 via autocrine and paracrine secretion,
respectively, in a serum-free environment. Such cytokines may also
promote the migration of intracellular CXCR4 to the cell surface.
However, such hypotheses require further investigation by future
studies.
In conclusion, the results of the present study
indicated that co-culturing AMSCs with AECs upregulated CXCR4
expression on the surfaces of AMSCs and improved the ability of
AMSCs to migrate along an SDF-1 gradient. These results may set the
foundation for improving the directional migration and homing
ability of AMSCs, and also provide a reliable theoretical basis for
the application of AMSCs in clinical practice as a novel strategy
to increase the success of hematopoietic stem cell
transplantation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 31260232), the
Research Institute Projects of Medical Research Units of Yunnan
(grant no. 2016NS048), the Training Program of Medical Discipline
Leader in Yunnan Province (grant no. 2010CI013) and the Joint Fund
of Department of Yunnan Provincial Science and Technology-Kunming
Medical University (grant no. 2017FE468-030).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MXS, YZ and SCW designed the present study; LJR and
DSZ collected the data; LJR performed the statistical analysis;
DSZ, JD, MH and SYL interpreted the data; LJR wrote and revised the
manuscript; MXS and JD revised the manuscript for important
intellectual content.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Kunming Medical
University and written informed consent was obtained from donors
for the use of human amniotic membranes.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AECs
|
amniotic epithelial cells
|
|
MSCs
|
mesenchymal stem cells
|
|
AMSCs
|
amniotic mesenchymal stem cells
|
|
CXCR4
|
C-X-C motif chemokine receptor 4
|
|
GVHD
|
graft versus host disease
|
|
BMSCs
|
bone marrow mesenchymal stem cells
|
|
IL
|
interleukin
|
References
|
1
|
Castro-Manrreza ME and Montesinos JJ:
Immunoregulation by mesenchymal stem cells: Biological aspects and
clinical applications. J Immunol Res. 2015:3949172015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bernardo ME and Locatelli F: Mesenchymal
stromal cells in hematopoietic stem cell transplantation. Methods
Mol Biol. 1416:3–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Larsen S and Lewis ID: Potential
therapeutic applications of mesenchymal stromal cells. Pathology.
43:592–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Manuelpillai U, Moodley Y, Borlongan CV
and Parolini O: Amniotic membrane and amniotic cells: Potential
therapeutic tools to combat tissue inflammation and fibrosis?
Placenta. 32 Suppl 4:S320–S325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi MX, Fang BJ, Liao LM, Yang SG, Liu YH
and Zhao CH: Flk1+ mesenchymal stem cells ameliorate carbon
tetrachloride-induced liver fibrosis in mice. Sheng Wu Gong Cheng
Xue Bao. 21:396–401. 2005.(In Chinese). PubMed/NCBI
|
|
6
|
Wu Y, Wang Z, Cao Y, Xu L, Li X, Liu P,
Yan P, Liu Z, Zhao D, Wang J, et al: Cotransplantation of
haploidentical hematopoietic and umbilical cord mesenchymal stem
cells with a myeloablative regimen for refractory/relapsed
hematologic malignancy. Ann Hematol. 92:1675–1684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakamura K, Inaba M, Sugiura K, Yoshimura
T, Kwon AH, Kamiyama Y and Ikehara S: Enhancement of allogeneic
hematopoietic stem cell engraftment and prevention of GVHD by
intra-bone marrow bone marrow transplantation plus donor lymphocyte
infusion. Stem Cells. 22:125–134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi M, Li J, Liao L, Chen B, Li B, Chen L,
Jia H and Zhao RC: Regulation of CXCR4 expression in human
mesenchymal stem cells by cytokine treatment: role in homing
efficiency in NOD/SCID mice. Haematologica. 92:897–904. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gong J, Meng HB, Hua J, Song ZS, He ZG,
Zhou B and Qian MP: The SDF-1/CXCR4 axis regulates migration of
transplanted bone marrow mesenchymal stem cells towards the
pancreas in rats with acute pancreatitis. Mol Med Rep. 9:1575–1582.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rider P, Carmi Y and Cohen I: Biologics
for targeting inflammatory cytokines, clinical uses, and
limitations. Int J Cell Biol. 2016:92596462016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang S, Sun HM, Yan JH, Xue H, Wu B, Dong
F, Li WS, Ji FQ and Zhou DS: Conditioned medium from human amniotic
epithelial cells may induce the differentiation of human umbilical
cord blood mesenchymal stem cells into dopaminergic neuron-like
cells. J Neurosci Res. 91:978–986. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tabatabaei M, Mosaffa N, Nikoo S,
Bozorgmehr M, Ghods R, Kazemnejad S, Rezania S, Keshavarzi B, Arefi
S, Ramezani-Tehrani F, et al: Isolation and partial
characterization of human amniotic epithelial cells: The effect of
trypsin. Avicenna J Med Biotechnol. 6:10–20. 2014.PubMed/NCBI
|
|
13
|
Fang B, Shi M, Liao L, Yang S, Liu Y and
Zhao RC: Multiorgan engraftment and multilineage differentiation by
human fetal bone marrow Flk1+/CD31-/CD34-Progenitors. J Hematother
Stem Cell Res. 12:603–613. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sawa M, Inoue M, Yabuki A, Kohyama M,
Miyoshi N, Setoguchi A and Yamato O: Rapid immunocytochemistry for
the detection of cytokeratin and vimentin: Assessment of its
diagnostic value in neoplastic diseases of dogs. J Vet Clin Pathol.
46:172–178. 2017. View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi M, Li W, Li B, Li J and Zhao C:
Multipotency of adult stem cells derived from human amnion. Sheng
Wu Gong Cheng Xue Bao. 25:754–760. 2009.(In Chinese). PubMed/NCBI
|
|
17
|
Vojdani Z, Babaei A, Vasaghi A, Habibagahi
M and Talaei-Khozani T: The effect of amniotic membrane extract on
umbilical cord blood mesenchymal stem cell expansion: Is there any
need to save the amniotic membrane besides the umbilical cord
blood? Iran J Basic Med Sci. 19:89–96. 2016.PubMed/NCBI
|
|
18
|
Capobianco V, Caterino M, Iaffaldano L,
Nardelli C, Sirico A, Del Vecchio L, Martinelli P, Pastore L, Pucci
P and Sacchetti L: Proteome analysis of human amniotic mesenchymal
stem cells (hA-MSCs) reveals impaired antioxidant ability,
cytoskeleton and metabolic functionality in maternal obesity. Sci
Rep. 6:252702016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou H, Zhang H, Yan Z and Xu R:
Transplantation of human amniotic mesenchymal stem cells promotes
neurological recovery in an intracerebral hemorrhage rat model.
Biochem Biophys Res Commun. 475:202–208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Herrmann RP and Sturm MJ: Adult human
mesenchymal stromal cells and the treatment of graft versus host
disease. Stem Cells Cloning. 7:45–52. 2014.PubMed/NCBI
|
|
21
|
Belmar-Lopez C, Mendoza G, Oberg D, Burnet
J, Simon C, Cervello I, Iglesias M, Ramirez JC, Lopez-Larrubia P,
Quintanilla M, Martin-Duque P, et al: Tissue-derived mesenchymal
stromal cells used as vehicles for anti-tumor therapy exert
different in vivo effects on migration capacity and tumor growth.
BMC Med. 11:1392013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sharma M, Afrin F, Tripathi R and
Gangenahalli G: Regulated expression of CXCR4 constitutive active
mutants revealed the up-modulated chemotaxis and up-regulation of
genes crucial for CXCR4 mediated homing and engraftment of
hematopoietic stem/progenitor cells. J Stem Cells Regen Med.
9:19–27. 2013.PubMed/NCBI
|
|
23
|
Li J, Guo W, Xiong M, Han H, Chen J, Mao
D, Tang B, Yu H and Zeng Y: Effect of SDF-1/CXCR4 axis on the
migration of transplanted bone mesenchymal stem cells mobilized by
erythropoietin toward lesion sites following spinal cord injury.
Int J Mol Med. 36:1205–1214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Díaz-Prado S, Muiños-López E,
Hermida-Gómez T, Rendal-Vázquez ME, Fuentes-Boquete I, de Toro FJ
and Blanco FJ: Multilineage differentiation potential of cells
isolated from the human amniotic membrane. J Cell Biochem.
111:846–857. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pelekanos RA, Ting MJ, Sardesai VS, Ryan
JM, Lim YC, Chan JK and Fisk NM: Intracellular trafficking and
endocytosis of CXCR4 in fetal mesenchymal stem/stromal cells. BMC
Cell Biol. 15:152014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Yu X, Lin S, Li X, Zhang S and Song
YH: Insulin-like growth factor 1 enhances the migratory capacity of
mesenchymal stem cells. Biochem Biophys Res Commun. 356:780–784.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li M, Yu J, Li Y, Li D, Yan D, Qu Z and
Ruan Q: CXCR4 positive bone mesenchymal stem cells migrate to human
endothelial cell stimulated by ox-LDL via SDF-1alpha/CXCR4
signaling axis. Exp Mol Pathol. 88:250–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hung CM, Hsu YC, Chen TY, Chang CC and Lee
MJ: Cyclophosphamide promotes breast cancer cell migration through
CXCR4 and matrix metalloproteinases. Cell Biol Int. 41:345–352.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sheng X, Zhong H, Wan H, Zhong J and Chen
F: Granulocyte colony-stimulating factor inhibits CXCR4/SDF-1α
signaling and overcomes stromal-mediated drug resistance in the
HL-60 cell line. Exp Ther Med. 12:396–404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen W, Chen J, Zhu T, Chen L, Zhang W,
Fang Z, Heng BC, Yin Z, Chen X, Ji J, et al: Intra-articular
injection of human meniscus stem/progenitor cells promotes meniscus
regeneration and ameliorates osteoarthritis through stromal
cell-derived factor-1/CXCR4-mediated homing. Stem Cells Transl Med.
3:387–394. 2014. View Article : Google Scholar : PubMed/NCBI
|