Introduction
Prostate cancer is the 2nd most commonly diagnosed
malignancy, as well as the 6th principal reason for malignancy
associated mortality worldwide (1,2).
Thanks to the progress in prostate-specific antigen (PSA) analysis
and management, prostate cancer without metastasis is a largely
treatable disease. However, existing therapeutics cannot
effectively treat metastatic prostate cancer. There were 28,170
prostate cancer patients who died of metastatic disease in 2012, as
estimated by the American Cancer Society (3–5). As
a hallmark capability of cancer, epithelial to mesenchymal
transition (EMT) has become more and more accepted as a key step in
promoting cancer cell invasion and metastasis into distant organs
(6), which also occurs in human
prostate cancer (5).
EMT causes epithelial cells to separate from their
neighbors and cross the basement membrane. They then move across
the extracellular matrix into different organ sites, or to distant
tissues. EMT also provides crucial understanding as to how tumor
cells achieve invasive capacity by losing epithelial properties,
including E-cadherin, and obtain mesenchymal characteristics, such
as vimentin and N-cadherin, to generate resistance against drugs
and apoptosis, encourage viability, and promote motility and
invasion into adjacent tissues (5,7–9).
Thus, it may be possible to regulate the malignant behaviors of
prostate cancer cells by reversing EMT, and improving our
understanding of the associated signaling pathways is essential to
the development of new effective therapies.
Many signal transduction pathways involved in EMT
are regulated by the ubiquitin proteasome system (UPS) (10), which maintains the homeostasis of
the cell cycle and tumor growth by controlling the degradation of
important regulatory proteins (11–13).
Proteasomal activity suppression is a promising strategy for
reversing tumor cell apoptosis resistance and augmenting cancer
cell sensitivity against chemotherapy, and is a novel specific
exclusive cancer therapeutic strategy (13–16).
The 26S proteasome is a multi-catalytic enzyme complex with a 20S
proteolytic core. The 20S proteasome particle includes three pairs
of proteolytic regions with specific substrates including
trypsin-like, peptidyl-glutamyl peptide hydrolyzing (PGPH) and
chymotrypsin (CT)-like activities (17–19).
Proteasomal CT-like activity is associated with the cell survival
of cancer cells (20,21). Therefore, down-regulation of
CT-like activity to reverse EMT may be an effective approach for
preventing prostate cancer metastasis.
Clinical studies have suggested that proteasome
inhibitors are prospective novel anticancer reagents. Matrine
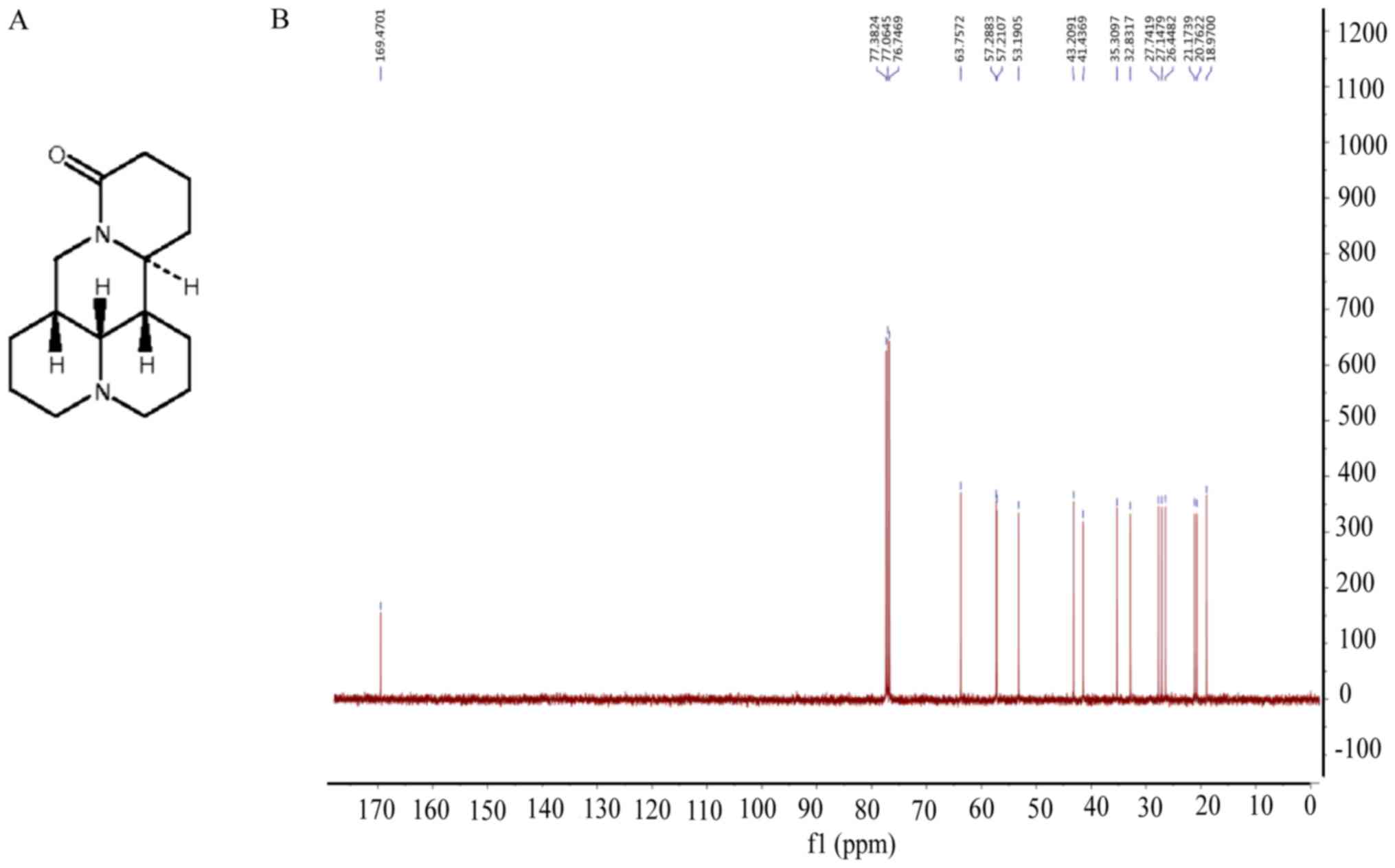
(Fig. 1A and B) is a natural
product derived from Sophora flavescens, the traditional
Chinese herbal medicine that has been shown to reduce the risk of
viral hepatitis (22) and atopic
dermatitis (23). The anticancer
activities of matrine have begun to be clarified recently (24), including in pancreatic cancer and
hepatoma (25,26). However, the potential function of
matrine in preventing prostate cancer metastasis, and the molecular
mechanism, are poorly understood.
Endoplasmic reticulum (ER) stress was found to be
involved in cytotoxicity due to proteasomal activity inhibition in
cancer cells (27), which was
induced by unfolded protein accumulation in the ER, known as the
unfolded protein response (UPR). If cell damage is sufficiently
severe, UPR/ER stress signaling will induce cell death by apoptosis
(28–31). ER stress also can control
oncogene-driven cell transformation (32). In mammals, there are three classes
of ER stress sensors (PERK, IRE1α and ATF6), which are inhibited by
the ER-specific chaperone BIP (33). PERK activation reduces general
protein synthesis by eukaryotic translation initiator factor 2α
(eIF2α) phosphorylation, which results in selective ATF4 mRNA
expression. ATF4 regulates the translation of essential genes
related to apoptosis and growth arrest, such as C/EBP-homologous
protein (CHOP). Continued ATF4 expression causes apoptosis,
possibly by up-regulating pro-apoptotic protein transcription, such
as Bax, and down-regulating the anti-apoptotic proteins of the
Bcl-2 family, such as Bcl-2 (30,34,35).
Therefore, UPR is a promising cancer treatment target because
increased ER stress can cause cell death.
The intention of the current study was to explore
the anticancer function of matrine in prostate cancer by reversing
EMT, as a result of proteasomal CT-like activity inhibition via
activation of UPR/ER stress both in vitro and in
vivo.
Materials and methods
Compounds and reagents
Matrine (cat. no. 519-02-8) was bought from the
Institute for Drug Control (Shanghai, China) and dissolved in
H2O. Z-Gly-Gly-Leu-AMC (cat. no. BML-ZW8505) was bought
from Enzo Life Sciences, Inc., (Farmingsale, NY, USA). Propidium
iodide (cat. no. P8080) and FITC Annexin V (cat. no. 556419) were
obtained from BD Biosciences (Franklin Lakes, NJ, USA). RIPA total
protein lysis buffer (P0013B), rabbit anti-Ki-67 monoclonal
antibody (cat. no. AF1738), and secondary antibodies of goat anti
mouse-HRP (cat. no. A0216) and rabbit-HRP (cat. no. A0208) were
bought from Beyotime Institute of Biotechnology (JiangSu, China).
Antibodies against ubiquitin (P4D1; cat. no. 3936c), Bip (C50B12;
cat. no. 3177), Phospho-eIF2α (Ser51) (D9G8; cat. no. 3398), eIF2α
(D7D3; cat. no. 5324), vimentin (5G3F10; cat. no. 3390), Bak (D4E4;
cat. no. 12105), Bcl-2 (cat. no. 2876), c-Myc (D84C12; cat. no.
5605), Cyclin B1 (D5C10; cat. no. 12231), Cyclin D1 (92G2; cat. no.
2978) and CDK1 (POH1; cat. no. 9116) were from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The cell cycle detection kit
was obtained from Key GENBioTECH (Nanjing, China). Antibodies
against E-cadherin (cat. no. 13-1700) and N-cadherin (cat. no.
33-3900) were from Invitrogen (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Antibodies against CREB-2 (ATF4; cat. no.
sc-390063) and GADD 153 (CHOP; cat. no. sc-575) were from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). Antibodies against
β-actin (Cat. no. sc-575) and 4-phenylbutyric acid (PBA; cat. no.
P21005) were from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
SuperSignal Chemiluminescent HRP substrate (cat. no. 34077) was
obtained from Thermo Fisher Scientific Inc. TRIzol reagent (cat.
no. 15596) was from Invitrogen; Thermo Fisher Scientific, Inc. SYBR
Premix Ex Taq II (cat. no. RR420L) and the PrimeScript RT reagent
kit (cat. no. RR037A) were from Takara Biotechnology Co., Ltd.
(Dalian, China). The TUNEL detection kit (cat. no. KGA7051) was
obtained from Key GENBioTECH.
Cell lines and cell culture
Human prostate cancer cells DU 145 and PC-3 were
purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA). DU 145 and PC-3 cells were cultured in F12
medium, purchased from Gibco; Thermo Fisher Scientific, Inc.,
containing streptomycin (100 µg/ml) and penicillin (100 U/ml), at
37°C with 5% CO2.
Proteasomal CT-like activity assay in
cultured living prostate cancer cells
To assess the inhibitory function of matrine on
proteasomal CT-like activity in prostate cancer cells, DU 145 or
PC-3 prostate cancer cells were seeded (4×104
cells/well) in 24-well cell culture plates at 37°C in a 5%
CO2 atmosphere for 24 h. Cells were starved for 12 h in
serum-free medium and incubated with different doses of matrine for
0–36 h, and then cultured for an additional 2 h with 20 µM
fluorogenic Z-Gly-Gly-Leu-AMC substrate to determine the
proteasomal CT-like activity. Cell medium (100 µl/sample) was then
collected for the measurement of free AMCs with a Varioskan Flash
Spectral Scanning Multimode Reader using an 380 nm excitation
filter and 460 nm emission (Thermo Fisher Scientific, Inc.), as
described previously (36).
Western blot assay
Total protein was isolated from prostate cancer
cells treated with 4 mM matrine for different times, with or
without pretreatment with PBA. To examine the protein expression
levels, proteins were isolated via SDS-PAGE and transferred onto
PVDF membranes, blotted using different antibodies and checked
using Super Signal West Matrineco Chemiluminescent Substrate, as
described previously (36).
Flow cytometric analysis
To determine the function of matrine on the cell
cycle and apoptosis, DU 145 or PC-3 prostate cancer cells were
exposed to matrine at the indicated dose and time. The cell cycle
was evaluated using the Cell Cycle Detection kit according to the
manufacturer's instructions, as described previously (36). Briefly, cells to be tested were
fixed overnight using cold 70% ethanol and washed using cold PBS,
then incubated with 40 µl RNaseA for 30 min at 37°C, with 160 µl
propidium iodide in a dark room at 4°C for an additional 30 min.
Cells were then measured using a flow cytometer (Accuri C6; BD
Biosciences, Franklin Lakes, NJ, USA). The distribution of the cell
cycle is represented as the cell percentages of
G0/G1, S and G2/M. The percentage
of apoptotic cells was also determined by flow cytometry after
staining with 5 µl FITC-labeled Annexin V (BD
Pharmingen; BD Biosciences) for 30 min at room
temperature, followed by 5 µl propidium iodide (50 µg/ml) (BD
Pharmingen; BD Biosciences) on ice for 5 min in a dark
room (36).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay
Briefly, 143B cells were seeded in chamber slides at
a density of 1×105 cells/ml, incubated for 24 h, and
then treated with PBS or matrine (2, 4, 8 mM) for 24 h. Cells were
fixed in 10% buffered formalin for 25 min at room temperature,
washed twice with PBS for 5 min and permeabilized by immersing the
slides in 0.2% Triton X-100 solution for 5 min. Subsequently, cells
were washed twice with PBS for 5 min. The TdT enzyme reaction mix
was added to the slides and incubated at 37°C for 60 min, washed
twice with PBS for 5 min. The streptavidin-FITC was added to the
slides and incubated 30 min. Finally, 4,6-diamidino-2-phenylindole
(DAPI) was added to stain the nucleus, and the apoptotic cells were
detected by fluorescence microscopy (DM4000B; Leica Microsystems
GmbH, Wetzlar, Germany).
Cell viability assay
Prostate cancer cells were harvested after culturing
with different doses of matrine for 48 h. Cell viability was
detected using a Countstar automated cell counter by loading 20 µl
cell suspension containing trypan blue (0.1%), as described
previously (36).
In vitro motility and invasion
assay
The migration of human prostate cancer cells was
measured using an xCELLigence RTCA DP system, as described
previously (36). Briefly, 165 µl
medium with 10% FBS was added into the lower chamber and 40 µl
medium without FBS was added into the upper chamber in each well of
the CIM-Plate 16. 100 µl cell suspension containing 40,000 cells
and different concentrations of matrine (0, 2, 4, or 8 mM) was
added into each upper chamber with a total volume of 140 µl medium.
Cell migration was then monitored continuously for 24 h. Cell
invasion was monitored continuously for 48 h using the same
conditions as the migration assay, except for the upper chambers
being pre-coated with Matrigel (1:40 dilution).
Xenograft of prostate cancer in nude
mice
Male BALB/c nude mice (6 weeks) were obtained from
Shanghai Slac Laboratory Animal Co. (Shanghai, China). All the
animal experiments, such as in vivo model preparation and
intervention, resection of xenograft cancer tissues to measure
tumor volume and tumor weight, and immunohistochemistry analysis,
were performed according to protocols approved by the Institutional
Animal Care and Use Committee of Shanghai University of TCM (no.
SZY2016004).
DU 145 cells (2×106 cells/100 µl) were
resuspended with sterile physiological saline and inoculated into
the right flank of the mice subcutaneously, then the mice were
divided at random into 2 groups with 7 mice in each group. On the
second day after inoculation, the animals started daily
intraperitoneal injections of: i) 100 µl saline in the control
group; or ii) 50 mg/kg/day matrine dissolved in saline for the
matrine group. The diameters of the tumors were estimated weekly
using vernier calipers. To calculate the tumor volumes, the
following principle was used: 0.5 × a × b (where a is the largest
dimension and the b is square of the smallest diameter). The body
weight of the mice was monitored every 3 days. Mice were euthanized
after 4 weeks' administration of matrine or saline to dissect the
tumor xenografts immediately for weighing, storing and fixing. The
following formula was used to calculate the inhibition rate of
tumor growth: (Tumor weight of saline treated group-tumor weight of
matrine treated group)/tumor weight of saline treated group
×100%.
Quantitative analysis of mRNA
levels
Total RNA was purified from the xenograft tumors
using TRIzol reagent. Primers for Bip were
5′-CCCGTGGCATAAACCCAGAT-3′ (forward), 5′-TGGTAGGCACCACTGTGTTC-3′
(reverse); ATF-4 were 5′-TTAAGCCATGGCGCTTCTCA-3′ (forward),
5′-TCCTTGCTGTTGTTGGAGGG-3′ (reverse); PARP were
5′-TTCAACAAGCAGCAAGTGCC-3′ (forward), 5′-CCTTTGGGGTTACCCACTCC-3′
(reverse); Bcl-2 were 5′-GGTGAACTGGGGGAGGATTG-3′ (forward),
5′-ATCACCAAGTGCACCTACCC-3′ (reverse); Cyclin B1 were
5′-TCTGCTGGGTGTAGGTCCTT-3′ (forward), 5′-ACCAATGTCCCCAAGAGCTG-3′
(reverse); vimentin were 5′-GGACCAGCTAACCAACGACA-3′ (forward),
5′-AAGGTCAAGACGTGCCAGAG-3′ (reverse) and GAPDH were
5′-TGTTGCCATCAATGACCCCTT-3′ (forward), 5′-CTCCACGACGTACTCAGCG-3′
(reverse). Reverse transcriptional PCR was performed with the
PrimeScript RT reagent kit. RT-PCR was performed using SYBR Premix
Ex Taq II in the Bio-Rad CFX 96 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) qPCR system. GAPDH was used as the internal
control, and 2−ΔΔCq was used to calculate the fold
changes. Each experiment was conducted in triplicate.
Immunohistochemical analyses
Xenograft tumor tissues, fixed in 10% neutral
buffered paraformaldehyde for 24 h at 4°C, were randomly selected,
embedded in paraffin, sliced (5-µm thick), deparaffinized and
rehydrated with PBS, and treated in 3% H2O2
for 10 min. Antigen retrieval was performed at 37°C for 10 min with
0.1% trypsin (M/V). The slices were stained with the indicated
primary antibodies at 4°C overnight after 5% BSA blocking, followed
by culture with the secondary antibody. Slides were counterstained
with hematoxylin after 5 min of staining with DAB, and then mounted
using neutral gum after permeabilizing using xylene. An image
autoanalysis system (Olympus BX50; Olympus Corporation, Tokyo,
Japan) was used to acquire images, and a representative image is
presented. Positive expression was indicated by strong brown
staining.
Statistical analysis
Representative data from triplicate experiments are
presented. To compare data from multiple groups, one-way ANOVA
followed by the Tukey-Kramer or Holm-Sidak tests was used. To
compare data from two groups, a Student's t-test was used.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Matrine inhibited the cellular
proteasomal CT-like activity of prostate cancer cells
Proteasomal CT-like activity is related to cancer
cell viability. Previous reports suggest that Chinese herbal
medicines with proteasome inhibitor activities mostly have
antipyretic-antioxidant effects. Matrine is an ethanol extract from
Sophora flavescens, a traditional Chinese herbal medicine
with antipyretic and antioxidant functions. We hypothesized that
matrine could potentially inhibit proteasomal activity and result
in the inhibition of prostate cancer. Thus, we investigated whether
matrine would reduce the proteasomal CT-like activity of prostate
cancer cells. It was revealed that matrine significantly inhibited
the proteasomal CT-like activity of both PC-3 and DU145 prostate
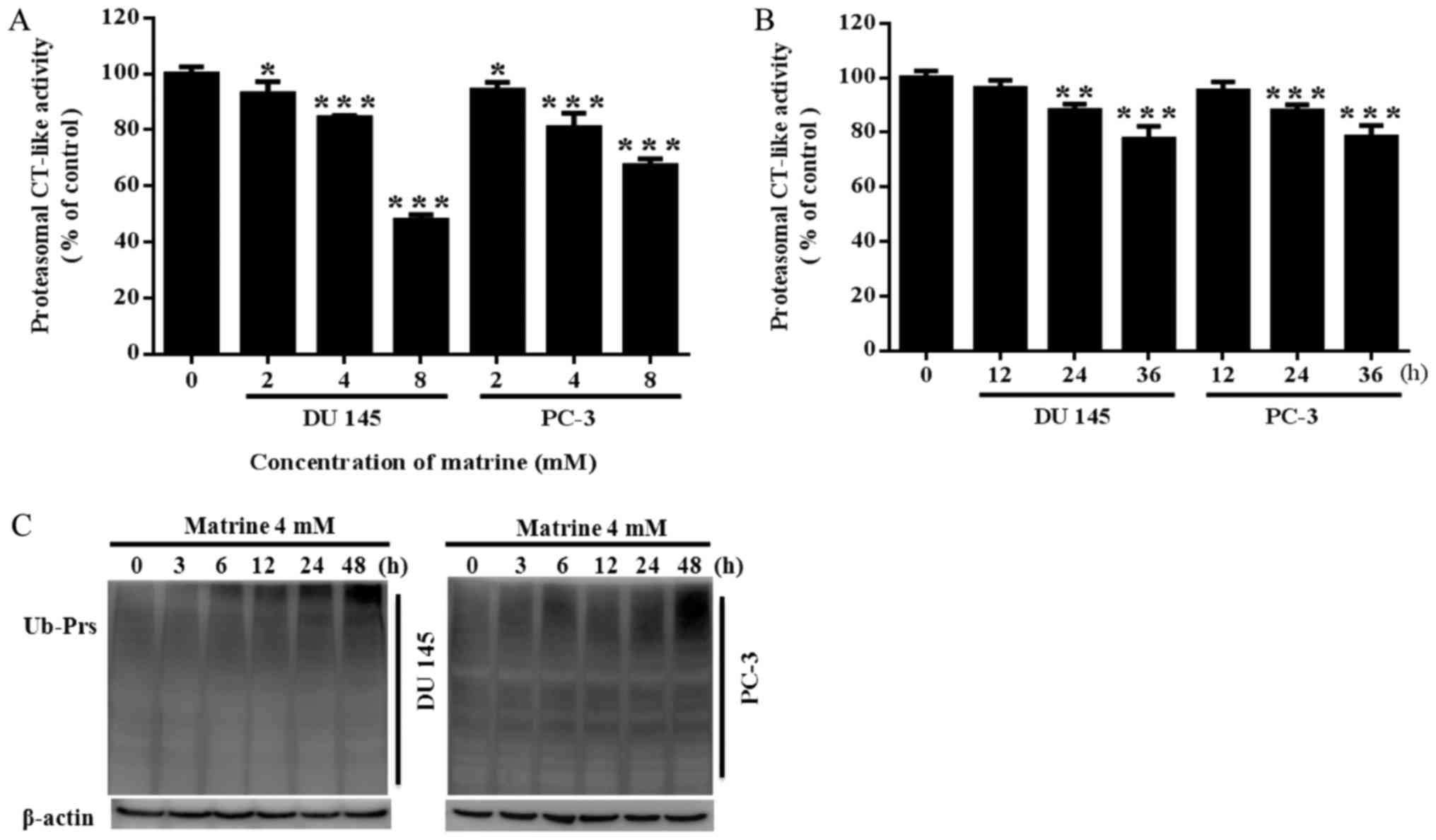
cancer cells in a concentration dependent (Fig. 2A) and time dependent manner
(Fig. 2B). Meanwhile, both DU145
and PC-3 prostate cancer cells showed a time-dependent accumulation
of ubiquitinated proteins (Ub-Prs) with 4 mM matrine treatment
(Fig. 2C).
Matrine reversed EMT and inhibited the
viability of prostate cancer cells
Prostate cancer cells showed EMT with characteristic
changes in vimentin, N-cadherin and E-cadherin expression.
Therefore, we proposed to investigate the function of matrine in
reversing EMT by measuring mesenchymal and epithelial markers. The
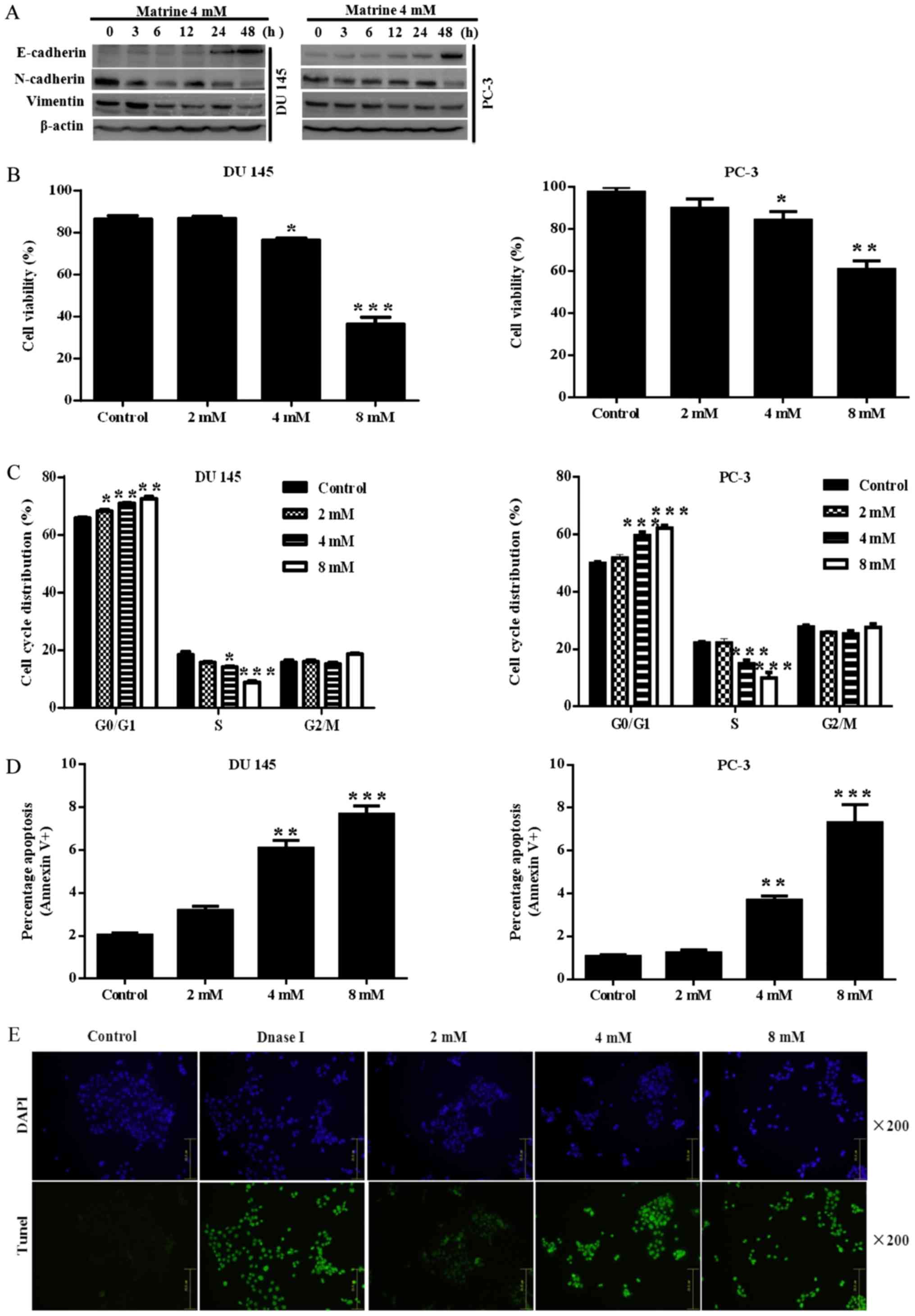
results showed that challenge with matrine led to up-regulation of
the epithelial marker E-cadherin, and down-regulation of the
mesenchymal markers vimentin and N-cadherin (Fig. 3A).
Taken together, our results indicate that, in
vitro, matrine negatively regulates EMT in prostate cancer
cells.
EMT-like status is also related to an increase in
cell viability under diverse conditions, including therapeutic
resistance. Thus, we further assessed the potential antitumor
effects of matrine on prostate cancer cells by detecting the
viability of prostate cancer cells. As assessed using the Countstar
automated cell counter, prostate cancer cells challenged with 2, 4
or 8 mM matrine for 72 h exhibited an inhibition of cell growth,
which was dose-dependent in DU145 (Fig. 3B, left panel) and PC-3 (Fig. 3B, right panel). To further
investigate in more detail, we conducted apoptosis and cell cycle
assays in DU145 and PC-3 cells challenged with 2, 4 or 8 mM matrine
for 24 h. Flow cytometric analysis was used to detect the cell
cycle distribution after propidium iodide staining. As shown in
Fig. 3C, prostate cancer cells
were arrested at the G0/G1 phase.
Furthermore, the higher the concentration of matrine used, the
greater the number of DU145 (Fig.
3C, left panel) and PC-3 (Fig.
3C, right panel) cells blocked at the
G0/G1 phase. Cell death was estimated by
examining the apoptotic percentage. As presented in Fig. 3D, flow cytometric analysis was used
to determine the percentage of apoptotic cells with FITC-labeled
Annexin-V and propidium iodide staining. Early apoptotic (only
stained with Annexin V-FITC) and late apoptotic (double stained
with propidium iodide and Annexin V-FITC) percentages were pooled
for analysis. Matrine dose-dependently caused increased apoptosis
in both DU145 (Fig. 3D, left
panel) and PC-3 (Fig. 3D, right
panel) cells, the apoptosis in DU145 cells was further confirmed by
TUNEL assay (Fig. 3E). These data
suggested that matrine could exert a significant growth inhibitory
effect on prostate cancer cells.
Matrine decreased the migration and
invasion of prostate cancer cells
The exchange of cadherin is a characteristic
EMT-like transformation which assists adhesion among homotypic
cells and is generally associated with invasion and migration, the
key processes for encouraging metastatic dissemination (37). To explore the functions of matrine
in reversing invasion and migration, the xCELLigence RTCA DP system
was used in DU145 cells. It was revealed that matrine treatment
significantly inhibited the capacity of DU145 cell migration
(Fig. 3F), invasion (Fig. 3G) and induced the morphological
changes (Fig. 3H) in a
concentration- and time-dependent manner compared with those cells
without matrine treatment.
Matrine activated the UPR/ER stress
signaling cascade and regulated the target genes involved in human
prostate cancer cell apoptosis and the cell cycle
The proteasome controls the degradation of misfolded
proteins. Proteasome inhibitors may increase unfolded protein
accumulation, leading to enhanced ER stress due to UPR. The Bcl-2
family and CHOP are the major mediators of ER stress-dependent
apoptosis (29). Therefore,
targeting factors associated with UPR-induced ER stress is a
possible approach for the management of malignancy. 4-phenylbutyric
acid (PBA) is a small-molecule chemical chaperone that stabilizes
the conformation of proteins and improves the folding ability of
proteins in the ER, as well as facilitating mutant protein
trafficking, while ER stress can be attenuated by PBA (38,39).
To further understand the molecular mechanism
related to matrine-stimulated changes in prostate cancer cell
survival and proteasomal activity inhibition. The key components of
the UPR/ER stress signaling pathway, and the target genes
associated with apoptosis and the cell cycle were investigated
using western blotting. We found that both DU145 and PC-3 prostate
cancer cells exposed to 4 mM of matrine exhibited time-dependent
activation of the PERK branch in UPR, as evidenced by increased BiP
expression, improved eIF2α phosphorylation, and up-regulated
GADD153 (CHOP) and ATF4 (Fig. 4A).
By detecting the associated target genes, we further found that
matrine inhibited anti-apoptotic protein expression, such as Bcl-2,
while increasing pro-apoptotic protein expression, such as Bax
(Fig. 4B). DU145 and PC-3 cells
exposed to matrine (4 mM) also exhibited time-dependent inhibition
of the expression of Cyclin D1, Cyclin B1, c-Myc and CDK1 (Fig. 4C), indicating that matrine break
down the complex of Cyclin/CDK1, which is important for
continuously reshuffling core elements following transition between
cell cycle phases after rapid division of cells (40), and resulted in
G0/G1 phase arrest in the prostate cancer
cell cycle (Fig. 3C). Meanwhile,
the activated UPR/ER stress signaling cascade (Fig. 4D) in prostate cancer cells caused
by matrine was attenuated by pretreatment of the DU 145 and PC-3
human prostate cancer cells with PBA (40 µM) for 24 h before
challenge with matrine.
Altogether, these data implied that matrine provoked
apoptosis and inhibited cell proliferation in DU 145 and PC-3
prostate cancer cells via specific up-regulation of UPR leading to
the activation of ER stress.
Matrine has therapeutic efficacy for
prostate cancer xenografts associated with activation of UPR/ER
stress signaling, thus reversing EMT, and inducing apoptosis and
cell cycle arrest in vivo
The data mentioned above clearly showed that matrine
could inhibit prostate cancer cell viability by inhibiting
proteasomal activity and UPR/ER stress signaling
activation-associated reversal of EMT, inducing apoptosis and cell
cycle arrest. To explore whether matrine had the same therapeutic
function and mechanism in prostate cancer in vivo, prostate
cancer DU 145 cells were subcutaneously inoculated into the right
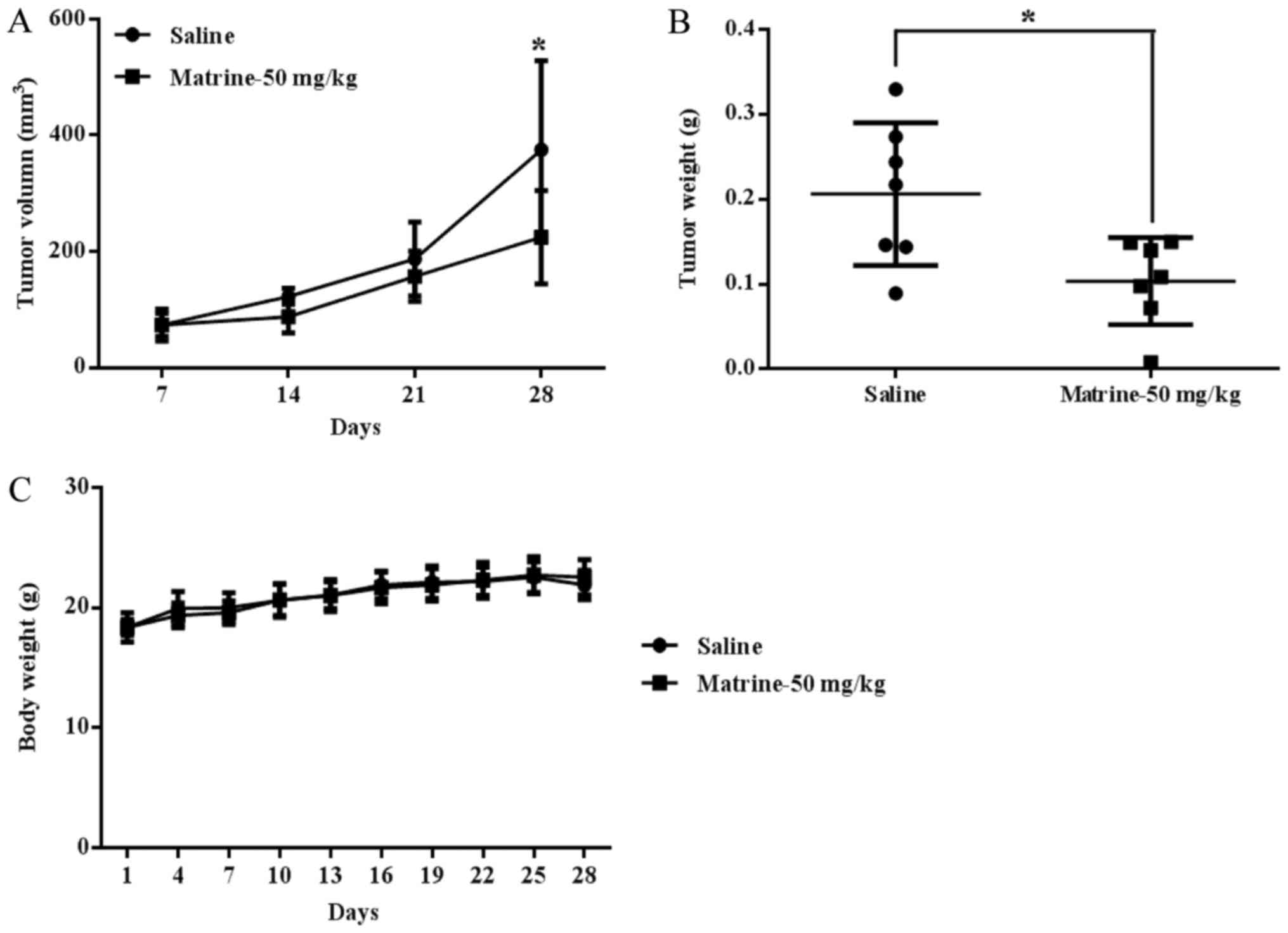
flank of each mouse. The results showed that matrine significantly
suppressed tumor growth in vivo, as evidenced by tumor
volume (Fig. 5A) and tumor weight
(Fig. 5B); the tumor growth
inhibition rate with matrine treatment was 49.77% relative to
control mice treated with the saline. Matrine caused no obvious
toxic effects on mice at a dose of 50 mg/kg/day, and the body
weights were not changed in the matrine treatment group vs. the
saline treatment group (Fig.
5C).
The in vitro data showed that matrine was a
potential natural proteasome inhibitor and caused Ub-Prs
accumulation in prostate cancer cells. To explore whether matrine
could also reduce proteasomal activity, and therefore stimulate
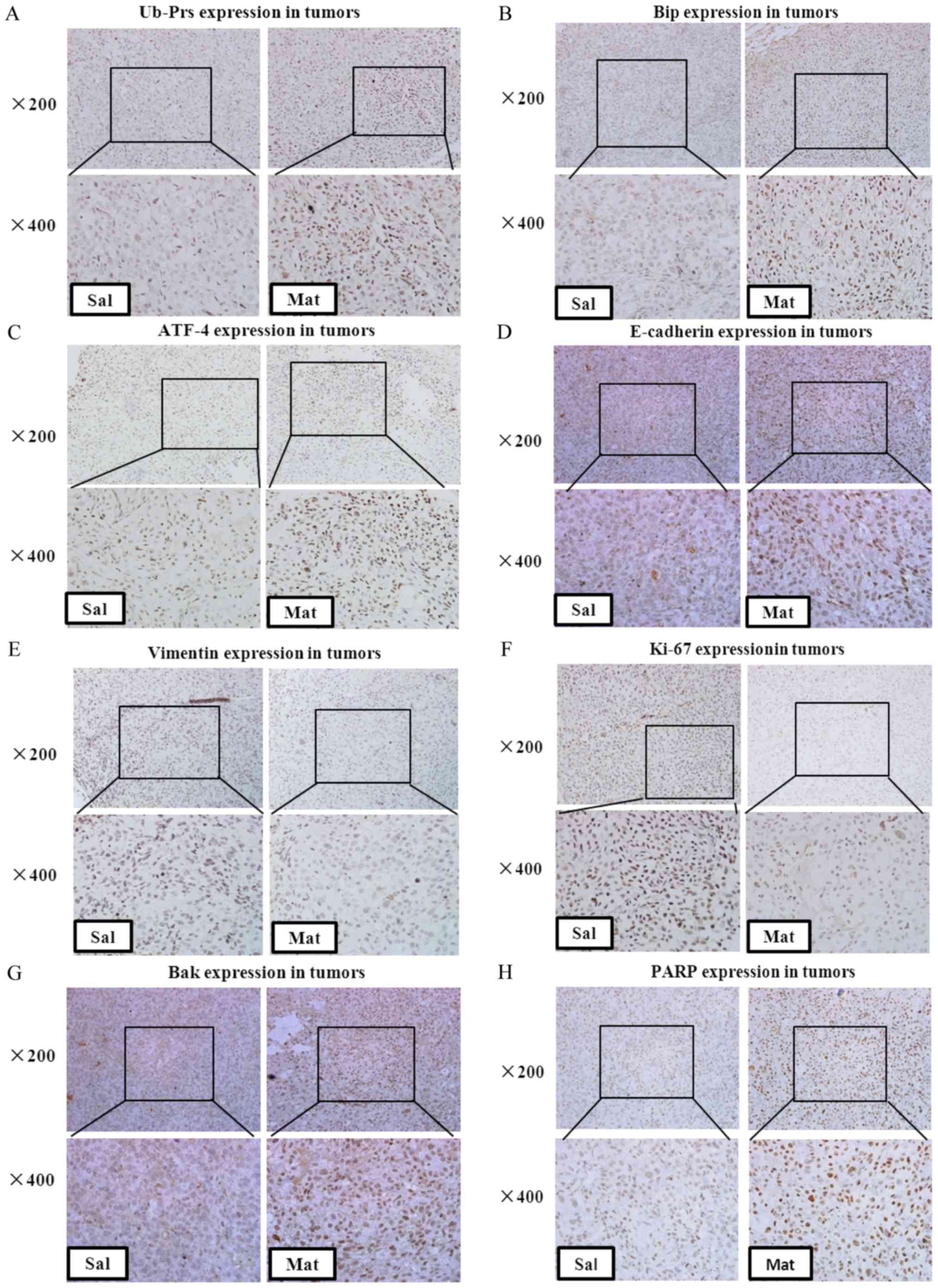
Ub-Prs accumulation in prostate cancer xenografts, the expression
of Ub-Prs in DU 145 xenografts was detected by immunohistochemistry
staining. A significant up-regulation in Ub-Prs was observed in the
prostate cancer xenografts after matrine treatment vs. the saline
treatment (Fig. 6A), indicating
that matrine also induced Ub-Prs accumulation in prostate cancer
xenografts; thus, intraperitoneal injections of matrine can inhibit
the proteasome in prostate tumors in vivo.
The constitutive activation of UPR/ER stress
signaling after treatment with matrine was further confirmed in
vivo by increased BiP and ATF4 expression, both at the protein
(Fig. 6B and C) and mRNA (Fig. 6I and J) levels, in the tissues from
matrine treated vs. saline treated prostate tumors. Molecular
changes associated with the reversal of EMT were also determined in
the tumor tissues from prostate cancer xenografts, which showed
significantly up-regulated E-cadherin (Fig. 6D) and down-regulated Vimentin
(Fig. 6E and K), indicating that
matrine decreases the in vivo metastasis of prostate
carcinoma by reversing EMT.
To further define the in vivo mechanism of
tumor growth inhibition, the expression of Ki-67 in xenograft tumor
tissues was identified by immunohistochemistry staining (Fig. 6F); it was shown that matrine
significantly decreased the expression of Ki-67 in prostate cancer
xenografts, which was indicated by stronger brown labeled nuclei.
Furthermore, the cell cycle and growth associated protein Cyclin B1
was found to be down-regulated in the tumor tissues from matrine
treated xenograft-bearing mice vs. saline-treated ones (Fig. 6L). These data further confirmed
that matrine could also inhibit human prostate cancer cell
proliferation in vivo.
Furthermore, up-regulated Bak (Fig. 6G) and PARP (Fig. 6H and M), and down-regulated Bcl-2
(Fig. 6N), were found in the
tumors of matrine-treated xenograft-bearing mice vs. the
saline-treated mice, suggesting the activation of apoptosis in DU
145 tumors in vivo by matrine.
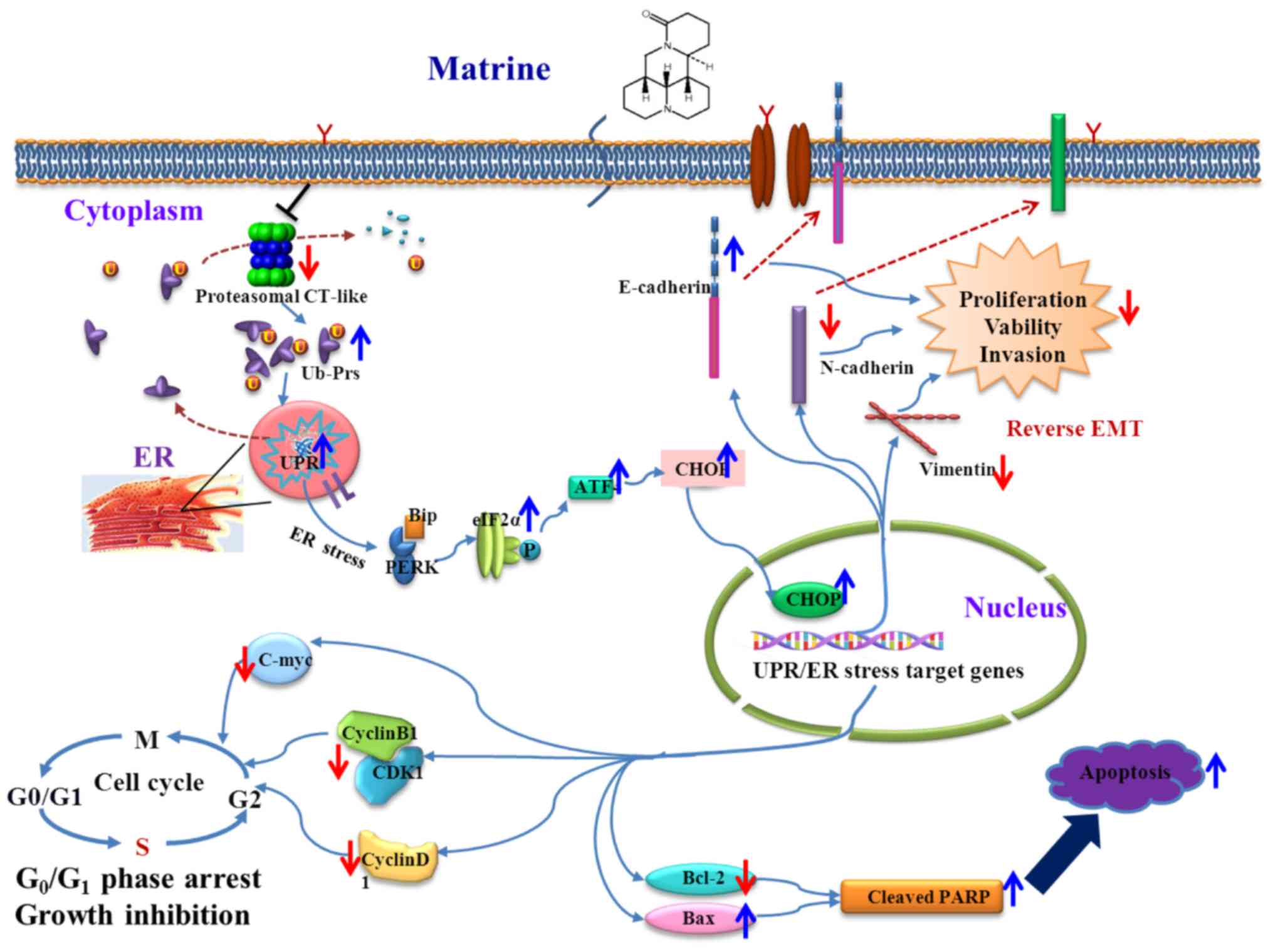
The schematic representation of the proposed
therapeutic mechanisms of matrine in human prostate cancer is shown
in Fig. 7.
Discussion
The lethality of prostate cancer is associated with
its metastasis to other organs. EMT is a well-established
biological procedure which converts epithelial cells to mesenchymal
cells, and this has a significant role in both normal organ
development and oncogenesis. EMT has attracted attention as a
theoretical standard to elucidate progression and metastatic
activity throughout cancer development. There is abundant proof
that EMT-like status is the crucial process for metastasis and
invasion in most epithelial cell source cancers, including prostate
cancer, and has become an extremely active field of research
(41). Therefore, using our
knowledge of EMT-like status is potential future therapeutic
avenue, and new compounds that can reverse EMT in prostate cancer
cells are urgently needed. A number of potential anti-EMT
compounds, including salinosporamide A (NPI-0052), the proteasome
inhibitor, have been reported (42). Meanwhile, conventional
chemotherapeutics cause side effects such as impaired renal
function, gonadal dysfunction, hearing loss and myelosuppression
(43).
Natural products and their derivatives have shown
potential for cancer prevention and treatment because of their
diverse structures, potential therapeutic effects and minimal side
effects. Matrine is a principal active drug monomer in Sophora
flavescens and has been used as an anti-cancer herbal medicine
for hundreds of years in China with a broad range of
pharmacological effects (22),
without apparent side effects or toxicity. More up to date studies
have found that matrine has effective anti-tumor activity, such as
in multiple myeloma (44).
In this study, we assessed the effects of matrine on
prostate cancer both in vitro and in vivo. The
results showed that matrine inhibited tumor growth and Ki-67
expression in xenograft-bearing nude mice. Matrine also showed the
ability to inhibit cell viability, arrest the cell cycle, induce
apoptosis and suppress invasion and migration in prostate cancer,
both in vivo and in vitro.
We conducted further experiments to explore the
mechanism of matrine in suppressing prostate cancer. We confirmed
that matrine inhibited intracellular proteasomal CT-like activity,
which resulted in an accumulation of Ub-Prs in both prostate cancer
cells and tumor tissues. We further found that matrine showed an
anti-EMT effect in both DU145 and PC-3 prostate cancer cells, and
the prostate tumor tissues of DU145 xenograft-bearing mice, as
evidenced by up-regulated E-cadherin and down-regulated Vimentin
and N-cadherin expression, which highlighted the anti-prostate
cancer potential of matrine.
Unrestrained proliferation is an important feature
of tumorigenesis, and the inhibition of proliferation leads to
tumor cell growth arrest. The cell cycle is also important in
regulating cell proliferation, cell division and cell growth. Cell
cycle arrest might result in the an inhibition of cell growth or
apoptotic cell death due to severe DNA damage (43).
We found that matrine caused cell cycle arrest at
the G0/G1 phase by time dependently
down-regulating Cyclin D1, Cyclin B1, c-Myc and CDK1 expression in
DU145 and PC-3 prostate cancer cells, and also down-regulating
Cyclin B1 in prostate cancer tumor tissues, suggesting that, both
in vitro and in vivo, cell cycle arrest might be a
mechanism underlying the anti-proliferative effect of matrine on
prostate cancer. Our results further confirmed that matrine
dose-dependently induced apoptosis and inhibited the survival of
DU145 and PC-3 prostate cancer cells. Our results further
demonstrated that matrine up-regulated BAX) and down-regulated
Bcl-2 expression in both prostate cancer cells and tumor tissues.
Therefore, our present study showed that matrine significantly
inhibited proliferation, and also induced apoptosis in human
prostate cancer in vitro and in vivo.
The ER is the center for protein folding and
maturation. Impairment of the ER causes ER stress, which is
activated by UPR and, in turn, alters cell activity and survival
through regulation of protein synthesis, folding and degradation
(45). UPR includes three key
pathways: The PERK-eIF2α-ATF4-CHOP pathway, the IRE1-XBP1 pathway
and the ATF6-chaperone pathway (46). The IRE1-XBP1 and ATF6-chaperone
pathways mainly regulate ER chaperones, for instance GRP78 and
p58IPK, thus promoting the ability of protein folding (47–50).
Early activation of PERK decreases the protein translation rate by
promoting eIF2α phosphorylation, which allows the ER to recover
from its stressed state. However, continued ER stress activates the
downstream factors of the PERK pathway, such as pro-apoptotic gene
C/EBP homologous protein (CHOP), and causes apoptosis and cell
death (46,51,52).
UPR can also potentiate the EMT of gastric cancer cells under
situations of severe hypoxia (53). In current study, it was found that
matrine time-dependently increased Bip, p-eIF2α, ATF4 and CHOP
expression in DU145 and PC-3 prostate cancer cells, and increased
Bip and ATF4 expression in prostate cancer tumor tissues. The
increased expression of p-eIF2α indicates the activation of PERK in
UPR, which indirectly inactivates eIF2 and reduces mRNA translation
(46). Meanwhile, p-eIF2α
activates transcription factor ATF4, and then stimulates its
downstream transcription factor CHOP, which is believed to regulate
the genes involved in apoptosis (46).
Our study showed that matrine inhibited EMT, and
induced cell cycle arrest and apoptosis both in human DU145 and
PC-3 prostate cancer cells and DU145 xenograft tissues from DU145
xenograft-bearing mice, suggesting that matrine delays prostate
cancer cell growth and induces apoptosis when persistent UPR/ER
stress appeared. The anti-prostate cancer function of matrine
might, at least partly, depend on the activation of UPR/ER
stress.
In conclusion, we have shown that matrine, derived
from Sophora flavescens, has potent and selective
anti-prostate cancer activity by inhibiting proteasomal activity,
reversing EMT, and inducing cell-cycle arrest and apoptosis by
specific activating the UPR/ER stress signaling pathway, both in
vitro and in vivo. Although further toxicological and
pharmacological studies are required, our conclusion highlights the
potential of matrine as a chemotherapeutic agent against prostate
cancer and other UPR/ER stress activation-associated
carcinomas.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Nature Science Foundation (grant nos. 81674006, 81603343, and
81102851); the Program for Innovative Research Team of Ministry of
Science and Technology of China (grant no. 2015RA4002); the Xinglin
Young Scholar, Xinglin Young Talent Program; and the Shanghai TCM
Medical Center of Chronic Disease (grant no. 2017ZZ01010).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY designed and coordinated the experiments; JC and
SH carried out most of the experiments; QS analyzed the
experimental results; WW and YL constructed and monitored the
xenograft-bearing nude mouse model; WZ, YW and SL collected
xenograft tissues; YY and YW wrote and edited the manuscript.
Ethics approval and consent to
participate
The ethics approval was obtained from the
Institutional Animal Care and Use Committee of Shanghai University
of TCM (ethics no. SZY2016004). All the animal experiments in this
study were performed according to this approved ethics
protocols.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xu S, Zhou W, Ge J and Zhang Z:
Prostaglandin E2 receptor EP4 is involved in the cell growth and
invasion of prostate cancer via the cAMP-PKA/PI3K-Akt signaling
pathway. Mol Med Rep. 17:4702–4712. 2018.PubMed/NCBI
|
|
2
|
Zhu Y, Shao S, Pan H, Cheng Z and Rui X:
MicroRNA-136 inhibits prostate cancer cell proliferation and
invasion by directly targeting mitogen-activated protein kinase
kinase 4. Mol Med Rep. 17:4803–4810. 2018.PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie D, Gore C, Liu J, Pong RC, Mason R,
Hao G, Long M, Kabbani W, Yu L, Zhang H, et al: Role of DAB2IP in
modulating epithelial-to-mesenchymal transition and prostate cancer
metastasis. Proc Natl Acad Sci USA. 107:2485–2490. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen X, Cheng H, Pan T, Liu Y, Su Y, Ren
C, Huang D, Zha X and Liang C: mTOR regulate EMT through RhoA and
Rac1 pathway in prostate cancer. Mol Carcinog. 54:1086–1095. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Voutsadakis IA: The ubiquitin-proteasome
system and signal transduction pathways regulating Epithelial
Mesenchymal transition of cancer. J Biomed Sci. 19:672012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adams J, Palombella VJ, Sausville EA,
Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S and
Elliott PJ: Proteasome inhibitors: A novel class of potent and
effective antitumor agents. Cancer Res. 59:2615–2622.
1999.PubMed/NCBI
|
|
12
|
Mani A and Gelmann EP: The
ubiquitin-proteasome pathway and its role in cancer. J Clin Oncol.
23:4776–4789. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu WK, Cho CH, Lee CW, Wu K, Fan D, Yu J
and Sung JJ: Proteasome inhibition: A new therapeutic strategy to
cancer treatment. Cancer Lett. 293:15–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang T, Zhu Y, Fang X, Chi Y, Kitamura M
and Yao J: Gap junctions sensitize cancer cells to proteasome
inhibitor MG132-induced apoptosis. Cancer Sci. 101:713–721. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seeger JM, Schmidt P, Brinkmann K, Hombach
AA, Coutelle O, Zigrino P, Wagner-Stippich D, Mauch C, Abken H,
Krönke M and Kashkar H: The proteasome inhibitor bortezomib
sensitizes melanoma cells toward adoptive CTL attack. Cancer Res.
70:1825–1834. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tagoug I, Jordheim LP, Herveau S, Matera
EL, Huber AL, Chettab K, Manié S and Dumontet C: Therapeutic
enhancement of ER stress by insulin-like growth factor I sensitizes
myeloma cells to proteasomal inhibitors. Clin Cancer Res.
19:3556–3566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Orlowski M and Wilk S: Catalytic
activities of the 20 S proteasome, a multicatalytic proteinase
complex. Arch Biochem Biophys. 383:1–16. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arendt CS and Hochstrasser M:
Identification of the yeast 20S proteasome catalytic centers and
subunit interactions required for active-site formation. Proc Natl
Acad Sci USA. 94:7156–7161. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dick TP, Nussbaum AK, Deeg M, Heinemeyer
W, Groll M, Schirle M, Keilholz W, Stevanović S, Wolf DH, Huber R,
et al: Contribution of proteasomal beta-subunits to the cleavage of
peptide substrates analyzed with yeast mutants. J Biol Chem.
273:25637–25646. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lopes UG, Erhardt P, Yao R and Cooper GM:
p53-dependent induction of apoptosis by proteasome inhibitors. J
Biol Chem. 272:12893–12896. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi G, Chen D, Zhai G, Chen MS, Cui QC,
Zhou Q, He B, Dou QP and Jiang G: The proteasome is a molecular
target of environmental toxic organotins. Environ Health Perspect.
117:379–386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Long Y, Lin XT, Zeng KL and Zhang L: Long
Y, Lin XT, Zeng KL and Zhang L: Efficacy of intramuscular matrine
in the treatment of chronic hepatitis B. Hepatobiliary Pancreat Dis
Int. 3:69–72. 2004.PubMed/NCBI
|
|
23
|
Liu JY, Hu JH, Zhu QG, Li FQ, Wang J and
Sun HJ: Effect of matrine on the expression of substance P receptor
and inflammatory cytokines production in human skin keratinocytes
and fibroblasts. Int Immunopharmacol. 7:816–823. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu P, Liu Q, Liu K, Yagasaki K, Wu E and
Zhang G: Matrine suppresses breast cancer cell proliferation and
invasion via VEGF-Akt-NF-kappaB signaling. Cytotechnology.
59:219–229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu T, Song Y, Chen H, Pan S and Sun X:
Matrine inhibits proliferation and induces apoptosis of pancreatic
cancer cells in vitro and in vivo. Biol Pharm Bull. 33:1740–1745.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nava MB, Rocco N, Catanuto G, Falco G,
Capalbo E, Marano L, Bordoni D, Spano A and Scaperrotta G: Impact
of contra-lateral breast reshaping on mammographic surveillance in
women undergoing breast reconstruction following mastectomy for
breast cancer. Breast. 24:434–439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Obeng EA, Carlson LM, Gutman DM,
Harrington WJ Jr, Lee KP and Boise LH: Proteasome inhibitors induce
a terminal unfolded protein response in multiple myeloma cells.
Blood. 107:4907–4916. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Walter P and Ron D: The unfolded protein
response: From stress pathway to homeostatic regulation. Science.
334:1081–1086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hetz C, Chevet E and Harding HP: Targeting
the unfolded protein response in disease. Nat Rev Drug Discov.
12:703–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan P, Griffith OL, Agboke FA, Anur P, Zou
X, McDaniel RE, Creswell K, Kim SH, Katzenellenbogen JA, Gray JW
and Jordan VC: c-Src modulates estrogen-induced stress and
apoptosis in estrogen-deprived breast cancer cells. Cancer Res.
73:4510–4520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huber AL, Lebeau J, Guillaumot P, Pétrilli
V, Malek M, Chilloux J, Fauvet F, Payen L, Kfoury A, Renno T, et
al: p58(IPK)-mediated attenuation of the proapoptotic PERK-CHOP
pathway allows malignant progression upon low glucose. Mol Cell.
49:1049–1059. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jwa M and Chang P: PARP16 is a
tail-anchored endoplasmic reticulum protein required for the PERK-
and IRE1α-mediated unfolded protein response. Nat Cell Biol.
14:1223–1230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McCullough KD, Martindale JL, Klotz LO, Aw
TY and Holbrook NJ: Gadd153 sensitizes cells to endoplasmic
reticulum stress by down-regulating Bcl2 and perturbing the
cellular redox state. Mol Cell Biol. 21:1249–1259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gallerne C, Prola A and Lemaire C: Hsp90
inhibition by PU-H71 induces apoptosis through endoplasmic
reticulum stress and mitochondrial pathway in cancer cells and
overcomes the resistance conferred by Bcl-2. Biochim Biophys Acta.
1833:1356–1366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang J, Wang H, Wang X, Zhao Y, Zhao D,
Wang C, Li Y, Yang Z, Lu S, Zeng Q, et al: Molecular mechanisms of
Polyphyllin I-induced apoptosis and reversal of the
epithelial-mesenchymal transition in human osteosarcoma cells. J
Ethnopharmacol. 170:117–127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shiota M, Bishop JL, Nip KM, Zardan A,
Takeuchi A, Cordonnier T, Beraldi E, Bazov J, Fazli L, Chi K, et
al: Hsp27 regulates epithelial mesenchymal transition, metastasis,
and circulating tumor cells in prostate cancer. Cancer Res.
73:3109–3119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dromparis P, Paulin R, Stenson TH, Haromy
A, Sutendra G and Michelakis ED: Attenuating endoplasmic reticulum
stress as a novel therapeutic strategy in pulmonary hypertension.
Circulation. 127:115–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Welch WJ and Brown CR: Influence of
molecular and chemical chaperones on protein folding. Cell Stress
Chaperones. 1:109–115. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rastogi N and Mishra DP: Therapeutic
targeting of cancer cell cycle using proteasome inhibitors. Cell
Div. 7:262012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nauseef JT and Henry MD:
Epithelial-to-mesenchymal transition in prostate cancer: Paradigm
or puzzle? Nat Rev Urol. 8:428–439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
King TE Jr, Pardo A and Selman M:
Idiopathic pulmonary fibrosis. Lancet. 378:1949–1961. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
van Rooij E and Olson EN: MicroRNA
therapeutics for cardiovascular disease: opportunities and
obstacles. Nat Rev Drug Discov. 11:860–872. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nicolopoulou A, Cortina KS, Ilgaz H, Cates
CB and de Sá AB: Using a narrative- and play-based activity to
promote low-income preschoolers' oral language, emergent literacy,
and social competence. Early Child Res Q. 31:147–162. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Catov JM, Abatemarco D, Althouse A, Davis
EM and Hubel C: Patterns of gestational weight gain related to
fetal growth among women with overweight and obesity. Obesity
(Silver Spring). 23:1071–1078. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Keating SE, Hackett DA, Parker HM,
O'Connor HT, Gerofi JA, Sainsbury A, Baker MK, Chuter VH, Caterson
ID, George J and Johnson NA: Effect of aerobic exercise training
dose on liver fat and visceral adiposity. J Hepatol. 63:174–182.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mortensen DS, Fultz KE, Xu S, Xu W,
Packard G, Khambatta G, Gamez JC, Leisten J, Zhao J, Apuy J, et al:
CC-223, a potent and selective inhibitor of mTOR kinase: In vitro
and in vivo characterization. Mol Cancer Ther. 14:1295–1305. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kruse CR, Nuutila K, Lee CC, Kiwanuka E,
Singh M, Caterson EJ, Eriksson E and Sørensen JA: The external
microenvironment of healing skin wounds. Wound Repair Regen.
23:456–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Woolcott OO, Richey JM, Kabir M, Chow RH,
Iyer MS, Kirkman EL, Stefanovski D, Lottati M, Kim SP, Harrison LN,
et al: High-fat diet-induced insulin resistance does not increase
plasma anandamide levels or potentiate anandamide insulinotropic
effect in isolated canine islets. PLoS One. 10:e01235582015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Merlino A, Caterino M, Krauss Russo I and
Vergara A: Missing gold atoms in lysozyme crystals used to grow
gold nanoparticles. Nat Nanotechnol. 10:2852015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zimarino M, Ricci F, Romanello M, Di
Nicola M, Corazzini A and De Caterina R: Complete myocardial
revascularization confers a larger clinical benefit when performed
with state-of-the-art techniques in high-risk patients with
multivessel coronary artery disease: A meta-analysis of randomized
and observational studies. Catheter Cardiovasc Interv. 87:3–12.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yan H, Catania C and Bazan GC:
Membrane-intercalating conjugated oligoelectrolytes: Impact on
bioelectrochemical systems. Adv Mater. 27:2958–3973. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Niemczyk NA, Catov JM, Barinas-Mitchell E,
McClure CK, Roberts JM, Tepper PG and Sutton-Tyrrell K: Nulliparity
is associated with less healthy markers of subclinical
cardiovascular disease in young women with overweight and obesity.
Obesity (Silver Spring). 23:1085–1091. 2015. View Article : Google Scholar : PubMed/NCBI
|