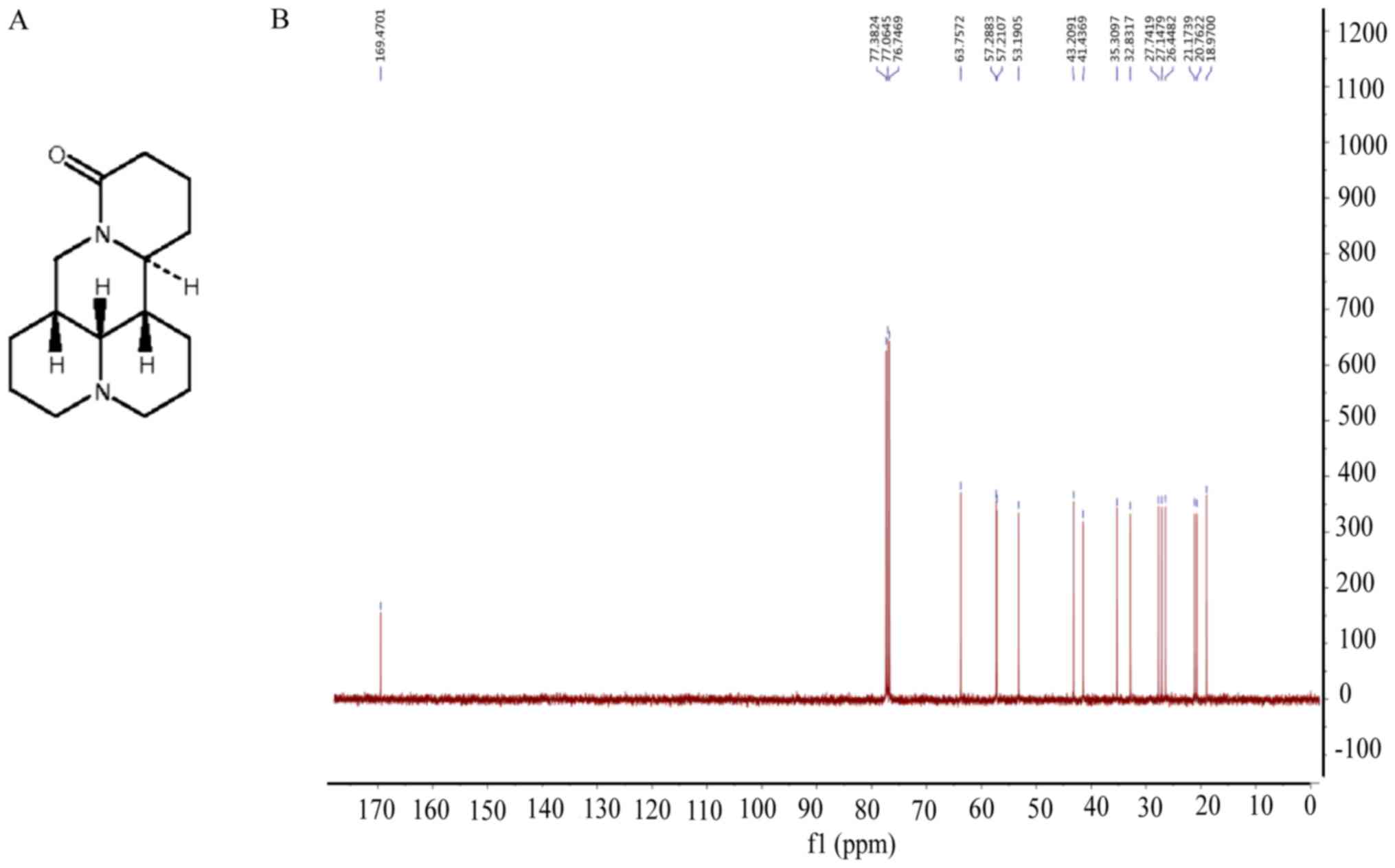
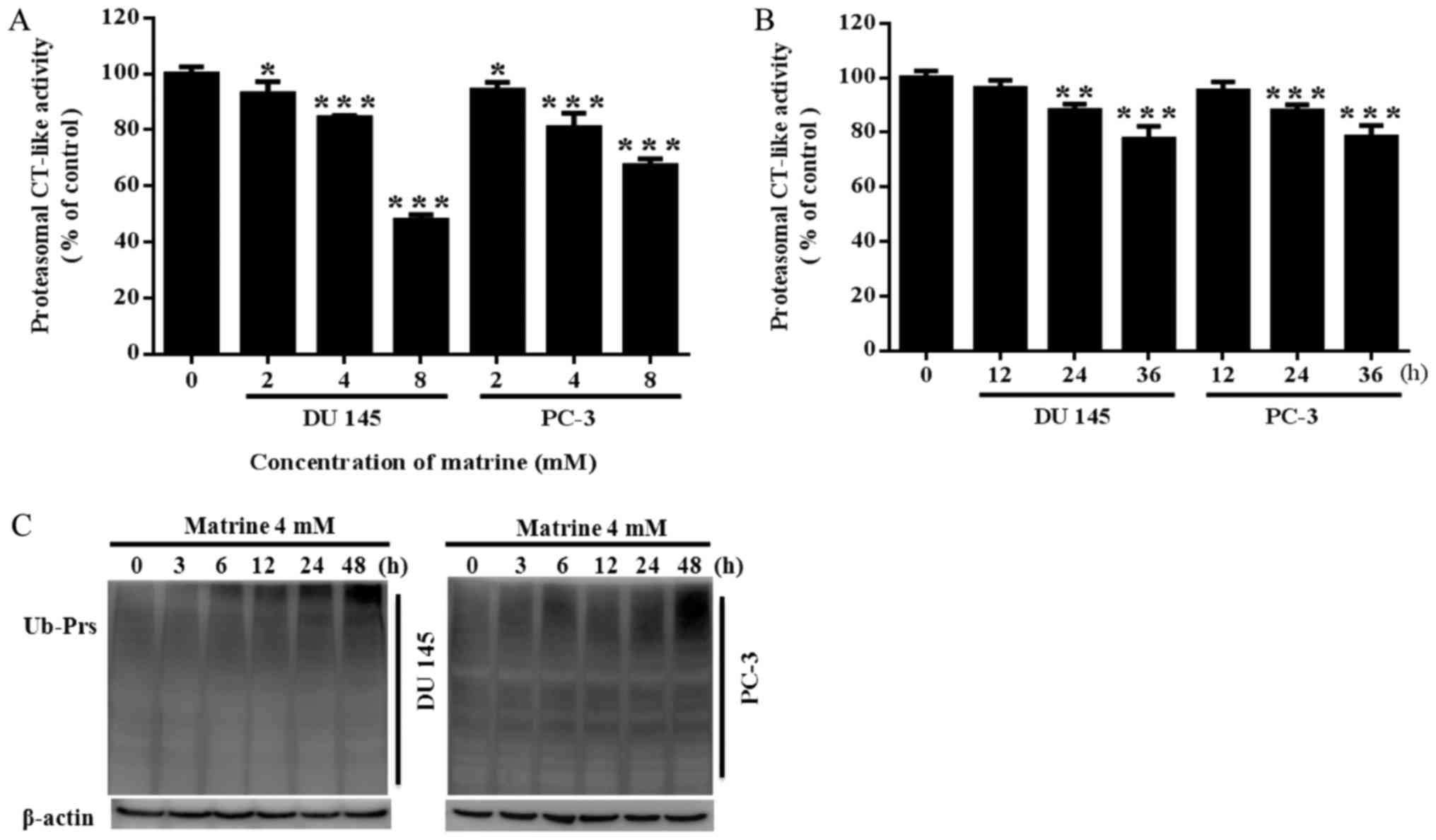
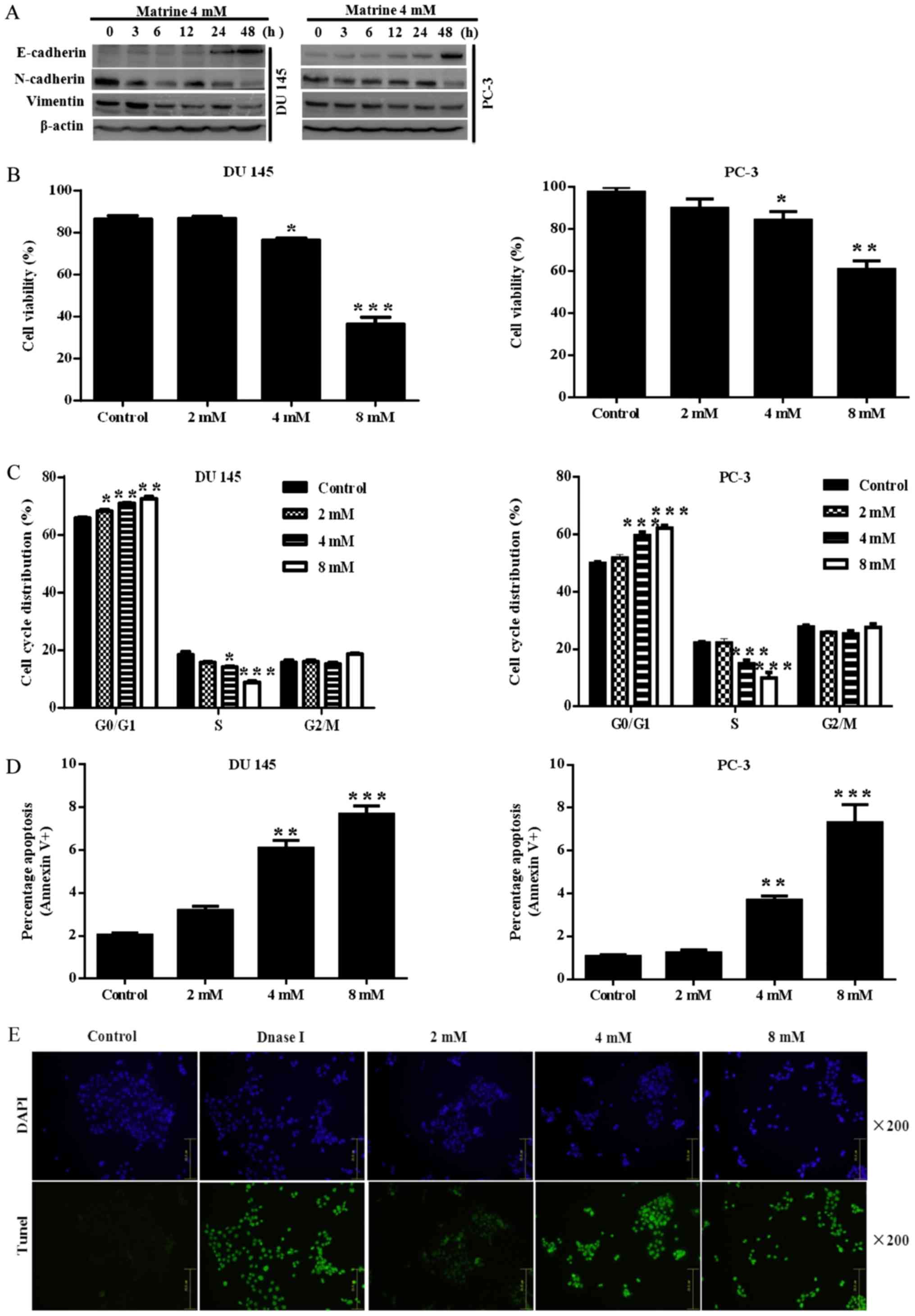
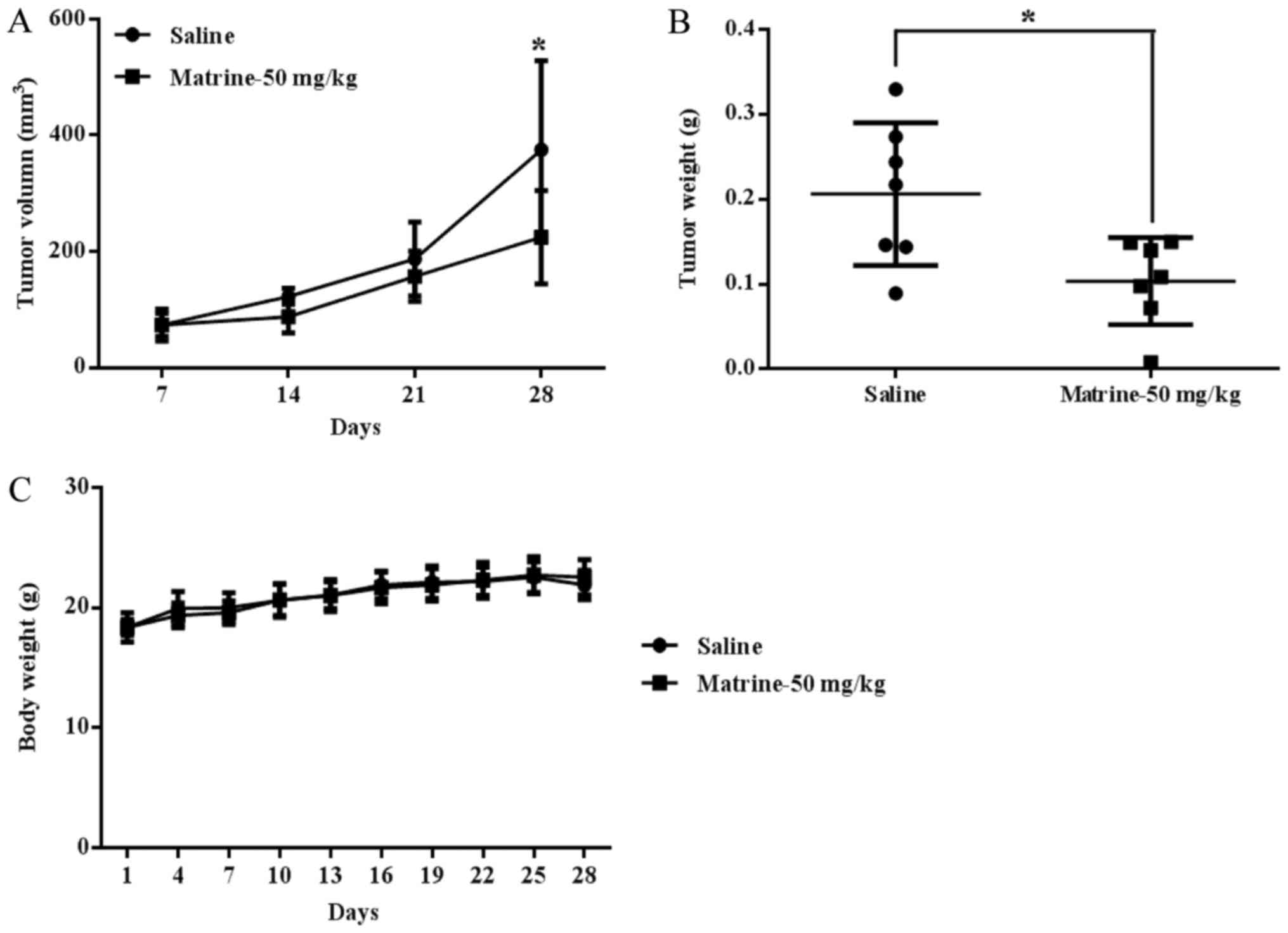
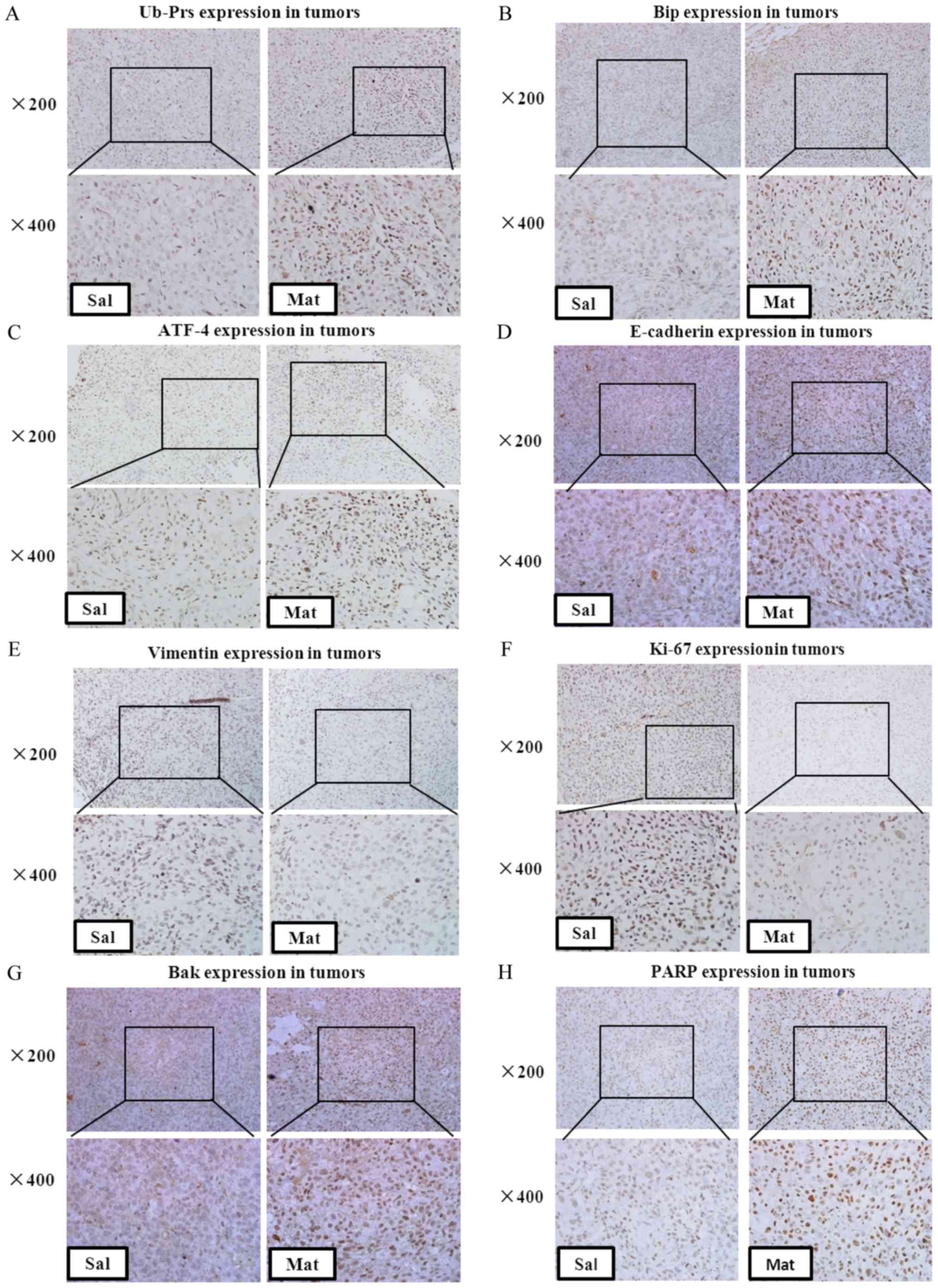
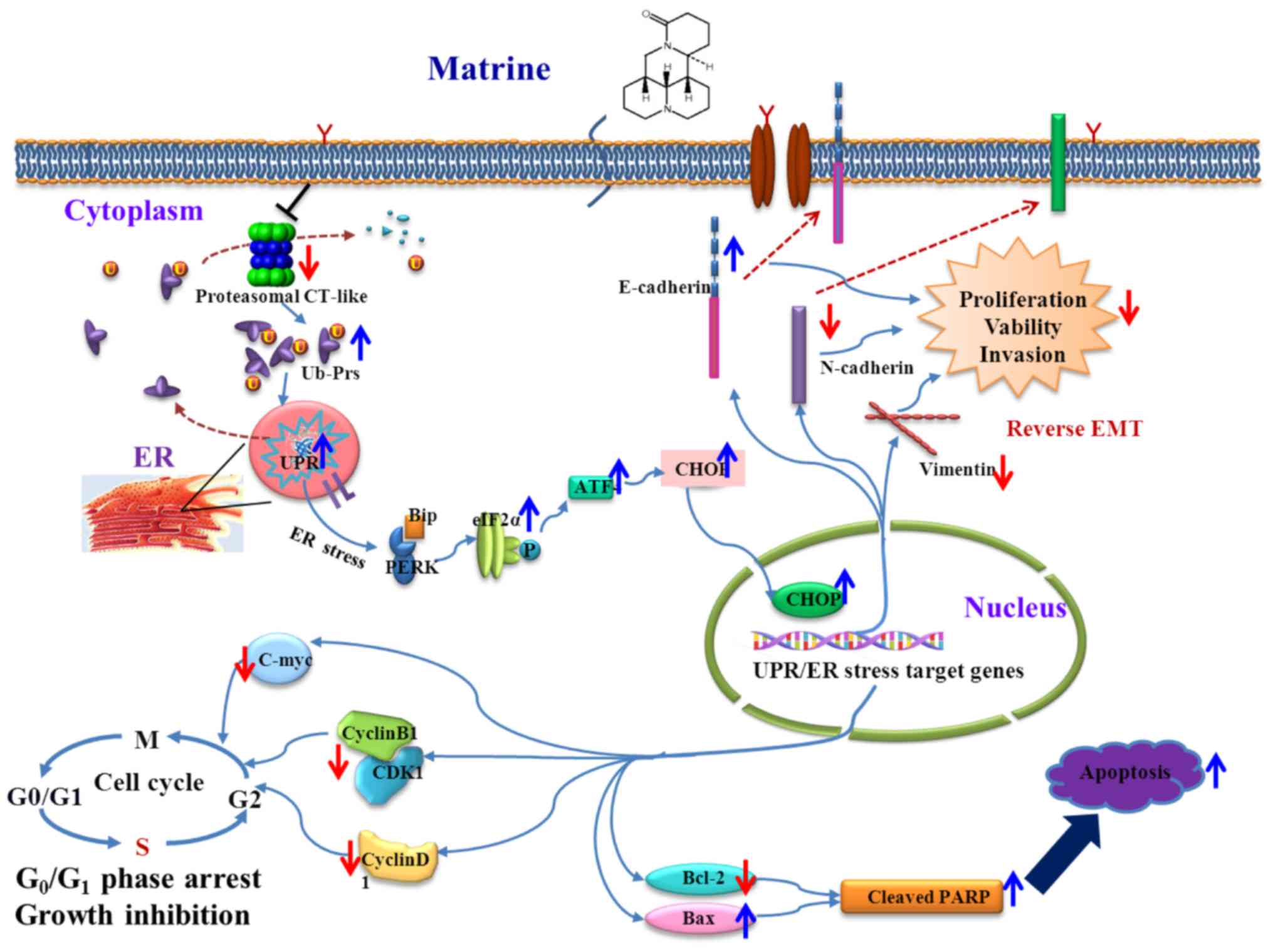
|
1
|
Xu S, Zhou W, Ge J and Zhang Z:
Prostaglandin E2 receptor EP4 is involved in the cell growth and
invasion of prostate cancer via the cAMP-PKA/PI3K-Akt signaling
pathway. Mol Med Rep. 17:4702–4712. 2018.PubMed/NCBI
|
|
2
|
Zhu Y, Shao S, Pan H, Cheng Z and Rui X:
MicroRNA-136 inhibits prostate cancer cell proliferation and
invasion by directly targeting mitogen-activated protein kinase
kinase 4. Mol Med Rep. 17:4803–4810. 2018.PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie D, Gore C, Liu J, Pong RC, Mason R,
Hao G, Long M, Kabbani W, Yu L, Zhang H, et al: Role of DAB2IP in
modulating epithelial-to-mesenchymal transition and prostate cancer
metastasis. Proc Natl Acad Sci USA. 107:2485–2490. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen X, Cheng H, Pan T, Liu Y, Su Y, Ren
C, Huang D, Zha X and Liang C: mTOR regulate EMT through RhoA and
Rac1 pathway in prostate cancer. Mol Carcinog. 54:1086–1095. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Voutsadakis IA: The ubiquitin-proteasome
system and signal transduction pathways regulating Epithelial
Mesenchymal transition of cancer. J Biomed Sci. 19:672012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adams J, Palombella VJ, Sausville EA,
Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S and
Elliott PJ: Proteasome inhibitors: A novel class of potent and
effective antitumor agents. Cancer Res. 59:2615–2622.
1999.PubMed/NCBI
|
|
12
|
Mani A and Gelmann EP: The
ubiquitin-proteasome pathway and its role in cancer. J Clin Oncol.
23:4776–4789. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu WK, Cho CH, Lee CW, Wu K, Fan D, Yu J
and Sung JJ: Proteasome inhibition: A new therapeutic strategy to
cancer treatment. Cancer Lett. 293:15–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang T, Zhu Y, Fang X, Chi Y, Kitamura M
and Yao J: Gap junctions sensitize cancer cells to proteasome
inhibitor MG132-induced apoptosis. Cancer Sci. 101:713–721. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seeger JM, Schmidt P, Brinkmann K, Hombach
AA, Coutelle O, Zigrino P, Wagner-Stippich D, Mauch C, Abken H,
Krönke M and Kashkar H: The proteasome inhibitor bortezomib
sensitizes melanoma cells toward adoptive CTL attack. Cancer Res.
70:1825–1834. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tagoug I, Jordheim LP, Herveau S, Matera
EL, Huber AL, Chettab K, Manié S and Dumontet C: Therapeutic
enhancement of ER stress by insulin-like growth factor I sensitizes
myeloma cells to proteasomal inhibitors. Clin Cancer Res.
19:3556–3566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Orlowski M and Wilk S: Catalytic
activities of the 20 S proteasome, a multicatalytic proteinase
complex. Arch Biochem Biophys. 383:1–16. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arendt CS and Hochstrasser M:
Identification of the yeast 20S proteasome catalytic centers and
subunit interactions required for active-site formation. Proc Natl
Acad Sci USA. 94:7156–7161. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dick TP, Nussbaum AK, Deeg M, Heinemeyer
W, Groll M, Schirle M, Keilholz W, Stevanović S, Wolf DH, Huber R,
et al: Contribution of proteasomal beta-subunits to the cleavage of
peptide substrates analyzed with yeast mutants. J Biol Chem.
273:25637–25646. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lopes UG, Erhardt P, Yao R and Cooper GM:
p53-dependent induction of apoptosis by proteasome inhibitors. J
Biol Chem. 272:12893–12896. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi G, Chen D, Zhai G, Chen MS, Cui QC,
Zhou Q, He B, Dou QP and Jiang G: The proteasome is a molecular
target of environmental toxic organotins. Environ Health Perspect.
117:379–386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Long Y, Lin XT, Zeng KL and Zhang L: Long
Y, Lin XT, Zeng KL and Zhang L: Efficacy of intramuscular matrine
in the treatment of chronic hepatitis B. Hepatobiliary Pancreat Dis
Int. 3:69–72. 2004.PubMed/NCBI
|
|
23
|
Liu JY, Hu JH, Zhu QG, Li FQ, Wang J and
Sun HJ: Effect of matrine on the expression of substance P receptor
and inflammatory cytokines production in human skin keratinocytes
and fibroblasts. Int Immunopharmacol. 7:816–823. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu P, Liu Q, Liu K, Yagasaki K, Wu E and
Zhang G: Matrine suppresses breast cancer cell proliferation and
invasion via VEGF-Akt-NF-kappaB signaling. Cytotechnology.
59:219–229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu T, Song Y, Chen H, Pan S and Sun X:
Matrine inhibits proliferation and induces apoptosis of pancreatic
cancer cells in vitro and in vivo. Biol Pharm Bull. 33:1740–1745.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nava MB, Rocco N, Catanuto G, Falco G,
Capalbo E, Marano L, Bordoni D, Spano A and Scaperrotta G: Impact
of contra-lateral breast reshaping on mammographic surveillance in
women undergoing breast reconstruction following mastectomy for
breast cancer. Breast. 24:434–439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Obeng EA, Carlson LM, Gutman DM,
Harrington WJ Jr, Lee KP and Boise LH: Proteasome inhibitors induce
a terminal unfolded protein response in multiple myeloma cells.
Blood. 107:4907–4916. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Walter P and Ron D: The unfolded protein
response: From stress pathway to homeostatic regulation. Science.
334:1081–1086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hetz C, Chevet E and Harding HP: Targeting
the unfolded protein response in disease. Nat Rev Drug Discov.
12:703–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan P, Griffith OL, Agboke FA, Anur P, Zou
X, McDaniel RE, Creswell K, Kim SH, Katzenellenbogen JA, Gray JW
and Jordan VC: c-Src modulates estrogen-induced stress and
apoptosis in estrogen-deprived breast cancer cells. Cancer Res.
73:4510–4520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huber AL, Lebeau J, Guillaumot P, Pétrilli
V, Malek M, Chilloux J, Fauvet F, Payen L, Kfoury A, Renno T, et
al: p58(IPK)-mediated attenuation of the proapoptotic PERK-CHOP
pathway allows malignant progression upon low glucose. Mol Cell.
49:1049–1059. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jwa M and Chang P: PARP16 is a
tail-anchored endoplasmic reticulum protein required for the PERK-
and IRE1α-mediated unfolded protein response. Nat Cell Biol.
14:1223–1230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McCullough KD, Martindale JL, Klotz LO, Aw
TY and Holbrook NJ: Gadd153 sensitizes cells to endoplasmic
reticulum stress by down-regulating Bcl2 and perturbing the
cellular redox state. Mol Cell Biol. 21:1249–1259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gallerne C, Prola A and Lemaire C: Hsp90
inhibition by PU-H71 induces apoptosis through endoplasmic
reticulum stress and mitochondrial pathway in cancer cells and
overcomes the resistance conferred by Bcl-2. Biochim Biophys Acta.
1833:1356–1366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang J, Wang H, Wang X, Zhao Y, Zhao D,
Wang C, Li Y, Yang Z, Lu S, Zeng Q, et al: Molecular mechanisms of
Polyphyllin I-induced apoptosis and reversal of the
epithelial-mesenchymal transition in human osteosarcoma cells. J
Ethnopharmacol. 170:117–127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shiota M, Bishop JL, Nip KM, Zardan A,
Takeuchi A, Cordonnier T, Beraldi E, Bazov J, Fazli L, Chi K, et
al: Hsp27 regulates epithelial mesenchymal transition, metastasis,
and circulating tumor cells in prostate cancer. Cancer Res.
73:3109–3119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dromparis P, Paulin R, Stenson TH, Haromy
A, Sutendra G and Michelakis ED: Attenuating endoplasmic reticulum
stress as a novel therapeutic strategy in pulmonary hypertension.
Circulation. 127:115–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Welch WJ and Brown CR: Influence of
molecular and chemical chaperones on protein folding. Cell Stress
Chaperones. 1:109–115. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rastogi N and Mishra DP: Therapeutic
targeting of cancer cell cycle using proteasome inhibitors. Cell
Div. 7:262012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nauseef JT and Henry MD:
Epithelial-to-mesenchymal transition in prostate cancer: Paradigm
or puzzle? Nat Rev Urol. 8:428–439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
King TE Jr, Pardo A and Selman M:
Idiopathic pulmonary fibrosis. Lancet. 378:1949–1961. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
van Rooij E and Olson EN: MicroRNA
therapeutics for cardiovascular disease: opportunities and
obstacles. Nat Rev Drug Discov. 11:860–872. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nicolopoulou A, Cortina KS, Ilgaz H, Cates
CB and de Sá AB: Using a narrative- and play-based activity to
promote low-income preschoolers' oral language, emergent literacy,
and social competence. Early Child Res Q. 31:147–162. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Catov JM, Abatemarco D, Althouse A, Davis
EM and Hubel C: Patterns of gestational weight gain related to
fetal growth among women with overweight and obesity. Obesity
(Silver Spring). 23:1071–1078. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Keating SE, Hackett DA, Parker HM,
O'Connor HT, Gerofi JA, Sainsbury A, Baker MK, Chuter VH, Caterson
ID, George J and Johnson NA: Effect of aerobic exercise training
dose on liver fat and visceral adiposity. J Hepatol. 63:174–182.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mortensen DS, Fultz KE, Xu S, Xu W,
Packard G, Khambatta G, Gamez JC, Leisten J, Zhao J, Apuy J, et al:
CC-223, a potent and selective inhibitor of mTOR kinase: In vitro
and in vivo characterization. Mol Cancer Ther. 14:1295–1305. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kruse CR, Nuutila K, Lee CC, Kiwanuka E,
Singh M, Caterson EJ, Eriksson E and Sørensen JA: The external
microenvironment of healing skin wounds. Wound Repair Regen.
23:456–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Woolcott OO, Richey JM, Kabir M, Chow RH,
Iyer MS, Kirkman EL, Stefanovski D, Lottati M, Kim SP, Harrison LN,
et al: High-fat diet-induced insulin resistance does not increase
plasma anandamide levels or potentiate anandamide insulinotropic
effect in isolated canine islets. PLoS One. 10:e01235582015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Merlino A, Caterino M, Krauss Russo I and
Vergara A: Missing gold atoms in lysozyme crystals used to grow
gold nanoparticles. Nat Nanotechnol. 10:2852015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zimarino M, Ricci F, Romanello M, Di
Nicola M, Corazzini A and De Caterina R: Complete myocardial
revascularization confers a larger clinical benefit when performed
with state-of-the-art techniques in high-risk patients with
multivessel coronary artery disease: A meta-analysis of randomized
and observational studies. Catheter Cardiovasc Interv. 87:3–12.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yan H, Catania C and Bazan GC:
Membrane-intercalating conjugated oligoelectrolytes: Impact on
bioelectrochemical systems. Adv Mater. 27:2958–3973. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Niemczyk NA, Catov JM, Barinas-Mitchell E,
McClure CK, Roberts JM, Tepper PG and Sutton-Tyrrell K: Nulliparity
is associated with less healthy markers of subclinical
cardiovascular disease in young women with overweight and obesity.
Obesity (Silver Spring). 23:1085–1091. 2015. View Article : Google Scholar : PubMed/NCBI
|