Introduction
Liver transplantation (LT) is the only effective
means of treating end-stage liver disease; however, advances in
liver transplantation are restricted by the shortage of donors
(1). Numerous livers donated after
cardiac death (DCD) are used in clinical practice (2,3),
however DCD livers are subject to prolonged warm ischemic injury,
which is detrimental to liver function and may affect prognosis
following transplantation (4–7).
Therefore, further study into improving the quality of the DCD
livers is required. Increasing evidence in liver transplant
research has suggested that hypothermic machine perfusion (HMP) may
be more beneficial for the quality of DCD livers than cold storage
(CS) (8,9); however, the specific mechanism
underlying HMP requires further investigation.
Nuclear factor erythroid 2-related factor 2 (Nrf2)
is a transcription factor associated with various intracellular
signaling pathways and is a sensor of oxidative stress; thus, Nrf2
serves an important role in the main defense mechanisms induced by
cellular oxidative stress (10,11).
Under physiological conditions, Nrf2 can achieve a stable balance
in combination with Kelch-like ECH-associated protein-1 (Keap1),
which can mediate the ubiquitination of the Nrf2/Keap1 complex
(12–14). Oxidative and/or electrophilic
stimuli may induce the dissociation of Nrf2 from Keap1. Nrf2 can
then rapidly translocate to the nucleus to function as a strong
transcriptional activator of the antioxidant response element (ARE)
regulating the transcription of genes, including heme oxygenase-1
(HO-1), glutathione-S-transferase-1 (GST-1), NAD(P)H:quinine
oxidoreductase 1 (NQO1) and glutamate cysteine ligase (GCL)
(15). Numerous studies have
demonstrated that the Nrf2-ARE signaling pathway serves an
important role in ischemia-reperfusion injury (IRI) (16–18).
In addition, Nrf2 can be activated by steady laminar flow (19,20).
Based on previous research (16–21),
the present study hypothesized that HMP may increase the
transcription of ARE-response genes via the binding of Nrf2, and
thus reduce IRI to DCD livers by attenuating oxidative stress; the
underlying mechanism was also investigated in the present
study.
Materials and methods
Ethics statement
The present study was conducted according to the
Experimental Animal Regulations of the People's Republic of China
and the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health and the Guide for the Care and Use of
Laboratory Animals of the USA (22), ensuring that all animals received
humane care. The present study was approved by the Ethics Committee
of Wuhan University (Wuhan, China).
Animals and experimental design
A total of 18 adult male Sprague-Dawley rats (age, 8
weeks; body weight, 250±10 g) were purchased from the Experimental
Animal Culture Center of Hubei Centers for Disease Control (Hubei,
China), and were maintained in the Animal Experimental Center of
Zhongnan Hospital of Wuhan University (Wuhan, China). The rats were
fed standard chow and water, and housed under standard experimental
conditions (temperature: 20–25°C, humidity: 50–70%) under a 12 h
light/dark cycle. To simulate DCD liver transplantation, 30 min of
warm ischemia was conducted in livers (n=12, CS and HMP groups)
in situ, followed by a rewarming period of 15 min prior to
being connected to the isolated perfused rat liver device (IPRL).
To investigate the protective effects of HMP on the DCD livers, the
extent of graft injury in healthy livers (non-DCD, no IRI) was
compared with DCD livers preserved by either CS or HMP (described
below). Following graft preservation, to simulate the period of
rewarming during re-implantation, all the livers were left
untouched on a petri dish at room temperature for ~15 min prior to
reperfusion (23).
Then, in order to analyze reperfusion injury, livers
were re-perfused in the isolated perfusion rat liver model for 1 h
following graft preservation within the CS and HMP groups, and the
same is true for the control group. For this purpose, the following
experimental groups were selected: i) Control group (n=6), livers
without warm ischemia and subsequent underwent 1 h reperfusion
prior to sample collection; ii) CS group (n=6), DCD livers that
underwent CS for 3 h, followed by 1 h reperfusion in vitro
and the iii) HMP group (n=6), in which DCD livers were connected to
the HMP system. HMP was performed via the portal vein for 3 h,
followed by 1 h reperfusion in vitro.
Modeling procedure
Rats were anesthetized via an intraperitoneal
injection of 1% sodium pentobarbital (30 mg/kg; Sinopharm Chemical
Reagent Co., Ltd., Shanghai, China). A midline incision was
conducted to provide entry into the abdominal cavity. The liver was
carefully separated from the attached round ligament. Subsequently,
the common bile duct was cannulated using a guided epidural tube
(Jiangsu Changfeng Medical Industry, Co., Ltd. (Jiangsu, China) to
collect bile during reperfusion. In the experimental groups, DCD
was induced by hypoxia via an incision of the diaphragm without
portal clamping prior to heparinization, as described below. The
onset of in situ-warm ischemia was determined to be the
point of the cardiac arrest, which was maintained for 30 min at
29±1.5°C. The control group did not experience the warm ischemia
and the other operations were the same as the experimental groups.
Subsequently, the hepatic artery was ligated and then 2 ml saline
containing 100 IU heparin (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China) was injected via the right iliac vein. A
total of 20 ml (4°C) histidine-tryptophan-ketoglutarate (HTK; Dr.
Franz Köhler Chemie GmbH, Bensheim, Germany) solution was used to
rinse the liver via the portal vein, which was cannulated using a
self-made polyethylene (PE) catheter (outer diameter, 2.1 mm, inner
diameter, 1.8 mm) (24). Finally,
the liver was harvested and a PE catheter (internal diameter, 3 mm)
was used to cannulate the suprahepatic inferior vena cava for
collection of hepatic effluent.
CS model establishment
Following the modeling procedure, the liver was
stored in the HTK solution at 0–4°C for 3 h to maintain a static
state without any treatment. Following preservation, the CS group
was simulated the period of rewarming for a 15 min and then
connected to the IPRL, which is the reperfusion device used to
assessment of IRI severity against livers (described below).
HMP and the IPRL model system
HMP and IPRL was performed as previously described
(25,26) with certain modifications. The HTK
solution served as a perfusate for HMP; the devices employed for
perfusion were maintained in an ice water solution (0–4°C).
Following collection of the livers, the samples from the HMP group
were connected to the perfusion device and perfused via the portal
vein. The perfusion flow was maintained at 0.23 ml/min/g (23). The perfusate was oxygenated with
air and recirculated for 3 h (perfusate volume, 100 ml). Following
preservation, the caudate lobe of the rat livers of the CS and HMP
groups was ligated and harvested to obtain samples prior to
reperfusion. The remaining rat liver tissues in the three groups
were then weighed and connected via the portal vein to the IPRL
system for reperfusion. IPRL is an in vitro system that
simulates physiological and pathophysiological conditions of
reperfusion in liver transplantation and is often used as a tool
for the assessment of IRI severity against livers (27).
The same perfusion device was used for HMP, as well
as for the 1 h reperfusion period (n=12, CS and HMP groups). A
detailed description of the HMP and IPRL system is given in as
described in a previous study (26). Krebs-Henseleit buffer (Macgene™
M&C Genetechnolgy, Beijing, China) with 4% dextran was used as
a perfusate for reperfusion (28).
The temperature of the perfusate was maintained at 36–37°C during
reperfusion and oxygenated to maintain PO2 >500 mmHg
under the effect of mixed gas (95% oxygen and 5% carbon dioxide).
The flow of portal venous perfusion was maintained at 3 ml/min/g
(29) and recirculated for 1 h
(perfusate volume, 250 ml).
Assessment of IRI using the IPRL
system
During reperfusion, which was performed for 1 h,
intrahepatic resistance (IHR) was recorded by the portal pressure
and portal flow and the perfusate was collected per 15 min.
Intrahepatic resistance was calculated according to the following
formula: Intrahepatic resistance (mmHg/ml/min/g liver)=portal
pressure (10.3 mm Hg)/portal flow (ml/min/g liver) (30). Hepatic effluent was obtained from
the perfusion fluid via the PE catheter every 15 min. Samples were
centrifuged at 14,000 × g and 4°C for 5–10 min and the supernatant
was collected and stored at −80°C prior to the determination of
aspartate aminotransferase (AST) and alanine aminotransferase (ALT)
activities. The enzyme activities were assessed using ALT (cat. no.
C009-2), AST (cat. no. C010-2) and LDH assay kits (cat. no. A020-2;
all Nanjing Jiancheng Bioengineering Institute), according to the
manufacturer's protocol. As the density of bile was equal to the
water (23,31), bile production was measured at 60
min by weighing the guided epidural tube in which bile was
collected from the common bile duct. Then, the bile flow was
gravimetrically estimated and expressed as µl/h/g liver (26).
Superoxide dismutase (SOD) activity
and malondialdehyde (MDA) content
Frozen liver samples were lysed with 0.05 M Tris.
HCl (Beijing Biotopped Science & Technology Co., Ltd., Being,
China) extraction buffer on ice. The cell lysates were centrifuged
at 4°C 12,000 × g for 10 min. The resulting cell lysates were used
to assess the SOD activity and MDA content. To measure total SOD
activity, a SOD kit (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China) was employed according to the manufacturer's
protocols. MDA content was assessed with an MDA assay kit (Nanjing
Jiancheng Bioengineering Institute) based on the products of
membrane lipid peroxidation, which are important indicators of
oxidative damage during hepatic IRI.
ATP extraction and measurement
The hepatic concentration of ATP served as an
indicator of the energy status of grafts following 1 h of ex
situ reperfusion. The ATP content of the tissue was measured
using an ATP assay kit (Nanjing Jiancheng Bioengineering Institute)
according to the manufacturer's protocols. ATP levels were
expressed as µmol/g protein.
Histological analysis
Following reperfusion, about 0.25 g liver samples
were fixed with 10% buffered formalin for 24 h at room temperature
(pH=7.2; cat. no. G2161; Beijing Solarbio Science & Technology,
Co., Ltd., Beijing, China), embedded in paraffin and cut into 5-µm
sections for histological analysis via hematoxylin-eosin staining
(H&E; hematoxylin staining for 5–15 min and eosin staining for
1–3 min; all performed at room temperature). Sections were analyzed
under a confocal microscope (magnification, ×200; Nikon A1R/A1;
Nikon Corporation, Tokyo, Japan) and images were obtained. A total
of 6 fields of view per section were randomly selected for the
assessment of liver damage. Numerical assessment of liver damage
was conducted according to the histological criteria for assessment
of liver damage (32).
Immunohistochemistry of Nrf2
Following reperfusion, a portion of the livers were
fixed with 10% buffered formalin for 24 h at room temperature
(pH=7.2; cat. no. G2161; Beijing Solarbio Science & Technology,
Co., Ltd., Beijing, China), and then embedded in paraffin, sliced,
dewaxed (Dewaxing was routinely performed at 60°C for 20 min, and
immediately xylene 1–3 for 10 min, respectively. However certain
sections prepared on the day could be treated at 60°C for 3–4 h),
and hydrated conventionally using an ethanol gradient (from high to
low). The tissues were cut into 4-µm sections and mounted on glass
slides. After 30 min of blocking at room temperature with 5% bovine
serum albumin (Beijing Solarbio Technology Co., Ltd., Beijing,
China), the samples were incubated with rabbit anti-Nrf2 antibody
(1:1,000; cat. no. 16396-1-AP; Wuhan Sanying Biotechnology, Wuhan,
China) overnight at 4°C. The samples were then incubated for 1 h at
room temperature with a horseradish peroxidase-conjugated goat
anti-rabbit IgG (1:3,000; cat. no. GB23303; Wuhan Goodbio
Technology Co., Ltd., Wuhan, China). Subsequently, the samples were
incubated with 3,3′-diaminobenzidine chromogen (DAB; Maixin-Bio
Ltd.) at room temperature for 5 min, and then blocked on a
coverslip. Any brown and yellow staining was considered to indicate
positive Nrf2 expression, as visualized under a light microscope
(magnification, ×200, Leica DM2000; Leica Microsystems GmbH,
Wetzlar, Germany). A total of 5 visual fields were randomly
selected for analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the liver specimens
using TRIzol® reagent (Thermo Fisher Scientific Inc.,
Waltham, MA, USA) according to the manufacturer's protocols. RNA
was reverse transcribed into cDNA using the Easy Script One-Step
gDNA Removal and cDNA Synthesis Super Mix (Beijing TransGen
Biotech, Co., Ltd., Beijing, China). Total RNA (1 µg), Random
primer (0.1 µg/µl), 2X ES Reaction Mix (10 µl), RI Enzyme Mix (1
µl), gDNA Remover (1 µl) were employed; the solution was made up to
20 µl with water (RNase-free). RT reactions were performed under
the following conditions: 42°C for 1 h and 75°C for 5 min. The
primers were synthesized by Shanghai ShineGene Molecular Biotech,
Inc. (Shanghai, China; Table I).
qPCR analysis was performed with the SYBR® Select Master
Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) in a
StepOnePlus Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) to determine the mRNA expression levels of Nrf2,
HO-1, NQO1, GST-1, GCL and β-actin. The thermocycling conditions
were as follows: 50°C for 2 min, 95°C for 10 min, followed by 40
cycles of 95°C for 10 sec and 60°C for 30 sec. Expression levels
were normalized to β-actin, which was measured on the same plate;
the differences were calculated via the 2−ΔΔCq method
(33,34).
 | Table I.Rat primer sequences used for reverse
transcription-quantitative-polymerase chain reaction. |
Table I.
Rat primer sequences used for reverse
transcription-quantitative-polymerase chain reaction.
| Gene | Sequence
(5′-3′) |
|---|
| NRF2 |
|
| F |
GAGATATACGCAGGAGAGGG |
| R |
CTTTTCAGAAGATGGAGGTTT |
| HO-1 |
|
| F |
GAAGGCTTTAAGCTGGTGATG |
| R |
GGCTGGTGTGTAAGGGATGG |
| NQO1 |
|
| F |
GGCTGGTTTGAGAGAGTGCT |
| R |
ACGTTCATGTCCCCGTGG |
| GST-1 |
|
| F |
CACAGAGACACAGCACAGC |
| R |
CCTTCCACCTCCAAAACAG |
| GCL |
|
| F |
GCAGCTCATTGGTTCATCT |
| R |
TCGTCCCTTCAAAGTCTTT |
| β-actin |
|
| F |
CCCTGGCTCCTAGCACCAT |
| R |
CACAGAGTACTTGCGCTCAGGA |
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick-end labeling
(TUNEL) assay
Apoptosis was determined via a TUNEL assay (One-Step
TUNEL Apoptosis assay kit, Beyotime Institute of Biotechnology,
Haimen, China) according to the manufacturer's protocols. Briefly,
4-µm thick paraffin sections, which contained the liver tissues
were deparaffinized and hydrated, then treated with proteinase K
for 20 min and subsequently incubated with a mixture of fluorescent
labeling solution and TdT enzyme at 37°C for 1 h in a humidified
atmosphere. The samples were washed in 1XPBS and mounted in
mounting media containing DAPI at room temperature for 10 min
without the light. Blue ray was chosen as the exciting light, the
wavelength is 420–485 nm. GFP was excited and emitted 515 nm green
fluorescence. Liver cells, which expressed GFP emitted green
fluorescent. The total hepatocytes and TUNEL-positive cells were
detected in 4–5 randomly selected fields (magnification, ×200) for
each liver section using a fluorescence microscope (Olympus X71;
Olympus Corporation, Tokyo, Japan). The apoptotic rate was
calculated according to the formula: Number of TUNEL positive
cells/number total cells × % (35,36).
The TUNEL positive cells were calculated by three different
authors.
Western blot analysis
Prior to and following reperfusion, all collected
liver tissues were rapidly dissected within 5 min and stored at
−80°C for cryopreservation. In order to extract the total proteins,
the liver tissue was thawed and homogenized in
radioimmunoprecipitation assay buffer containing a protease
inhibitor (cat. no. G2002; Wuhan Servicebio Co., Ltd., Wuhan,
China) and then centrifuged at 20,000 × g for 10 min at 4°C.
Following collection of the supernatants and measuring the total
protein concentration, 30 mg protein, which was calculated by the
bicinchoninic acid kit (cat. no. G2026; Wuhan Servicebio Co., Ltd.,
Wuhan, China) was separated by 10% SDS-PAGE and transferred to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked in 5% skimmed milk for 1 h at room
temperature. The blots were then incubated in 4°C for 12 h with the
following antibodies: Rabbit anti-Nrf2 antibody (1:1,000; cat. no.
16396-1-AP; Wuhan Sanying Biotechnology), rabbit anti-HO-1 antibody
(1:2,000; cat. no. 27282-1-AP Wuhan Sanying Biotechnology), rabbit
anti-NQO1 antibody (1:200; cat. no. bs-23407R Beijing Biosynthesis
Biotechnology Co., Ltd., Beijing, China), rabbit anti-Toll-like
receptor 4 (TLR4) antibody (cat. no. 19811-1-AP, 1:1,000; Wuhan
Sanying Biotechnology), rabbit anti-B-cell lymphoma 2 (Bcl-2)
antibody (1:1,000; cat. no. 10435-1-AP; Wuhan Sanying
Biotechnology), rabbit anti-Bcl-2-associated X (Bax) antibody
(1:1,000; cat. no. 60267-1-Ig; Wuhan Sanying Biotechnology) and
anti-β-actin antibody (1:3,000; ProteinTech Group, Inc., Chicago,
IL, USA). Following incubation for 1 h at room temperature with
horseradish peroxidase-conjugated goat anti-rabbit IgG (1:3,000;
cat. no. GB23303; Wuhan Goodbio Technology Co. Ltd., Wuhan, China),
the proteins were detected using an enhanced chemiluminescence
reagent (cat. no. G2020; Wuhan Servicebio Co., Ltd., Wuhan, China)
followed by exposure to X-ray film. Quantification of protein bands
was performed using ImageJ v1.42q software (National Institutes of
Health, Bethesda, MA, USA).
Statistical analysis
All data were analyzed using SPSS 16.0 statistical
software for Windows (SPSS, Inc., Chicago, IL, USA) by one-way
analysis of variance with Tukey's post-hoc test. All results are
presented as the mean ± standard deviation (n=6 for each
experiment). P<0.05 was considered to indicate a statistically
significant difference.
Results
HMP leads to decreased enzymatic
levels, reduced intrahepatic resistance and improved liver function
compared with the CS group
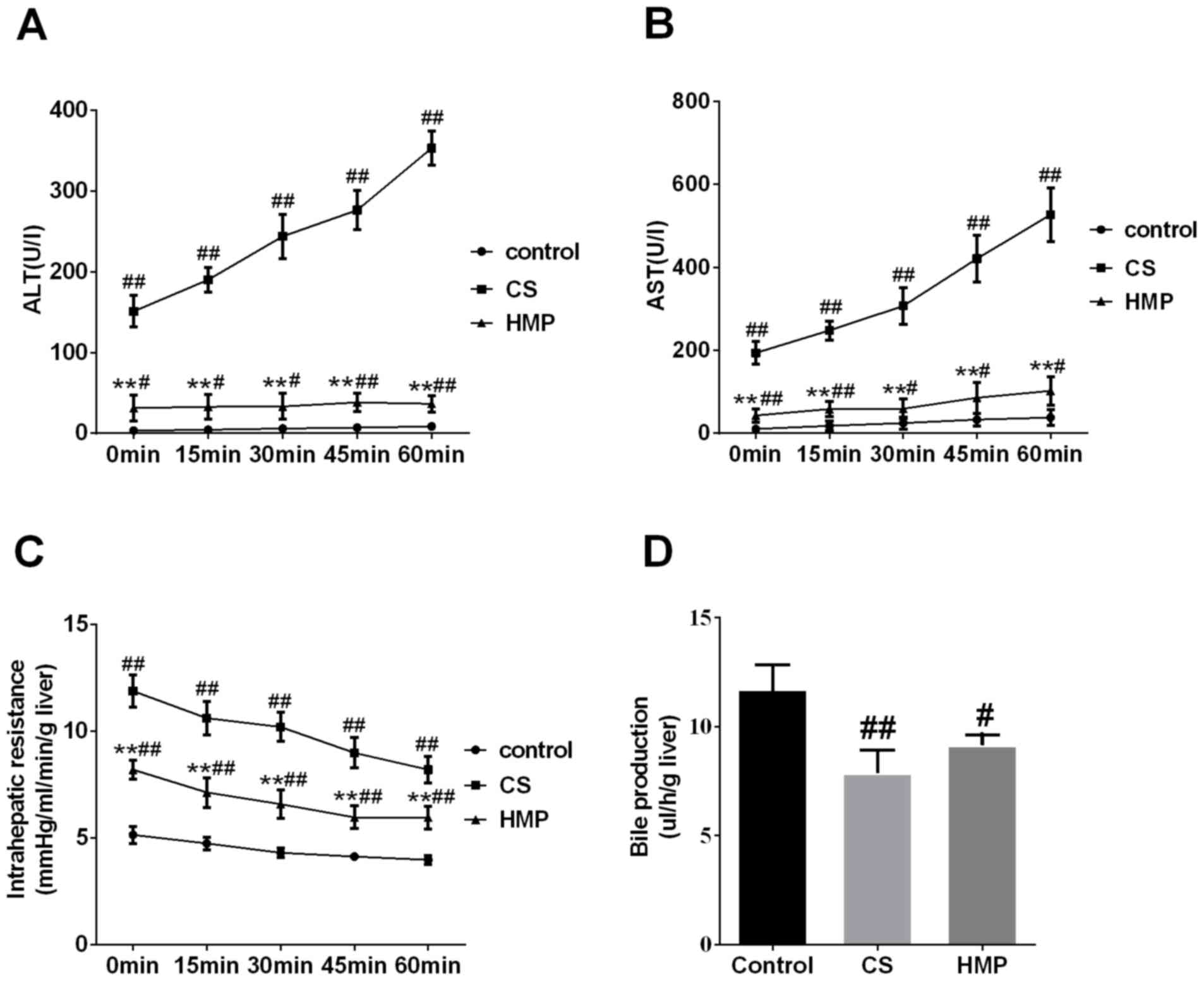
The activities of ALT and AST enzymes in the
perfusate were used to assess the severity of IRI to DCD livers. In
the present study, the levels of liver enzymes ALT and AST in the
perfusate of the CS group increased significantly compared with in
the control group (P<0.01; Fig. 1A
and B, respectively). The HMP group exhibited significantly
decreased levels of ALT and AST compared with in the CS group
(P<0.01; Fig. 1A and B).
Additionally, the intrahepatic resistance of livers in the control
group was low during reperfusion; however, in the CS group, it was
significantly higher compared with in the HMP group at each
analyzed time point (P<0.01; Fig.
1C). In addition, bile production in the CS group was
significantly lower compared with in the control group (P<0.01;
Fig. 1D). Furthermore, bile
production decreased in the HMP group; however, no significant
difference was observed compared with in the CS group (P>0.05).
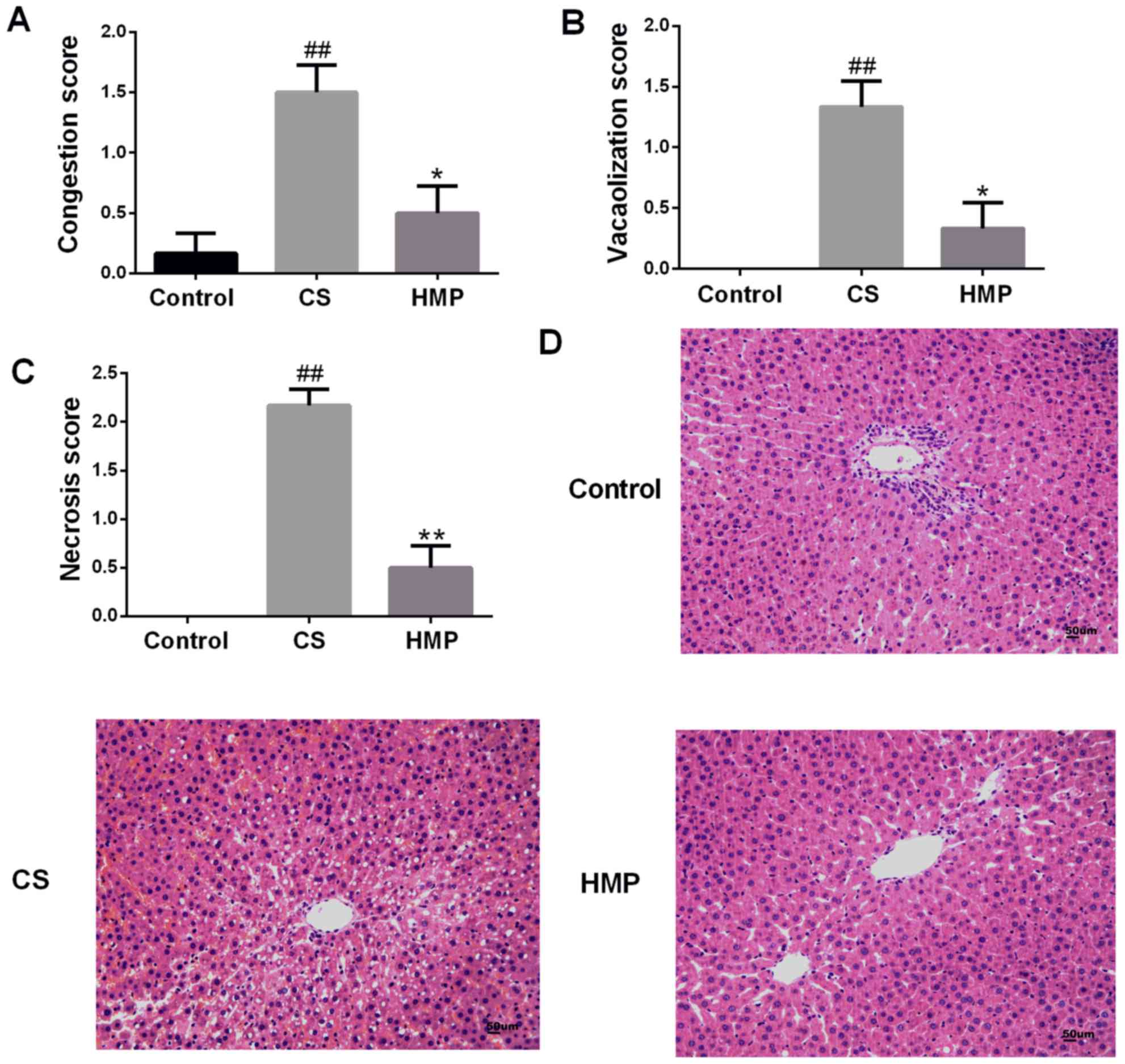
Visual and numerical analyses of the histological images indicated
that there were few observable abnormalities in the livers of the
control group (Fig. 2).
Conversely, in the CS group, histological analysis revealed
significant congestion of the hepatic sinusoid, vacuolar
degeneration and necrosis (P<0.01; Fig. 2A-C). Additionally, significantly
attenuated liver damage was observed in the HMP group compared with
in the CS group (P<0.05; Fig.
2A-C).
HMP reduces hepatic oxidative stress,
inflammation and apoptosis
The present study analyzed the typical biochemical
markers of oxidative stress to further investigate the effects of
HMP on the IRI livers. Oxidative stress has been considered to be
an important factor leading to IRI. The SOD activities and
expression levels of MDA of the tissues were presented in Fig. 3A and B. Compared with the control
group, SOD activity in the CS group was significantly decreased
(P<0.01), and the expression levels of MDA were significantly
increased (P<0.01). The results of the present study indicated
that HMP treatment may significantly decrease the MDA expression
levels (P<0.01) and significantly increase the SOD activity
(P<0.01); opposite trends to those observed in the CS group.
These results suggested that HMP may attenuate oxidative stress. In
addition, the ATP content in the liver was also measured to analyze
liver function. As presented in Fig.
3C, within the CS group, the ATP content was significantly
lower compared with the control group (P<0.01); however, HMP
treatment significantly increased the ATP content compared with in
the CS group. (P<0.01).
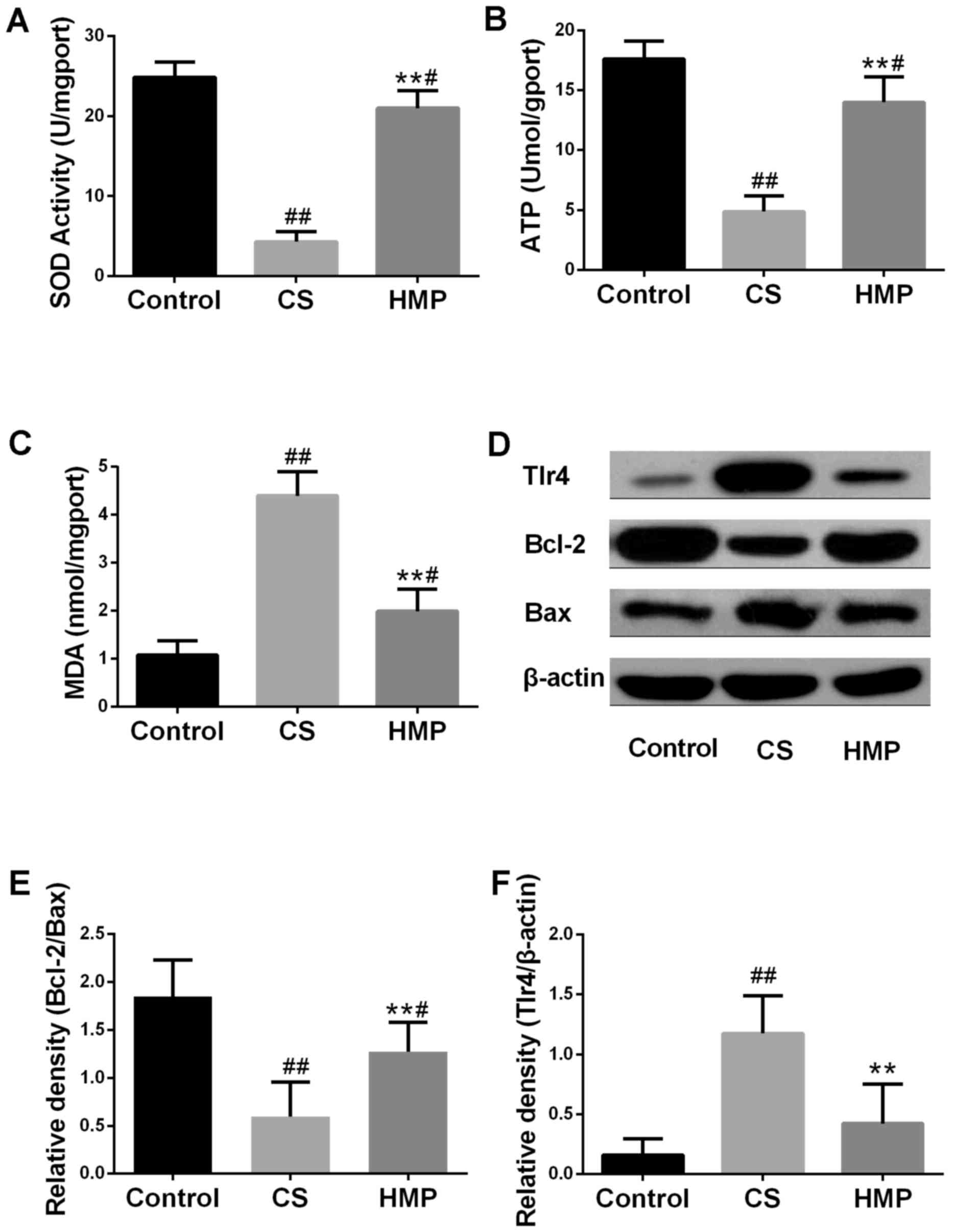 | Figure 3.Effects of HMP on the index of
inflammation and apoptosis. Following reperfusion, (A) SOD
activity, (B) MDA expression levels and (C) ATP content in the
liver tissues of each group were assessed using respective
commercial kits. (D) Protein expression levels of Tlr4, Bcl-2 and
Bax in the livers of each group were analyzed by western blotting.
β-actin served as an internal control. Compared with in the CS
group, the (E) Bcl-2/Bax ratio was significantly increased;
however, the expression levels of (F) TLR4 protein were decreased.
#P<0.05, ##P<0.01 vs. the control
group; **P<0.01 vs. the CS group. Bcl-2, B-cell lymphoma-2; Bax,
Bcl-2 associated X; CS, cold storage; DCD, donated after cardiac
death; HMP, hypothermic machine perfusion; MDA, malondialdehyde;
SOD, superoxide dismutase; TLR4, Toll like receptor 4. |
Oxidative stress can also induce hepatic
inflammation and apoptosis (37,38).
Therefore, the present study investigated the expression levels
apoptosis-associated proteins in the liver (Fig. 3D). The ratio of Bcl-2 to Bax is
also important in determining susceptibility of cells to apoptosis
(39). The overexpression of Bax
revealed that apoptosis was promoted in response to a
death-inducing signal, which suggested its role as an apoptosis
agonist (40). Bcl-2
overexpression has been associated with heterodimerization with Bax
and reduced levels of apoptosis (41). In addition, TLR4 has been reported
to be an important marker of inflammation (42). As presented in Fig. 3E, the ratio of Bcl-2 to Bax was
significantly downregulated in the CS group and significantly
upregulated in the HMP group (P<0.01). The protein expression
levels of TLR4 were significantly increased in the CS group and
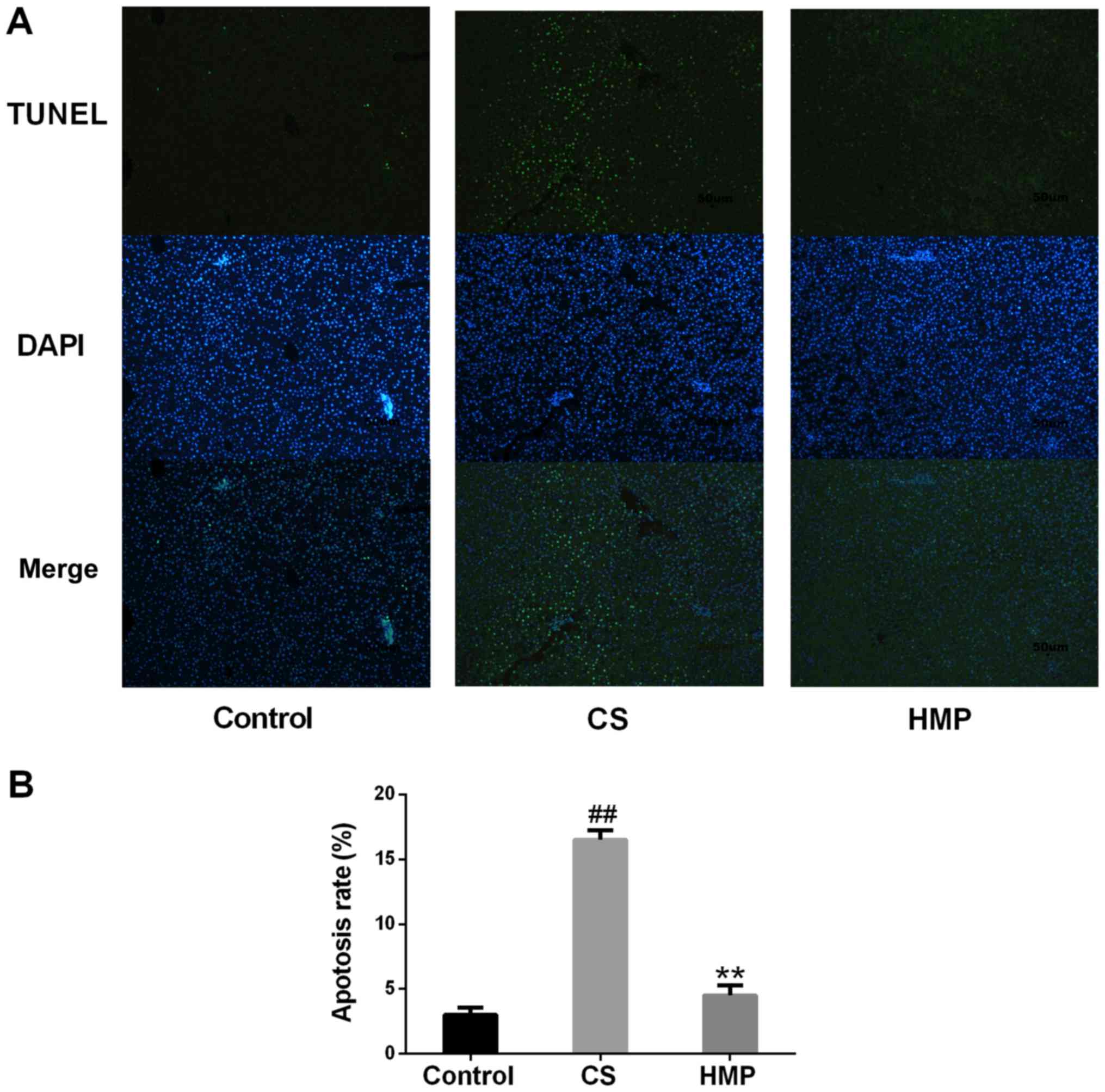
significantly decreased in the HMP group (P<0.01; Fig. 3F). Furthermore, the present study
revealed that, compared with the control group, the rate of
apoptosis in the CS group was significantly increased (P<0.01;
Fig. 4); however, treatment with
HMP was associated with significantly reduced rates of
apoptosis.
HMP activates the Keap1/Nrf2-ARE
antioxidant pathway in DCD rat livers
Nrf2 serves an important role in the main defense
mechanisms induced by cellular oxidative stress (10,11).
Therefore, the present study investigated whether HMP conferred
protection against IRI to rat DCD livers via alterations in Nrf2
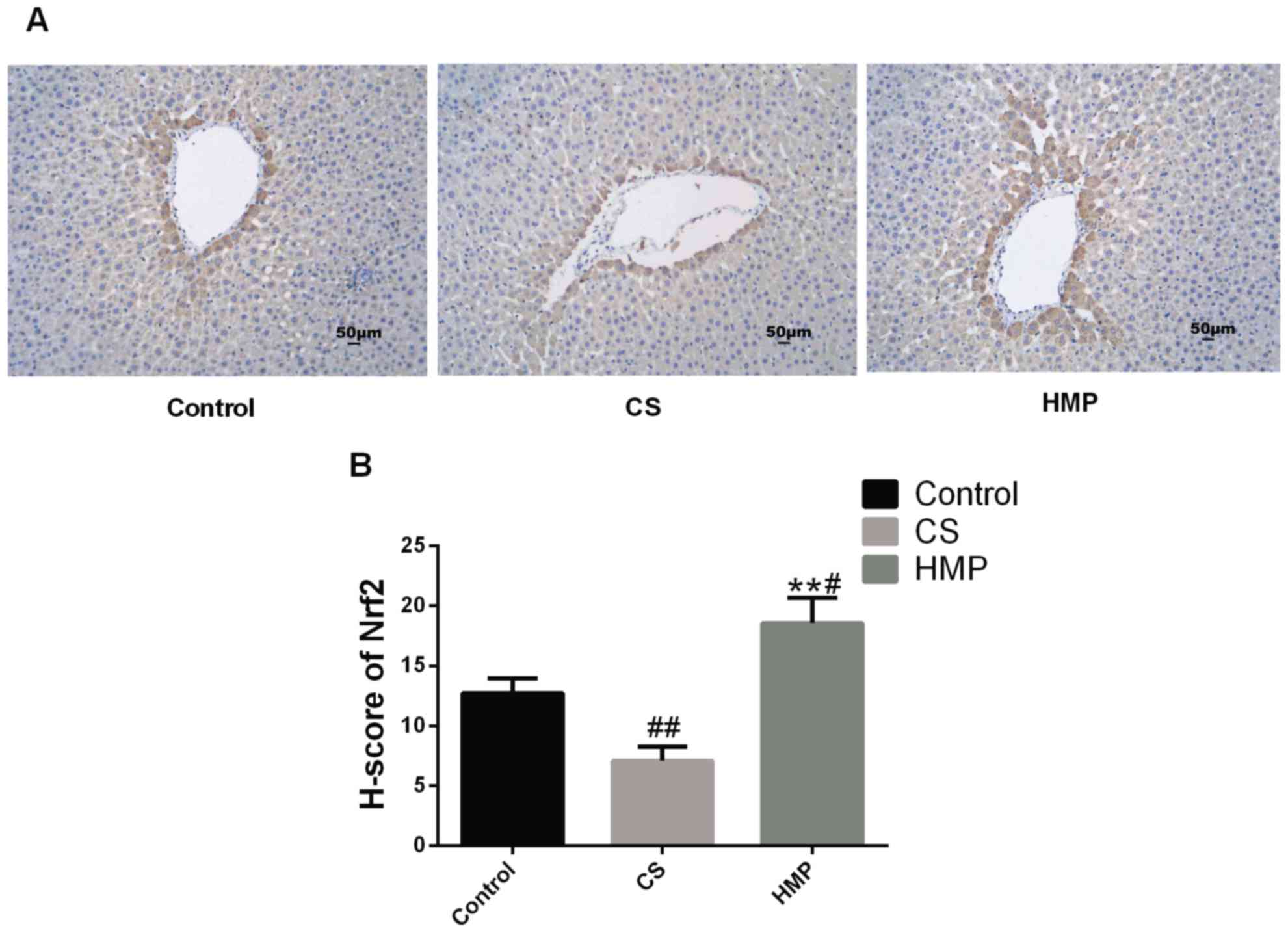
expression. The results reveled that compared with the control
group, the expression levels of Nrf2 in the CS group were
significantly decreased (P<0.05); however, the HMP group
exhibited a significant increase compared with in the CS group
(P<0.01; Fig. 5A and B). In
addition to the expression levels of Nrf2 protein, NQO1 protein
expression levels in the CS and HMP groups demonstrated opposing
expression patterns (P<0.05; Fig.
5A and B). Notably, despite the significant increase in HO-1
protein expression levels in the CS group compared with the
control, the HMP group exhibited a significant decrease compared
with the CS group (P<0.01; Fig. 5A
and B).
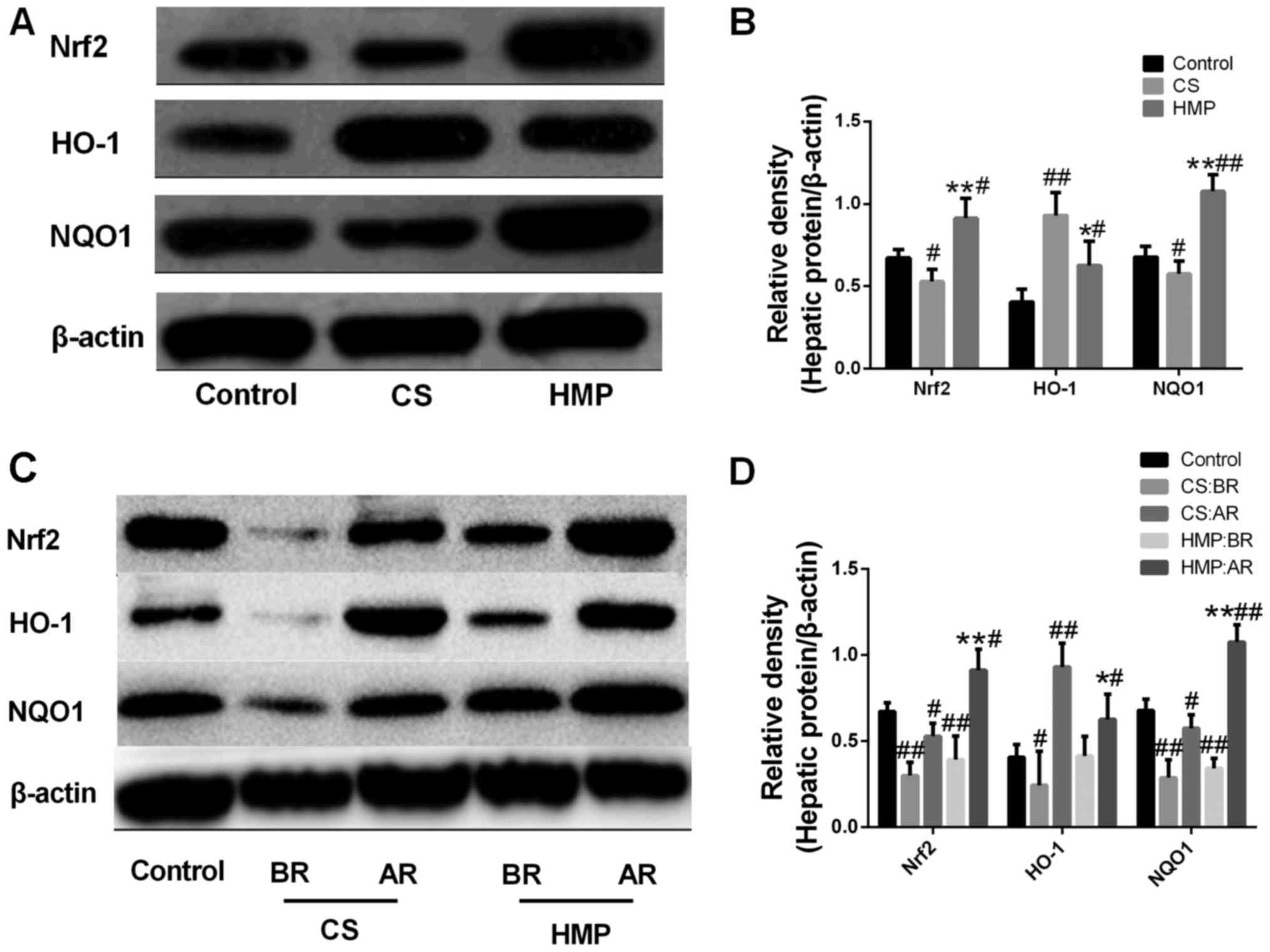 | Figure 5.Effect of HMP on Nrf2, HO-1 and NQO1
protein expression levels in donated after cardiac death rat liver
models. (A and B) Following reperfusion, the protein expression
levels of Nrf2, HO-1 and NQO1 in the livers of the control, CS and
HMP groups were analyzed by western blotting. β-actin served as an
internal control. #P<0.05, ##P<0.01 vs.
the control group, *P<0.05, **P<0.01 vs. the CS group. (C and
D) Western blotting and the quantitative analysis revealed the
expression levels of the components associated with the Nrf2-ARE
pathway in the livers of the CS and HMP groups prior to and
following reperfusion. #P<0.05,
##P<0.01, the CS group vs. the BR CS group; and HMP
group vs. the BR HMP group, respectively. AR, after reperfusion;
BR, before reperfusion; CS, cold storage; HMP, hypothermic machine
pressure; HO-1, heme oxygenase-1; NQO1, NAD(P)H:quinine
oxidoreductase 1; Nrf2, nuclear factor erythroid 2-related factor
2. |
Reperfusion injury due to toxic reactive oxygen
species generated upon reintroduction of blood flow and oxygen
supply to ischemic tissues is the main cause of DCD liver injury
(43). Therefore, the expression
levels of proteins in the presence or absence of reperfusion were
investigated in the present study to determine whether HMP may
activate the Nrf2-ARE signaling pathway.
The results of the present study revealed that the
expression levels of Nrf2, NQO-1 and HO-1 were all been
significantly upregulated during reperfusion in both the CS and HMP
groups (P<0.05; Fig. 5C and D).
In addition, the induction of Nrf2 (0.392±0.137 vs. 0.912±0.122;
P<0.0001) and NQO-1 (0.342±0.057 vs. 1.076±0.102; P<0.0001)
expression in the presence of IRI was significantly higher within
the HMP group compared with the induction of Nrf2 (0.300±0.079 vs.
0.529±0.075; P=0.007) and NQO-1 (0.287±0.104 vs. 0.575±0.078;
P<0.0001) expression in the CS group. All this suggested that
the effects of HMP on oxidative stress may occur via activation of
the Nrf2-ARE signaling pathway (P<0.05; Fig. 5C and D).
Additionally, the mRNA expression levels of Nrf2,
HO-1, NQO1, GST-1, and GCL in the liver were investigated via
RT-qPCR (Fig. 6). The results of
the present study revealed that except for HO-1, the mRNA
expression levels of the other ARE-regulated genes were
significantly enhanced in the HMP group compared with the CS group
(P<0.05; Fig. 6). This
suggested that additional regulatory elements may be involved in
the activation of HO-1 in the CS group; however, the activation of
Nrf2 and other ARE-regulated genes, as well as the inhibition of
oxidative stress markers, including MDA in the HMP group, suggested
that HMP may be responsible for the increase in antioxidative
ability of cells by activating the Nrf2-ARE signaling pathway.
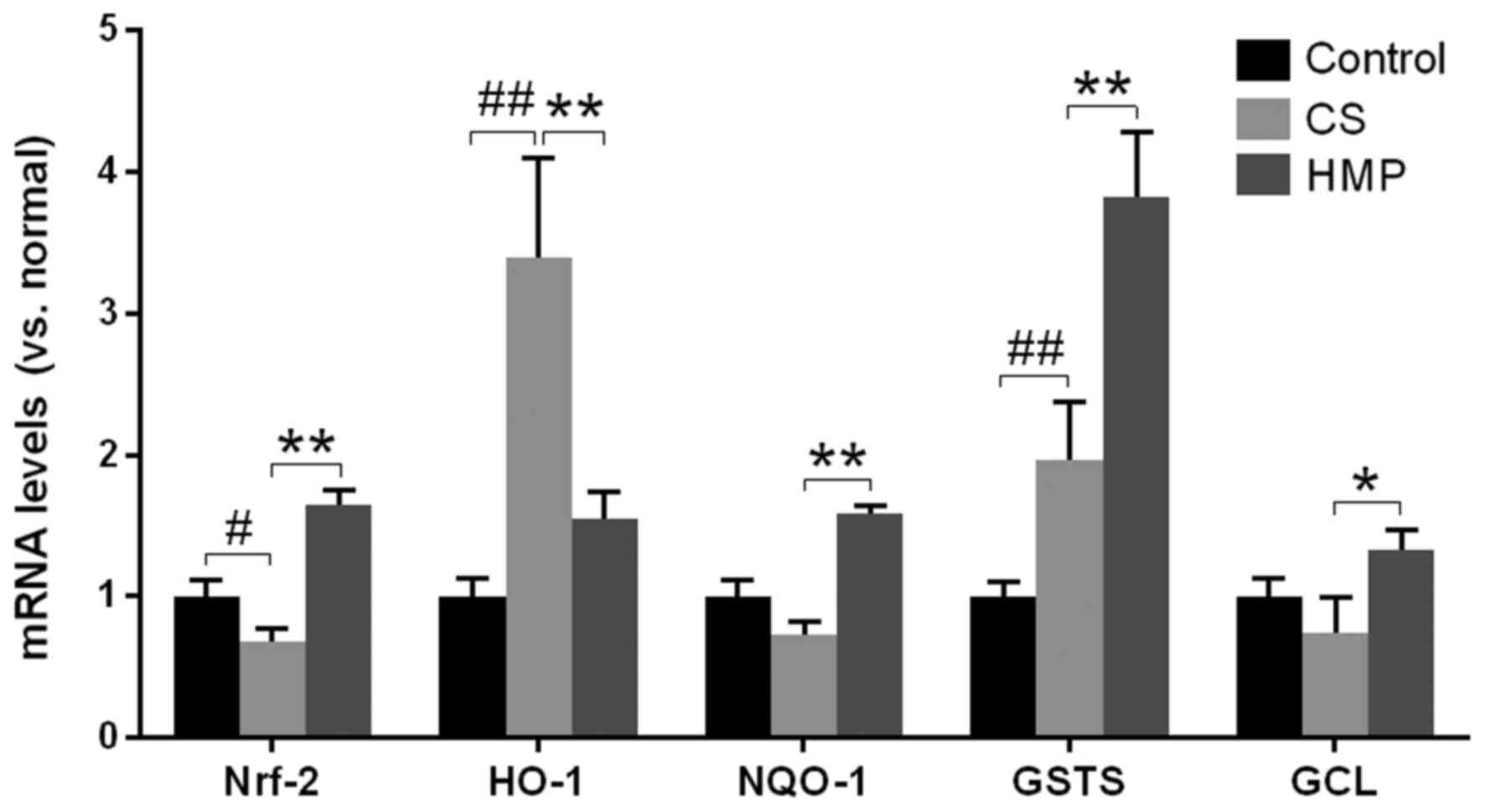 | Figure 6.Fold alterations in Nrf-2, HO-1,
NQO1, GST-1, and GCL mRNA expression levels following reperfusion
of rat livers. Reverse transcription-quantitative polymerase chain
reaction revealed that the expression of antioxidant response
element-containing genes (HO-1, NQO1, GST-1 and GCL) exhibited
significant alterations between the CS and HMP groups. The
quantification cycle values were quantified by the ratio of target
relative to housekeeping gene β-actin and the differences were
calculated by the 2−ΔΔCq method. #P<0.05,
##P<0.01 vs. the control group; *P<0.05,
**P<0.01 vs. the CS group. CS, cold storage; GCL, glutamate
cysteine ligase; GST-1, glutathione-S-transferase-1; HO-1, heme
oxygenase-1; HMP, hypothermic machine pressure; NQO1, NADPH
NAD(P)H:quinine oxidoreductase 1; Nrf2, nuclear factor erythroid
2-related factor 2. |
Furthermore, previous studies have confirmed that
Nrf2 may be activated in endothelial cells by steady laminar flow
(19,20). Thus, immunohistochemistry was
conducted to assess whether steady laminar flow provided by HMP may
induce the expression levels of Nrf2 (Fig. 7). The results revealed that within
the HMP group, Nrf2 expression was significantly increased in the
periportal regions of livers when compared with the CS group
(P<0.01), This suggested that Nrf2 in hepatocytes and liver
sinusoidal endothelial cells around periportal regions may be
induced by steady laminar flow.
Discussion
HMP has become a topic of interest in the past
decade, with regards to maintaining the quality of liver grafts,
particularly DCD livers (44);
however, the protective mechanisms of HMP require further
investigation. The present study investigated the benefits of HMP
in mitigating injuries from preservation methods with a specific
focus on oxidative stress in DCD livers. The Nrf2 signaling pathway
is an important mediator of the antioxidant system in mammals
(15). In the present study, it
was demonstrated that HMP may reduce IRI to DCD livers via
activation of the Nrf2-ARE signaling pathway.
Additionally, the present study demonstrated that,
compared with the CS group, the HMP group revealed improved
hepatocellular functions and overall tissue viability, as indicated
by improved ATP and bile production, and lower ALT and AST levels.
Bile production between the CS group and HMP group did not exhibit
significant differences under the HMP condition, and this may have
been due to the delivery of nutrients and oxygen via the portal
vein rather than the hepatic artery, which is the only way oxygen
is delivered in the physiological state (23,31).
In HMP group the liver was perfused through portal vein, therefore
cholangiocytes may be more sensitive to ischemic injury and would
influence the production of bile. The congestion and vacuolar
degeneration observed in the histological sections were considered
to be possible causes for microcirculation dysfunction. In addition
to the improved histological findings, the levels of intrahepatic
resistance in the HMP group were significantly lower compared with
in the CS group.
Numerous mechanisms underlying IRI in the DCD liver
grafts have been reported, including oxidative stress (45). In the present study, the levels of
SOD activity represented the antioxidant capacity and MDA
expression levels reflected the degree of oxidative damage to
cells. The present study observed a significant reduction in the
levels of MDA within the HMP group, indicating that oxidative
damage to the graft may be attenuated following HMP preservation
compared with in the CS group. Furthermore, an increase in the
activity of SOD following reperfusion was observed during HMP
preservation, which indicated a cellular attempt to ameliorate
oxidative damage. Overall, the findings of the present study
suggested that HMP may be a better strategy of preservation than CS
and may exert protective effects against IRI on the DCD liver.
Oxidative stress may also induce hepatic
inflammation and apoptosis (37–39).
In addition, TLR4 is an important marker of inflammation and is
activated during hepatic IR, as well as IRI in multiple organs; the
importance of TLR4 in IRI has also been verified in a transplant
model (46). The results of the
present study indicated that the protein expression levels of TLR4
in the CS group were significantly higher than the control group;
however, the HMP group exhibited lower expression levels of TLR4
than the CS group. This result suggested that reductions in
oxidative stress may reduce the inflammation of DCD liver. In
addition, Bax and Bcl-2 are important markers of apoptosis
(40); Bax is a proapoptotic
protein, while Bcl-2 is an antiapoptotic protein (41). Thus, the protein expression levels
of Bcl-2 and Bax following reperfusion were investigated in the
present study to determine whether apoptosis may be inhibited. The
alterations of the Bcl-2/Bax ratio in the CS and HMP groups
indicated that HMP may attenuate hepatocyte apoptosis. TUNEL
staining also indicated the extent of hepatocyte apoptosis in the
present study. When investigations were performed with HMP instead
of CS to preserve the liver, the rate of hepatocyte apoptosis
decreased significantly in the present study. This indicated that,
with the decline of liver oxidative stress, liver inflammation and
apoptosis may be attenuated.
Previous studies have suggested a role of Nrf2 in
antioxidative and anti-apoptotic process functions in IRI (16,47).
Therefore, the present study proposed that the activity of Nrf2 may
serve a role in the protective effects of HMP against IRI; the
protein and mRNA expression levels of Nrf2 between the HMP and CS
groups were investigated. Significantly higher expression levels
were observed in the HMP group compared with in the CS, which
indicated that HMP may have induced the expression of Nrf2.
Additionally, immunohistochemistry analysis demonstrated that the
expression of Nrf2 in the pericentral region was notably increased
following HMP treatment. The Nrf2 signaling pathway mainly involves
Nrf2, Keap1 and ARE. Following dissociation from Keap1, activated
Nrf2 enters the nucleus and binds to the ARE sequence by combining
with MAF protein to form heterodimers. Through this binding
process, Nrf2 regulates the transcription of
antioxidative-associated genes (15). Following reperfusion, the
expression of antioxidant molecules, including GST-1, NQO1 and GCL,
which are the target genes of Nrf2, are increased in the livers of
the HMP group. As described in the previous results, compared with
the control group, the expression levels of HO-1 protein appeared
to increase more than in the HMP group. The reason for this
phenomenon may be that the regulation of HO-1 gene expression
occurs at the transcriptional level. The regulatory elements of the
gene include not only components associated with the Nrf2-ARE
signaling pathway, but also the activator protein-1 binding site,
nuclear factor-κB binding site, a heat-shock element and hypoxic
response elements. Activation of the HO-1 gene results from the
binding of various transcription factors to these regulatory
elements (48–50). There may be other potential
mechanisms underlying the increased protein expression levels of
HO-1 in the CS group.
Providing that reperfusion is the main cause of DCD
liver injury, the present study also investigated the expression of
proteins associated with the Nrf2-ARE signaling pathway prior to
and following reperfusion to determine whether HMP may activate
this signaling pathway. The protein expression levels of the
Nrf2-ARE signaling pathway were significantly different prior to
and following reperfusion. In addition, the higher end protein
level quantification values of Nrf2 and NQO1 in the HMP group
compared with the CS group following reperfusion indicated that the
effect of HMP was more notable than CS treatment. In addition, the
present study revealed that HMP may be able to reduce the extent of
apoptosis and inflammation in accordance with alterations in Nrf2
expression. This indicated that the Nrf2-ARE signaling pathway may
serve an important role in the molecular mechanism underlying the
protective effects of HMP.
Furthermore, previous studies have confirmed that
Nrf2 may be activated by steady laminar flow (19,20,51).
When endothelial cells come into contact with the flowing blood,
they experience different types of stress. In contrast with
disturbed blood flow, steady laminar flow serves positive roles,
and in this situation the activation of protective factors
including Nrf2 is dominant (20).
Based on previous studies, steady laminar flow leads to sustained
high shear stress, and flow shear stress were considered to produce
antioxidant, antiapoptotic, anti-inflammatory, and
antiproliferative effects (20,51).
Therefore, the results of the present study that HMP demonstrated
decreased enzymatic levels, reduced intrahepatic resistance and
improved histological findings compared with CS, suggest that
steady laminar flow may provides a supply of metabolic substrates
and removes byproducts, recreating the normal circulation, which
corresponds with the previous studies (52). Then, Hsieh et al (51) demonstrated that shear stress can
increase Nrf2 protein expression and induce Nrf2 translocation into
nuclei; in addition, shear stress also increased the ARE-binding
activity of Nrf2, and a number of antioxidant genes, including
HO-1, NQO1 and GST-1, are upregulated in endothelial cells under
laminar shear stress. The activation of Nrf2 is essential for the
antioxidant function of shear stress, and the effector proteins
HO-1 and NQO1 have been demonstrated to respond to flow shear
stress in vascular endothelial cells (19). However the detailed mechanism of
the activation of Nrf2 and how the antioxidant ability of flow
shear stress is regulated remains to be studied. Additionally, as
this perspective suggests, immunohistochemistry analysis in our
study also demonstrated that the expression of Nrf2 which may be
activated in endothelial cells by steady laminar flow, was notably
increased in the pericentral region following HMP treatment. As a
result of all this information, HMP preservation was concluded to
dilate the intrahepatic vasculature and improve microcirculation,
resulting in steady laminar flow during reperfusion, which was the
activator of Nrf2-ARE pathway.
In the present study, the alterations of NRF2-ARE
pathway by different storage method during simulated DCD liver
transplantation were investigated. NRF2 is a key molecule in
anti-oxidative stress (10,11).
Combined with the authors' studies, this may suggest that the
NRF2-ARE signaling pathway may be a novel pharmacological target
for improving DCD liver quality. However, the present study also
has some deficiencies. Due to the lack of a murine IPRL system, the
present study failed to use knockout mice, which may need further
study. Organ preservation has always been a bottleneck that
affected the development of transplantation. How to preserve organs
more effectively has always been a study hotspot in the field of
transplantation. In addition, although HMP is the routine method
for clinical donated kidney protection, which kind of preservation
method is more effective in the liver remains unknown. It was
demonstrated that the possible mechanism of HMP to protect the DCD
liver maybe result from the activation of NRF2-ARE pathway, but the
specific mechanism needs to be further research by using the
knockout mice. In addition, clinical trials may focus on drugs
associated with NRF2 pathway to improve DCD liver and the outcome
of the transplant. These are possible future research areas. In
conclusion, the protective effects of HMP on the DCD liver may
arise from alterations in Nrf2 expression levels. Therefore, via
the activation of Nrf2 and its function of binding to the ARE
sequence, HMP may serve a role against oxidative stress to protect
the DCD liver from IRI.
Acknowledgements
The authors would like to thank the professors and
students from Zhongnan Hospital of Wuhan University and Institute
of Hepatobiliary Diseases of Wuhan University, who participated in
this study.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. U1403222).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SX and QY contributed the central idea, analyzed
most of the data, and wrote the initial draft of the paper. WH, XZ,
ZZ, YX and YW contributed to refining the ideas. SX, QY, WH, XZ, ZT
and SF carried out additional analyses and finalizing this
paper.
Ethics approval and consent to
participate
The present study was conducted according to the
Experimental Animal Regulations of the People's Republic of China
and the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health, ensuring that all animals received
humane care. The present study was approved by the Ethics Committee
of Wuhan University (Wuhan, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
O'Leary JG, Lepe R and Davis GL:
Indications for liver transplantation. Gastroenterology.
134:1764–1776. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Monbaliu D, Pirenne J and Talbot D: Liver
transplantation using donation after cardiac death donors. J
Hepatol. 56:474–485. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neyrinck A, Van Raemdonck D and Monbaliu
D: Donation after circulatory death: Current status. Curr Opin
Anaesthesiol. 26:382–390. 2013.PubMed/NCBI
|
|
4
|
Jay CL, Lyuksemburg V, Ladner DP, Wang E,
Caicedo JC, Holl JL, Abecassis MM and Skaro AI: Ischemic
cholangiopathy after controlled donation after cardiac death liver
transplantation: A meta-analysis. Ann Surg. 253:259–264. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fujita S, Mizuno S, Fujikawa T, Reed AI,
Kim RD, Howard RJ and Hemming AW: Liver transplantation from
donation after cardiac death: A single center experience.
Transplantation. 84:46–49. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Manzarbeitia CY, Ortiz JA, Jeon H,
Rothstein KD, Martinez O, Araya VR, Munoz SJ and Reich DJ:
Long-term outcome of controlled, non-heart-beating donor liver
transplantation. Transplantation. 78:211–215. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morrissey PE and Monaco AP: Donation after
circulatory death: Current practices, ongoing challenges, and
potential improvements. Transplantation. 97:258–264. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Henry SD and Guarrera JV: Protective
effects of hypothermic ex vivo perfusion on ischemia/reperfusion
injury and transplant outcomes. Transplantation Rev (Orlando).
26:163–175. 2012. View Article : Google Scholar
|
|
9
|
Schreinemachers MJ, Doorschodt BM and van
Gulik TM: Machine perfusion preservation of the liver: A worthwhile
clinical activity? Curr Opin Organ Transplant. 12:224–230. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma Q and He X: Molecular Basis of
Electrophilic and Oxidative Defense: Promises and Perils of Nrf2.
Pharmacol Rev. 64:1055–1081. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kensler TW, Wakabayashi N and Biswal S:
Cell survival responses to environmental stresses via the
Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Itoh K, Wakabayashi N, Katoh Y, Ishii T,
Igarashi K, Engel JD and Yamamoto M: Keap1 represses nuclear
activation of antioxidant responsive elements by Nrf2 through
binding to the amino-terminal Neh2 domain. Genes Dev. 13:76–86.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang MI, Kobayashi A, Wakabayashi N, Kim
SG and Yamamoto M: Scaffolding of Keap1 to the actin cytoskeleton
controls the function of Nrf2 as key regulator of cytoprotective
phase 2 genes. Proc Natl Acad Sci USA. 101:2046–2051. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang DD and Hannink M: Distinct cysteine
residues in keap1 are required for Keap1-dependent ubiquitination
of Nrf2 and for stabilization of Nrf2 by chemopreventiveagents and
oxidative stress. Mol Cell Biol. 23:8137–8151. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kurokawa H, Motohashi H, Sueno S, Kimura
M, Takagawa H, Kanno Y, Yamamoto M and Tanaka T: Structural basis
of alternative DNA recognition by Maf transcription factors. Mol
Cell Biol. 29:6232–6244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kudoh K, Uchinami H, Yoshioka M, Seki E
and Yamamoto Y: Nrf2 activation protects the liver from
ischemia/reperfusion injury in mice. Ann Surg. 260:118–127. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee LY, Harberg C, Matkowskyj KA, Cook S,
Roenneburg D, Werner S, Johnson J and Foley DP: Overactivation of
the nuclear factor (erythroid-derived 2)-like 2-antioxidant
response element pathway in hepatocytes decreases hepatic
ischemia/reperfusion injury in mice. Liver Transplant. 22:91–102.
2016. View
Article : Google Scholar
|
|
18
|
Tanaka Y, Maher JM, Chen C and Klaassen
CD: Hepatic ischemia-reperfusion induces renal heme oxygenase-1 via
NF-E2-related factor 2 in rats and mice. Mol Pharmacol. 71:817–825.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fukuda Y, Kaishima M, Ohnishi T, Tohyama
K, Chisaki I, Nakayama Y, Ogasawara-Shimizu M and Kawamata Y: Fluid
shear stress stimulates MATE2-K expression via Nrf2 pathway
activation. Biochem Biophys Res Commun. 484:358–364. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nigro P, Abe J and Berk BC: Flow shear
stress and atherosclerosis: A matter of site specificity. Antioxid
Redox Signal. 15:1405–1414. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zakkar M, Angelini GD and Emanueli C:
Regulation of vascular endothelium inflammatory signalling by shear
stress. Curr Vasc Pharmacol. 14:181–186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
National Research Council, . Guide for the
care and use of laboratory animals. 8th edition. The National
Academies press; Washington, DC: 2011, PubMed/NCBI
|
|
23
|
Carnevale ME, Balaban CL, Guibert EE,
Bottai H and Rodriguez JV: Hypothermic machine perfusion versus
cold storage in the rescuing of livers from non-heart-beating donor
rats. Artif Organs. 37:985–991. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rodriguez J, Guibert EE, Quintana A,
Scandizzi A and Almada L: Role of sodium nitroprusside in the
improvement of rat liver preservation in University of Wisconsin
solution: A study in the isolated perfused liver model. J Surg Res.
87:201–208. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dutkowski P, Schönfeld S, Odermatt B,
Heinrich T and Junginger T: Rat liver preservation by hypothermic
oscillating liver perfusion compared to simple cold storage.
Cryobiology. 36:61–70. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zeng C, Hu X, He W, Wang Y, Li L, Xiong Y
and Ye Q: Hypothermic machine perfusion ameliorates inflammation
during ischemia-reperfusion injury via sirtuin-1-mediated
deacetylation of nuclear factor-κB p65 in rat livers donated after
circulatory death. Mol Med Rep. 16:8649–8656. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schlegel A, Kron P, Graf R, Dutkowski P
and Clavien PA: Warm vs. cold perfusion techniques to rescue rodent
liver grafts. J Hepatol. 61:1267–1275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pizarro MD, Rodriguez JV, Mamprin ME,
Fuller BJ, Mann BE, Motterlini R and Guibert EE: Protective effects
of a carbon monoxide-releasing molecule (CORM-3) during hepatic
cold preservation. Cryobiology. 58:248–255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Srinivasan PK, Yagi S, Doorschodt B, Nagai
K, Afify M, Uemoto S and Tolba R: Impact ofvenous systemic oxygen
persufflation supplemented with nitric oxide gas on cold-stored,
warm ischemia-damaged experimental liver grafts. Liver Transpl.
18:219–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Minor T and Manekeller S: Assessment of
hepatic integrity after ischemic preservation by isolated perfusion
in vitro: The role of albumin. Cryobiology. 54:188–195. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boyer JL: Bile formation and secretion.
Compr Physiol. 3:1035–1078. 2013.PubMed/NCBI
|
|
32
|
Suzuki S, Toledo-Pereyra LH, Rodriguez FJ
and Cejalvo D: Neutrophil Infiltration As An Important Factor in
Liver Ischemia and Reperfusion Injury. Transplantation.
55:1265–1272. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Funel N, Giovannetti E, Chiaro MD, Pollina
L, Mosca F, Peters G, Campani D and Boggi U: Molecular mechanisms
underlying the synergistic interaction of the novel anticancer drug
celandine with gemcitabine in preclinical models of pancreatic
cancer. Polski Tygodnik Lekarski. 11:23–40. 2011.
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun K, Liu ZS and Sun Q: Role of
mitochondria in cell apoptosis during hepaticischemia-reperfusion
injury and protective effect of ischemic postconditioning. World J
Gastroenterol. 10:1934–1938. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang L, Li C, Quan R and Xie S: The
effect of electroacupuncture on neuronal apoptosis and related
functions in rats with acute spinal cord injury. Chin Med.
5:199–210. 2014. View Article : Google Scholar
|
|
37
|
Radak Z, Zhao Z, Koltai E, Ohno H and
Atalay M: Oxygen consumption and usage during physical exercise:
The balance between oxidative stress and ROS-dependent adaptive
signaling. Antioxid Redox Signal. 18:1208–1246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cardoso AR, Kakimoto PA and Kowaltowski
AJ: Diet-sensitive sources of reactive oxygen species in liver
mitochondria: Role of very long chain Acyl-CoA dehydrogenases. PLoS
One. 8:e770882013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: How are
they linked? Free Radic Biol Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chao DT and Korsmeyer SJ: BCL-2 family:
Regulators of cell death. Annu Rev Immunol. 16:395–419. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Klune JR and Tsung A: Molecular biology of
liver ischemia/reperfusion injury: Established mechanisms and
recent advancements. Surg Clin North Am. 90:665–677. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Weigand K, Brost S, Steinebrunner N,
Büchler M, Schemmer P and Müller M: Ischemia/reperfusion injury in
liver surgery and transplantation: Pathophysiology. HPB Surg.
2012:1767232012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Marecki H, Bozorgzadeh A, Porte RJ,
Leuvenink HG, Uygun K and Martins PN: Liver ex situ machine
perfusion preservation: A review of the methodology and results of
large animal studies and clinical trials. Liver Transpl.
23:679–695. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Montalvo-Jave EE, Escalante-Tattersfield
T, Ortega-Salgado JA, Piña E and Geller DA: Factors in the
pathophysiology of the liver ischemia-reperfusion injury. J Surg
Res. 147:153–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kaczorowski DJ, Tsung A and Billiar TR:
Innate immune mechanisms in ischemia/reperfusion. Front Biosci
(Elite Ed). 1:91–98. 2009.PubMed/NCBI
|
|
47
|
Zeng XP, Li XJ, Zhang QY, Liu QW, Li L,
Xiong Y, He CX, Wang YF and Ye QF: Tert-Butylhydroquinone protects
liver against ischemia/reperfusion injury in rats through
Nrf2-activating anti-oxidative activity. Transplant Proc.
49:366–372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tao X, Sun X, Xu L, Yin L, Han X, Qi Y, Xu
Y, Zhao Y, Wang C and Peng J: Total flavonoids from Rosa laevigata
Michx fruit ameliorates hepatic ischemia/reperfusion injury through
inhibition of oxidative stress and inflammation in rats. Nutrients.
8:E4182016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chou YH, Ho FM, Liu DZ, Lin SY, Tsai LH,
Chen CH, Ho YS, Hung LF and Liang YC: The possible role of heat
shock factor-1 in the negative regulation of heme oxygenase-1. Int
J Biochem Cell Biol. 37:604–615. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pastukh V, Ruchko M, Gorodnya O, Wilson GL
and Gillespie MN: Sequence-specific oxidative base modifications in
hypoxia-inducible genes. Free Radic Biol Med. 43:1616–1626. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hsieh CY, Hsiao HY, Wu WY, Liu CA, Tsai
YC, Chao YJ, Wang DL and Hsieh HJ: Regulation of shear-induced
nuclear translocation of the Nrf2 transcription factor in
endothelial cells. J Biomed Sci. 16:122009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
St Peter SD, Imber CJ and Friend PJ: Liver
and kidney preservation by perfusion. Lancet. 359:604–613. 2002.
View Article : Google Scholar : PubMed/NCBI
|