Introduction
Vanadium is a grey metal that exists in different
states of oxidation (ranging from −1 to +5) of which vanadium
pentoxide (V2O5) is the most usual form.
All vanadium compounds have been considered toxic.
The exposure limit to V2O5 dust and fumes in
workplace air (8 h work day/40 h work week) has been fixed by the
Occupational Safety and Health Administration in 0.05 and 0.1
mg/m3, respectively (1).
The National Institute for Occupational Safety and
Health (NIOSH) sets to 35 mg/m3 the dose of vanadium
exposure that may cause seriously health issues up to death
(1).
Toxic effects of vanadium are reflected mainly on
respiratory system, while the effect on the gastrointestinal system
is less relevant because of the minimal gut absorption rate of the
substance (2–4). Unfortunately, no sufficient data are
available in order to determine the reference range of a subchronic
or chronic inhaled dose.
Studies conducted on rat models showed the toxic
effects (resulted from an oral, or inhaled, vanadium exposures) on
serum parameters (5,6), liver (7), nervous (8) and other tissues development (9).
Vanadium workers (NIOSH 1983) showed an increased
prevalence of skin rashes, such as atopic dermatitis.
Until now no in vivo, or in vitro,
studies were carried out to evaluate the effect of exposure to
vanadium in dermal fibroblasts.
Here, we evaluate the effect of
V2O5 on viability and proliferation, and
secretion of chemokine (C-X-C motif) ligand (CXCL)8, or CXCL11 [an
interferon (IFN)γ dependent chemokine, of the same class of CXCL9,
and CXCL10] in dermal human fibroblasts.
Materials and methods
Fibroblast cell cultures
We have obtained fibroblasts from derma of six
patients who underwent an operation for thyroid nodular goiter
(discard dermal material; all females, age range 57–76 years,
euthyroid, without other disorders or diseases, and not treated
with any kind of drugs).
Involved subjects gave their informed consent and
the study was approved by the University of Pisa (Pisa, Italy)
Ethics Committee. Tissue explants were firstly minced and then
placed in culture dishes, allowing the fibroblasts proliferation
(as previously described) (10).
Fibroblasts were propagated in 199 medium [with 20% FBS (Gibco;
Thermo Fisher Scientific, Waltham, MA, USA), gentamycin (20 µg/ml),
penicillin (100 U/ml)], in a 37°C humidified incubator with 5%
CO2; and maintained subsequently in a 199 medium with
10% FBS (and antibiotics) (11).
The cells were all used at the 4th passage, and were tested for
purity by immunocytochemistry (12).
Proliferation and viability
We have done the WST-1 (Roche Diagnostics, Almere,
The Netherlands) assay (that uses
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide, in
the MTT assay) to evaluate cell viability and proliferation
(13–16).
Firstly fibroblasts were seeded in each well of
96-well plates at a concentration of 35,000 cells/ml (in a final
volume of 100 µl).
Subsequently V2O5 effect on
fibroblasts viability and proliferation was determined exposing
cells for 24 h with increased concentrations of the compound (1,
10, 100 nM).
Fibroblasts were plated and treated with
V2O5 or with its vehicle alone (for 24 h),
performing all experiments in triplicate for each cell
preparation.
As the cell viability and proliferation WST-1 assay
may have limitations on evaluating cellular proliferation (17), fibroblasts proliferation was
determined also by cell number counting (13–16).
Chemokine secretion assay and
ELISA
To perform the CXCL8 and CXCL11 secretion assays,
30,000 cells/ml were seeded in 96-well plates, in a final volume of
100 µl per well, in growth medium, that was removed after 24 h.
After cells were washed in PBS, and incubated (24 h) in phenol red
and serum-free medium containing IFNγ (500, 1,000, 5,000, 10,000
IU/ml) and/or 10 ng/ml TNFα (all R&D Systems, Minneapolis, MN,
USA), alone or in combination (10). The TNFα concentration to obtain the
highest secretion was selected in preliminary experiments. After 1
day the supernatants were collected and then kept frozen at −20°C
(until chemokine assay).
We treated fibroblasts, for 24 h, with increasing
concentrations of V2O5 (1, 10, 100 nM), in
presence/absence of IFNγ (1,000 IU/ml), and/or TNFα (10 ng/ml), in
order to evaluate the effect of V2O5 on the
chemokine secretion induced by IFNγ.
CXCL8 and CXCL11 concentrations were measured in the
supernatants using the ELISA assay. The experiments were carried
out three times, for each different cell preparation.
Chemokines levels were measured in culture
supernatants, using commercially kits (R&D Systems). The mean
minimum detectable dose was 2.7 pg/ml for CXCL8 and 3.2 pg/ml for
CXCL11; the intra- and inter-assay coefficients of variation were
3.5 and 6.5% for CXCL8, 4.7 and 8.5% for CXCL11. Quality control
pools of low, normal, or high concentration for all parameters were
included in each assay.
Statistical analysis
For normally distributed variables values are given
in text as mean (±SD), or mean (±SEM) in figures, otherwise as
median [and interquartile range]. Mean group values are compared by
one-way analysis of variance (ANOVA) for variables normally
distributed, or with the Kruskal-Wallis test, or Mann-Whitney U
test. Proportions are compared by the Chi-Square. We have used the
Bonferroni-Dunn test for post hoc comparison of normally
distributed variables.
Results
Cell proliferation of dermal
fibroblasts
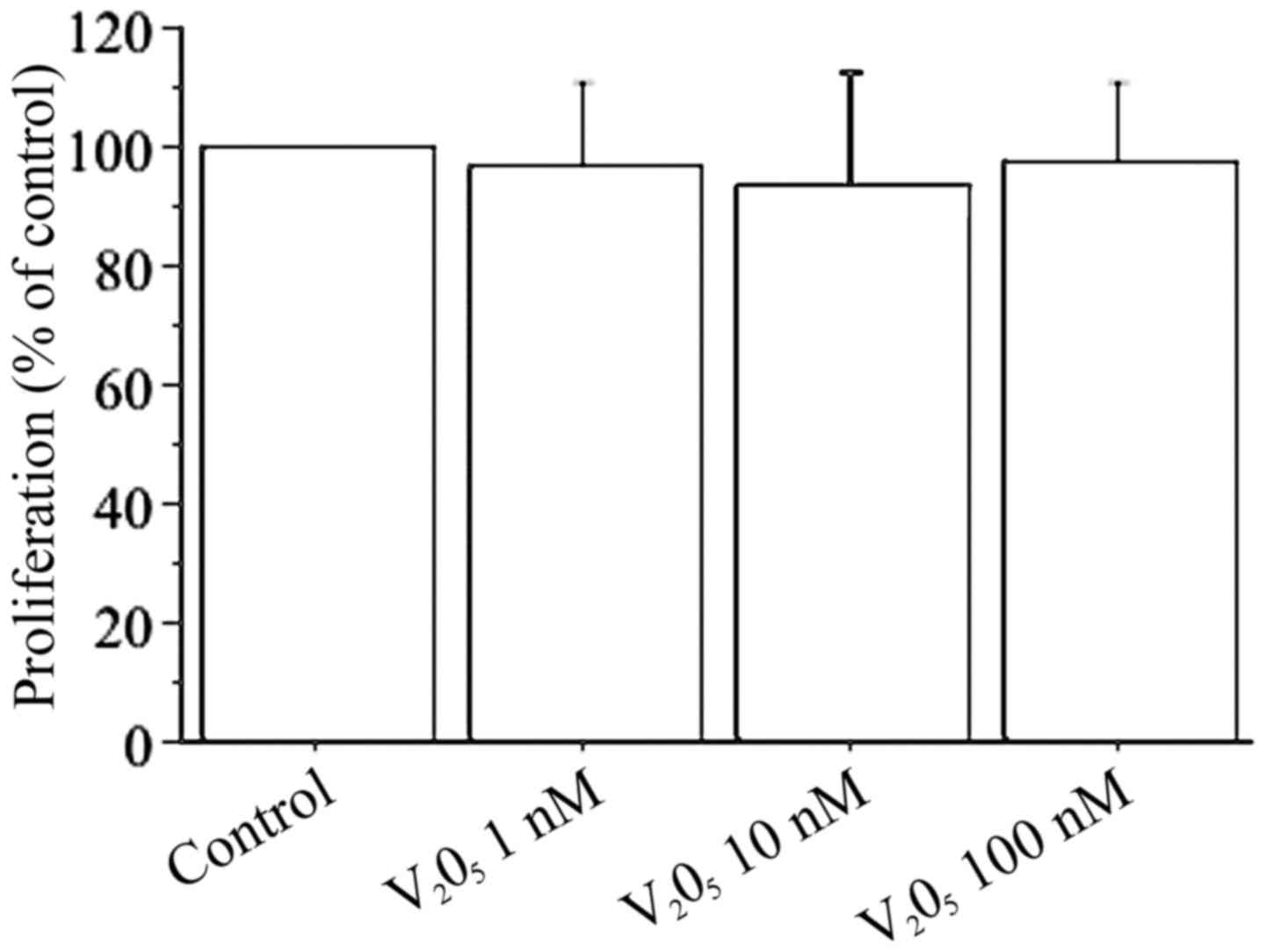
Cell counting shows that V2O5
(1, 10, 100 nM) does not change viability or proliferation of
dermal fibroblasts (Fig. 1). The
results of WST-1 assay in dermal fibroblasts with
V2O5 (1, 10, 100 nM) confirmed the cell
counting data: with V2O5 1 nM it was 99% with
respect to the control; with V2O5 10 nM it
was 97% with respect to the control; and with
V2O5 100 nM it was 98% with respect to the
control.
Fibroblast secretion of CXCL8
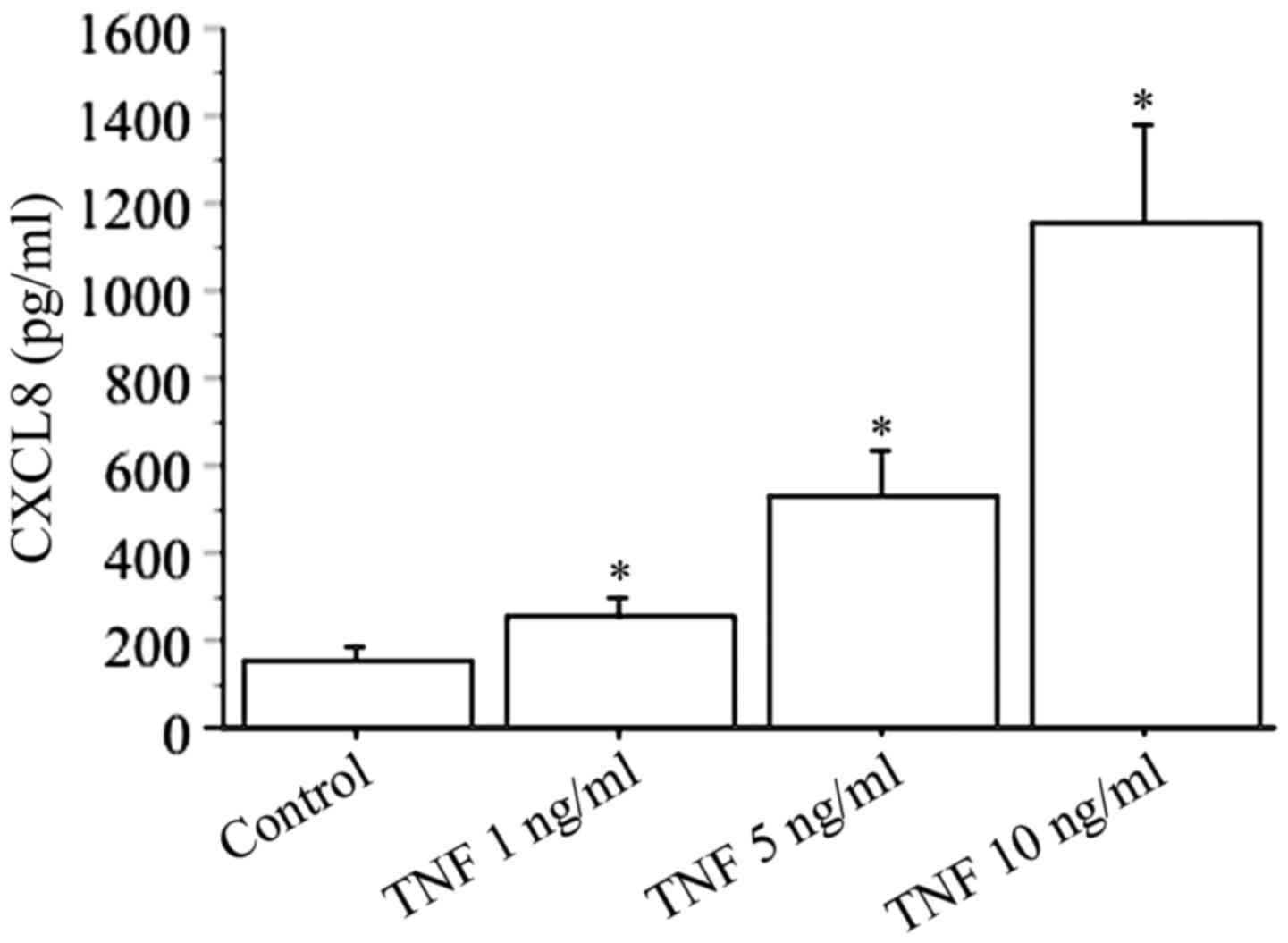
In basal conditions, the secretion of CXCL8 (range,
51–213 pg/ml) was measured in all preparations of cultured dermal
fibroblasts (Fig. 2).
CXCL8 secretion increased in a dose-dependent manner
using different concentrations of TNFα (1, 5, 10 ng/ml), with the
highest response reached with 10 ng/ml TNFα (basal 156±46 pg/ml vs.
TNFα 1154±321 pg/ml; P<0.01) (Fig.
2).
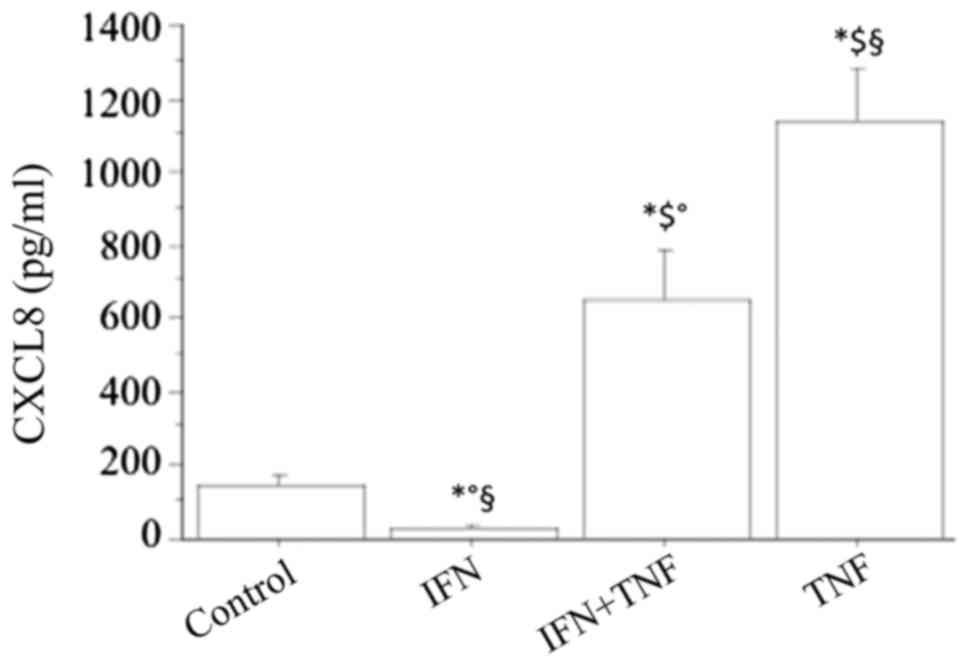
The basal CXCL8 secretion was significantly
inhibited by IFNγ in a dose-dependent manner (CXCL8: 84±37, 34±25,
pg/ml; respectively, with IFNγ 500 or 1,000 IU/ml; ANOVA,
P<0.05), while TNFα alone (10 ng/ml) significantly stimulated
the CXCL8 secretion (P<0.01) (Fig.
3). Combining IFNγ with TNFα led to a significant reversal of
the stimulating effect of TNFα (TNFα+IFNγ 661±176 pg/ml vs. TNFα
1154±321 pg/ml; P<0.05) (Fig.
3). However, the stimulating effect of TNFα on the secretion of
CXCL8 was not completely reversed by IFNγ, because the
concentration of this chemokine was still significantly higher than
in basal conditions (TNFα+IFNγ vs. basal; P<0.01).
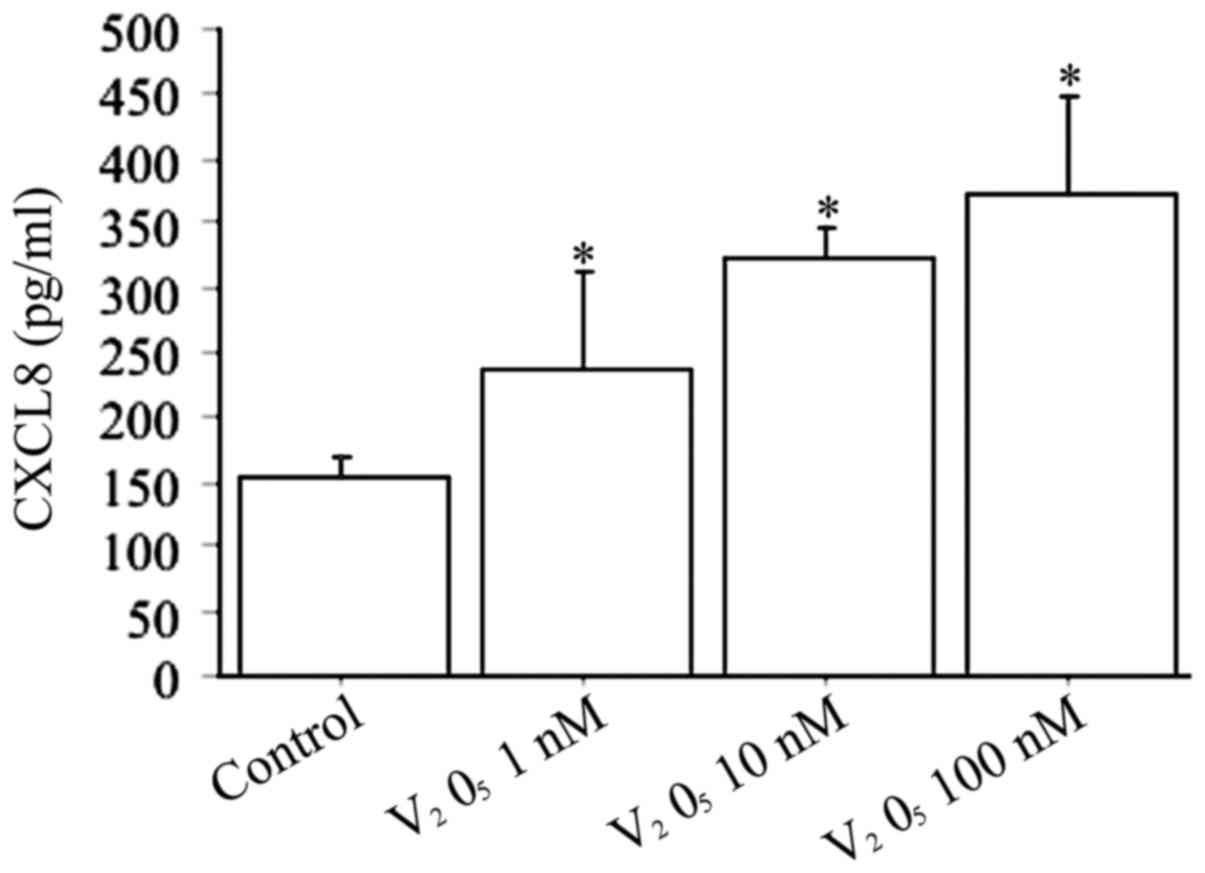
When fibroblasts were treated with increased
V2O5 concentrations (1, 10, 100 nM) the CXCL8
release was dose-dependently stimulated (P<0.0001, by ANOVA)
(Fig. 4).
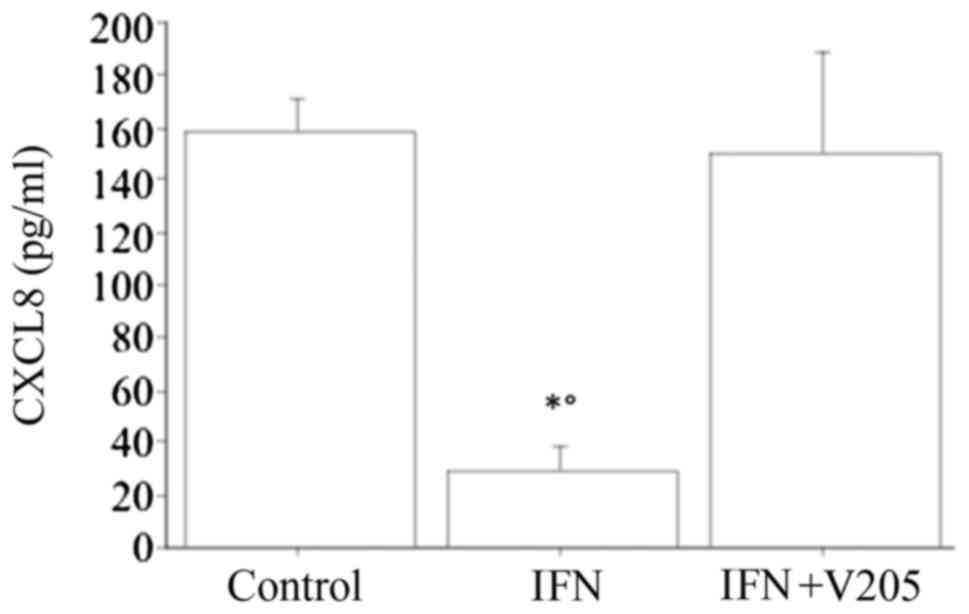
When treating dermal fibroblasts with
V2O5 (100 nM), together with IFNγ, CXCL8
release was not significantly changed with respect to the basal
condition, and IFNγ suppressed the V2O5
stimulating effect, but it stills increased it compared to IFNγ
alone (Fig. 5).
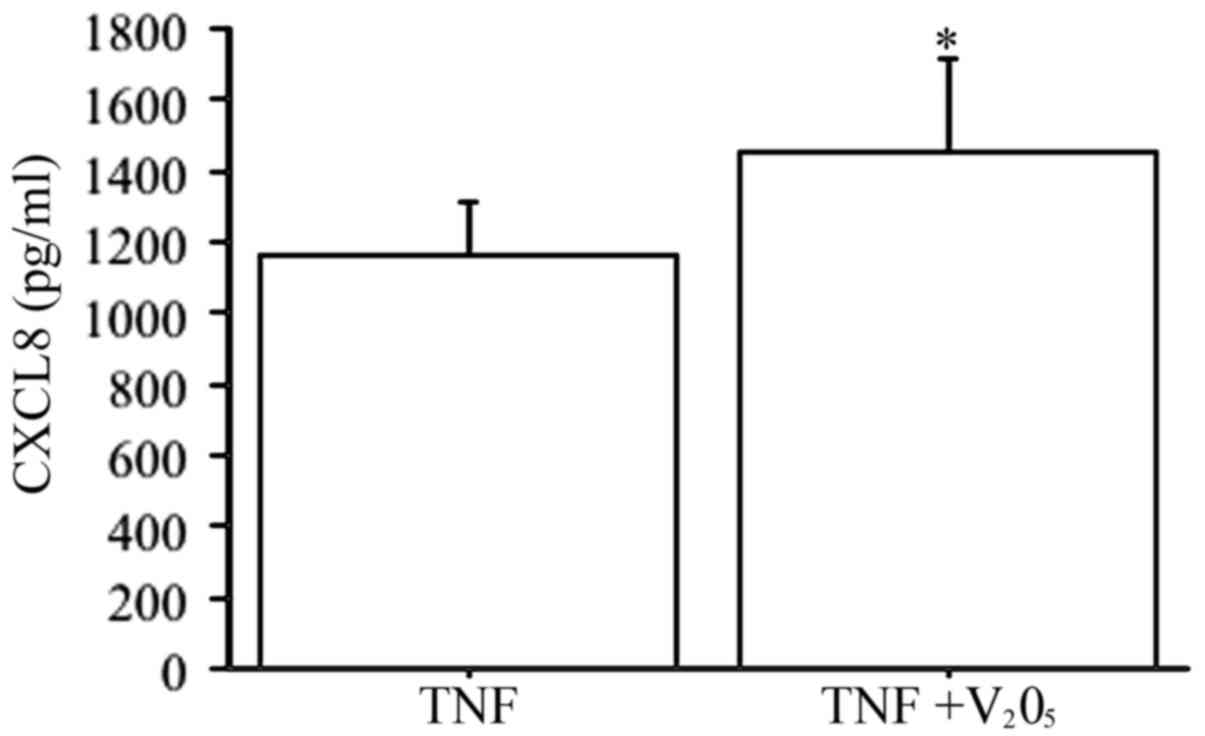
V2O5 (100 nM) plus TNFα
elicited a synergistic effect on CXCL8 secretion (P<0.0001, by
ANOVA), compared to TNFα alone (Fig.
6).
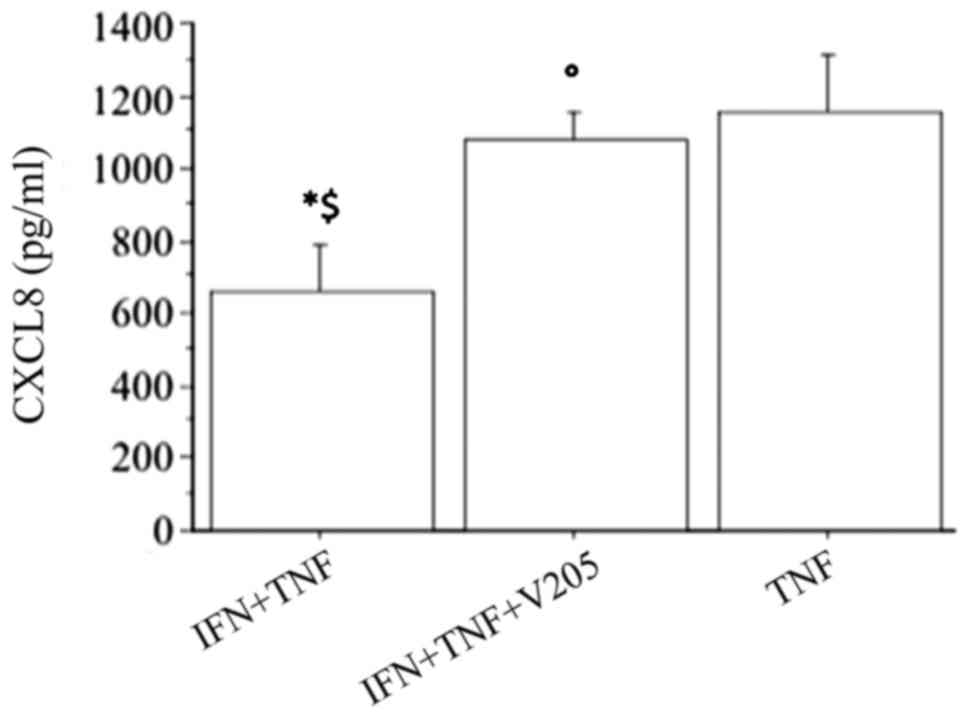
The CXCL8 release synergistically increased
(P<0.0001, by ANOVA), when fibroblasts were treated with
V2O5 (100 nM) with the combination of IFNγ
and TNFα, abolishing the inhibitory effect of IFNγ (Fig. 7).
Fibroblast secretion of CXCL11
CXCL11 release was inducted by IFNγ in a
dose-dependent manner (CXCL11: 0, 31±17, 87±35, 123±47, 187±52
pg/ml; respectively, with IFNγ 0, 500, 1,000, 5,000, 10,000 IU/ml;
ANOVA, P<0.001).
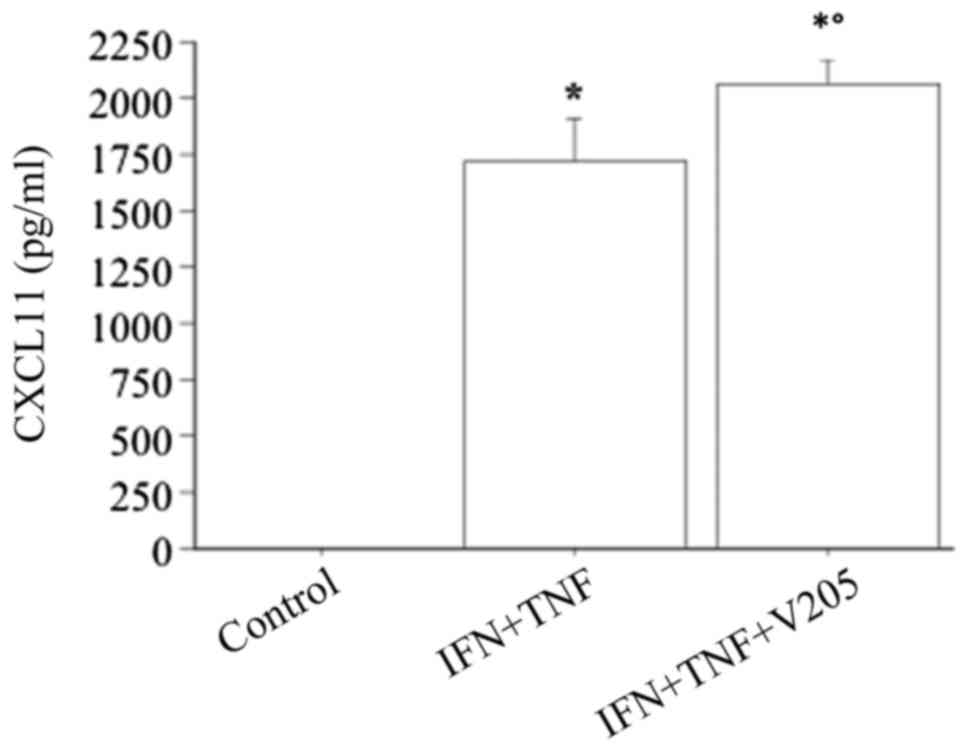
TNFα alone had no effect (chemokine remaining
undetectable), while the combination of IFNγ and TNFα had a
significant synergistic effect on the CXCL11 secretion (CXCL11,
1724±252 vs. 87±35 pg/ml with IFNγ alone, ANOVA, P<0.0001).
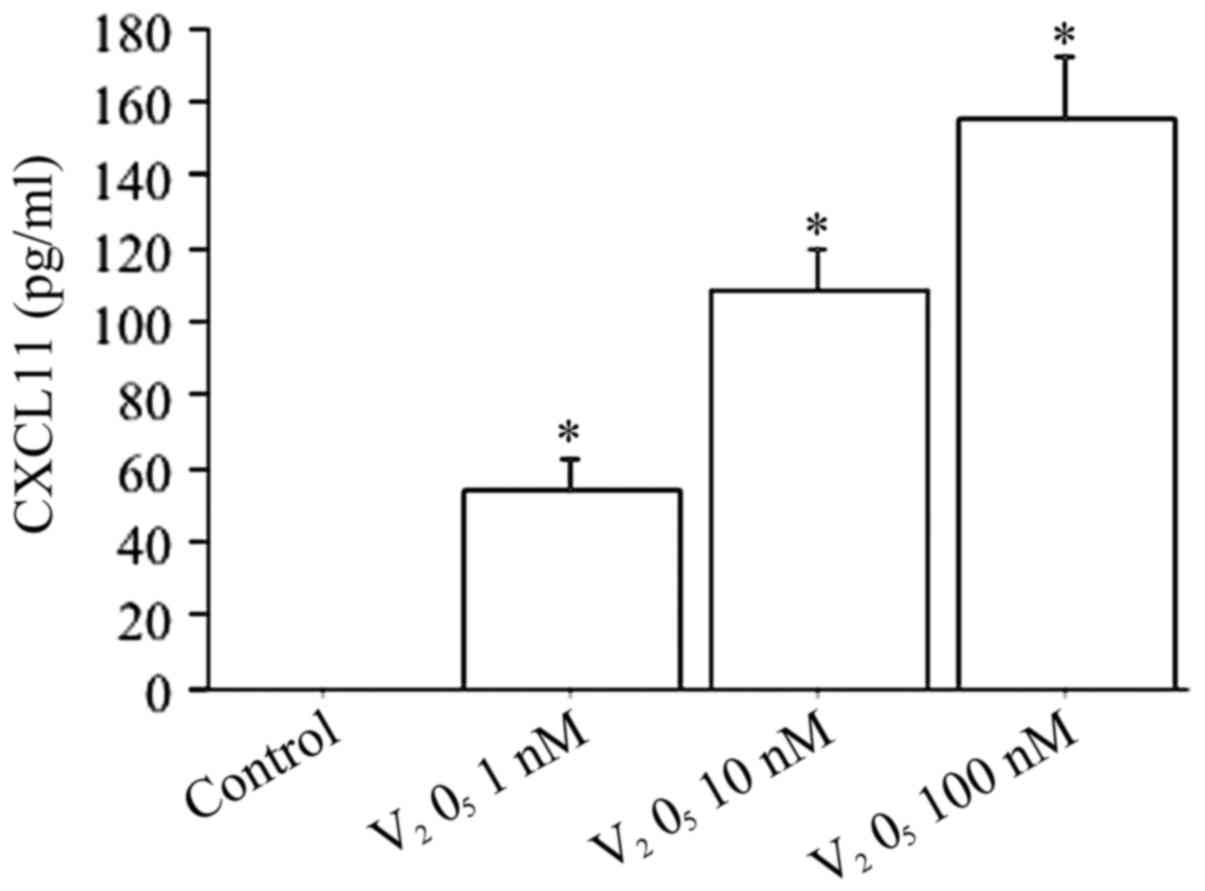
When fibroblasts were treated with increased
V2O5 concentrations (1, 10, 100 nM) the
CXCL11 release was dose-dependently stimulated (ANOVA, P<0.0001)
(Fig. 8).
CXCL11 release was not significantly changed
treating cells with V2O5 (100 nM), together
with TNFα, with respect to V2O5 alone (data
not shown).
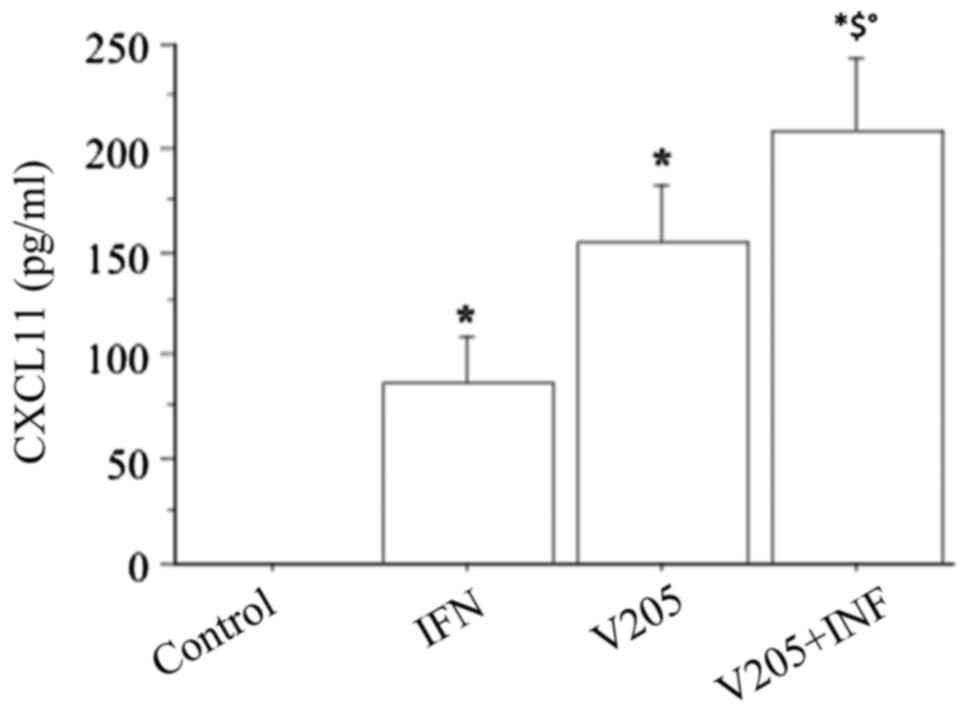
When treating fibroblasts with
V2O5 (100 nM), plus IFNγ, CXCL11
release synergistically increased (P<0.0001, by ANOVA), compared
to both IFN or V2O5 alone (Fig. 9).
CXCL11 release was synergistically increased (ANOVA,
P<0.0001) when fibroblasts were treated with
V2O5 (100 nM), together with IFNγ and TNFα
stimulation, compared to IFNγ+TNFα (Fig. 10).
Discussion
Our results demonstrate that
V2O5 stimulates the secretion of the CXCL8
chemokine, and of the IFNγ dependent chemokine CXCL11, in dermal
fibroblasts, without altering their viability and proliferation.
Moreover, our study confirms that IFNγ and TNFα stimulated in a
different way, the secretion of CXCL8, or CXCL11, chemokines as
expected (18). Interestingly,
V2O5 can synergize with IFNγ and TNFα,
furtherly increasing CXCL11 secretion. In addition,
V2O5 combined with TNFα, elicited a
synergistic influence on CXCL8 chemokine production, abolishing the
inhibitory effect of IFNγ.
These results, on the whole, agreed with the view
that V2O5 is able to induce and perpetuate an
inflammatory disorder in the dermal tissue inducing inflammatory
chemokines secretion (13).
Our findings regarding TNFα, and IFNγ effect in
fibroblasts are in line with the results of another study in a
different type of cells. In fact, it has been recently investigated
if CXCL8 and CXCL10 chemokines secretion by normal human thyrocytes
is dependent upon specific proinflammatory stimuli. CXCL8, but not
CXCL10 (an IFNγ inducible chemokine, of the same class of CXCL11),
was detected in basal conditions. The two chemokines showed
differences in their response to proinflammatory cytokines.
Actually, IFNγ induced a significant CXCL10
secretion, not obtained with TNFα; whereas CXCL8 was secreted in
response to TNFα, being instead inhibited by IFNγ. The combination
of TNFα plus IFNγ synergistically increased the IFNγ-induced
CXCL10 secretion, while reversed the TNFα-induced CXCL8 secretion
(19).
IFNγ-inducible CXC chemokines can be produced by
several types of normal mammalian cells, such as thyrocytes,
fibroblasts, colon epithelial cells, islet cells, and others
(10,13,14,10). However, these cells are not able
to produce the CXC chemokines in basal condition, but only when
stimulated by cytokines, such as IFNγ and TNFα, that are released
in a T-helper 1 (Th1) type inflammatory site, such as the thyroid
at the beginning of Graves' disease, by Th1 activated lymphocytes.
It has been suggested that this process can be involved in the
initiation and the perpetuation of the inflammation in several
autoimmune diseases (10,13,14,10), and considering our results it
can be applied to the thyroid, too.
Our findings about vanadium stimulation of
chemokines agree with those of other studies conducted in different
cell types. V2O5 exposure is a cause of
occupational bronchitis; a study evaluated gene expression profiles
in human lung fibroblasts (in cultures) after
V2O5 exposure with the aim to identify genes
that could be implicated in the bronchial inflammation, repair, and
fibrosis in the pathogenesis of bronchitis. Among the 10 genes
overexpressed by V2O5, also CXCL8,
CXCL9 and CXCL10 were induced (26).
Another study reports that fibroblasts have a role
in the innate immune response to vanadium-induced oxidative stress
through the synthesis of IFNβ and the activation of STAT-1 that
cause an increase of CXCL10 levels (27).
Interestingly vanadium can increase chemokine
secretion in a dose range, from 1 to 100 nM. It could be observed
that normal blood levels of vanadium are ranging from 0.45 to 18.4
nM, and that 100 nM is a dose that might mimick an abnormally high
exposure (28). So we could
hypothesize that the induction of an inflammatory reaction into the
dermal tissue could predispose to the appearance of skin rashes, or
atopic dermatitis.
In conclusion our study shows that
V2O5 can induce CXCL8, and CXCL11 chemokines
secretion into the dermal fibroblasts. Interestingly,
V2O5 synergistically increased the effect of
the IFNγ on CXCL11 secretion. Moreover, V2O5
synergistically increased the effect of the TNFα on CXCL8
secretion, abolishing the inhibitory effect of IFNγ. Overall CXCL8,
and CXCL11 chemokines induction by V2O5 could
lead to the appearance and perpetuation of an inflammatory reaction
into the dermal tissue. Further studies are needed to evaluate
dermal integrity, and manifestations in subjects occupationally
exposed, or living in polluted areas.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PF, SB, AA and SMF made substantial contributions to
the conception and design of the study and to the acquisition of
data. All authors analyzed the data. PF, SB, AA and SMF drafted the
manuscript. AA revised the manuscript. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the study are appropriately investigated and resolved. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all study
participants and the study was approved by the University of Pisa
Ethics Committee.
Consent for publication
Written informed consent was obtained from all
participants for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Occupational Safety and Health
Administration: Occupational Safety and Health Guidelines for
Vanadium Pentoxide. https://www.osha.gov/SLTC/metalsheavy/vanadium.htmlOctober
29–2017
|
|
2
|
Sax NI: Dangerous Properties of Industrial
Materials. 6th edition. Van Nostrand Reinhold Company; New York,
NY: pp. 2717–2720. 1984
|
|
3
|
Ress NB, Chou BJ, Renne RA, Dill JA,
Miller RA, Roycroft JH, Hailey JR, Haseman JK and Bucher JR:
Carcinogenicity of inhaled vanadium pentoxide in F344/N rats and
B6C3F1 mice. Toxicol Sci. 74:287–296. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wörle-Knirsch JM, Kern K, Schleh C,
Adelhelm C, Feldmann C and Krug HF: Nanoparticulate vanadium oxide
potentiated vanadium toxicity in human lung cells. Environ Sci
Technol. 41:331–336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scibior A, Zaporowska H and Ostrowski J:
Selected haematological and biochemical parameters of blood in rats
after subchronic administration of vanadium and/or magnesium in
drinking water. Arch Environ Contam Toxicol. 51:287–295. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
González-Villalva A, Fortoul TI,
Avila-Costa MR, Piñón-Zarate G, Rodriguez-Laraa V, Martínez-Levy G,
Rojas-Lemus M, Bizarro-Nevarez P, Díaz-Bech P, Mussali-Galante P
and Colin-Barenque L: Thrombocytosis induced in mice after subacute
and subchronic V2O5 inhalation. Toxicol Ind Health. 22:113–116.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kobayashi K, Himeno S, Satoh M, Kuroda J,
Shibata N, Seko Y and Hasegawa T: Pentavalent vanadium induces
hepatic metallothionein through interleukin-6-dependent and
-independent mechanisms. Toxicology. 228:162–170. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soazo M and Garcia GB: Vanadium exposure
through lactation produces behavioral alterations and CNS myelin
deficit in neonatal rats. Neurotoxicol Teratol. 29:503–510. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barceloux DG: Vanadium. J Toxicol Clin
Toxicol. 37:265–278. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Antonelli A, Ferri C, Fallahi P, Ferrari
SM, Frascerra S, Sebastiani M, Franzoni F, Galetta F and Ferrannini
E: High values of CXCL10 serum levels in patients with hepatitis C
associated mixed cryoglobulinemia in presence or absence of
autoimmune thyroiditis. Cytokine. 42:137–143. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valyasevi RW, Harteneck DA, Dutton CM and
Bahn RS: Stimulation of adipogenesis, peroxisome
proliferator-activated receptor-gamma (PPARgamma), and thyrotropin
receptor by PPARgamma agonist in human orbital preadipocyte
fibroblasts. J Clin Endocrinol Metab. 87:2352–2358. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Luo J and He S: Induction of MMP-9
release from human dermal fibroblasts by thrombin: Involvement of
JAK/STAT3 signaling pathway in MMP-9 release. BMC Cell Biol.
8:142007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Antonelli A, Rotondi M, Fallahi P,
Romagnani P, Ferrari SM, Buonamano A, Ferrannini E and Serio M:
High levels of circulating CXC chemokine ligand 10 are associated
with chronic autoimmune thyroiditis and hypothyroidism. J Clin
Endocrinol Metab. 89:5496–5499. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kemp EH, Metcalfe RA, Smith KA, Woodroofe
MN, Watson PF and Weetman AP: Detection and localization of
chemokine gene expression in autoimmune thyroid disease. Clin
Endocrinol (Oxf). 59:207–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Antonelli A, Bocci G, La Motta C, Ferrari
SM, Fallahi P, Fioravanti A, Sartini S, Minuto M, Piaggi S, Corti
A, et al: Novel pyrazolopyrimidine derivatives as tyrosine kinase
inhibitors with antitumoral activity in vitro and in vivo in
papillary dedifferentiated thyroid cancer. J Clin Endocrinol Metab.
96:E288–E296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Antonelli A, Ferrari SM, Fallahi P, Berti
P, Materazzi G, Barani L, Marchetti I, Ferrannini E and Miccoli P:
Primary cell cultures from anaplastic thyroid cancer obtained by
fine-needle aspiration used for chemosensitivity tests. Clin
Endocrinol (Oxf). 69:148–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Quent VM, Loessner D, Friis T, Reichert JC
and Hutmacher DW: Discrepancies between metabolic activity and DNA
content as tool to assess cell proliferation in cancer research. J
Cell Mol Med. 14:1003–1013. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Antonelli A, Ferrari SM, Fallahi P,
Frascerra S, Santini E, Franceschini SS and Ferrannini E: Monokine
induced by interferon gamma (IFNgamma) (CXCL9) and IFNgamma
inducible T-cell alpha-chemoattractant (CXCL11) involvement in
Graves' disease and ophthalmopathy: Modulation by peroxisome
proliferator-activated receptor-gamma agonists. J Clin Endocrinol
Metab. 94:1803–1809. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rotondi M, Coperchini F, Pignatti P,
Sideri R, Groppelli G, Leporati P, La Manna L, Magri F, Mariotti S
and Chiovato L: Interferon-γ and tumor necrosis factor-α sustain
secretion of specific CXC chemokines in human thyrocytes: A first
step toward a differentiation between autoimmune and tumor-related
inflammation? J Clin Endocrinol Metab. 98:308–313. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Antonelli A, Ferrari SM, Frascerra S,
Pupilli C, Mancusi C, Metelli MR, Orlando C, Ferrannini E and
Fallahi P: CXCL9 and CXCL11 chemokines modulation by peroxisome
proliferator-activated receptor-alpha agonists secretion in Graves'
and normal thyrocytes. J Clin Endocrinol Metab. 95:E413–E420. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garcià-Lòpez MA, Sancho D, Sànchez-Madrid
F and Marazuela M: Thyrocytes from autoimmune thyroid disorders
produce the chemokines IP-10 and Mig and attract CXCR3+
lymphocytes. J Clin Endocrinol Metab. 86:5008–5016. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Antonelli A, Ferrari SM, Corrado A,
Ferrannini E and Fallahi P: CXCR3, CXCL10 and type 1 diabetes.
Cytokine Growth Factor Rev. 25:57–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Antonelli A, Ferrari SM, Giuggioli D,
Ferrannini E, Ferri C and Fallahi P: Chemokine (C-X-C motif) ligand
(CXCL)10 in autoimmune diseases. Autoimmun Rev. 13:272–280. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Antonelli A, Fallahi P, Delle Sedie A,
Ferrari SM, Maccheroni M, Bombardieri S, Riente L and Ferrannini E:
High values of Th1 (CXCL10) and Th2 (CCL2) chemokines in patients
with psoriatic arthtritis. Clin Exp Rheumatol. 27:22–27.
2009.PubMed/NCBI
|
|
25
|
Fallahi P, Ferrari SM, Ruffilli I, Elia G,
Biricotti M, Vita R, Benvenga S and Antonelli A: The association of
other autoimmune diseases in patients with autoimmune thyroiditis:
Review of the literature and report of a large series of patients.
Autoimmun Rev. 15:1125–1128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ingram JL, Antao-Menezes A, Turpin EA,
Wallace DG, Mangum JB, Pluta LJ, Thomas RS and Bonner JC: Genomic
analysis of human lung fibroblasts exposed to vanadium pentoxide to
identify candidate genes for occupational bronchitis. Respir Res.
8:342007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Antao-Menezes A, Turpin EA, Bost PC,
Ryman-Rasmussen JP and Bonner JC: STAT-1 signaling in human lung
fibroblasts is induced by vanadium pentoxide through an IFN-beta
autocrine loop. J Immunol. 180:4200–4207. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sabbioni E, Kuèera J, Pietra R and
Vesterberg O: A critical review on normal concentrations of
vanadium in human blood, serum, and urine. Sci Total Environ.
188:49–58. 1996. View Article : Google Scholar : PubMed/NCBI
|