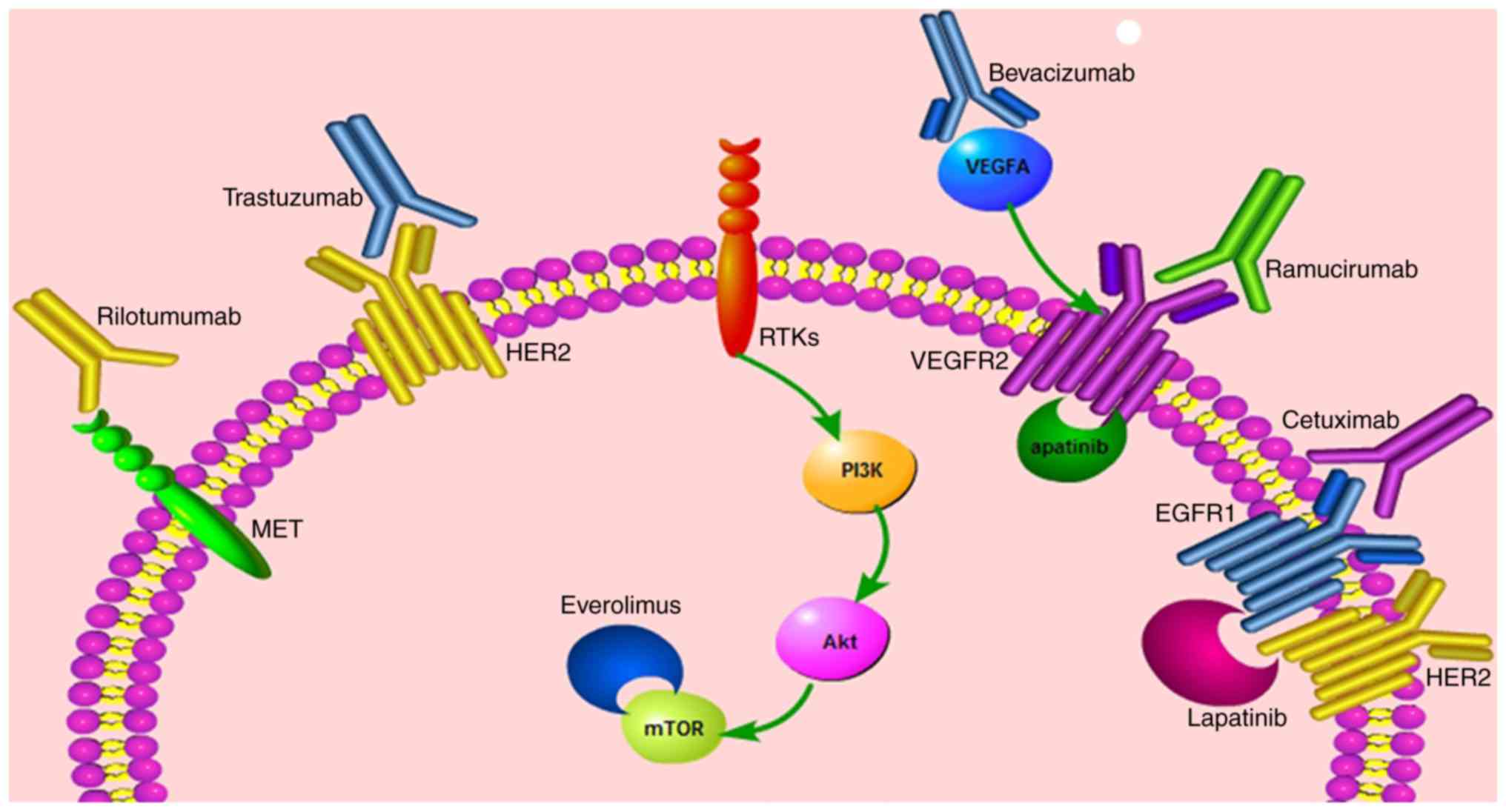
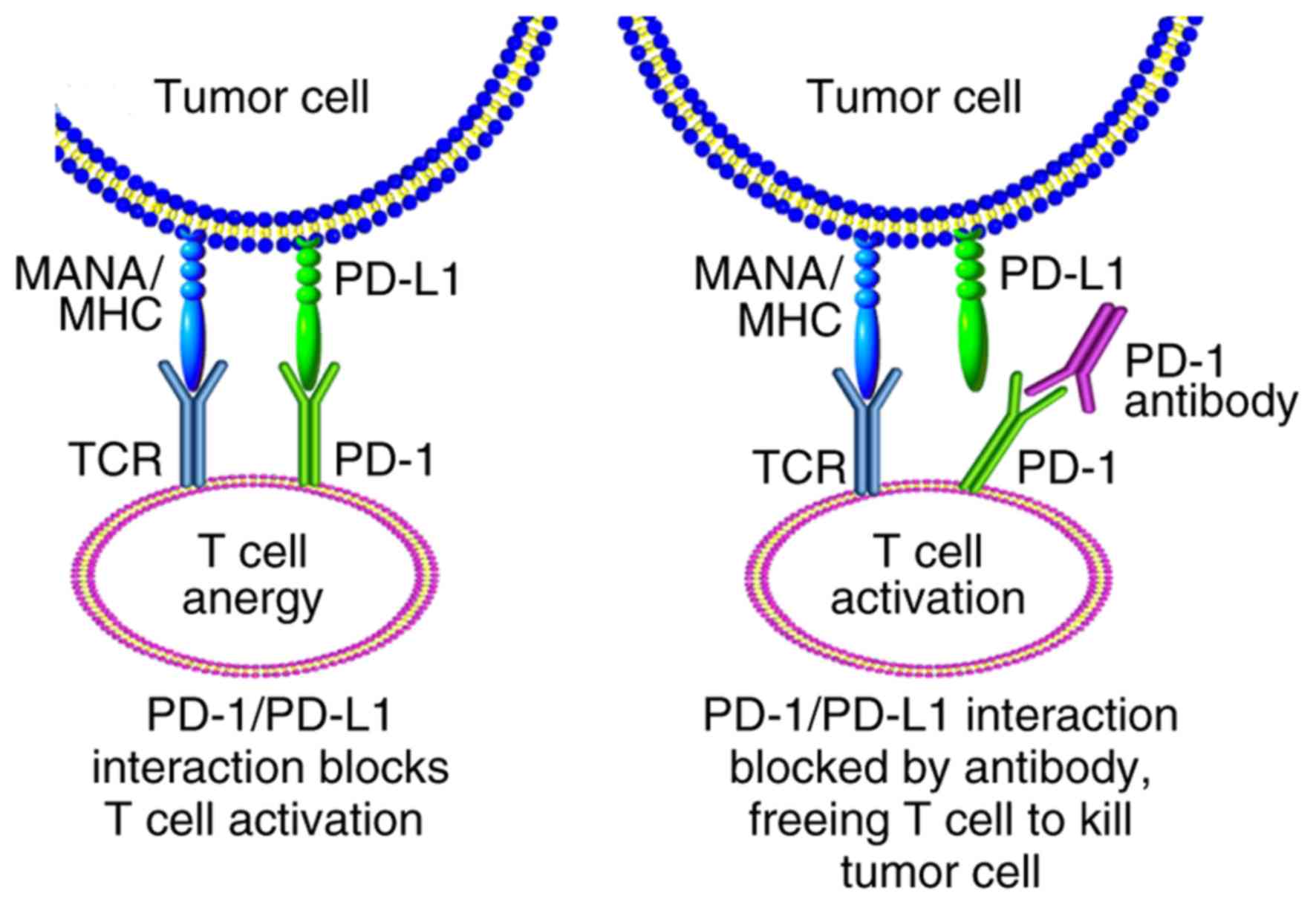
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ajani JA, Mayer RJ, Ota DM, Steele GD,
Evans D, Roh M, Sugarbaker DJ, Dumas P, Gray C, Vena DA, et al:
Preoperative and postoperative combination chemotherapy for
potentially resectable gastric carcinoma. J Natl Cancer Inst.
85:1839–1844. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leichman L, Silberman H, Leichman CG,
Spears CP, Ray M, Muggia FM, Kiyabu M, Radin R, Laine L, Stain S,
et al: Preoperative systemic chemotherapy followed by adjuvant
postoperative intraperitoneal therapy for gastric cancer: A
University of Southern California pilot program. J Clin Oncol.
10:1933–1942. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chau I, Norman AR, Cunningham D, Waters
JS, Oates J and Ross PJ: Multivariate prognostic factor analysis in
locally advanced and metastatic esophago-gastric cancer-pooled
analysis from three multicenter, randomized, controlled trials
using individual patient data. J Clin Oncol. 22:2395–2403. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Livshits Z, Rao RB and Smith SW: An
approach to chemotherapy-associated toxicity. Emerg Med Clin North
Am. 32:167–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mangia A, Caldarola L, Dell'Endice S,
Scarpi E, Saragoni L, Monti M, Santini D, Brunetti O, Simone G and
Silvestris N: The potential predictive role of nuclear NHERF1
expression in advanced gastric cancer patients treated with
epirubicin/oxaliplatin/capecitabine first line chemotherapy. Cancer
Biol Ther. 16:1140–1147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tebbutt NC, Parry MM, Zannino D,
Strickland AH, Van Hazel GA, Pavlakis N, Ganju V, Mellor D,
Dobrovic A and Gebski VJ: Australasian Gastro-Intestinal Trials
Group (AGITG): Docetaxel plus cetuximab as second-line treatment
for docetaxel-refractory oesophagogastric cancer: The AGITG ATTAX2
trial. Br J Cancer. 108:771–774. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang ZD, Kong Y, Yang W, Zhang B, Zhang
YL, Ma EM, Liu HX, Chen XB and Hua YW: Clinical evaluation of
cetuximab combined with an S-1 and oxaliplatin regimen for Chinese
patients with advanced gastric cancer. World J Surg Oncol.
12:1152014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lordick F, Kang YK, Chung HC, Salman P, Oh
SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, et
al: Capecitabine and cisplatin with or without cetuximab for
patients with previously untreated advanced gastric cancer
(EXPAND): A randomised, open-label phase 3 trial. Lancet Oncol.
14:490–499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Waddell T, Chau I, Cunningham D, Gonzalez
D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G,
Wadsley J, et al: Epirubicin, oxaliplatin, and capecitabine with or
without panitumumab for patients with previously untreated advanced
oesophagogastric cancer (REAL3): A randomised, open-label phase 3
trial. Lancet Oncol. 14:481–489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hotz B, Keilholz U, Fusi A, Buhr HJ and
Hotz HG: In vitro and in vivo antitumor activity of cetuximab in
human gastric cancer cell lines in relation to epidermal growth
factor receptor (EGFR) expression and mutational phenotype. Gastric
Cancer. 15:252–264. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moehler M, Mueller A, Trarbach T, Lordick
F, Seufferlein T, Kubicka S, Geissler M, Schwarz S, Galle PR and
Kanzler S: German Arbeitsgemeinschaft Internistische Onkologie:
Cetuximab with irinotecan, folinic acid and 5-fluorouracil as
first-line treatment in advanced gastroesophageal cancer: A
prospective multi-center biomarker-oriented phase II study. Ann
Oncol. 22:1358–1366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi AH, Kim J and Chao J: Perioperative
chemotherapy for resectable gastric cancer: MAGIC and beyond. World
J Gastroenterol. 21:7343–7348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pietrantonio F, De Braud F, Da Prat V,
Perrone F, Pierotti MA, Gariboldi M, Fanetti G, Biondani P,
Pellegrinelli A, Bossi I and Di Bartolomeo M: A review on
biomarkers for prediction of treatment outcome in gastric cancer.
Anticancer Res. 33:1257–1266. 2013.PubMed/NCBI
|
|
16
|
Gomez-Martin C, Plaza JC, Pazo-Cid R,
Salud A, Pons F, Fonseca P, Leon A, Alsina M, Visa L, Rivera F, et
al: Level of HER2 gene amplification predicts response and overall
survival in HER2-positive advanced gastric cancer treated with
trastuzumab. J Clin Oncol. 31:4445–4452. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ock CY, Lee KW, Kim JW, Kim JS, Kim TY,
Lee KH, Han SW, Im SA, Kim TY, Kim WH, et al: Optimal patient
selection for trastuzumab treatment in HER2-positive advanced
gastric cancer. Clin Cancer Res. 21:2520–2529. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JY, Hong M, Kim ST, Park SH, Kang WK,
Kim KM and Lee J: The impact of concomitant genomic alterations on
treatment outcome for trastuzumab therapy in HER2-positive gastric
cancer. Sci Rep. 5:92892015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oyama K, Fushida S, Tsukada T, Kinoshita
J, Watanabe T, Shoji M, Nakanuma S, Okamoto K, Sakai S, Makino I,
et al: Evaluation of serum HER2-ECD levels in patients with gastric
cancer. J Gastroenterol. 50:41–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Azambuja E, Holmes AP, Piccart-Gebhart
M, Holmes E, Di Cosimo S, Swaby RF, Untch M, Jackisch C, Lang I,
Smith I, et al: Lapatinib with trastuzumab for HER2-positive early
breast cancer (NeoALTTO): Survival outcomes of a randomised,
open-label, multicentre, phase 3 trial and their association with
pathological complete response. Lancet Oncol. 15:1137–1146. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hecht JR, Bang YJ, Qin SK, Chung HC, Xu
JM, Park JO, Jeziorski K, Shparyk Y, Hoff PM, Sobrero A, et al:
Lapatinib in combination with capecitabine plus oxaliplatin in
human epidermal growth factor receptor 2-positive advanced or
metastatic gastric, esophageal, or gastroesophageal adenocarcinoma:
TRIO-013/LOGiC-A randomized phase III trial. J Clin Oncol.
34:443–451. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Satoh T, Xu RH, Chung HC, Sun GP, Doi T,
Xu JM, Tsuji A, Omuro Y, Li J, Wang JW, et al: Lapatinib plus
paclitaxel versus paclitaxel alone in the second-line treatment of
HER2-amplified advanced gastric cancer in Asian populations:
TyTAN-a randomized, phase III study. J Clin Oncol. 32:2039–2049.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oshima Y, Tanaka H, Murakami H, Ito Y,
Furuya T, Kondo E, Kodera Y and Nakanishi H: Lapatinib
sensitivities of two novel trastuzumab-resistant HER2
gene-amplified gastric cancer cell lines. Gastric Cancer.
17:450–462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eto K, Iwatsuki M, Watanabe M, Ida S,
Ishimoto T, Iwagami S, Baba Y, Sakamoto Y, Miyamoto Y, Yoshida N
and Baba H: The microRNA-21/PTEN pathway regulates the sensitivity
of HER2-positive gastric cancer cells to trastuzumab. Ann Surg
Oncol. 21:343–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang X, Park JS, Park KH, Kim KH, Jung M,
Chung HC, Rha SY and Kim HS: PTEN deficiency as a predictive
biomarker of resistance to HER2-targeted therapy in advanced
gastric cancer. Oncology. 88:76–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hong YS, Kim J, Pectasides E, Fox C, Hong
SW, Ma Q, Wong GS, Peng S, Stachler MD, Thorner AR, et al: Src
mutation induces acquired lapatinib resistance in ERBB2-amplified
human gastroesophageal adenocarcinoma models. PLoS One.
9:e1094402014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sanford M: Trastuzumab: A review of its
use in HER2-positive advanced gastric cancer. Drugs. 73:1605–1615.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gomez-Martin C, Lopez-Rios F, Aparicio J,
Barriuso J, García-Carbonero R, Pazo R, Rivera F, Salgado M, Salud
A, Vázquez-Sequeiros E and Lordick F: A critical review of
HER2-positive gastric cancer evaluation and treatment: from
trastuzumab, and beyond. Cancer Lett. 351:30–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sanford M: Subcutaneous trastuzumab: A
review of its use in HER2-positive breast cancer. Target Oncol.
9:85–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim HP, Han SW, Song SH, Jeong EG, Lee MY,
Hwang D, Im SA, Bang YJ and Kim TY: Testican-1-mediated
epithelial-mesenchymal transition signaling confers acquired
resistance to lapatinib in HER2-positive gastric cancer. Oncogene.
33:3334–3341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
De Silva N, Schulz L, Paterson A, Qain W,
Secrier M, Godfrey E, Cheow H, O'Donovan M, Lao-Sirieix P,
Jobanputra M, et al: Molecular effects of Lapatinib in the
treatment of HER2 overexpressing oesophago-gastric adenocarcinoma.
Br J Cancer. 113:1305–1312. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abdel-Rahman O: Targeting vascular
endothelial growth factor (VEGF) pathway in gastric cancer:
Preclinical and clinical aspects. Crit Rev Oncol Hematol. 93:18–27.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Escudier B, Pluzanska A, Koralewski P,
Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar
B, Bajetta E, et al: Bevacizumab plus interferon alfa-2a for
treatment of metastatic renal cell carcinoma: A randomised,
double-blind phase III trial. Lancet. 370:2103–2111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qu CY, Zheng Y, Zhou M, Zhang Y, Shen F,
Cao J and Xu LM: Value of bevacizumab in treatment of colorectal
cancer: A meta-analysis. World J Gastroenterol. 21:5072–5080. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lauro S, Onesti CE, Righini R and
Marchetti P: The use of bevacizumab in non-small cell lung cancer:
An update. Anticancer Res. 34:1537–1545. 2014.PubMed/NCBI
|
|
36
|
Ohtsu A, Shah MA, Van Cutsem E, Rha SY,
Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, et al:
Bevacizumab in combination with chemotherapy as first-line therapy
in advanced gastric cancer: A randomized, double-blind,
placebo-controlled phase III study. J Clin Oncol. 29:3968–3976.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shen L, Li J, Xu J, Pan H, Dai G, Qin S,
Wang L, Wang J, Yang Z, Shu Y, et al: Bevacizumab plus capecitabine
and cisplatin in Chinese patients with inoperable locally advanced
or metastatic gastric or gastroesophageal junction cancer:
Randomized, double-blind, phase III study (AVATAR study). Gastric
Cancer. 18:168–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamashita-Kashima Y, Fujimoto-Ouchi K,
Yorozu K, Kurasawa M, Yanagisawa M, Yasuno H and Mori K: Biomarkers
for antitumor activity of bevacizumab in gastric cancer models. BMC
Cancer. 12:372012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Van Cutsem E, de Haas S, Kang YK, Ohtsu A,
Tebbutt NC, Xu Ming J, Yong Peng W, Langer B, Delmar P, Scherer SJ
and Shah MA: Bevacizumab in combination with chemotherapy as
first-line therapy in advanced gastric cancer: A biomarker
evaluation from the AVAGAST randomized phase III trial. J Clin
Oncol. 30:2119–2127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Han K, Jin J, Maia M, Lowe J, Sersch MA
and Allison DE: Lower exposure and faster clearance of bevacizumab
in gastric cancer and the impact of patient variables: Analysis of
individual data from AVAGAST phase III trial. AAPS J. 16:1056–1063.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hacker UT, Escalona-Espinosa L, Consalvo
N, Goede V, Schiffmann L, Scherer SJ, Hedge P, Van Cutsem E,
Coutelle O and Büning H: Evaluation of Angiopoietin-2 as a
biomarker in gastric cancer: results from the randomised phase III
AVAGAST trial. Br J Cancer. 114:855–862. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Poole RM and Vaidya A: Ramucirumab: First
global approval. Drugs. 74:1047–1058. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fuchs CS, Tomasek J, Yong CJ, Dumitru F,
Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry
DR, et al: Ramucirumab monotherapy for previously treated advanced
gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An
international, randomised, multicentre, placebo-controlled, phase 3
trial. Lancet. 383:31–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Iacovelli R, Pietrantonio F, Farcomeni A,
Maggi C, Palazzo A, Ricchini F, de Braud F and Di Bartolomeo M:
Chemotherapy or targeted therapy as second-line treatment of
advanced gastric cancer. A systematic review and meta-analysis of
published studies. PLoS One. 9:e1089402014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y,
Liu W, Tong J, Liu Y, Xu R, et al: Randomized, double-blind,
placebo-controlled phase III trial of apatinib in patients with
chemotherapy-refractory advanced or metastatic adenocarcinoma of
the stomach or gastroesophageal junction. J Clin Oncol.
34:1448–1454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fioroni I, Dell'Aquila E, Pantano F,
Intagliata S, Caricato M, Vincenzi B, Coppola R, Santini D and
Tonini G: Role of c-mesenchymal-epithelial transition pathway in
gastric cancer. Expert Opin Pharmacother. 16:1195–1207. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang Y, Doshi S and Zhu M:
Pharmacokinetics and pharmacodynamics of rilotumumab: a decade of
experience in preclinical and clinical cancer research. Br J Clin
Pharmacol. 80:957–964. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Iveson T, Donehower RC, Davidenko I,
Tjulandin S, Deptala A, Harrison M, Nirni S, Lakshmaiah K, Thomas
A, Jiang Y, et al: Rilotumumab in combination with epirubicin,
cisplatin, and capecitabine as first-line treatment for gastric or
oesophagogastric junction adenocarcinoma: An open-label, dose
de-escalation phase 1b study and a double-blind, randomised phase 2
study. Lancet Oncol. 15:1007–1018. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Catenacci DVT, Tebbutt NC, Davidenko I,
Murad AM, Al-Batran SE, Ilson DH, Tjulandin S, Gotovkin E,
Karaszewska B, Bondarenko I, et al: Rilotumumab plus epirubicin,
cisplatin, and capecitabine as first-line therapy in advanced
MET-positive gastric or gastro-oesophageal junction cancer
(RILOMET-1): A randomised, double-blind, placebo-controlled, phase
3 trial. Lancet Oncol. 18:1467–1482. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shah MA, Bang YJ, Lordick F, Alsina M,
Chen M, Hack SP, Bruey JM, Smith D, McCaffery I, Shames DS, et al:
Effect of fluorouracil, leucovorin, and oxaliplatin with or without
onartuzumab in HER2-negative, MET-positive gastroesophageal
adenocarcinoma: The METGastric randomized clinical trial. JAMA
Oncol. 3:620–627. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shah MA, Wainberg ZA, Catenacci DV,
Hochster HS, Ford J, Kunz P, Lee FC, Kallender H, Cecchi F, Rabe
DC, et al: Phase II study evaluating 2 dosing schedules of oral
foretinib (GSK1363089), cMET/VEGFR2 inhibitor, in patients with
metastatic gastric cancer. PLoS One. 8:e540142013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu Z, Zhang Z, Ge X, Lin Y, Dai C, Chang
J, Liu X, Geng R, Wang C, Chen H, et al: Identification of
short-form RON as a novel intrinsic resistance mechanism for
anti-MET therapy in MET-positive gastric cancer. Oncotarget.
6:40519–40534. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kwak EL, Ahronian LG, Siravegna G,
Mussolin B, Godfrey JT, Clark JW, Blaszkowsky LS, Ryan DP, Lennerz
JK, Iafrate AJ, et al: Molecular heterogeneity and receptor
coamplification drive resistance to targeted therapy in
MET-amplified esophagogastric cancer. Cancer Discov. 5:1271–1281.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ji F, Liu X, Wu Y, Fang X and Huang G:
Overexpression of PI3K p110α contributes to acquired resistance to
MET inhibitor, in MET-amplified SNU-5 gastric xenografts. Drug Des
Devel Ther. 9:5697–5704. 2015.PubMed/NCBI
|
|
56
|
Musiani D, Konda JD, Pavan S, Torchiaro E,
Sassi F, Noghero A, Erriquez J, Perera T, Olivero M and Di Renzo
MF: Heat-shock protein 27 (HSP27, HSPB1) is up-regulated by MET
kinase inhibitors and confers resistance to MET-targeted therapy.
FASEB J. 28:4055–4067. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen CT, Kim H, Liska D, Gao S,
Christensen JG and Weiser MR: MET activation mediates resistance to
lapatinib inhibition of HER2-amplified gastric cancer cells. Mol
Cancer Ther. 11:660–669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sukawa Y, Yamamoto H, Nosho K, Ito M,
Igarashi H, Naito T, Mitsuhashi K, Matsunaga Y, Takahashi T, Mikami
M, et al: HER2 expression and PI3K-Akt pathway alterations in
gastric cancer. Digestion. 89:12–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
An X, Wang F, Shao Q, Wang FH, Wang ZQ,
Wang ZQ, Chen C, Li C, Luo HY, Zhang DS, et al: MET amplification
is not rare and predicts unfavorable clinical outcomes in patients
with recurrent/metastatic gastric cancer after chemotherapy.
Cancer. 120:675–682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Xu K, Liu P and Wei W: mTOR signaling in
tumorigenesis. Biochim Biophys Acta. 1846:638–654. 2014.PubMed/NCBI
|
|
61
|
Hasskarl J: Everolimus. Recent Results
Cancer Res. 201:373–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung
HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K, et al: Everolimus
for previously treated advanced gastric cancer: results of the
randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol.
31:3935–3943. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wainberg ZA, Soares HP, Patel R, DiCarlo
B, Park DJ, Liem A, Wang HJ, Yonemoto L, Martinez D, Laux I, et al:
Phase II trial of everolimus in patients with refractory metastatic
adenocarcinoma of the esophagus, gastroesophageal junction and
stomach: Possible role for predictive biomarkers. Cancer Chemother
Pharmacol. 76:61–67. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhu Y, Tian T, Zou J, Wang Q, Li Z, Li Y,
Liu X, Dong B, Li N, Gao J and Shen L: Dual PI3K/mTOR inhibitor
BEZ235 exerts extensive antitumor activity in HER2-positive gastric
cancer. BMC Cancer. 15:8942015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Fuereder T, Jaeger-Lansky A, Hoeflmayer D,
Preusser M, Strommer S, Cejka D, Koehrer S, Crevenna R and Wacheck
V: mTOR inhibition by everolimus counteracts VEGF induction by
sunitinib and improves anti-tumor activity against gastric cancer
in vivo. Cancer Lett. 296:249–256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jaeger-Lansky A, Cejka D, Ying L, Preusser
M, Hoeflmayer D, Fuereder T, Koehrer S and Wacheck V: Effects of
vatalanib on tumor growth can be potentiated by mTOR blockade in
vivo. Cancer Biol Ther. 9:919–927. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Muro K, Chung HC, Shankaran V, Geva R,
Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, et al:
Pembrolizumab for patients with PD-L1-positive advanced gastric
cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial.
Lancet Oncol. 17:717–726. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T,
Machado M, Sun W, Jalal SI, Shah MA, Metges JP, et al: Safety and
efficacy of pembrolizumab monotherapy in patients with previously
treated advanced gastric and gastroesophageal junction cancer:
Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. Mar 15–2018.(Epub
ahead of print). View Article : Google Scholar :
|
|
71
|
Ohtsu A, Tabernero J, Bang YJ, Fuchs CS,
Sun L, Wang Z, Csiki I, Koshiji and Cutsem EV: Pembrolizumab versus
paclitaxel as second-line therapy for advanced gastric or
gastroesophageal junction (GEJ) adenocarcinoma: Phase 3 KEYNOTE-061
study. J Clin Oncol. 34 15 Suppl:TPS41372016. View Article : Google Scholar
|
|
72
|
Tabernero J, Bang YJ, Fuchs CS, Ohtsu A,
Kher U, Lam B, Koshiji M and Cutsem V: KEYNOTE-062: Phase III study
of pembrolizumab alone or in combination with chemotherapy versus
chemotherapy alone as first-line therapy for advanced gastric or
gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol. 34 4
Suppl:TPS1852016. View Article : Google Scholar
|
|
73
|
Camacho LH: CTLA-4 blockade with
ipilimumab: Biology, safety, efficacy, and future considerations.
Cancer Med. 4:661–672. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Moehler MH, Janjigian YY, Adenis A, Aucoin
JS, Boku N, Chau I, Cleary JM, Feeney KT, Franke FA, Mendez GA, et
al: CheckMate 649: A randomized, multicenter, open-label, phase 3
study of nivolumab (nivo) + ipilimumab (ipi) or nivo + chemotherapy
(CTX) vs CTX alone in pts with previously untreated advanced (adv)
gastric (G) or gastroesophageal junction (GEJ) cancer. J Clin
Oncol. 35 15 Suppl:TPS41322017.
|