Introduction
Colorectal cancer (CRC) is the third most common
type of malignant tumor in the digestive tract and poses a
substantial threat to human health worldwide (1). In addition, CRC is the third highest
contributor to cancer-associated mortality worldwide, with ~26,580
and 24,790 mortalities annually for males and females, respectively
(2). It is reported that ~20% of
all patients with CRC are diagnosed with rectal cancer, while ~45%
of patients with rectal cancer are further diagnosed with locally
advanced rectal cancer (LARC) (3,4).
Local recurrence is common in patients with LARC.
Neoadjuvant chemoradiotherapy (nCRT) may prevent local recurrence
and improve overall survival, and has therefore emerged as the
standard treatment for patients with LARC, followed by surgical
resection (5). Overall, following
nCRT, ~15–20% of patients with LARC demonstrate a complete response
(CR), with complete tumor eradication (6–8)
while 20% of patients exhibit a poor response to nCRT (9–11).
Patients with a CR to nCRT subsequently undergo a simple
watch-and-wait procedure or endoscopic resection (12). Patients with a poor response to
nCRT are associated with substantial adverse effects and high
medical costs (13). Thus,
identifying valid predictive biomarkers is of high importance.
MicroRNAs (miRNAs/miR) have been proposed as
potential biomarkers for predicting the response to treatment and
prognosis in rectal cancers. The expression of miR-143 and miR-145
is reported to be significantly upregulated in rectal tumor tissues
following treatment, and miR-145 expression demonstrated a close
association with tumor regression in patients with rectal cancer
(14). Additionally, the distinct
expression signatures of miR-16, miR-590-5p and miR-153 in
pretreated rectal cancer tissue may predict the response to
treatment, miR-519c-3p and miR-561 accurately predict a good or
poor response to nCRT in patients with rectal cancer (15). Furthermore, miR-21-5p was
demonstrated to be overexpressed in patients with CR (16). However, the role of these
biomarkers has not been fully clarified.
Della Vittoria Scarpati et al (17), reported a set of 13 miRNAs with
specific signatures that were associated with a pathological CR
(pCR) to nCRT based on microarray data (accession no. GSE29298).
However, the mechanisms of the biomarkers remain unclear.
Therefore, the present study downloaded this data from the Gene
Expression Omnibus (GEO) database. Based on the miRNA expression
profile, differentially expressed miRNAs were identified between
pCR and no pCR (incomplete response) groups, and their target genes
were predicted, followed by Kyoto Encyclopedia of Genes and Genomes
(KEGG) pathway enrichment analysis, miRNA co-regulatory network
construction and disease-associated gene analysis. The aim of the
current study was to investigate specific miRNA signatures as
potential biomarkers for predicting a CR to nCRT in rectal cancer
and to determine the potential mechanisms of the miRNAs.
Materials and methods
Data acquisition
The miRNA expression profile of GSE29298 (17) was downloaded from the GEO
(http://www.ncbi.nlm.nih.gov/geo/), which
was sequenced on an Agilent-021827 Human miRNA Microarray platform
(Agilent Technologies, Inc., Santa Clara, CA, USA). The miRNA
expression profile was analyzed by microarray using fresh frozen
biopsies. A total of 38 patients with LARC (cT3-4/N+) were treated
with capecitabine-oxaliplatin and pelvic conformal radiotherapy
(45cGy) followed by surgery (after 6–8 weeks). Pathological
responses were scored according to the tumor regression grade
(TRG), as described by Mandard (9). The number of patients with TRG1,
TRG2, TRG3 and TRG4 was 9, 16, 10 and 3, respectively. TRG1
indicates pCR, which represents complete tumor regression, while
TRG >1 represents an incomplete response (no pCR) with residual
tumor cells. Patients were divided into two groups: pCR group
(TRG1, n=9) and no pCR group (TRG >1, n=29).
Data preprocessing
Microarray data were preprocessed using the
Bioconductor package (version 3.5) Limma (version 3.28.21)
(18), which involved background
correction, normalization and expression calculation. The miRNA ID
was transformed from the probe ID according to the probe annotation
file. The most recent human miRNA annotation file was downloaded
from the miRBase Database (19,20)
to obtain the miRNA name from the miRNA ID.
Identification of differentially
expressed miRNAs
Differentially expressed miRNAs in the pCR group
compared with the no pCR group were identified by non-paired
Student's t test analysis and linear models using the Bioconductor
package Limma. P<0.05 and|log2 fold change (FC)|≥0.58
were set as the cutoff criteria for differentially expressed
miRNAs.
Prediction of target genes for
differentially expressed miRNAs
Target genes regulated by the differentially
expressed miRNAs were predicted by ‘validated target module’ using
the miRWalk 2.0 online tool (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2)
(21).
KEGG pathway enrichment analysis
KEGG pathways enriched in miRNA target genes were
analyzed using the clusterProfiler (version 3.3.1) of R package
(version 3.3.2) (22). Pathways
involving miRNAs were determined. The P-value was calculated using
a hypergeometric distribution statistical approach based on the
KEGG.db annotation package (version 3.2.3) (23). The cutoff value for a significant
pathway was set at P<0.05.
miRNA co-regulatory network
According to the association between miRNAs and
target genes, miRNA-miRNA pairs that regulate the same target genes
were screened and an miRNA co-regulatory network was constructed by
Cytoscape software (version 3.2.0) (24).
Disease-associated gene analysis
Marker or therapeutic genes associated with
colorectal neoplasms were downloaded from the Comparative
Toxicogenomics Database (25).
Subsequently, the regulatory network between differentially
expressed miRNAs and disease-associated genes was constructed by
Cytoscape software (version 3.2.0) (24).
Results
Differentially expressed miRNAs
Following preprocessing, 835 miRNAs were identified
and subjected to non-paired Student's t test analysis. According to
P<0.05 and |log2FC| ≥0.58, a total of 41 miRNAs were
differentially expressed in the pCR group compared with the no pCR
group, including 36 upregulated and 5 downregulated miRNAs. The
heat map demonstrated that the two groups were distinguished by the
expression profiles of 41 differentially expressed miRNAs (Fig. 1).
Target genes regulated by
differentially expressed miRNAs
As indicated in Table
I, 3,989 target genes were predicted to be regulated by 41
differentially expressed miRNAs. The results revealed that a single
miRNA regulates multiple genes. miR-548c-5p, miR-548d-5p and
miR-663a were demonstrated to regulate the greatest number of
genes, and were reported to regulate 274, 277 and 127 genes,
respectively.
 | Table I.Target genes regulated by microRNAs
in pathological complete response group compared with the
pathological incomplete response group. |
Table I.
Target genes regulated by microRNAs
in pathological complete response group compared with the
pathological incomplete response group.
| A, Upregulated
miRNAs |
|---|
|
|---|
| miRNA | Target gene
count |
|---|
| hsa-miR-1183 | 108 |
|
hsa-miR-1224-5p | 84 |
| hsa-miR-1246 | 52 |
|
hsa-miR-125a-3p | 292 |
| hsa-miR-1268a | 49 |
| hsa-miR-134-5p | 95 |
| hsa-miR-1471 |
3 |
| hsa-miR-188-5p | 136 |
| hsa-miR-483-5p | 145 |
|
hsa-miR-548c-5p | 274 |
|
hsa-miR-548d-5p | 277 |
| hsa-miR-575 | 141 |
| hsa-miR-622 | 118 |
| hsa-miR-630 | 83 |
| hsa-miR-659-3p | 68 |
| hsa-miR-663a | 127 |
| hsa-miR-671-5p | 164 |
| hsa-miR-765 | 355 |
| hsa-miR-1182 | 82 |
|
hsa-miR-1207-5p | 223 |
|
hsa-miR-1225-5p | 49 |
|
hsa-miR-1226-5p | 78 |
| hsa-miR-1275 | 155 |
| hsa-miR-1299 | 84 |
| hsa-miR-149-3p | 733 |
| hsa-miR-150-3p | 44 |
|
hsa-miR-1909-5p | 50 |
|
hsa-miR-1914-3p | 117 |
| hsa-miR-202-3p | 253 |
| hsa-miR-370-3p | 111 |
|
hsa-miR-371a-5p | 324 |
| hsa-miR-494-3p | 154 |
| hsa-miR-557 | 207 |
| hsa-miR-584-5p | 79 |
| hsa-miR-601 | 18 |
| hsa-miR-623 | 262 |
|
| B, Downregulated
miRNAs |
|
| miRNA | Target gene
count |
|
| hsa-let-7e-5p | 711 |
| hsa-miR-1260a | 170 |
| hsa-miR-192-3p | 149 |
| hsa-miR-26a-5p | 541 |
| hsa-miR-30b-5p | 477 |
Significant KEGG pathways of
differentially expressed miRNAs
Significant pathways involving the top 11
differentially expressed miRNAs are illustrated in Fig. 2. For miR-548c-5p and miR-548d-5p,
the significantly enriched pathways included ‘signaling pathways
regulating pluripotency of stem cells’ and ‘ubiquitin-mediated
proteolysis’.
miRNAs and their co-regulatory target
genes
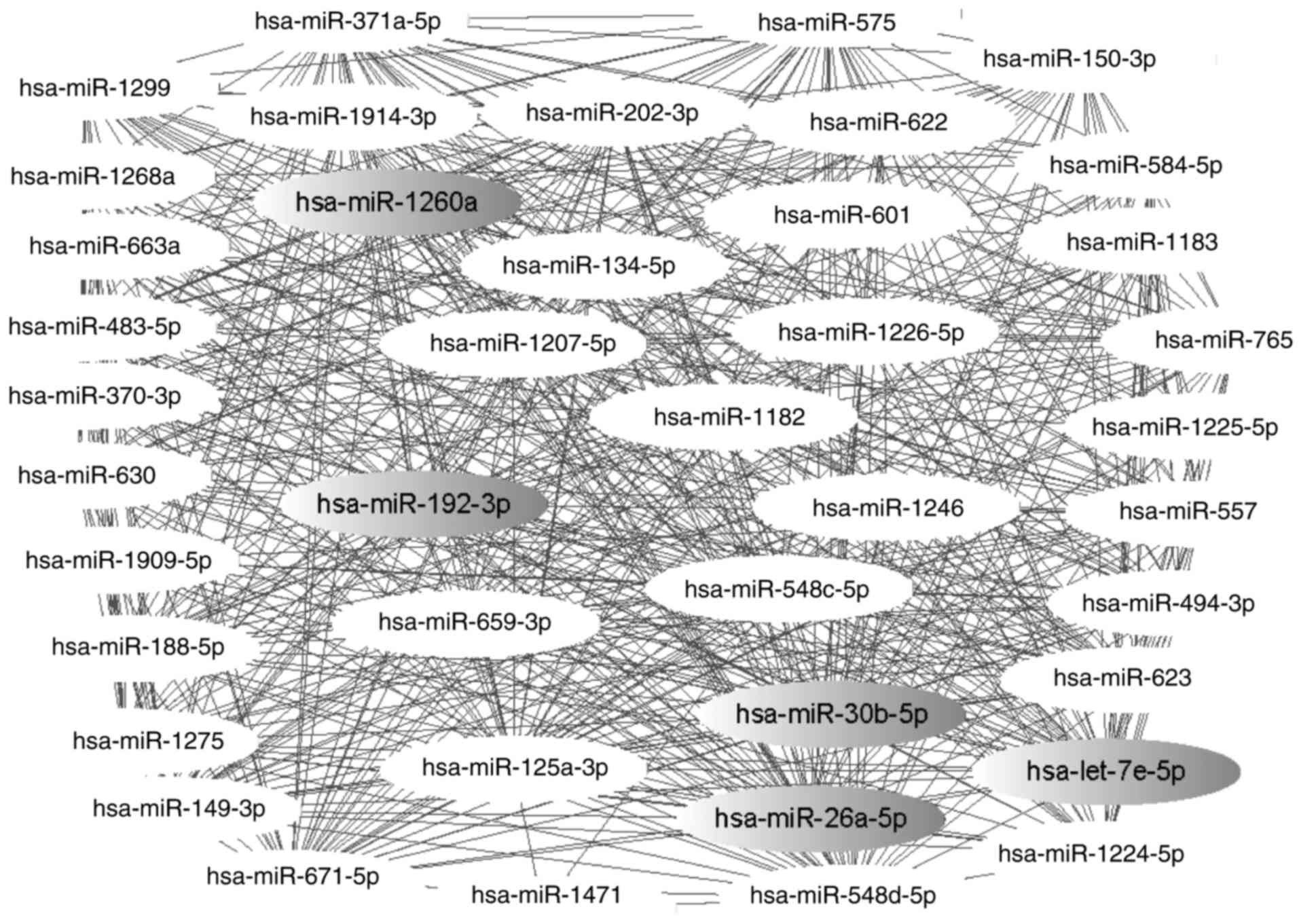
A co-regulatory network with 41 nodes and 596 edges
was constructed, as demonstrated in Fig. 3. The results indicated that the
miR-202-3p/let-7e-5p pairing is responsible for the co-regulation
156 genes, while the miR-548c-5p/miR-548d-5p complex was
demonstrated to co-regulate 183 genes, including interleukin (IL)-6
signal transducer (IL6ST), cell cycle checkpoint kinase 2 (CHEK2),
mutated in colorectal cancers (MCC), marker of proliferation Ki-67
(MKI6), damage-specific DNA binding protein 1 and actinin α4.
Rectal cancer-associated genes
A total 33 differentially expressed miRNAs
associated with the regulation of rectal cancer-associated genes
were analyzed. The regulatory network between 33 miRNAs and 80
marker or therapeutic genes associated with colorectal neoplasms
was constructed (Fig. 4). Four
genes, including cyclin-dependent kinase inhibitor 1B (CDKN1B),
adenomatous polyposis coli (APC), MCC and interferon β1 (IFNB1),
were demonstrated to be associated with rectal cancer. The
following seven miRNAs were identified as regulators of the four
rectal cancer-associated genes: miR-548c-5p/miR-548d-5p (MCC),
miR-663a (APC), miR-149-3p (CDKN1B), miR-1909-5p (CDKN1B),
miR-494-3p (MCC) and miR-26a-5p (IFNB1). Specifically, the
miR-548c-5p/miR-548d-5p complex was reported to co-regulate IL6ST,
CHEK2, MCC and MKI67, and miR-663a was demonstrated to regulate
cadherin 7 (CDH7), calreticulin (CALR), APC and transforming growth
factor (TGF) β1 (TGFB1).
 | Figure 4.Regulatory network between miRNAs and
target genes associated with colorectal neoplasms. Diamonds
represent target genes, with pink diamonds representing genes
associated with rectal cancer and all other genes being associated
with colorectal neoplasms. Grey filled ovals represent
downregulated miRNA and non-filled ovals represent upregulated
miRNA in the pCR group compared with the no pCR group. miR/miRNA,
microRNA; nCRT, neoadjuvant chemoradiotherapy; pCR, complete
response to nCRT; no pCR, incomplete response to nCRT; MCC, mutated
in colorectal cancers; CDKN1B, cyclin-dependent kinase inhibitor
1B; IFNB1, interferon β1; APC, adenomatous polyposis coli; MKI67,
marker of proliferation Ki-67; IL6ST, interleukin-6 signal
transducer; CHEK2, cell cycle checkpoint kinase 2; CDH7, cadherin
7; CALR, calreticulin; TGFB1, transforming growth factor β1. |
Discussion
It was previously reported that nCRT improves the
outcome of patients with rectal cancer; however, not all patients
benefit from this treatment (15).
Thus, identifying predictive molecular biomarkers is of clinical
importance (16). The present
study aimed to identify specific miRNAs as predictive biomarkers
based on their expression profiles. Furthermore, the potential
underlying mechanisms of the biomarkers involved in the response to
nCRT were investigated. In the current study, 36 upregulated and 5
downregulated miRNAs were detected between pCR and no pCR
(incomplete response) groups.
KEGG pathway analysis demonstrated that the
differentially expressed miR-134-5p and miR-630 were significantly
associated with the ‘HIF-1 signaling pathway’. Hypoxia-inducible
factor (HIF)-1 is a transcription factor that affects gene
expression by regulating oxygen delivery and deprivation (26). In addition, the activation of the
HIF pathway is reported to contribute to tumor growth. Clinical
evidence demonstrated that HIF-1 was overexpressed in patients with
a poor prognosis in various cancer types (27). In the present study, miR-134-5p and
miR-630 were demonstrated to be significantly upregulated in
patients that exhibited a CR to nCRT. The expression of miRNA is
negatively associated with the expression of its target genes.
Thus, the target genes involved in the HIF-1 signaling pathway were
significantly downregulated in CR cases, which may inhibit the
HIF-1 signaling pathway and contribute to tumor regression. These
results indicated that the bioinformatic findings were promising in
the present study. Additionally, three miRNAs among the top 11
miRNAs, including miR-548c-5p, miR-548d-5p and miR-663a, were
demonstrated to regulate genes associated with colorectal
neoplasms.
It has been previously reported that miR-548c-5p
inhibited the proliferation, migration and invasion, and promoted
the apoptosis, of liver cancer stem-like cells (28). In addition, miRNA-548d-5p was
reported to have a complementary role in supporting oncogenicity in
cervical cancer (29). However,
few studies have reported the association between these two miRNAs
and rectal cancer. Co-regulatory network analysis indicated that
miR-548c-5p and miR-548d-5p may function as a complex to
co-regulate four genes associated with colorectal neoplasms,
including IL6ST, CHEK2, MKI67 and MCC. High IL-6
cytokine levels were previously reported to be associated with an
advanced stage of disease and decreased survival of patients with
CRC (30). A previous study
demonstrated that IL6ST mediated tumor cell proliferation
and apoptosis through glycoprotein 130 activation, with subsequent
signaling through signal transducer and activator of transcription
3 in CRC (31). CHEK2 is a
tumor suppressor that functions in the p53 pathway of the DNA
damage response (32). The
CHEK2 variants I157T and 1100delC were reported to increase
the risk of CRC (33,34). MKI67 encodes a nuclear
protein that is strictly associated with cellular proliferation,
which means it is regarded as a proliferative marker (35). Antigen Ki-67, encoded by
MKI67, is reported to be highly expressed in proliferative
cells (36) and a previous study
demonstrated that increased Ki-67 expression post-therapy was
associated with improved disease-free survival in patients with
rectal cancer (37). In addition,
another study reported that the extent of the tumor response to
nCRT was closely associated with the expression level of Ki-67
(38). Therefore, the expression
of Ki-67 may be a potential biomarker for predicting the
radiosensitivity of tumors for CRT in rectal cancers. A colorectal
mutant cancer protein encoded by MCC, located on chromosome
5q21, was identified as a tumor suppressor gene and is considered
to negatively regulate cell cycle progression (39). Allele loss in MCC is common
in CRC (40). These findings
indicate that miR-548c-5p and miR-548d-5p may be involved in the
mechanism of the CR to nCRT by targeting genes associated with cell
proliferation, DNA damage and the cell cycle.
Furthermore, KEGG pathway analysis revealed that
‘signaling pathways regulating pluripotency of stem cells’ and
‘ubiquitin-mediated proteolysis’ signaling pathways were
significantly enriched for the miR-548c-5p/miR-548d-5p complex.
Cancer stem cells have the capacity for tumor initiation by
differentiating into heterogeneous lineages of cancer cells
(41). The ubiquitin-dependent
proteolysis system is involved in the regulation β-catenin turnover
(42) and it is established that
the accumulation of β-catenin leads to the transcription of
pre-oncogenes (43). These
findings indicate that the miR-548c-5p/miR-548d-5p complex may
contribute to the CR to nCRT through the pluripotency of stem cells
and ubiquitin-mediated proteolysis pathways.
It was previously reported that miR-663 exhibited an
anti-cancer effect by targeting the TGFB1 transcript through
the TGFβ signaling pathway in SW480 human colon cancer cells
(44), which is consistent with
the results of the present study. In the present study, three other
genes associated with colorectal neoplasms were demonstrated to be
regulated by miR-663a, including CDH7, CALR and APC.
Furthermore, E-cadherin (CDH1), a member of the CDH family,
determines cell-cell adhesion and increases the nuclear
translocation of β-catenin (44,45).
It is well-document that the APC protein, encoded by APC,
prevents the accumulation of β-catenin, a key factor in cancer
(46). CDH7 is also a member of
the CDH family (47). Thus, it can
be suggested that miR-663a may function by altering the levels of
β-catenin through targeting CDH7 and APC.
Calreticulin, encoded by CALR, was reported to be associated
with the infiltration of T-cells, and low expression of CALR
may represent a novel mechanism underlying immune escape in colon
cancer (48). Thus, miR-663a may
be closely associated with a CR to nCRT by targeting these four
genes.
The aforementioned results support the predictive
roles of miR-548c-5p, miR-548d-5p and miR-663a in the response of
patients with rectal cancer to nCRT. The present study further
analyzed the miRNA microarray data developed from patients treated
with capecitabine-oxaliplatin and pelvic conformal radiotherapy (45
cGy) followed by surgery (after 6–8 weeks). Due to the lack of the
clinical samples under the same conditions, experimental
validations were not available, which is a limitation of the
present study. Thus, evaluations in larger, prospective trials are
required to validate these biomarkers.
In conclusion, miR-548c-5p, miR-548d-5p and miR-663a
are potential biomarkers for predicting a CR to nCRT in rectal
cancer. The miR-548c-5p/miR-548d-5p complex may function by
targeting IL6ST, CHEK2, MKI67 and MCC. In addition,
this complex may function through the pluripotency of stem cells
and ubiquitin-mediated proteolysis signaling pathways. miR-663a may
affect the tumor response to treatment by regulating CDH7, CALR,
APC and TGFB1. Furthermore, targeting these miRNAs with
oligonucleotides may represent a potential therapy for improving a
poor response to nCRT in patients with rectal cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Bureau of
Science and Technology of Innovation and Entrepreneurship Project
(Lanzhou, China; grant no. 2015-RC-37).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BD and WZ were responsible for the conception and
design of the present study. JW and LC acquired the data. XW and DW
analyzed and interpreted the data. TW, XS and DW performed the
statistical analyses. XY, DW and LC were involved in conception of
the research, participated in its design and coordination and aided
the writing of the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zheng S and Cai S: Colorectal cancer
epidemiology and prevention study in China. Chinese-German J
Clinical Oncol. 2:72–75. 2003. View Article : Google Scholar
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dusek L, Muzik J, Kubasek M, Koptikova J,
Zaloudik J and Vyzula R: Epidemiology of Malignant Tumours in the
Czech Republic. http://www.svod.czSeptember 12–2013
|
|
5
|
Krook JE, Moertel CG, Gunderson LL, Wieand
HS, Collins RT, Beart RW, Kubista TP, Poon MA, Meyers WC, Mailliard
JA, et al: Effective surgical adjuvant therapy for high-risk rectal
carcinoma. N Engl J Med. 324:709–715. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Valentini V, Aristei C, Glimelius B,
Minsky BD, Beets-Tan R, Borras JM, Haustermans K, Maingon P,
Overgaard J, Pahlman L, et al: Multidisciplinary rectal cancer
management: 2nd european rectal cancer consensus conference
(EURECA-CC2). Radiother Oncol. 92:148–163. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gérard JP, Conroy T, Bonnetain F, Bouché
O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E,
Maurel J, et al: Preoperative radiotherapy with or without
concurrent fluorouracil and leucovorin in T3-4 rectal cancers:
Results of FFCD 9203. J Clin Oncol. 24:4620–4625. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Engels B, Gevaert T, Sermeus A and Ridder
MD: Current status of intensified neo-adjuvant systemic therapy in
locally advanced rectal cancer. Front Oncol. 2:472012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mandard AM, Dalibard F, Mandard JC, Marnay
J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G,
et al: Pathologic assessment of tumor regression after preoperative
chemoradiotherapy of esophageal carcinoma. Clinicopathologic
correlations. Cancer. 73:2680–2686. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martin ST, Heneghan HM and Winter DC:
Systematic review and meta-analysis of outcomes following
pathological complete response to neoadjuvant chemoradiotherapy for
rectal cancer. Br J Surg. 99:918–928. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suárez J, Vera R, Balén E, Gómez M, Arias
F, Lera JM, Herrera J and Zazpe C: Pathologic response assessed by
Mandard grade is a better prognostic factor than down staging for
disease-free survival after preoperative radiochemotherapy for
advanced rectal cancer. Colorectal Dis. 10:563–568. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Azizian A, Gruber J, Ghadimi BM and
Gaedcke J: MicroRNA in rectal cancer. World J Gastrointest Oncol.
8:416–426. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Svoboda M, Sana J, Fabian P, Kocakova I,
Gombosova J, Nekvindova J, Radova L, Vyzula R and Slaby O: MicroRNA
expression profile associated with response to neoadjuvant
chemoradiotherapy in locally advanced rectal cancer patients.
Radiat Oncol. 7:1952012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Drebber U, Lay M, Wedemeyer I, Vallböhmer
D, Bollschweiler E, Brabender J, Mönig SP, Hölscher AH, Dienes HP
and Odenthal M: Altered levels of the onco-microRNA 21 and the
tumor-supressor microRNAs 143 and 145 in advanced rectal cancer
indicate successful neoadjuvant chemoradiotherapy. Int J Oncol.
39:409–415. 2011.PubMed/NCBI
|
|
15
|
Kheirelseid EA, Miller N, Chang KH, Curran
C, Hennessey E, Sheehan M, Newell J, Lemetre C, Balls G and Kerin
MJ: miRNA expressions in rectal cancer as predictors of response to
neoadjuvant chemoradiation therapy. Int J Colorectal Dis.
28:247–260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lopes-Ramos CM, Habr-Gama A, Bde Quevedo
S, Felício NM, Bettoni F, Koyama FC, Asprino PF, Galante PA,
Gama-Rodrigues J, Camargo AA, et al: Overexpression of miR-21-5p as
a predictive marker for complete tumor regression to neoadjuvant
chemoradiotherapy in rectal cancer patients. BMC Med Genomics.
7:682014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Della Vittoria Scarpati G, Falcetta F,
Carlomagno C, Ubezio P, Marchini S, De Stefano A, Singh VK,
D'Incalci M, De Placido S and Pepe S: A Specific miRNA signature
correlates with complete pathological response to neoadjuvant
chemoradiotherapy in locally advanced rectal cancer. Int J Radiat
Oncol Biol Phys. 83:1113–1119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smyth BGK: Limma: Linear models for
microarray dataBioinformatics and Computational Biology Solutions
Using R and Bioconductor. Springer; pp. 397–420. 2005, View Article : Google Scholar
|
|
19
|
Griffiths-Jones S: miRBase: The microRNA
sequence database. Methods Mol Biol. 342:129–138. 2006.PubMed/NCBI
|
|
20
|
Griffiths-Jones S: miRBase: microRNA
sequences and annotation. Curr Protoc Bioinformatics.
29:12.9.1–12.9.10. 2010.
|
|
21
|
Dweep H and Gretz N: miRWalk2.0: A
comprehensive atlas of microRNA-target interactions. Nat Methods.
12:6972015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maqbool A, Lattke M, Wirth T and Baumann
B: Sustained, neuron-specific IKK/NF-κB activation generates a
selective neuroinflammatory response promoting local
neurodegeneration with aging. Mol Neurodegener. 8:402013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kohl M, Wiese S and Warscheid B:
Cytoscape: Software for visualization and analysis of biological
networks. Methods Mol Biol. 696:291–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Davis AP, Grondin CJ, Lennonhopkins K,
Saraceni-Richards C, Sciaky D, King BL, Wiegers TC and Mattingly
CJ: The Comparative Toxicogenomics Database's 10th year
anniversary: Update 2015. Nucleic Acids Res. 43:(Database issue).
D914–D920. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Semenza GL: HIF-1 and mechanisms of
hypoxia sensing. Curr Opin Cell Biol. 13:167–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jaakkola P, Mole DR, Tian YM, Wilson MI,
Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji
M, Schofield CJ, et al: Targeting of HIF-alpha to the von
Hippel-Lindau ubiquitylation complex by O2-regulated prolyl
hydroxylation. Science. 292:468–472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fang L, Zhang HB, Li H, Fu Y and Yang GS:
miR-548c-5p inhibits proliferation and migration and promotes
apoptosis in CD90(+) HepG2 cells. Radiol Oncol. 46:233–241.
2015.
|
|
29
|
Mandal P, Bhattacharjee B, Das Ghosh D,
Mondal NR, Chowdhury Roy R, Roy S and Sengupta S: Differential
expression of HPV16 L2 gene in cervical cancers harboring episomal
HPV16 genomes: Influence of synonymous and non-coding region
variations. PLoS One. 8:e656472013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Knüpfer H and Preiss R: Serum
interleukin-6 levels in colorectal cancer patients-a summary of
published results. Int J Colorectal Dis. 25:135–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Waldner MJ, Foersch S and Neurath MF:
Interleukin-6-A Key regulator of colorectal cancer development. Int
J Biol Sci. 8:1248–1253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kilpivaara O, Laiho P, Aaltonen LA and
Nevanlinna H: CHEK2 1100delC and colorectal cancer. J Med Genet.
40:e1102003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meijers-Heijboer H, Wijnen J, Vasen H,
Wasielewski M, Wagner A, Hollestelle A, Elstrodt F, van den Bos R,
de Snoo A, Fat GT, et al: The CHEK2 1100delC mutation identifies
families with a hereditary breast and colorectal cancer phenotype.
Am J Hum Genet. 72:1308–1314. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kilpivaara O, Alhopuro P, Vahteristo P,
Aaltonen LA and Nevanlinna H: CHEK2 I157T associates with familial
and sporadic colorectal cancer. J Med Genet. 43:e342006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hofstädter F, Knüchel R and Rüschoff J:
Cell proliferation assessment in oncology. Virchows Arch.
427:323–341. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dalerba P, Kalisky T, Sahoo D, Rajendran
PS, Rothenberg ME, Leyrat AA, Sim S, Okamoto J, Johnston DM, Qian
D, et al: Single-cell dissection of transcriptional heterogeneity
in human colon tumors. Nat Biotechnol. 29:1120–1127. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rau B, Sturm I, Lage H, Berger S,
Schneider U, Hauptmann S, Wust P, Riess H, Schlag PM, Dörken B and
Daniel PT: Dynamic Expression Profile of p21WAF1/CIP1 and Ki-67
predicts survival in rectal carcinoma treated with preoperative
radiochemotherapy. J Clin Onco. 21:3391–3401. 2003. View Article : Google Scholar
|
|
38
|
Kim NK, Park JK, Lee KY, Yang WI, Yun SH,
Sung J and Min JS: p53, BCL-2, and Ki-67 expression according to
tumor response after concurrent chemoradiotherapy for advanced
rectal cancer. Ann Surg Oncol. 8:418–424. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kinzler KW, Nilbert MC, Vogelstein B,
Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hamilton SR, Hedge P,
Markham A, et al: Identification of a gene located at chromosome
5q21 that is mutated in colorectal cancers. Science. 251:1366–1370.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cawkwell L, Lewis FA and Quirke P:
Frequency of allele loss of DCC, p53, RBI, WT1, NF1, NM23 and
APC/MCC in colorectal cancer assayed by fluorescent multiplex
polymerase chain reaction. Br J Cancer. 70:813–818. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kong D, Li Y, Wang Z and Sarkar FH: Cancer
stem cells and epithelial-to-mesenchymal transition
(EMT)-phenotypic cells: Are they cousins or twins? Cancers (Basel).
3:716–729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aberle H, Bauer A, Stappert J, Kispert A
and Kemler R: beta-catenin is a target for the ubiquitin-
proteasome pathway. EMBO J. 16:3797–3804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Morin PJ, Sparks AB, Korinek V, Barker N,
Clevers H, Vogelstein B and Kinzler KW: Activation of
beta-catenin-Tcf signaling in colon cancer by mutations in
beta-catenin or APC. Science. 275:1787–1790. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu L, Chen H, Zhou D, Li D, Bai R, Zheng
S and Ge W: MicroRNA-9 up-regulation is involved in colorectal
cancer metastasis via promoting cell motility. Med Oncol.
29:1037–1043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schmalhofer O, Brabletz S and Brabletz T:
E-cadherin, beta-catenin, and ZEB1 in malignant progression of
cancer. Cancer Metastasis Rev. 28:151–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Munemitsu S, Albert I, Souza B, Rubinfeld
B and Polakis P: Regulation of intracellular beta-catenin levels by
the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc
Natl Acad Sci USA. 92:3046–3050. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gul IS, Hulpiau P, Saeys Y and Van RF:
Evolution and diversity of cadherins and catenins. Exp Cell Res.
358:3–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Peng RQ, Chen YB, Ding Y, Zhang R, Zhang
X, Yu XJ, Zhou ZW, Zeng YX and Zhang XS: Expression of calreticulin
is associated with infiltration of T-cells in stage IIIB colon
cancer. World J Gastroenterol. 16:2428–2434. 2010. View Article : Google Scholar : PubMed/NCBI
|