Introduction
Breast cancer is the most common cancer and the
second leading cause of cancer-associated mortality among women
worldwide, with ~1.7 million cases and 521,900 fatalities occurring
in 2012 (1). Breast cancer is a
highly heterogeneous type of cancer; this heterogeneity is evident
with regards to tumor morphology and at the transcriptome and
proteome levels (2,3). It is difficult to identify specific
and sensitive diagnostic and therapeutic targets; therefore,
understanding the molecular and cellular mechanisms of tumor
heterogeneity that are relevant to the diagnosis, prognosis and
treatment of breast cancer is a primary research concern (4,5).
For the study of breast cancer heterogeneity, it has
been acknowledged that breast cancer is categorized into three
basic therapeutic groups, based on the expression levels of
estrogen receptor (ER), human epidermal growth factor receptor 2
(HER2; also termed ERBB2) and progesterone receptor (PR), which can
also be used to classify breast cancer phenotype heterogeneity.
Recently, a study from Sweden reported a comparative analysis of
ER, PR, HRR2 and Ki67 status between the primary tumor and
corresponding relapse and found that the discordance of four
receptors status was 14.2, 39.6, 9.6 and 36.3%, respectively. Loss
of ER or PR in the relapse resulted in a significantly increased
risk of mortality [hazard ratio (HR) 3.62; 95% confidence interval
(CI), 1.65–7.94] and (HR 2.34; 95% CI, 1.01–5.47) compared with
patients with stable ER or PR positive tumors (6). It is also established that there is
no available effective treatment available for triple-negative
breast cancer, which does not express ER, HER2 or PR. Therefore,
the identification of specific tumor targets for breast cancer is
required.
The development of genome sequencing technology has
resulted in more objective ways to identify tumor heterogenetic
genes, such as variant molecular subtypes and critical mutations
genes, in order to aid understanding of the molecular function and
mechanisms of the disease (7). For
example, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic
subunit alpha (PIK3CA) and p53 have been identified as the two most
frequently mutated genes in breast cancer (8). When the oncogenic PIK3CA-H1047R
mutant is expressed at physiological levels in basal cells, it
induces the formation of luminal ER-positive/PR-positive tumors in
animal models, whereas its expression in luminal cells gives rise
to luminal ER+PR+ tumors or basal-like
ER−PR− tumors (9). In addition, Koren et al
(10) demonstrated that
concomitant expression of PIK3CA-H1047R and deletion of p53
accelerates tumor development, inducing more aggressive mammary
tumors. These findings provided information regarding the molecular
mechanisms underlying tumor heterogeneity and suggested the
potential therapeutic applications of blocking key oncogenes, such
as PIK3CA. There are currently no tumor-specific genes or proteins
that have been identified for all breast cancer subtypes;
therefore, the present study aimed to analyze ‘breast
cancer-associated genes’ using high-throughput molecular profiling
data, in order to identify potential genes for diagnosis and
therapy. Genomic data for breast cancer were obtained from The
Cancer Genome Atlas (TCGA), and it was observed that excision
repair cross-complementation group 6 like (ERCC6L) was highly
expressed in 91.51% of breast cancers, although the role of ERCC6L
in breast cancer remains unclear. Functional studies were also
performed to further confirm that ERCC6L may act as an oncogene
involved in tumor development and progression, thus suggesting that
ERCC6L may be a potential target for breast cancer treatment.
Materials and methods
Data acquisition
For breast cancer, there are 1,097 available data
samples which contains 106 pairs of data. In the present study, the
gene expression data were from 106 breast cancer samples (18 were
stage I, 61 stage II and 27 stage III–IV) and were downloaded from
TCGA (http://cancergenome.nih.gov; RNA-Seq
Version and RNA-Seq Version 2). All data standardization sets were
processed using the Trimmed Mean of M-values normalization
method.
Cell culture
The breast cancer cell line MDA-MB-231 was obtained
from the Institute of Biochemistry and Cell Biology of the Chinese
Academy of Science (Shanghai, China). MDA-MB-231 cells were
maintained in Dulbecco's modified Eagle's medium (DMEM; HyClone, GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and 1% penicillin/streptomycin (Gibco; Thermo
Fisher Scientific, Inc.) in a 5% CO2 incubator at
37°C.
Lentivirus construction and cell
transduction
All lentiviral constructs were prepared by Shanghai
GeneChem Co., Ltd. (Shanghai, China). Briefly, the ERCC6L-specific
short hairpin (sh)RNA (shERCC6L) and negative control (NC) shRNA
were designed and synthesized by Shanghai GeneChem Co., Ltd. and
their sequences were as follows: 5′-TCATGCCAACCAATCTTAT-3′ and
5′-TTCTCCGAACGTGTCACGT-3′, respectively. These fragments were
inserted into the AgeI/EcoRI site of a lentivirus
linearized hU6-MSC-CMV-puror vector (GV115, 7.5 kb;
GeneChem Co., Ltd.) carrying green fluorescent protein (GFP). For
lentivirus packaging, approximately 24 h before transfection,
5×106 HEK 293 cells were seeded into 10 cm tissue
culture plates in 10 ml of growth medium and incubated at 37°C in
5% CO2 overnight. The cells were 70% confluent at the
time of transfection. Then, 2 h prior to transfection, the medium
was replaced with serum-free DMEM. Then the transfection medium (15
µg pHelper plasmids, 10 µg pHelper 2.0 plasmids, 20 µg lentivirus
packing vector GV115 and Lipofectamine® 2000 (volumes as
recommended by the manufacturer, Invitrogen; Thermo Fisher
Scientific, Inc.) was respectively added into a sterilized tube,
then mixed and adjusted to 1 ml, and incubated at room temperature
for 15 min. Finally, the transfection medium was added to the HEK
293 cells and incubated at 37°C for 6 h. Then the medium was
removed and replaced with new culture medium and culture continued
for an additional 48-h. The lentiviral supernatants were harvested
and filtered through a 0.45 µm low protein binding filter to remove
cellular debris. shRNA lentiviruses were concentrated by
ultracentrifugation (2 h at 50,000 × g) and subsequently purified
on a sucrose 20% gradient (2 h at 46,000 × g) for future use. The
virus titer of shERCC6L and shCtrl was 4×108 TU/ml and
5×108 TU/ml, respectively.
For cell transduction, 2×106 MDA-MB-231
cells per well were seeded in a 6 well-plate overnight and then
infected with lentiviruses [multiplicity of infection (MOI) 20].
Following incubation for 72 h at 37°C in a 5% CO2
incubator, most cells expressed green fluorescent protein (GFP)
under fluorescence microscopy. When the infection rate of
MDA-MB-231 cells reached more than 70%, the cells were used in the
following experiments. Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blotting were used
to determine the effectiveness of the shRNA on ERCC6L
knockdown.
Cell Proliferation
Following transfection with shERCC6L or shCtrl
lentivirus for 72 h, MDA-MB-231 cells were seeded at a density of
2×103 cells (200 µl/per well) in 96-well plates and
cultured at 37°C in a 5% CO2 incubator for 5 days. A
Celigo Image cytometer (Nexcelom Bioscience, Lawrence, MA) was used
to detect the growth number of MDA-MB-231 cells at fixed time
points.
RNA extraction and RT-qPCR
Cells were collected at 48 h post-transduction and
total RNA was extracted using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). RT was performed with
random nucleotide primers using an M-MLV RT system (Promega
Corporation, Madison, WI, USA), according to the manufacturer's
protocol. RT-qPCR with gene-specific primers was performed using
GoTaq qPCR master mix (Promega Corporation). GAPDH was used as the
internal control. The real-time PCR program consisted of 30 sec at
95°C, 40 cycles of 5 sec at 95°C, 1 min at 60°C and a dissociation
stage at the end of the run from 60 to 95°C. It was performed in a
Roche LightCycler480 Real-Time PCR system. The relative mRNA
expression levels were determined by the cycle quantification (Cq)
normalized against GAPDH using the 2−ΔΔCq formula
(11). The primers used were as
follows: ERCC6L, forward 5′-CTCTGGCTTGCTACTTTATCGAG-3′, reverse
5′-TGCATCAAACATACCGGAAAGG-3′; GAPDH, forward
5′-TGACTTCAACAGCGACACCCA-3′, and reverse
5′-TGCATCAAACATACCGGAAAGG-3′.
Western blotting
Cells were lysed with radioimmunoprecipitation assay
buffer (RIPA; cat. no. P0013B; Beyotime Institute of Biotechnology,
Haimen, China) and proteins were quantified using a Bicinchoninic
Protein Assay kit (cat.no. P0010S, Beyotime Institute of
Biotechnology). A total of 20–50 µg total protein was separated by
8% SDS-PAGE and subsequently transferred onto polyvinylidene
difluoride membranes (cat. no. IPVH00010; EMD Millipore, Billerica,
MA, USA). Following blocking in TBST (Tris-buffered saline with
0.5% Tween-20) which contained 5% non-fat milk for 60 min at room
temperature, the membranes were incubated with the primary antibody
overnight at 4°C on a shaker. The primary antibody includes mouse
anti-ERCC6L (1:1,000; cat. no. SAB1407576; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) and mouse anti-GAPDH (1:2,500; cat. no.
sc-47724; Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Following washing with 1% TBST buffer for 10 min 3 times, the
membranes were incubated with the HRP-linked goat anti-mouse IgG
secondary antibodies (1:2,000 dilution; cat. no. 7060; Cell
Signaling Technology, Inc., Danvers, MA, USA). Following incubation
in enhanced chemiluminescence solution (cat. no. M3121; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocols.
The proteins on the membranes were detected using Bio-Rad Universal
Hood III, and analyzed by Image Lab™ software version 2.0 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Apoptosis assay and cell cycle
analysis
For apoptosis analysis, 1×106/ml
MDA-MB-231 cells were harvested at 48 h post-transduction, and the
level of apoptosis was determined using an Annexin V apoptosis
detection kit (eBioscience; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. As Annexin V is an
allophycocyanin (APC) dye, the apoptosis analysis was performed on
a fluorescence-activated cell sorting (FACS) machine (EMD
Millipore) with an APC channel. For cell cycle analysis,
1×106/ml MDA-MB-231 cells were collected following
transduction and fixed with cold 70% alcohol at 4°C overnight.
Alcohol was removed and cells were washed with cold PBS. Cells were
stained with propidium iodide solution (Sigma-Aldrich; Merck KGaA)
containing 20 µg/ml RNase A (Fermentas; Thermo Fisher Scientific,
Inc.) and incubated at room temperature for 30 min. Following
filtration with a nylon mesh filter, cell cycle analysis was
performed on a fluorescence-activated cell sorting (FACS) machine
(EMD Millipore). DNA content analysis was performed with ModFit LT
software (Verity Software House, Inc., Topsham, ME, USA) to
calculate cell cycle phase.
Statistical analysis
Each experiment was performed ≥3 times. All data are
expressed as the means ± standard deviation. Unless otherwise
noted, the differences of continuous variables between two groups
were analyzed by Student's t-test or Wilcoxon rank-sum test. All
analyses were performed using SPSS software version 17.0 (Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
ERCC6L is overexpressed in breast
cancer tissues
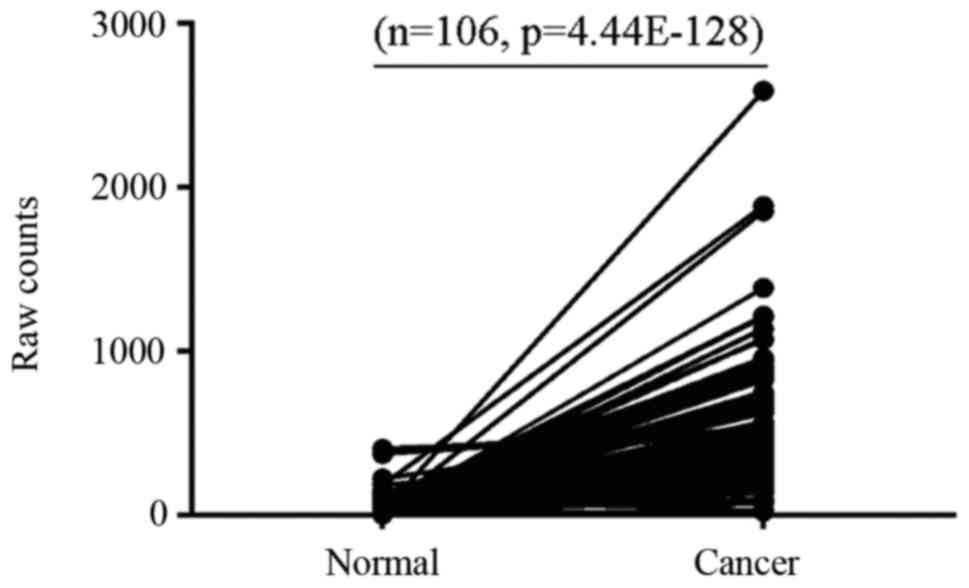
The present study analyzed data from 106 paired
breast cancer samples at different pathological stages from TCGA
and demonstrated that ERCC6L expression was higher in 91.51%
(97/106), unchanged in 7.54% (8/106) and decreased in 0.94% (1/106)
of breast cancer samples compared with in matched controls
(Fig. 1). These results suggested
that abnormal expression of ERCC6L may be involved in breast cancer
development.
Effects of ERCC6L silencing breast
cancer cells
In order to investigate the role of ERCC6L in breast
cancer, the present study constructed and evaluated the knockdown
effects of a shERCC6L-lentivirus. As demonstrated in Fig. 2A, fluorescence microscopy revealed
that the transduction efficiency of the GFP-containing
shERCC6L-lentivirus in MDA-MB-231 cells following transfection for
72 h was >80%, and the virus MOI was 20. Data from RT-qPCR
revealed that the knockdown effect of the shERCC6L-lentivirus in
MDA-MB-231 cells was >91.50% (Fig.
2B) and the western blotting results simultaneously indicated
that the shERCC6L-lentivirus decreased the expression levels of
ERCC6L in MDA-MB-231 cells (Fig.
2C). These data suggested that the shERCC6L lentivirus was
successfully constructed.
ERCC6L shRNA inhibits breast cancer
cell proliferation
To determine the effects of ERCC6L on the
proliferation of MDA-MB-231 cells, a Celigo Image Cytometry system
was used to detect MDA-MB-231 cell growth post-transduction with
shERCC6L-lentivirus or NC-lentivirus, at a specific time-point. As
demonstrated in Fig. 3A, the
number of fluorescent MDA-MB-231 cells post-transduction with
shERCC6L-lentivirus was decreased compared with cells transduced
with NC-lentivirus. In addition, the number (Fig. 3B) and growth (Fig. 3C) of MDA-MB-231 cells was markedly
decreased compared with the NC cells. These findings indicated that
silencing ERCC6L may suppress breast cancer cell proliferation.
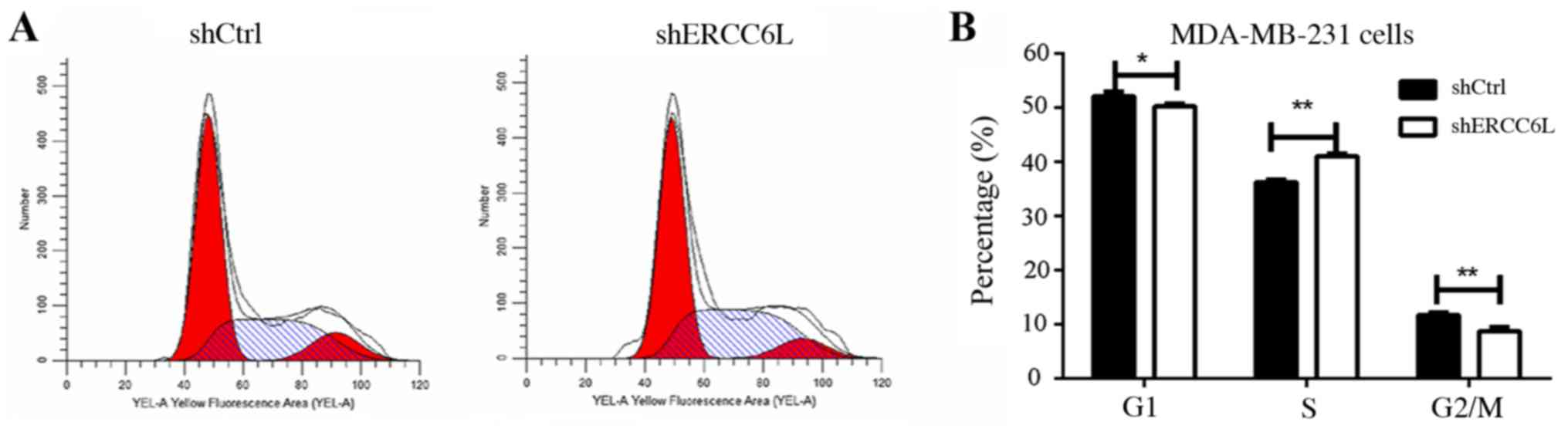
ERCC6L affects the cell cycle
distribution of MDA-MB-231 cells
It is well known that cell proliferation and
apoptosis are linked by cell cycle regulation (12,13),
therefore the present study aimed to identify whether ERCC6L
affects the cell cycle. Cell cycle analysis indicated that the S
phase population of MDA-MB-231 was increased post-transduction with
the shERCC6L-lentivirus, whereas G1 and G2/M phase populations were
decreased compared with in the NC group (P<0.01; Fig. 4). These results suggested that
ERCC6L may be involved in the DNA synthesis process in breast
cancer cells.
Knockdown of ERCC6L expression induces
MDA-MB-231 cell apoptosis
In addition to cell cycle analysis, the present
study investigated whether knockdown of ERCC6L expression in
MDA-MB-231 cells induced cell apoptosis. FACS was used to analyze
the apoptosis rate of MDA-MB-M231 cells which were passaged and
cultured for 2 days following infection for 72 h. FACS analysis
demonstrated that the apoptosis rate of shERCC6L-MDA-MB-231 cells
cultured for 2 days was increased to 12.16±0.1462% compared with
the negative control rate (4.86±0.204%; Fig. 5). These findings demonstrated that
knockdown of ERCC6L expression levels suppressed MDA-MB-231 cell
growth by affecting the cell cycle and by inducing apoptosis.
Discussion
To the best of the authors' knowledge, breast cancer
is the first type of cancer that has been reported to exhibit
phenotypic heterogeneity, and tumor heterogeneity is currently one
of the most highly investigated areas in cancer research (14). Tumor heterogeneity is typically
observed in cellular morphology, and is present at the
transcriptome and proteome levels, resulting in difficulties in
identifying effective diagnostic markers and therapeutic treatments
(4). The development of
genome-wide technology has provided the opportunity to
comprehensively understand disease mechanisms (15). Elucidation of the molecular and
cellular mechanisms underlying tumor heterogeneity that are
relevant to the development of treatment resistance is a major area
of research.
The present study conducted an in-depth data
analysis with high-throughput molecular profiling data to identify
potential and useful targets for the development of novel
therapies. Briefly, data from 106 paired breast cancer samples at
different pathological stages from TCGA were analyzed, and it was
demonstrated that ERCC6L was highly expressed in 91.51% (97/106),
unchanged in 7.54% (8/106) and decreased in 0.94% (1/106) of breast
cancer samples compared with matched controls. This result was in
agreement with that reported by Pu et al (16). Previous studies also suggested that
ERCC6L is highly expressed in several types of tumor, including
bladder, kidney, oral and gastric cancers (17–20).
Previous studies also indicated that ERCC6L genetic polymorphisms
are associated with cancer susceptibility. Abbasi et al
(19) reported that laryngeal
cancer risk is associated with genetic polymorphisms in ERCC5,
ERCC6 and RAD23 homolog B, nucleotide excision repair protein. Liu
et al (20) also revealed
that the DNA repair gene ERCC6 rs1917799 polymorphism is associated
with gastric cancer risk in the Chinese population. It has also
been identified that ERCC6 polymorphisms are associated with
susceptibility to oral, lung, bladder and colorectal cancers
(21–23). These findings suggested that ERCC6L
may act as an oncogene involved in tumor progression, and may be
considered a potential target to aid the efficient diagnosis and
development of therapies against breast cancer.
ERCC6L is a DNA helicase, also termed polo-like
kinase 1-interacting checkpoint ‘helicase’, and is an embryonic
development-associated protein (12). A previous study reported that
ERCC6L is overexpressed in the embryonic heart, brain and other
tissues; however, it is rarely expressed in adult tissues (24). Another study also demonstrated that
ERCC6L is critical to embryonic development (17). DNA helicases are important
components of DNA replication, recombination and repair in all
eukaryotes and bacteria; in response to aberrant ERCC6L
functioning, DNA damage and genetic instability are induced, which
in turn may enhance cancer development (19). Pu et al (16) reported that ERCC6L knockdown
induces G0/G1 cell cycle arrest and inhibits cell proliferation,
but does not increase apoptosis of MCF-7 breast cancer cells.
However, in the present study, it was demonstrated that
downregulation of ERCC6L in breast cancer cells using a
shRNA-containing lentivirus, caused cell growth inhibition,
apoptosis and cell phase distribution. The difference between these
findings may be due to cell line differences or culture
conditions.
In conclusion, the present results suggested that
ERCC6L may act as an oncogene involved in breast cancer development
and progression. However, further in vitro and in
vivo studies are required to fully reveal the effects and the
underlying mechanisms of ERCC6L on tumorigenesis, treatment
resistance and tumor differentiation. Silencing of ERCC6L inhibited
breast cancer growth in vitro, thus indicating that ERCC6L
may be a potential target for the treatment of breast cancer;
however, this requires validation.
Acknowledgements
The authors thank Xiaoyun Shen from the Sir Run Run
Shaw hospital affiliated to Zhejiang University School of Medicine
for providing general support and Xu Liu from Zhongshan Hospital,
Fudan University for providing assistance in writing the
manuscript.
Funding
This study was supported by a grant from the Youth
Science Foundation of Zhongshan Hospital, Fudan University (grant
no. 2017ZSQN35).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JL performed the experiments, analyzed the data, and
drafted the manuscript. JS participated in the design of the study,
helped to perform the analysis, provided valued discussions and
helped to draft the manuscript. QZ helped in the sequence
alignment. ZZ contributed to the conception and design of the
study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TCGA
|
The Cancer Genome Atlas
|
|
ERCC6L
|
excision repair cross-complementation
group 6 like
|
|
ER
|
estrogen receptor
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
PR
|
progesterone receptor
|
References
|
1
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johann DJ, Rodriguez-Canales J, Mukherjee
S, Prieto DA, Hanson JC, Emmert-Buck M and Blonder J: Approaching
solid tumor heterogeneity on a cellular basis by tissue proteomics
using laser capture microdissection and biological mass
spectrometry. J Proteome Res. 8:2310–2318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Polyak K: Heterogeneity in breast cancer.
J Clin Invest. 121:3786–3788. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park SY, Gönen M, Kim HJ, Michor F and
Polyak K: Cellular and genetic diversity in the progression of in
situ human breast carcinomas to an invasive phenotype. J Clin
Invest. 120:636–644. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beca F and Polyak K: Intratumor
heterogeneity in breast cancer. Adv Exp Med Biol. 882:169–189.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karlsson E, Appelgren J, Solterbeck A,
Bergenheim M, Alvariza V and Bergh J: Breast cancer during
follow-up and progression - A population based cohort on new
cancers and changed biology. Eur J Cancer. 50:2916–2924. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Badve S and Gökmen-Polar Y: Tumor
heterogeneity in breast cancer. Adv Anat Pathol. 22:294–302. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bai X, Zhang E, Ye H, Nandakumar V, Wang
Z, Chen L, Tang C, Li J, Li H, Zhang W, et al: PIK3CA and TP53 gene
mutations in human breast cancer tumors frequently detected by ion
torrent DNA sequencing. PLoS One. 9:e993062014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van Keymeulen A, Lee MY, Ousset M, Brohée
S, Rorive S, Giraddi RR, Wuidart A, Bouvencourt G, Dubois C, Salmon
I, et al: Reactivation of multipotency by oncogenic PIK3CA induces
breast tumour heterogeneity. Nature. 525:119–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koren S, Reavie L, Couto JP, De Silva D,
Stadler MB, Roloff T, Britschgi A, Eichlisberger T, Kohler H, Aina
O, et al: PIK3CA(H1047R) induces multipotency and multi-lineage
mammary tumours. Nature. 525:114–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
King KL and Cidlowski JA: Cell cycle
regulation and apoptosis. Annu Rev Physiol. 60:601–617. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alenzi FQ: Links between apoptosis,
proliferation and the cell cycle. Br J Biomed Sci. 61:99–102. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Foote FW Jr and Stewart FW: A histologic
classification of carcinoma of the breast. Surgery. 19:74–99.
1946.PubMed/NCBI
|
|
15
|
McGranahan N and Swanton C: Biological and
therapeutic impact of intratumor heterogeneity in cancer evolution.
Cancer Cell. 27:15–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pu SY, Yu Q, Wu H, Jiang JJ, Chen XQ, He
YH and Kong QP: ERCC6L, a DNA helicase, is involved in cell
proliferation and associated with survival and progress in breast
and kidney cancers. Oncotarget. 8:42116–42124. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baumann C, Körner R, Hofmann K and Nigg
EA: PICH, a centromere-associated SNF2 family ATPase, is regulated
by Plk1 and required for the spindle checkpoint. Cell. 128:101–114.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin Z, Zhang X, Tuo J, Guo Y, Green B,
Chan CC, Tan W, Huang Y, Ling W, Kadlubar FF, et al: A variant of
the Cockayne syndrome B gene ERCC6 confers risk of lung cancer. Hum
Mutat. 29:113–122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abbasi R, Ramroth H, Becher H, Dietz A,
Schmezer P and Popanda O: Laryngeal cancer risk associated with
smoking and alcohol consumption is modified by genetic
polymorphisms in ERCC5, ERCC6 and RAD23B but not by polymorphisms
in five other nucleotide excision repair genes. Int J Cancer.
125:1431–1439. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu JW, He CY, Sun LP, Xu Q, Xing CZ and
Yuan Y: The DNA repair gene ERCC6 rs1917799 polymorphism is
associated with gastric cancer risk in Chinese. Asian Pac J Cancer
Prev. 14:6103–6108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang CH, Chiu CF, Wang HC, Wu HC, Tsai
RY, Tsai CW, Wang RF, Wang CH, Tsou YA and Bau DT: Significant
association of ERCC6 single nucleotide polymorphisms with bladder
cancer susceptibility in Taiwan. Anticancer Res. 29:5121–5124.
2009.PubMed/NCBI
|
|
22
|
Ma H, Hu Z, Wang H, Jin G, Wang Y, Sun W,
Chen D, Tian T, Jin L, Wei Q, et al: ERCC6/CSB gene polymorphisms
and lung cancer risk. Cancer Lett. 273:172–176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ramaniuk VP, Nikitchenko NV, Savina NV,
Kuzhir TD, Rolevich AI, Krasny SA, Sushinsky VE and Goncharova RI:
Polymorphism of DNA repair genes OGG1, XRCC1, XPD and ERCC6 in
bladder cancer in Belarus. Biomarkers. 19:509–516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yin Y, Tang L, Zhang J, Tang B and Li Z:
Molecular cloning and gene expression analysis of Ercc6l in Sika
Deer (Cervus nippon hortulorum). PLoS One. 6:e209292011. View Article : Google Scholar : PubMed/NCBI
|