Introduction
Ocular alkali burn is a common intractable ocular
disease in the clinic, which may cause blindness (1). Infiltrating leukocytes following
alkali burn release proteolytic enzymes and a variety of
inflammatory mediators, which leads to non-specific damage on the
corneal tissue, seriously affecting the structure and function of
the cornea. Corneal alkali burns cause serious conditions, such as
corneal melting, neovascularization and ulcer perforation (2). Severe alkali burns specifically
affect the vision of these patients (3). Chemical burns, particularly alkali
burns, are a common cause of corneal neovascularization (CNV), and
CNV is closely associated with vision loss. Numerous studies have
investigated the therapeutic methods that may cure corneal alkali
burns quickly and effectively (4,5).
Mesenchymal stem cells (MSCs) are derived from adult
stem cells in the mesoderm, which is an important cellular
component of the hematopoietic microenvironment (6,7).
MSCs contribute to proliferation and differentiation of a variety
of tissues, such as bone, cartilage, muscle, ligaments, tendons and
adipose stromal cells, and have low immunogenicity. Therefore, MSCs
are considered to be an ideal cellular source for tissue
engineering (8,9). In addition, MSCs are easily
transfected and carry exogenous genes. Furthermore, MSCs have a
wide range of applications in cell and gene therapy (10). Currently, clinical trials indicate
that MSCs may be used to repair genetic deficiency diseases of
mesenchymal tissue, which presents broad clinical application
possibilities (11,12).
Micro RNA (miRNA) is a class of non-coding,
single-stranded miRNA (18–24 bp), which incorporates into the
RNA-induced silencing complex, adjusting the stability and
translational efficiency of target molecules, and effectively
inhibiting gene expression (13,14).
In recent years, studies have demonstrated that miRNA is
particularly important in post-transcriptional regulation of
embryonic development, phylogeny, tissue differentiation and
evolution of disease, for example, cancer, cardiovascular disease
and neurological diseases (15).
In the process of MSC osteogenic differentiation, miRNAs are also
vital.
In the current study, MSCs were genetically modified
using a replication lentivirus over- or under-expressing miR146a
genes. We hypothesize that the MSCs over-expressing miR146a are
able to effectively repair the corneal alkali burn. The present
study may provide a promising method for corneal alkali burn
treatment.
Materials and methods
Isolation and culture of MSCs
A total of one six-week-old, female Sprague-Dawley
(SD; 100 g) rat was purchased from Animal Experimental Center of
Wenzhou Medical University (Wenzhou, China) and sacrificed
immediately to obtain the bone marrow. Bone marrow cells were
obtained by flushing the femurs and tibias with Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA; cat. no. 11965118). The cells were cultured in
culture flasks in complete DMEM with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.; cat. no. 16000044) and
penicillin/gentamycin (10 mg/ml; Gibco; Thermo Fisher Scientific,
Inc.; cat. no. 15070063) at 37°C. After 72 h, the nonadherent cells
were removed by replacing the DMEM. The DMEM was refreshed every
three days, and the cells were transferred upon reaching 80%
confluence. MSCs were isolated using flow cytometry, and MSCs from
the third passage were collected and resuspended in
phosphate-buffered saline (PBS) with 10% FBS. Monoclonal antibodies
were added for 30 min at 4°C, including cluster of differentiation
(CD)90 (Abcam, Cambridge, UK; 1:1,000; cat. no. EPR3132); CD45
(Abcam; 1:600; cat. no. ab10558); CD34 (Abcam; 1:3,000; cat. no.
ab81289); CD73 (Abcam; 1:600; cat. no. ab175396). The
phycoerythrin-conjugated antibodies against cluster of
differentiation CD90, CD45, CD34 and CD73 were purchased from
Biolegend, Inc. (San Diego, CA, USA). PBS served as a negative
control.
Animal model
A total of 24 female SD rats were used in the
current study, rats (age, 8–12 weeks; weight, 200–220 g) were
purchased and housed in an environmentally-controlled breeding room
of Wenzhou Medical University (Wenzhou, China), at a temperature of
20±2°C, a relative humidity of 60±5% and under a 12-h light/dark
cycle. The rats were anesthetized by intraperitoneal injection of
chloral hydrate (10%; 4 ml/kg). Sodium hydroxide (NaOH; 4 µl, 1
mol/l) was applied to a piece of filter paper (diameter, 3 mm) and
placed in the center of the cornea for 40 sec. The cornea was
immediately rinsed with saline for 1 min. The animals were
administered a subconjunctival one-off injection of MSCs
(1×107 cells) through the tail vein. The present study
was approved by the veterinary ethics committee of Zhejiang
(Wenzhou, China; ID: LY16H120004).
Quantitative polymerase chain reaction
(qPCR)
qPCR was performed as previously described (16). mRNA was obtained from the MSCs
using an RNeasy kit (Qiagen China Co., Ltd., Shanghai, China) and
cDNA was synthesized from total RNA (TaqMan™ Fast Reagent Starter
kit, cat. no. 4352407; Thermo Fisher Scientific, Inc.). PCR was
performed with the following thermocycling conditions: An initial 5
min at 95°C, followed by 40 cycles of 95°C for 30 sec, 55°C for 30
sec and 72°C for 30 sec. The primer sequences were as follows:
Forward, 5′-ACCACACCTTCTACAATGA-3′ and reverse,
5′-ATAGCACAGCCTGGATAG-3′ for β-actin, which were designed with
primer premier 6.0 (Premier Biosoft International, Palo Alto, CA,
USA). qPCR was conducted with an Applied Biosystems 7500 real-time
PCR system. Results were analyzed using the Light Cycler Software
version 3.5. Housekeeping gene β-actin was used as an internal
reference to normalize the results. All experiments were performed
in triplicate. Finally, the 2−ΔΔCq method was performed
to calculate the relative expression (17).
MTT assay
Evaluation of cell viability of MSCs was performed
via MTT assay. MSCs (2×105 in 100 µl) were seeded into
96-well cell plates and cultured in a cell incubator at 37°C
overnight. Then, 10 µl MTT (5 mg/ml) was added to each well and the
plates were incubated for 3 h at room temperature in the dark. The
dimethyl sulfoxide (DMSO) was added and the medium was discarded.
The absorbance 490 (nm) was tested 3 min later using an ELISA
microplate reader.
Apoptosis analysis of MSCs
Apoptosis analysis was performed as described
previously (18). Briefly, MSCs
were harvested from the cell culture flasks at approximately 80–90%
confluence and centrifuged at 300 × g for 10 min at 4°C. The medium
was discarded and cells were washed once with 3 ml PBS on ice. Each
cell pellet was resuspended in 100 µl PBS, stained with 10 µl
propidium iodide (PI) and 4 µl Annexin V-fluorescein isothiocyanate
(20 µg/ml) and incubated on ice for 30 min in the dark. Cell
apoptosis was analyzed by flow cytometry (Epics XL; Beckman
Coulter, Inc., Brea, CA, USA), and data were analyzed using a
FlowJo Software 7.6 (Tree Star, Inc., Ashland, OR, USA).
Western blot analysis
MSCs were harvested and lysed in RIPA buffer
(Sigma-Aldrich; Merck KGaA; cat. no. 20-188) at 4°C. Following
centrifugation (14,000 × g for 15 min at 4°C), the protein
concentration was determined using the Bradford method (Beyotime
Institute of Biotechnology, Nantong, China) according to the
manufacturer's protocol. A total of 20 µg total protein sample was
separated by 10% SDS-PAGE (Hangzhou Fude Biological Technology Co.,
Ltd., Hangzhou, China) and transferred onto a nitrocellulose
membrane. The nitrocellulose membrane was blocked with 5% nonfat
milk for 1 h at room temperature then incubated with antibodies
against p65 NF-κB (Abcam; cat. no. ab16502; 0.5 µg/ml), PCNA
(Abcam; cat. no. ab18197; 1 µg/ml) and Fas (Abcam; cat. no.
ab82419; 1:1,000) at 4°C overnight. The nitrocellulose membrane was
then incubated with anti-rabbit IgG (Abcam; cat. no. ab191866;
1:500) secondary antibodies, for 1 h at room temperature. Finally,
protein was detected using a 3,3′-diaminobenzidine kit (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China). GAPDH
served as the internal control.
ELISA
The expression levels of VEGF (R&D Systems,
Inc., Minneapolis, MN, USA; cat. no. RRV00), CD45 (G-Biosciences,
St Louis, MO, USA; cat. no. 50-148-9078), IL-10 (Invitrogen; Thermo
Fisher Scientific, Inc.; cat. no. ERIL10) and INF-γ (R&D
Systems, Inc.; cat. no. RIF00) in the aqueous humor of rats were
determined using an ELISA kit. Measurements were conducted
according to the manufacturer's protocol. A microplate reader was
used to determine the optical densities and data were presented as
means of triplicate wells.
Histological analysis
Histological analysis of corneal tissue samples was
performed by hematoxylin and eosin (H&E) staining. Rats were
administered with a subconjunctival injection of MSCs
(1×107 cells), or PBS for the control, through the tail
vein. The rats were subsequently sacrificed, following 4 weeks of
treatment, for histological examination. Corneal tissue samples
were preserved in 4% formaldehyde solution at room temperature,
dehydrated and embedded in paraffin. The 5-µm paraffin sections
were immersed in distilled water according to routine strategy.
H&E staining was conducted as follows: Washing with running
water for 30 min, dehydration in 90% alcohol for 5 min and eosin
staining for 3 min at room temperature. Then pictures were captured
under ×10 magnification using a light microscope (Leica Upright
Microscope; Leica Microsystems GmbH, Wetzlar, Germany).
Statistical analysis
Each experiment was repeated three times
independently. Data are presented as means ± standard deviation.
Data analyses were conducted using GraphPad Prism 6.0 software
(GraphPad Software, Inc., La Jolla, CA, USA). Statistical
significance was determined by using an analysis of variance
followed by least significant difference post hoc assessment
(α=0.05) and P<0.05 was considered to indicate a statistically
significant difference.
Results
miR146a expression levels in MSCs from
each group
As shown in Table
I, the results of qPCR indicate that there was no significant
difference between the Normal MSCs group and the Control group, and
the expression level of miR146a in the miR146a-low MSCs was
significantly decreased to 67.5±9.1% (P<0.001 vs. Normal MSCs),
the expression level of miR146a in miR146a-high MSCs was increased
to 137.5±15.9% (P<0.01 vs. Normal MSCs).
 | Table I.Expression levels of miR146a in each
group (means ± standard deviation). |
Table I.
Expression levels of miR146a in each
group (means ± standard deviation).
| Group | Control, % | Normal MSCs, % | miR146a-low MSCs,
% | miR146a-high MSCs,
% |
|---|
| miR146a | 100.0±11.2 | 97.9±10.5 | 67.5±9.1a |
137.5±15.9b |
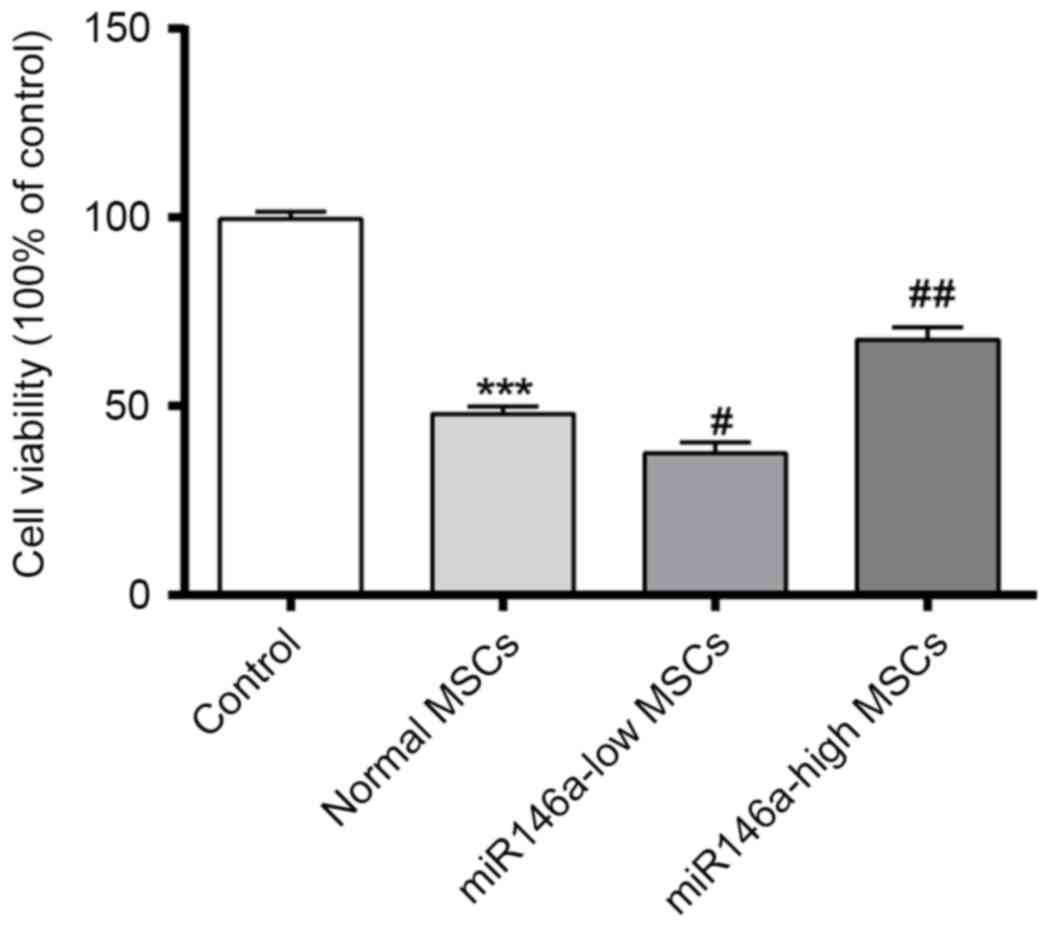
miR146a-high MSCs improve the cell
viability of MSCs following alkali burn
An MTT assay was performed to confirm the cell
viability of each group. As shown in Fig. 1, cell viability was significantly
decreased following alkali burn, while the cell viability of the
miR146a-low MSCs group was further decreased when compared with
that of Normal MSCs. However, the cell viability of the
miR146a-high MSCs group was restored, and significantly greater
than that of the Normal MSCs group.
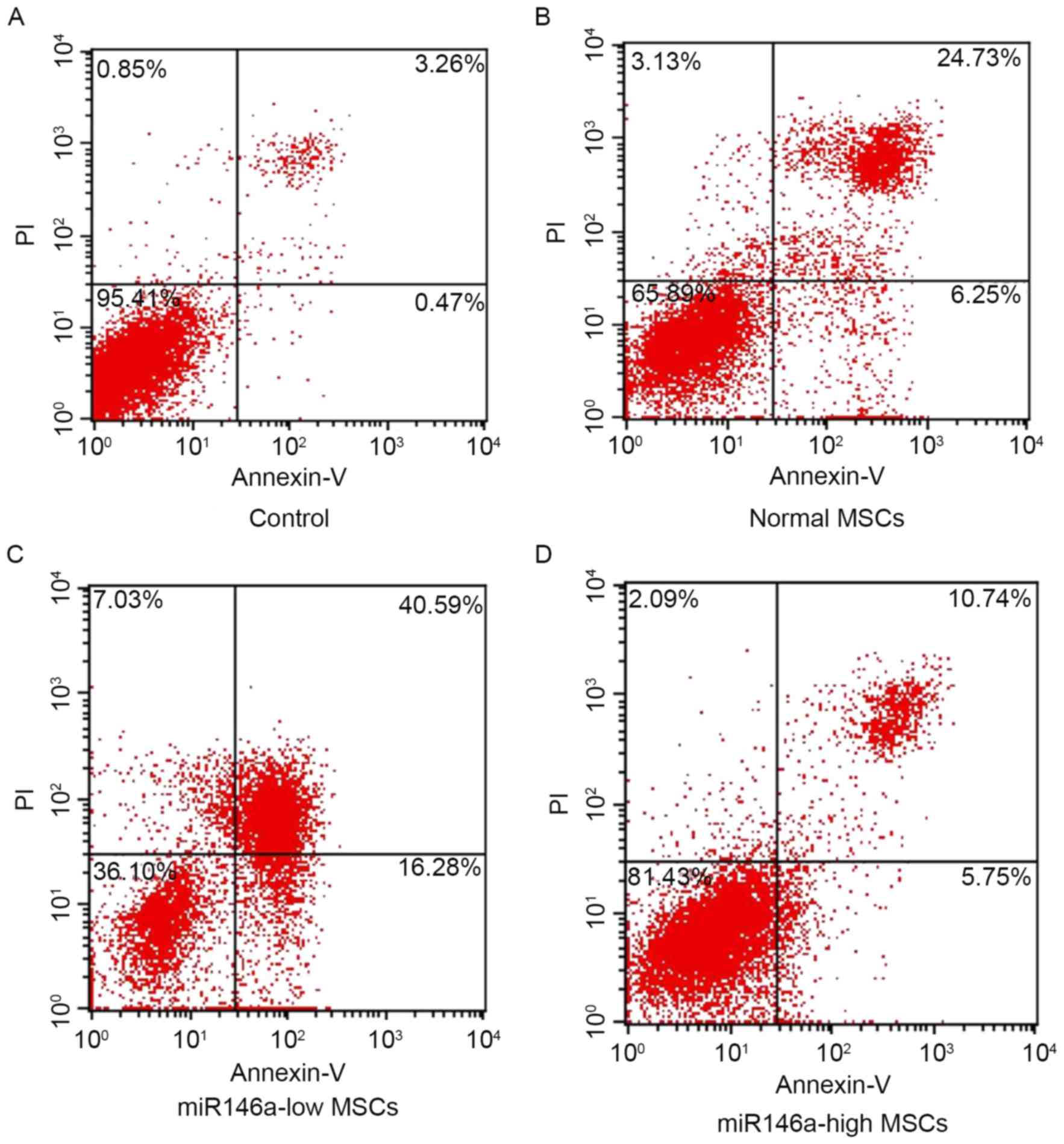
miR146a-high MSCs inhibited apoptosis
of MSCs following alkali burn
As shown in Fig. 2,
the percentage of apoptosis was detected using Annexin V/PI double
staining in each group. The results indicate that cells are
significantly apoptotic following alkali burn; furthermore, the
percentage of apoptotic cells in the miR146a-low MSCs group was
significantly increased. However, the apoptosis ratio of the
miR146a-high MSCs group was suppressed, and significantly reduced
when compared with the Normal MSCs group.
Expression levels of p65 NF-κB, PCNA
and Fas in MSCs following alkali burn
As shown in Fig. 3A and
B, western blot results demonstrated that expression levels of
p65 NF-κB and PCNA were decreased in MSCs following alkali burn,
although the expression level of Fas appears to be increased. p65
NF-κB and PCNA were significantly decreased in the miR146a-low MSCs
group, and Fas expression was increased to a certain degree,
although the difference was not significant when compared with the
Normal MSCs group. The levels of expression of p65NF-κB and PCNA in
the miR146a-high MSCs group were recovered to a certain degree,
while the expression level of Fas was suppressed; the differences
were significant when compared with the Normal MSCs group.
miR146a-high MSCs group demonstrated
improved corneal opacity and enhanced the inhibition of
neovascularization
The score of corneal opacity severity indicated
that, after 1, 2, 3 and 4 weeks of treatment, the degree of corneal
opacity in the Normal MSCs group was significantly improved,
although the cornea opacity in the miR146a-low MSCs group was
suppressed, and the miR146a-high MSCs group demonstrated enhanced
improvement of corneal opacity (Table
II). The results of the neovascularization show that after
weeks 1–4 of treatment, the neovascularization was normal. MSCs was
significantly inhibited, but the inhibition of neovascularization
was weakened in the miR146a-low MSCs group, and the miR146a-high
MSCs group demonstrated enhanced inhibition of neovascularization
(Table III).
 | Table II.Corneal opacity severity score of rats
in each group. |
Table II.
Corneal opacity severity score of rats
in each group.
|
| Corneal opacity
severity score |
|---|
|
|
|
|---|
| Group | 1 week | 2 weeks | 3 weeks | 4 weeks |
|---|
| Control | 3.91±0.32 | 3.88±0.31 | 3.24±0.28 | 2.92±0.24 |
| Normal MSCs |
3.01±0.22a |
2.75±0.26a |
2.10±0.36a |
1.98±0.21a |
| miR146a-low MSCs |
3.38±0.37b |
3.16±0.25b |
2.69±0.27b |
2.33±0.34b |
| miR146a-high
MSCs |
2.54±0.23b |
2.18±0.34b |
1.89±0.19b |
1.55±0.16b |
 | Table III.Comparison of the neovascularization
area of rats in each group. |
Table III.
Comparison of the neovascularization
area of rats in each group.
|
| Neovascularization
area, mm2 |
|---|
|
|
|
|---|
| Group | 1 week | 2 weeks | 3 weeks | 4 weeks |
|---|
| Control | 10.2±1.22 | 18.3±2.01 | 16.7±1.78 | 15.5±1.68 |
| Normal MSCs |
8.23±1.74a |
13.4±1.24b |
10.6±1.20b |
8.74±1.09b |
| miR146a-low
MSCs |
9.26±1.18c |
15.4±1.66c |
13.1±1.54c |
11.1±1.07c |
| miR146a-high
MSCs |
6.64±0.77d |
10.2±1.04c |
6.36±0.87d |
3.96±0.49d |
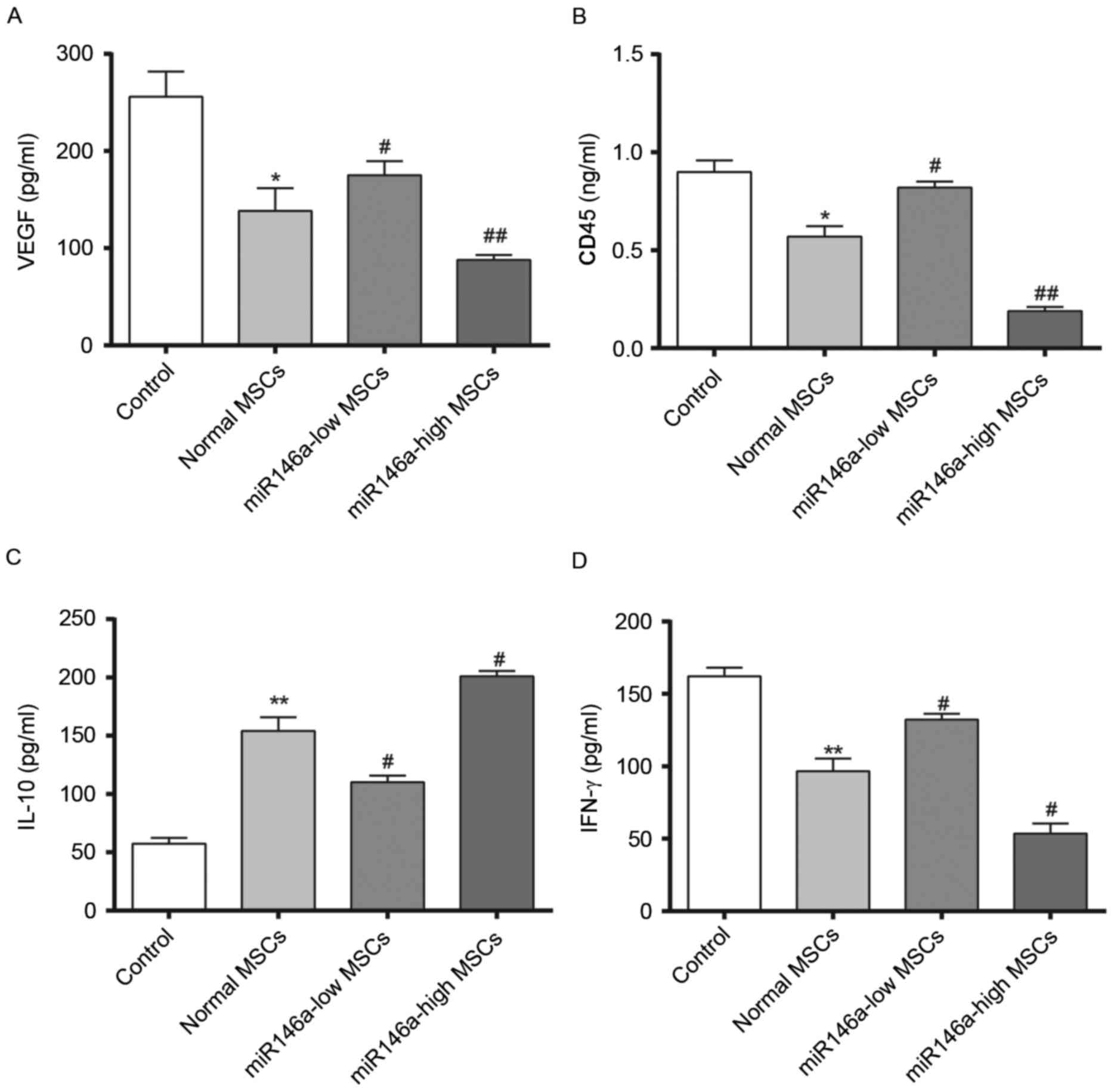
Levels of VEGF secretion in the
aqueous humour and inflammation-associated cytokine expression
levels in rat corneal tissue samples following alkali burn
Fig. 4 demonstrates
results of 4 weeks following the alkali burn surgery being
performed. The content of VEGF was significantly decreased in the
Normal MSCs group and the content of VEGF was significantly higher
in the miR146a-low MSCs group compared with the Normal MSCs group,
while the content of VEGF in the miR146a-high MSCs group was
significantly lower than in the Normal MSCs group. In addition,
evaluation of the corneal tissue inflammation factors, CD45 and
IFN-γ demonstrated a consistent trend, whereas the change in the
concentration of IL-10 was the opposite (indicating the special
status of IL-10, which requires further investigation). This
indicated that miR146a may inhibit the expression levels of VEGF,
CD45 and IFN-γ, while enhancing the expression level of IL-10.
These results indicate that the miR146a-high MSCs group exerted a
better effect on inhibiting the inflammation when compared with the
Normal MSCs group.
Histological observation of the rat
corneas following treatment
After four weeks of treatment, morphological
observation of corneal tissue samples was conducted by H&E
staining (Fig. 5). In the control
group, the corneal surface was covered with epithelial cells, no
edema in the stroma, collagen fibers neatly arranged and a large
number of visible novel blood vessels are evident in the corneal
stroma. In the normal MSCs group the epithelial corneal wound was
healed, there was no edema in the stroma and the collagen fibers
were neatly arranged, however only a few of visible novel blood
vessels are visible in the corneal stroma. In the miR146a-low MSCs
group, the corneal surface was covered with epithelial cells with
no edema in the stroma, the collagen fibers neatly arranged and a
mass of visible novel blood vessels present in the corneal stroma.
In the miR146a-high MSCs group, the epithelial corneal wound was
healed, there was no edema in stroma, collagen fibers were tightly
arranged and there were only a few visible novel blood vessels in
the corneal stroma.
Discussion
Corneal alkali burn is a common clinical
ophthalmology disease, the treatment of which is quite difficult
and the prognosis is poor. Previous studies of the underlying
mechanism of the injury have been performed (19,20).
MSCs are an important cellular component of the hematopoietic
microenvironment, which proliferate and differentiate into a
variety of tissues, and have low immunogenicity. Furthermore, MSCs
have a high degree of proliferation, self-renewal and pluripotency
(21). Clinical trials confirmed
that MSCs may be used in tissue repair (22). For the osteogenic differentiation
of MSCs, multiple cytokines and signaling pathways are involved in
the regulation of differentiation.
In the current study, MSCs were transfected with
lentiviral recombined miR146a genes. In the current assay, SD rats
were used to establish corneal alkali burn models to evaluate the
effects of miR146a-high MSCs. The data demonstrated that
miR146a-high MSCs inhibited cell apoptosis in corneal alkali burn
rats. In addition, miR146a-high MSCs inhibited the expression of
p65 NF-κB and PCNA, and promoted the expression of Fas in corneal
alkali burn rats. The result implied that miR146a-high MSCs
produced a strong repair effect and provided protection (23). These results demonstrated that
genetically modified miR146a-high MSCs represent a promising
strategy for corneal alkali burn therapeutic strategies.
One or four weeks after transplantation, corneal
haze and the neovascular situation were observed under a slit lamp
(the growth time, length and number of CNV were recorded, and the
length and area of CNV were calculated as described previously
(24). The corneal alkali burn
scoring (cornea scoring criteria following alkali burn) was also
evaluated according to the standard reference (25). Strong inhibition of CNV in rats
treated with miR146a-high MSCs was observed. Thus, it was inferred
that miR146a-high MSCs result in high repair effects, which may be
due to the miR146a. Thus, further studies are required to determine
the role of miR146a. As shown in Fig.
4, decreased expression levels of VEGF, CD45 and IFN-γ were
observed in the miR146a-high MSCs group rats. CD45 and IFN-γ have
been demonstrated to be inflammation-associated cytokines (26,27).
Therefore, miR146a promotes repair of tissues via inhibited
secretion of CD45 and IFN-γ. Whereas the expression level of IL-10
was the opposite, which was a notable finding.
In conclusion, the present study indicated that
miR146a in MSCs induced a powerful protective and repair effect.
Notably, miR146a directly decreased the expression level of p65
NF-κB and PCNA, and inhibited apoptosis, inflammatory cytokine
secretion and CNV in corneal alkali burn rats. The level of corneal
opacity also improved significantly in rats treated with
miR146a-high MSCs. These results imply that MSCs genetically
modified with miR146a may serve as an effective therapeutic
strategy for corneal alkali burn. The present study still has
limitations, for example, the detailed signaling pathway requires
further investigation, and large animal experiments also are
required to determine the role of miR146a-high MSCs in treating
corneal alkali burns.
Acknowledgements
Not applicable.
Funding
The present study was supported by Zhejiang
Provincial Natural Science Foundation of China (grant nos.
LY16H120004, LY16H110002 and LY14H020005).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
ZJ designed this study. XL and JL performed all the
experiments. LY helped to collect data. JP helped to organize
figures. YZ helped with analysis and interpretation of data.
Ethics approval and consent to
participate
The present study was approved by the veterinary
ethics committee of Zhejiang (Wenzhou, China).
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Welling JD, Pike EC and Mauger TF: Alkali
burn of the ocular surface associated with a commonly used antifog
agent for eyewear: Two cases and a review of previous reports.
Cornea. 35:289–291. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saud EE, Moraes HV Jr, Marculino LG, Gomes
JA, Allodi S and Miguel NC: Clinical and histopathological outcomes
of subconjunctival triamcinolone injection for the treatment of
acute ocular alkali burn in rabbits. Cornea. 31:181–187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hua MT and Betz P: Descemet membrane
detachment after alkali ocular surface burn. Bull Soc Belge
Ophtalmol. 85–86. 2010.PubMed/NCBI
|
|
4
|
Nishiwaki-Dantas MC, Dantas PE and Reggi
JR: Ipsilateral limbal translocation for treatment of partial
limbal deficiency secondary to ocular alkali burn. Br J Ophthalmol.
85:1031–1033. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ke Y, Wu Y, Cui X, Liu X, Yu M, Yang C and
Li X: Polysaccharide hydrogel combined with mesenchymal stem cells
promotes the healing of corneal alkali burn in rats. Plos One.
10:e01197252015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cox CD, Nakayama Y, Nomura T and Martinac
B: The evolutionary ‘tinkering’ of MscS-like channels: Generation
of structural and functional diversity. Pflugers Arch. 467:3–13.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dumitru CA, Hemeda H, Jakob M, Lang S and
Brandau S: Stimulation of mesenchymal stromal cells (MSCs) via TLR3
reveals a novel mechanism of autocrine priming. Faseb J.
28:3856–3866. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lemos DR, Eisner C, Hopkins CI and Rossi
FMV: Skeletal muscle-resident MSCs and bone formation. Bone.
80:19–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rowe I, Anishkin A, Kamaraju K, Yoshimura
K and Sukharev S: The cytoplasmic cage domain of the
mechanosensitive channel MscS is a sensor of macromolecular
crowding. J Gen Physiol. 143:543–557. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhoj M, Zhang C and Green DW: A first step
in de novo synthesis of a living pulp tissue replacement using
dental pulp MSCs and tissue growth factors, encapsulated within a
bioinspired alginate hydrogel. J Endod. 41:1100–1107. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Langroudi L, Hassan ZM, Soleimani M and
Hashemi SM: Tumor associated mesenchymal stromal cells show higher
immunosuppressive and angiogenic properties compared to adipose
derived MSCs. Iran J Immunol. 12:226–239. 2015.PubMed/NCBI
|
|
12
|
Bajpai I, Kim DY, Kyong-Jin J, Song IH and
Kim S: Response of human bone marrow-derived MSCs on triphasic Ca-P
substrate with various HA/TCP ratio. J Biomed Mater Res B Appl
Biomater. 105:72–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Naqvi AR, Zhong S, Dang H, Fordham JB,
Nares S and Khan A: Expression profiling of LPS responsive miRNA in
primary human macrophages. J Microb Biochem Technol. 8:136–143.
2016.PubMed/NCBI
|
|
14
|
Li D, Mou W, Luo Z, Li L, Limwachiranon J,
Mao L and Ying T: Developmental and stress regulation on expression
of a novel miRNA, Fan-miR73, and its target ABI5 in strawberry. Sci
Rep. 6:283852016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang P, Xu J, Hou Z, Wang F, Song Y, Wang
J, Zhu H and Jin H: miRNA-34a promotes proliferation of human
pulmonary artery smooth muscle cells by targeting PDGFRA. Cell
Prolif. 49:484–493. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zibara K, Awada Z, Dib L, El-Saghir J,
Al-Ghadban S, Ibrik A, El-Zein N and El-Sabban M: Anti-angiogenesis
therapy and gap junction inhibition reduce MDA-MB-231 breast cancer
cell invasion and metastasis in vitro and in vivo. Sci Rep.
5:125982015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ji YB and Yu L: In vitro analysis of the
role of the mitochondrial apoptosis pathway in CSBE therapy against
human gastric cancer. Exp Ther Med. 10:2403–2409. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giacomini C, Ferrari G, Bignami F and Rama
P: Alkali burn versus suture-induced corneal neovascularization in
C57BL/6 mice: An overview of two common animal models of corneal
neovascularization. Exp Eye Res. 121:1–4. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anderson C, Zhou Q and Wang S: An
alkali-burn injury model of corneal neovascularization in the
mouse. J Vis Exp. 86:e511592014.
|
|
21
|
Nikiforou M, Willburger C, De Jong AE,
Kloosterboer N, Jellema RK, Ophelders DR, Steinbusch HW, Kramer BW
and Wolfs TG: Global hypoxia-ischemia induced inflammation and
structural changes in the preterm ovine gut which were not
ameliorated by mesenchymal stem cell treatment. Mol Med. 22:2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang X, Zhang L, Wang S, Han Q and Zhao
RC: Exosomes secreted by mesenchymal stem cells promote endothelial
cell angiogenesis by transferring miR-125a. J Cell Sci.
129:2182–2189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu X, Yan C, Kossmann BR and Ivanov I:
Secondary interaction interfaces with PCNA control conformational
switching of DNA polymerase PolB from polymerization to editing. J
Phys Chem B. 120:8379–8388. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su P, Wang Y, Cooper DN, Zhu W, Huang D,
Férec C, Wang Y and Chen JM: Disclosing the hidden structure and
underlying mutational mechanism of a novel type of duplication CNV
responsible for hereditary multiple osteochondromas. Hum Mutat.
36:758–763. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moro C, Cornette R, Vieaud A, Bruneau N,
Gourichon D, Bed'hom B and Tixier-Boichard M: Quantitative effect
of a CNV on a morphological trait in chickens. Plos One.
10:e01187062015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ikeda H, Old LJ and Schreiber RD: The
roles of IFN gamma in protection against tumor development and
cancer immunoediting. Cytokine Growth Factor Rev. 13:95–109. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baer C, Squadrito ML, Laoui D, Thompson D,
Hansen SK, Kiialainen A, Hoves S, Ries CH, Ooi CH and De Palma M:
Suppression of microRNA activity amplifies IFN-gamma-induced
macrophage activation and promotes anti-tumour immunity. Nat Cell
Biol. 18:790–802. 2016. View
Article : Google Scholar : PubMed/NCBI
|