Introduction
The vascular endothelium has an important function
in maintaining homeostasis among a number of endothelial-derived
factors including nitric oxide (NO), prostacyclin, endothelin-1 and
angiotensin II. NO is now considered the most efficient endogenous
vasodilator and it can exert vasoprotective effects by inhibiting
platelet aggregation, inflammation, oxidative stress, fibrosis,
vascular smooth muscle cell migration, proliferation, and leukocyte
adhesion (1,2). However these functions of the
endothelium can be weakened or eliminated by exposure to
atherosclerosis risk factors. Endothelial dysfunction is a key
factor in the initiation, progression and complications of
atherosclerosis, and is now regarded as a clinical syndrome
(3–5). Endothelial NO synthase (eNOS) is the
key enzyme for NO generation. Tetrahydrobiopterin (THB) is a
required cofactor for eNOS that is primarily regulated by its
rate-limiting enzyme, guanosine triphosphate cyclohydrolase I
(GCH1) (6,7).
Glucagon-like peptide-1 (GLP-1) is a potent
glucose-dependent insulin tropic hormone that is released from
intestinal L-cells. It has been observed that GLP-1 also has
cardioprotective effects that are distinct from its glucose and
weight-loss effects (8,9). The short half-life of GLP-1 and its
effects on metabolites limit its clinical application (10).
Exendin-4 (Ex4; Food and Drug Administration
approval number BYETTA; NDA; 021773; www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&applno=021773),
a GLP-1 analog, is a highly potent agonist of the GLP-1 receptor.
As a result of its shared effects with GLP-1 and as it resists
degradation by dipeptidyl peptidase 4, Ex4 has been approved by the
United States Federal Drug Administration as an adjunct therapy to
improve glycemic control in patients with type 2 diabetes
mellitus.
Previous studies demonstrated that Ex4 has a
protective effect against cardiovascular disease. For example, Ex4
exerts cardioprotective effects by improving endothelial function
and acting as an antihypertensive, and these effects are
independent of its hypoglycemic and weight-loss functions (11–13).
d'Uscio et al (14) in 2016
demonstrated that exenatide treatment improved the coronary flow
velocity reserve of patients with type 2 diabetes. Further study of
human umbilical vein endothelial cells revealed that Ex4 increased
NO production, GCH1 levels and eNOS activation via GLP-1R/cyclic
adenosine monophosphate (cAMP) signaling pathways (15).
Apolipoprotein E knockout (APOE-KO) mice can develop
severe hypercholesterolemia, atherosclerosis and associated
endothelial dysfunction (14).
Evidence has demonstrated that THB is involved in maintaining the
normal function of eNOS (6,7),
suggesting that THB and NOS may be associated. Phosphorylation of
Ser1177 is associated with the activation of eNOS and enhances NO
generation. Several studies have demonstrated that Ex4 improves
endothelial function (16,17), but few studies have investigated
the involvement of the GCH1/THB signaling pathway in endothelial
dysfunction in mice fed a high-cholesterol diet (18).
It was hypothesized that Ex4 treatment may increase
GCH1 and THB levels, thereby preserving normal eNOS function, to
produce increased NO production (19,20,21).
In the present study, the association between THB levels and eNOS
function was examined. Furthermore, the effect of Ex4 on
dysfunctional endothelium in the thoracic aorta isolated from
APOE-KO mice given a high-cholesterol diet was investigated.
Materials and methods
All experiments were performed in accordance with
protocols approved by the Guangxi Medical University Animal Ethics
Committee (Nanning, China).
Animals
Genetically wild-type (WT) C57BL/6 mice (n=30;
weight, 17.22±0.79 g) and APOE-KO mice of C57BL/6 background (n=30;
weight, 20.12±0.96 g) were purchased from Charles River
Laboratories, Inc., (Beijing, China). All of the mice were male and
6-weeks-old. The WT mice and the APOE-KO mice were each randomly
and equally allocated to be treated either with Ex 4 (Ex4-treated)
or not (controls). Therefore, 4 groups of 15 mice were created: i)
WT control; ii) WT+Ex4; iii) APOE-KO control; and iv) APOE-KO+Ex4.
The mice in the two treatment groups (WT+Ex4 and APOE-KO+Ex4) were
intraperitoneally injected (IP) with Ex4 (1.0
nmol·kg−1·day−1, twice daily) for 8 weeks.
The control groups were IP injected with an equal volume of
physiological saline.
All the mice in all the groups were fed a
high-cholesterol diet (1.25% cholesterol) starting at age 6 weeks
and throughout the entire experiment. All mice were provided free
access to food and water and atmosphere were maintained at a
temperature of 23±2°C, 40–70% humidity and a 12-h
light/dark-cycle.
Tissue procurement
This investigation conforms to the Guide for the
Care and Use of Laboratory Animals published by the United States
National Institutes of Health (22) and was approved by the Laboratory
Animal Ethical Committee of Guangxi Medical University.
Following 8 weeks of treatment, the mice were
anesthetized by pentobarbital (1%; 80 mg drug/kg animal body
weight; IP) and euthanized by exsanguination. Blood samples were
harvested from the heart (0.8–1 ml/mouse). The entire length of the
aorta of each mouse was carefully dissected, from the aortic valve
to the iliac bifurcation. The aorta was immediately put into a dish
with ice-cold Krebs buffer (NaCl 120 mM, NaHCO3 25 mM,
KCl 4.8 mM, NaH2PO4 1.2 mM, MgSO4
1.2 mM, dextrose 11.0 mM and CaCl2 1.8 mM aerated with
95% O2, pH=7.4). The fat and loose connective tissue was
separated carefully under a light microscope and the gross specimen
was observed.
The aorta was fixed in 10% formalin at room
temperature of 25°C for 24 h and embedded in paraffin. Serial 5-µm
sections were cut at the aortic arch and descending aorta and
stained with hematoxylin/eosin (H&E) 1–3 min at room
temperature for histological analysis. Aortas were frozen in liquid
nitrogen for subsequent western blotting and high-performance
liquid chromatography (HPLC), or prepared for the next vasomotor
function experiment.
Pathology and morphological study
The aortic arch of each mouse was employed for the
pathological and morphological study. The isolated arteries were
observed under a dissecting microscope to study the gross
appearance. Photographs of the gross specimen were captured with a
NIKON camera (D5000; Nikon Corporation, Tokyo, Japan; Fig. 1). The arteries indicated in
Fig. 1 were fixed with 4%
paraformaldehyde for 24 h at room temperature and processed for
paraffin embedding. Serial 4-µm transverse sections were taken at
the aortic arch and stained with H&E for pathological study
(Fig. 2) as described previously
(23).
Plasma
The plasma glucose of each mouse was measured by the
glucose oxidase method performed as described previously (24). (GOD-POD kit, Applygen Technologies
Inc., Beijing, China). Insulin levels were monitored by
radioimmunoassay as described previously (25) (Insulin RIA Kit, BNIBT, Beijing,
China). Plasma triglyceride (TG), total cholesterol (TC),
low-density lipoprotein cholesterol (LDL-C) and high-density
lipoprotein cholesterol (HDL-C) were determined using an automated
clinical biochemistry analyzer (Hitachi 7600; Hitachi, Ltd., Tokyo,
Japan).
Artery endothelial function
Measurement of vascular
relaxation
The descending thoracic aorta of each mouse was
studied. The aorta was crosscut into 3–4 mm vascular rings,
avoiding the intercostal artery branches and one side was mounted
on a hook in an organ chamber containing 20 ml Krebs buffer (NaCl,
120 mM; NaHCO3, 25 mM; KCl, 4.8 mM; NaH2PO4, 1.2 mM; MgSO4, 1.2 mM;
glucose, 11.1 mM; CaCl2, 1.8 mM, aerated with 95% O2 and 5% CO2,
pH=7.4.) maintained at 37°C. The other side was connected with a
force transducer to collect isometric tension data. The resting
tension of the rings was increased gradually to 5 mN and
equilibrated for 60 min. During equilibration, the Krebs buffer in
the organ chamber was refreshed every 20 min.
Following equilibration, Krebs buffer with 60 mmol/l
KCl was added into the chamber, 3 times, to determine the maximal
depolarization-induced contractions. The rings were washed with
Krebs buffer 3 times and re-equilibrated for 30 min.
Once vessels were pre-contracted at 37°C with
L-phenylephrine (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany;
10−6 mol/l), the relaxation response to acetylcholine
(10−5 mol/l; Sigma-Aldrich; Merck KGaA) was tested to
confirm the integrity of the endothelium and then was equilibrated.
The rings were pre-contracted with phenylephrine (1 M) and when
they reached a stable contraction plateau, endothelium-dependent
relaxation responses to cumulative concentrations of acetylcholine
(10−9 to 10−5 mol/l) were measured.
Following another 3 washes with Krebs buffer and a
30 min equilibration, endothelium-independent relaxation responses
to sodium nitroprusside (Sigma-Aldrich; Merck KGaA; 10−9
to 10−5 mol/l) were determined.
Western blot analysis
The snap-frozen aorta of each mouse was lysed with
Radioimmunoprecipitation Assay buffer (Beyotime Institute of
Biotechnology, Haimen, China) with 1 mmol/l fresh
phenylmethylsulfonyl fluoride (Beyotime Institute of
Biotechnology,) and a complete phosphatase inhibitor cocktail
tablet ((Roche Diagnostics GmbH, Mannheim, Germany). The protein
content was determined by a Bicinchoninic protein assay (Beyotime
Institute of Biotechnology). Aorta protein extracts were denatured
and 80 µg sample was loaded onto 10%SDS-PAGE gel and transferred.
The resolved proteins in the gel were electro-transferred to a
polyvinylidene fluoride membrane. The membrane was blocked with 5%
(w/v) nonfat dry milk (dissolved in Tris-buffered saline with
0.05%Tween) for 1 h at room temperature.
Membranes were incubated serially overnight at 4°C
with the following primary antibodies, diluted appropriately in
primary antibody dilution buffer: Anti-eNOS (1:1,000; cat. no.
sc654 Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-phosphorylated (p)-eNOS ser-1177 (1:1,000; cat. no. sc12972
Santa Cruz Biotechnology, Inc., Dallas, TX, USA); and anti-GCH1
(1:1,000; cat. no. ab236387 Abcam, Cambridge, UK).
The membranes were then washed in TBST 3 times for
10 min, incubated with horseradish peroxidase-conjugated goat
anti-rabbit IgG (1:10,000; ZSGB-BIO; OriGene Technologies, Inc.,
Beijing, China.) for 1 h at room temperature and washed in TBST 3
times for 10 min. The immunoblots were visualized using a DAB
chemiluminescence kit (Beyotime Institute of Biotechnology). Equal
loading of protein was confirmed by blotting for GAPDH (1:1,000;
cat. no. ta309157; ZSGB-BIO, Beijing, China). Protein levels were
normalized with reference to GAPDH. p-eNOS ratios were normalized
according to the total eNOS levels of corresponding groups.
Relative band densitometry was analyzed by ImageJ software (ImageJ
1.44p; National Institutes of Health, Bethesda, MD, USA).
Measurement of THB and biopterin
Measurement of THB levels in aortas was performed
using internal standard HPLC with florescence detection, as
described previously (26). A
total of three snap-frozen aortas from each group were lysed in
ice-cold extraction buffer (50 mmol/l Tris-HCl, pH 7.4, 1 mmol/l
dithiothreitol and 1 mmol/l EDTA), centrifuged at 13,000 × g for 10
min at 4°C and the supernatant was collected.
A protein assay was performed using a Bicinchoninic
protein assay (Beyotime Institute of Biotechnology). The proteins
from the supernatant were removed by adding 10 µl of a 1:1 mixture
of 1.5 mol/l HClO4 and 2 mol/l
H3PO4, to 90 µl extracts, and centrifuged at
13,000 × g for 10 min at 4°C. The supernatant was employed for THB
measurement.
Total biopterin (THB, dihydropterin and biopterin)
was determined by acidic oxidation using 10 µl of 1% iodine and 2%
KI dissolved in 1 mol/l HCL, added to 90 µl protein-free
supernatant. BH2+B was determined by alkaline oxidation.
This was kept at room temperature in the dark for 1 h. A total of
20 µl, 1 mol/l H3PO4 was added to acidify the
alkaline-oxidation samples. A total of 5 µl fresh ascorbic acid (20
mg/ml) was added into each sample to remove the iodine. Samples
were centrifuged at 13,000 × g for 10 min at 4°C.
Biopterin (Sigma-Aldrich; Merck KGaA) was used for
internal standard. A total of 20 µl of supernatant was loaded into
a 250-mm long, 4.6-mm inner diameter Spherisorb ODS-1 column
(Guangzhou Research and Creativity Biotechnology, Co., Ltd.,
Guangzhou, China) with a methanol-to-water (5:95, v/v) mobile phase
running at a flow rate of 1.0 ml/min, column temperature was 25°C
and detected fluorometrically at wavelengths of 350 nm for
excitation and 440 nm for emission (LC-20 series, Shimadzu Corp.,
Kyoto, Japan).
Statistical analysis
In vitro experiments were repeated 3 times.
Data are expressed as the mean ± standard deviation. One-way
analysis of variance was used to examine the differences among the
4 groups of mice and the Student-Newman-Keuls post hoc test was
used for a further pairwise comparison. All statistical analyses
were performed using SPSS 16.0 software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Plasma analyses and body weight of
mice
Plasma TG, HDL-C, glucose and insulin levels were
not significantly different among the 4 groups (Table I). The TC in the APOE-KO and
APOE-KO+Ex4 groups was ~15-fold higher compared with the WT control
and WT+Ex4 groups, and LDL-C levels were also significantly
increased in the APOE-KO (P<0.01) and APOE-KO+Ex4 (P<0.01)
groups. However, the TC and LDL-C levels were not statistically
different between APOE-KO and APOE-KO+Ex4 groups.
 | Table I.Plasma lipids, glucose, insulin and
NO levels. |
Table I.
Plasma lipids, glucose, insulin and
NO levels.
| Factor | WT | WT+Ex4 | APOE-KO | APOE-KO+Ex4 |
|---|
| TC, mmol/l | 2.64±0.46 | 2.34±0.62 |
37.61±7.72b |
33.75±7.38b |
| TG, mmol/l | 0.65±0.20 | 0.87±0.32 | 1.11±0.24b |
1.16±0.25b |
| HDL-C, mmol/l | 2.19±0.34 | 2.57±1.37 | 4.73±2.00 | 5.13±2.88 |
| LDL-C, mmol/l | 0.65±0.14 | 0.72±0.11 |
16.87±6.01b |
13.08±4.55b |
| Glucose,
mmol/l | 6.59±1.78 | 6.90±2.25 | 6.26±1.63 | 4.14±1.16 |
| Insulin,
pmol/l | 17.16±4.98 | 15.06±3.94 | 12.53±2.74 | 11.26±2.23 |
| NO, µmol/l | 27.11±7.44 |
65.33±11.72a | 20.00±7.37 |
37.33±12.6c |
There was a trend in the WT control group toward
higher NO levels compared with the APOE-KO group, but the
difference was not significant (Table
I). However, NO levels in the APOE-KO+Ex4 mice were
significantly increased to 37.3±12.6 µmol/l, which was nearly
2-fold higher compared with the APOE-KO group (20.0±7.4 µmol/l;
P=0.015; both groups). The NO levels were also significantly 2-fold
higher in the WT+Ex4 group (65.3±11.7 µmol/l) compared with the WT
control group (27.1±7.4 µmol/l; P<0.001, both groups).
The body weights of mice were recorded and analyzed
(Table II). The data regarding
body weights demonstrated no significant difference between the
APOE-KO and APOE-KO+Ex4 groups at 6- and 14-weeks-old. The body
weights of the WT control and WT+Ex4 groups were also comparable at
6- and 14-weeks-old.
 | Table II.Body weight of mice in the present
study. |
Table II.
Body weight of mice in the present
study.
| Age group
(weeks) | WT (g) | WT+Ex4 (g) | APOE-KO (g) | APOE-KO+Ex4
(g) |
|---|
| 6 | 17.22±0.79 | 17.78±0.83 | 20.12±0.96 | 20.48±1.59 |
| 14 | 26.88±1.05 | 25.78±1.54 | 29.12±0.97 | 28.36±0.66 |
Histopathology and morphological
changes of the arteries
Regarding general gross observation of the artery
specimens, an atheromatous plaque was undetectable in the WT
control (Fig. 1A) and WT+Ex4
(Fig. 1B) groups, and there was no
vascular stenosis; they were normal in morphology. By contrast, in
the untreated APOE-KO mice (Fig.
1C) specifically, the histopathology and morphological results
demonstrated severe atherosclerosis of the aorta in the APOE-KO
mice (Figs. 1 and 2). The arterial lumen was substantially
stenosed by an atheromatous plaque, particularly at the aortic
arch, the predilection site of arteriosclerotic lesions and also in
certain principal branches. However, atherosclerosis was not
grossly observed in the APOE-KO+Ex4 (Fig. 1D) group; the lumen and edge of the
artery were clearly observed and appeared similar to that of the WT
mice.
On microscopic examination of the sections stained
with H&E, arteries of the WT control and WT+Ex4 (Fig. 2A and B) groups were similar in
morphology. The intimae of the arteries were smooth, and the
endothelia and muscle were arranged regularly. Atheromatous lesions
could not be observed. Typical atheromatous lesions were
demonstrated easily in the arteries of the APOE-KO (Fig. 2C) group, with a number of
macrophages and foam cells in atheromatous plaques. The arterial
wall was thickened homogeneously. In the APOE-KO+Ex4 (Fig. 2D) group, foam cells were also
present in the subendothelium. Atherosclerotic lesions were not
diffused so widely and thickening of the arterial wall was
inconspicuous when compared with the APOE-KO group.
Endothelium-dependent vasodilation
mediated by acetylcholine
Once arterial rings were pre-contracted with
10−6 mol/l phenylephrine, concentration-dependent
acetylcholine-induced vasodilation was observed (Fig. 3). The maximum
acetylcholine-induced-vasodilation of the arteries of the
non-treated APOE-KO group (53.9±4.3%) was ~1.5 times that of the WT
control group and was significantly different (77.9±8.7%;
P<0.05; Fig. 3A). The maximum
acetylcholine-induced-vasodilation of the arteries of the WT+Ex4
group was 98.9±2.1%, which was significantly higher compared with
the WT control group (77.9±8.7%; P<0.05; both groups; Fig. 3B). By contrast, the maximum
acetylcholine-induced-vasodilation in the APOE-KO+Ex4 mice was
87.8±3.6%, which was significantly increased compared with the
APOE-KO group (53.9±4.3%; P<0.01; Fig. 3C). The 4 groups were comparable
regarding the degree of sodium nitroprusside-induced
endothelium-independent vasodilation (Fig. 3D).
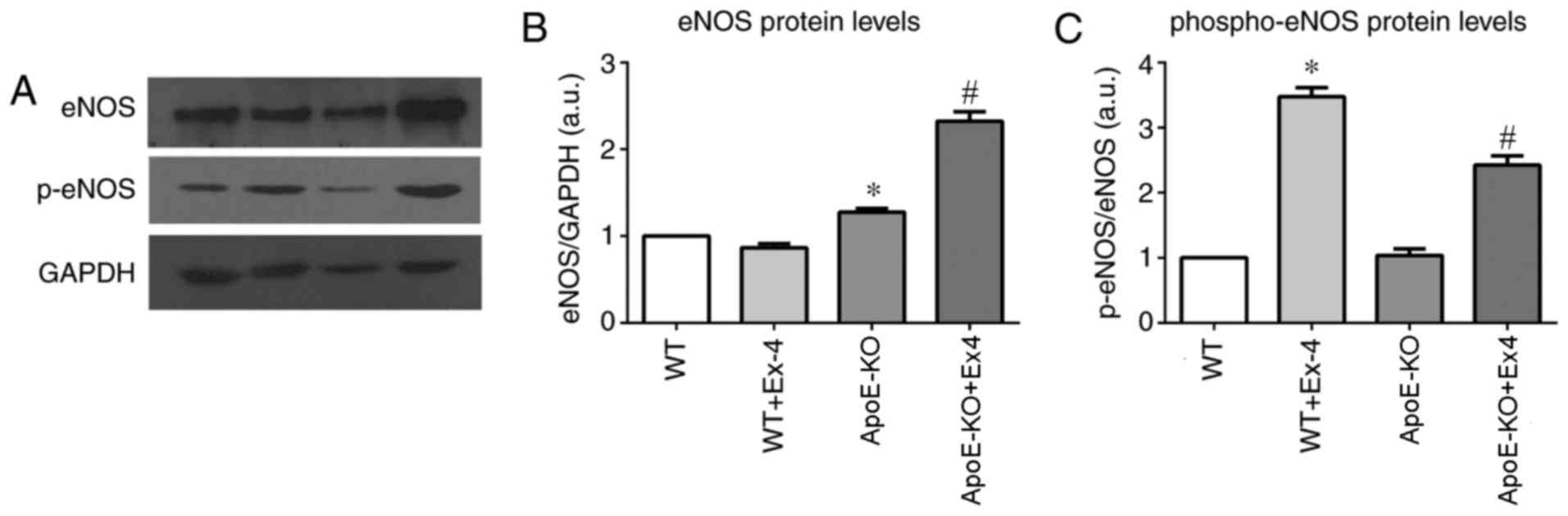
eNOS and p-eNOS protein levels
Western blotting bands are presented in Fig. 4A. The results indicated that total
eNOS protein levels in the arteries of the APOE-KO group were
significantly increased compared the WT group (P=0.014). Ex4
treatment was associated with ~2-fold higher total eNOS protein
levels in the APOE-KO+Ex4 group compared with the APOE-KO group
(P<0.05). However, the total eNOS of the WT+Ex4 group was
similar to that of the WT group (P=0.154; Fig. 4B).
The arterial p-eNOS protein levels were also
investigated, and the WT control and APOE-KO groups were
comparable. Ex4 treatment in the WT+Ex4 group was associated with a
3.5-fold increase in p-eNOS levels compared with the WT non-treated
group (P<0.05) and with a 2-fold increase in the APOE-KO+Ex4
mice compared with the non-treated APOE-KO group (P<0.05;
Fig. 4C)
GCH1 protein and THB levels
Western blotting analysis was employed to measure
the vascular GCH1 protein level (Fig.
5A). GCH1 of the WT+Ex4 group were significantly higher when
compared with the WT group (2.5-fold; P<0.05). The GCH1 level of
the APOE-KO+Ex4 group were nearly 2-fold higher compared with the
APOE-KO group (P=0.002; Fig.
5B).
Production of THB was detected by HPLC (Fig. 5C). The differences in arterial THB
levels among the 4 groups were similar to the patterns observed in
GCH1 protein and p-eNOS level. In the WT+Ex4 group, THB protein
(1.34±0.03 pmol/mg), was significantly higher compared with the WT
group (0.37±0.13 pmol/mg; P<0.05). THB protein of the
APOE-KO+Ex4 group (0.75±0.14 pmol/mg) was 7-fold higher than that
of the APOE-KO group (P<0.05). There was no difference in total
biopterin among the 4 groups (Fig.
5C).
The ratio of BH4 to total biopterin was higher in
the WT+Ex4 group (59.6±14.6%) when compared with the WT group
(35.3±22%; P=0.065), although the difference was not significant.
The ratio of BH4 to total biopterin in the APOE-KO+Ex4 group
(34.3±9%) was significantly higher when compared with the APOE-KO
group (5.4±2.3%; P=0.035; Fig.
5D).
Discussion
In this present study, it was demonstrated that a
high-cholesterol diet was associated with higher TC and LDL-C in
the APOE-KO mice compared with WT control mice. Also, the results
of the present study demonstrated that Ex4 treatment was associated
with improved endothelial dysfunction of the aortae in APOE-KO mice
fed a high-cholesterol diet, without affecting blood lipid and
glucose. The effect of Ex4 treatment was independent of TC, TG,
LDL-C or glucose. A THB-suppressant was not used to demonstrate a
link between THB and eNOS directly, since specific studies have
investigated this previously (27,28).
The acetylcholine-stimulated endothelium-dependent vasodilation was
decreased in the APOE-KO mice when compared the WT control group.
However, the condition of the aorta of the APOE-KO+ Ex4 group was
improved. Similar improvement to the vascular condition was also
observed in the WT+Ex4 mice compared with the non-treated WT
control mice.
Similar to the results of the present study, a
previous study demonstrated that Ex4 treatment directly elicited
significant and concentration-dependent vasorelaxation in the rat
aortas (29). Ozyazgan et
al (29) used Ex4 for the
long-term treatment of type 2 diabetic rats and demonstrated that
Ex4 treatment returned the acetylcholine-induced relaxation
response to approximately that of the control group, which is
similar to the results of the present study. Different results of
endothelial dysfunction of rat arteries demonstrated neither a
direct protective effect on endothelial dysfunction nor
vasorelaxation effects of Ex4 (30). However, this may be explained by
the endothelial protective effect of Ex4 is due to multi-factors
in vivo rather than rapidly in vitro.
In the present study, it was demonstrated that the
NO levels in the APOE-KO mice were lower when compared with the WT
control mice. This indicated that the production of NO was reduced,
or its half-life had been shortened. By contrast, in the Ex4
intervention groups the plasma NO level was higher by 2-fold
(WT+Ex4) and 1.5-fold (APOE-KO+Ex4) compared with non-treated
groups. The mechanism may involve cAMP/5′-AMP-activated protein
kinase-Associated signaling pathways and enhancement of eNOS
activity (16). It is hypothesized
that the positive effect on endothelial function of Ex4 may involve
eNOS.
Acetylcholine-stimulated endothelium-dependent
vasodilation was impaired in APOE-KO mice; it was significantly
lower than that of the WT control group. However, the aorta
condition of the APOE-KO+Ex4 group was better and their arteries
performed well in acetylcholine-induced endothelium-dependent
vasodilation. Similar improvement of vascular condition was also
observed in the WT+Ex4 mice compared with the non-treated WT
control mice. A previous isolated vessel study on standard rats
demonstrated that Ex4 treatment directly elicited significant and
concentration-dependent vasorelaxation in rat aortas (31). However the maximal relaxation only
reached ~30%. This may be associated with the healthy animal model.
the scale of the improvement seems less significant compare with
APOE-KO mice which were suffering more severe endothelial
injury.
Kuhlencordt et al (32) observed the atherosclerotic lesion
area of ApoE/eNOS-double knockout mice and demonstrated that
atherosclerosis was more severe in eNOS-deficient mice compared
with the APOE-KO mice. This may indicate that eNOS has a protective
cardiovascular effect. Notably, in the study by Ozaki et al
(28), the atherosclerotic lesion
areas of the aorta were unexpectedly increased in
APOE-KO/eNOS-transgene (Tg) mice (eNOS-overexpressing) compared
with APOE-KO mice. A previous study demonstrated that endothelial
cells incubated with exenatide can increase eNOS protein levels but
did not increase the expression of eNOS mRNA (33). This may indicate that Ex-4
regulates eNOS expression without affecting eNOS gene
transcription. It is hypothesized that Ex4 treatment led to a more
normalized eNOS function in the APOE-KO+Ex4 group relative to the
non-treated APOE-KO mice.
It was established that the phosphorylation of eNOS
on serine 1,177 can activate the enzyme and lead to NO production
(34,35). The present study demonstrated that
p-eNOS levels were increased in mice treated with Ex4, for the
APOE-KO and WT control mice, and the treated groups exhibited
higher plasma NO. These results suggested that Ex4 may have a
protective effect towards eNOS activity. This is similar to
previous in vitro studies, which suggests that Ex4
upregulated p-eNOS in human umbilical vein endothelial cells and
human coronary artery endothelial cells (17,19,33).
THB, a cofactor of NOS regulates eNOS activity
(20,36). And GCH1 is the first and
rate-limiting enzyme for THB de novo synthetic pathway
(21), so THB is mainly determined
by the content and bioactivity of GCH1. The results of the present
study demonstrated that vascular GCH1 and THB protein levels were
decreased in the APOE-KO group compared with the WT control group.
Cai et al (20)
demonstrated that GCH1 gene transfer in endothelial cells notably
increased THB, enhanced eNOS homodimerization and increased NO
production, suggesting that GCH1 may be a reasonable target to
augment endothelial THB and normalize eNOS activity in endothelial
dysfunction. The present study hypothesized that the severe
abnormal function and uncoupling of eNOS in APOE-KO mice was due in
part to the absence of THB, and ultimately led to a lower NO
level.
Hattori et al (37) treated APOE-KO mice with oral THB
and observed that continuous THB availability improved NO-mediated
endothelial function and limited the progression of
atherosclerosis. In the present study, it was also demonstrated
that arterial GCH1 protein level were increased in the APOE-KO+Ex4
(~2-fold) and the WT+Ex4 (2.5-fold) groups, compared with their
respective non-treated groups.
The THB content of the 2 intervention groups were
correspondingly higher and the increase in the APOE-KO+Ex4 group
was ~7-fold compared with the non-treated APOE-KO mice. Similar to
previous studies, Bendall et al (38) demonstrated that eNOS protein was
significantly increased in the eNOS-Tg and eNOS/GCH1-transgene
mice. However, the ratio of eNOS dimer-to-monomer was significantly
decreased in eNOS-Tg mice and its superoxide production was
significantly increased. By contrast, with sufficient THB levels of
superoxide the function of eNOS was normal in eNOS/GCH-Tg mice.
Takaya et al (39) reported
that atherosclerotic lesion formation was increased in
APOE-KO/eNOS-Tg mice compared with APOE-KO mice, while
atherosclerotic lesions were reduced in APOE-KO/eNOS-Tg/GCH-Tg
mice, which had higher vascular THB compared with APOE-KO/eNOS-Tg
mice. The present study demonstrated a similar phenomenon.
Apart from the GCH1-THB-eNOS signaling pathway, one
of the important mechanisms of Ex4 on cardiovascular protection,
previous studies have demonstrated that Ex4 can improve myocardial
function in heart failure by reversing cardiac remodeling (40–42).
Evidence from a previous study indicated that Ex4 reduced
monocyte/macrophage accumulation in the arterial wall by inhibiting
the inflammatory response in macrophages and may contribute to the
attenuation of atherosclerotic lesions (43). This effect may also help preserve
the bioactivity of THB and eNOS, and is good for endothelial
function and cardiovascular disease.
Although the sample size of the present study was
small, the statistical power in this study remain valid. Also, the
present study, to the best of our knowledge, is the first study
using the flow-mediated dilation technique to suggest that
long-term administration of Ex4 improves arterial dilation.
In conclusion, models of endothelial dysfunction in
APOE-KO mice were established and the results provide strong
evidence that systemic application of Ex4 can reverse endothelial
dysfunction in APOE-KO mice induced by a high-cholesterol diet. The
present study also suggested that the protective mechanisms
associated with Ex4 treatment may primarily concern the promotion
of GCH1 expression and increase of THB level. Furthermore, adequate
THB may help maintain normal eNOS function and preserve eNOS
phosphorylation to improve endothelial function and achieve
cardiovascular protection.
Acknowledgements
Authors would like to thank Qiaoyun Tang (Life
Science Institute of Guangxi Medical University) for the excellent
technical support.
Funding
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81260059).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
ZT, LL, YG, GD and MC performed experiments. LL
provided animal experiment technical assistance. ZT analyzed and
interpreted data. ZT drafted the manuscript. ZT, YG and JW edited
and revised manuscript. JW and ZT contributed to conception and
design of research. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments were performed in accordance with
protocols approved by the Guangxi Medical University Animal Ethics
Committee (Nanning, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Zhou X, Chen X, Cai JJ, Chen LZ, Gong YS,
Wang LX, Gao Z, Zhang HQ, Huang WJ and Zhou H: Relaxin inhibits
cardiac fibrosis and endothelial-mesenchymal transition via the
Notch pathway. Drug Des Devel Ther. 9:4599–4611. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao Y, Vanhoutte PM and Leung SW:
Vascular nitric oxide: Beyond eNOS. J Pharmacol Sci. 129:83–94.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park KH and Park WJ: Endothelial
dysfunction: Clinical implications in cardiovascular disease and
therapeutic approaches. J Korean Med Sci. 30:1213–1225. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bonetti PO, Lerman LO and Lerman A:
Endothelial dysfunction: A marker of atherosclerotic risk.
Arterioscler Thromb Vasc Biol. 23:168–175. 2002. View Article : Google Scholar
|
|
5
|
Husain K, Hernandez W, Ansari RA and
Ferder L: Inflammation, oxidative stress and renin angiotensin
system in atherosclerosis. World J Biol Chem. 6:209–217. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alkaitis MS and Crabtree MJ: Recoupling
the cardiac nitric oxide synthases: Tetrahydrobiopterin synthesis
and recycling. Curr Heart Fail Rep. 9:200–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fukai T: Endothelial GTPCH in eNOS
uncoupling and atherosclerosis. Arterioscler Thromb Vasc Biol.
27:1493–1495. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS,
Drucker DJ and Husain M: Cardioprotective and vasodilatory actions
of glucagon-like peptide 1 receptor are mediated through both
glucagon-like peptide 1 receptor-dependent and -independent
pathways. Circulation. 117:2340–2350. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ussher JR and Drucker DJ: Cardiovascular
biology of the incretin system. Endocr Rev. 33:187–215. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nadkarni P, Chepurny OG and Holz GG:
Regulation of glucose homeostasis by GLP-1. Prog Mol Biol Transl
Sci. 121:23–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chiquette E, Toth PP, Ramirez G, Cobble M
and Chilton R: Treatment with exenatide once weekly or twice daily
for 30 weeks is associated with changes in several cardiovascular
risk markers. Vasc Health Risk Manag. 8:621–629. 2012.PubMed/NCBI
|
|
12
|
Simo R, Guerci B, Schernthaner G, Gallwitz
B, Rosas-Guzman J, Dotta F, Festa A, Zhou M and Kiljanski J:
Long-term changes in cardiovascular risk markers during
administration of exenatide twice daily or glimepiride: Results
from the European exenatide study. Cardiovasc Diabetol. 14:1162015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seufert J and Gallwitz B: The
extra-pancreatic effects of GLP-1 receptor agonists a focus on the
cardiovascular, gastrointestinal and central nervous systems.
Diabetes Obes Metab. 16:673–688. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
d'Uscio LV, Baker TA, Mantilla CB, Smith
L, Weiler D, Sieck GC and Katusic ZS: Mechanism of endothelial
dysfunction in apolipoprotein E-deficient mice. Arterioscler Thromb
Vasc Biol. 21:1017–1022. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei R, Ma S, Wang C, Ke J, Yang J, Li W,
Liu Y, Hou W, Feng X, Wang G and Hong T: Exenatide exerts direct
protective effects on endothelial cells through the AMPK/Akt/eNOS
pathway in a GLP-1 receptor-dependent manner. Am J Physiol
Endocrinol Metab. 310:E947–E57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han L, Yu Y, Sun X and Wang B: Exendin-4
directly improves endothelial dysfunction in isolated aortas from
obese rats through the cAMP or AMPK-eNOS pathways. Diabetes Res
Clin Pract. 97:453–460. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Erdogdu O, Eriksson L, Xu H, Sjoholm A,
Zhang Q and Nystrom T: Exendin-4 protects endothelial cells from
lipoapoptosis by PKA, PI3K, eNOS, p38 MAPK, and JNK pathways. J Mol
Endocrinol. 50:229–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alp NJ, McAteer MA, Khoo J, Choudhury RP
and Channon KM: Increased endothelial tetrahydrobiopterin synthesis
by targeted transgenic GTP-cyclohydrolase i overexpression reduces
endothelial dysfunction and atherosclerosis in ApoE-knockout mice.
Arterioscler Thromb Vasc Biol. 24:445–450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Erdogdu O, Nathanson D, Sjoholm A, Nystrom
T and Zhang Q: Exendin-4 stimulates proliferation of human coronary
artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent
pathways and requires GLP-1 receptor. Mol Cell Endocrinol.
325:26–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cai S, Alp NJ, McDonald D, Smith I, Kay J,
Canevari L, Heales S and Channon KM: GTP cyclohydrolase I gene
transfer augments intracellular tetrahydrobiopterin in human
endothelial cells: effects on nitric oxide synthase activity,
protein levels and dimerisation. Cardiovasc Res. 55:838–849. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moens AL and Kass DA: Tetrahydrobiopterin
and Cardiovascular Disease. Arterioscler Thromb Vasc Biol.
26:2439–2444. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
National Research Council: Guide for the
Care and Use of laboratory animals. Washington, DC: The National
Academies Press; 1996
|
|
23
|
Fischer AH, Jacobson KA, Rose J and Zeller
R: Hematoxylin and eosin staining of tissue and cell sections. CSH
Protoc. 2008:2008.
|
|
24
|
Burrin JM and Price CP: Performance of
three enzymic methods for filter paper glucose determination. Ann
Clin Biochem. 21:411–416. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jones PM, Salmon DM and Howell SL: Protein
phosphorylation in electrically permeabilized islets of Langerhans.
Efects of Ca2+, cyclic AMP, a phorbol ester and noradrenaline.
Biochem J. 254:397–403. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fukushima T and Nixon JC: Analysis of
reduced forms of biopterin in biological tissues and fluids. Anal
Biochem. 102:176–188. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alp NJ, McAteer MA, Khoo J, Choudhury RP
and Channon KM: Increased endothelial tetrahydrobiopterin synthesis
by targeted transgenic GTP-cyclohydrolase I overexpression reduces
endothelial dysfunction and atherosclerosis in ApoE-knockout mice.
Arterioscler Thromb Vasc Biol. 24:445–450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ozaki M, Kawashima S, Yamashita T, Hirase
T, Namiki M, Inoue N, Hirata K-i, Yasui H, Sakurai H, Yoshida Y, et
al: Overexpression of endothelial nitric oxide synthase accelerates
atherosclerotic lesion formation in apoE-deficient mice. J Clin
Invest. 110:331–340. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ozyazgan S, Kutluata N, Afsar S, Ozdas SB
and Akkan AG: Effect of glucagon-like peptide-1(7–36) and exendin-4
on the vascular reactivity in streptozotocin/nicotinamide-induced
diabetic rats. Pharmacology. 74:119–126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nathanson D, Erdogdu O, Pernow J, Zhang Q
and Nystrom T: Endothelial dysfunction induced by triglycerides is
not restored by exenatide in rat conduit arteries ex vivo. Regul
Pept. 157:8–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Green BD, Hand KV, Dougan JE, McDonnell
BM, Cassidy RS and Grieve DJ: GLP-1 and related peptides cause
concentration-dependent relaxation of rat aorta through a pathway
involving KATP and cAMP. Arch Biochem Biophys. 478:136–142. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuhlencordt PJ, Gyurko R, Han F,
Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH and Huang PL:
Accelerated atherosclerosis, aortic aneurysm formation, and
ischemic heart disease in apolipoprotein E/endothelial nitric oxide
synthase double-knockout mice. Circulation. 104:448–454. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ding L and Zhang J: Glucagon-like
peptide-1 activates endothelial nitric oxide synthase in human
umbilical vein endothelial cells. Acta Pharmacol Sin. 33:75–81.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Michell BJ, Griffiths JE and Mitchelhill
KI: The Akt kinase signals directly to endothelial nitric oxide.
Current Biology. 9:845–848. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matsumoto S, Shimabukuro M, Fukuda D,
Soeki T, Yamakawa K, Masuzaki H and Sata M: Azilsartan, an
angiotensin II type 1 receptor blocker, restores endothelial
function by reducing vascular inflammation and by increasing the
phosphorylation ratio Ser1177/Thr497 of endothelial nitric oxide
synthase in diabetic mice. Cardiovascular Diabetology. 13:1–10.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Du YH, Guan YY, Alp NJ, Channon KM and
Chen AF: Endothelium-Specific GTP Cyclohydrolase I Overexpression
Attenuates Blood Pressure Progression in Salt-Sensitive Low-Renin
Hypertension. Circulation. 117:1045–1054. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hattori Y, Hattori S, Wang X, Satoh H,
Nakanishi N and Kasai K: Oral administration of tetrahydrobiopterin
slows the progression of atherosclerosis in apolipoprotein
E-knockout mice. Arterioscler Thromb Vasc Biol. 27:865–870. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bendall JK, Alp NJ, Warrick N, Cai S,
Adlam D, Rockett K, Yokoyama M, Kawashima S and Channon KM:
Stoichiometric relationships between endothelial
tetrahydrobiopterin, endothelial NO synthase (eNOS) activity, and
eNOS coupling in vivo: Insights from transgenic mice with
endothelial-targeted GTP cyclohydrolase 1 and eNOS overexpression.
Circ Res. 97:864–871. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takaya T, Hirata K, Yamashita T, Shinohara
M, Sasaki N, Inoue N, Yada T, Goto M, Fukatsu A, Hayashi T, et al:
A specific role for eNOS-derived reactive oxygen species in
atherosclerosis progression. Arterioscler Thromb Vasc Biol.
27:1632–1637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lorber D: GLP-1 receptor agonists: Effects
on cardiovascular risk reduction. Cardiovasc Ther. 31:238–249.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Okerson T and Chilton RJ: The
cardiovascular effects of GLP-1 receptor agonists. Cardiovasc Ther.
30:e146–155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Monji A, Mitsui T, Bando YK, Aoyama M,
Shigeta T and Murohara T: Glucagon-like peptide-1 receptor
activation reverses cardiac remodeling via normalizing cardiac
steatosis and oxidative stress in type 2 diabetes. Am J Physiol
Heart Circ Physiol. 305:H295–H304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Arakawa M, Mita T, Azuma K, Ebato C, Goto
H, Nomiyama T, Fujitani Y, Hirose T, Kawamori R and Watada H:
Inhibition of monocyte adhesion to endothelial cells and
attenuation of atherosclerotic lesion by a glucagon-like peptide-1
receptor agonist, exendin-4. Diabetes. 59:1030–1037. 2010.
View Article : Google Scholar : PubMed/NCBI
|