Introduction
Ulcerative colitis (UC) is a chronic, relapsing and
non-specific immunological-mediated disorder of the
gastrointestinal tract. Together with Crohn's disease (CD), it is a
type of inflammatory bowel disease (IBD) (1). Individuals suffering from UC are at a
high risk of developing colitis-associated cancer, which causes up
to 15% of IBD-associated cases of mortality (2). The pathogenesis of UC is complex and
has not been clearly elucidated at present. However, genetic,
environmental and immunological factors are all thought to be
contributors (3). It has been
identified that unbalanced cytokine secretion leads to intestinal
tissue damage and epithelial barrier disruption in patients with UC
and experimental models of colitis (4). Oxidative stress is thought to be a
key factor in the development of UC as a regulator of
oxidant/antioxidant balance (5).
Nuclear erythroid 2-related factor 2 (Nrf2) plays an
important role in antioxidant reactions. It can regulate the
transcription of several enzymes in detoxification and antioxidant
responses (6). In general, Nrf2 is
located in the cytosol, bound to Kelch-like ECH-associated protein
1. When exposed to oxidative stress, Nrf2 enters the nucleus and
combines with antioxidant responsive element to regulate the
expression of Phase II enzymes, including heme oxygenase 1 (HO-1)
and NAD(P)H quinine oxidoreductases (NQOs). These enzymes are
critical for maintaining optimal cellular functions (7,8).
Sinomenine hydrochloride (SIN, purity >97%) is an
active alkaloid originally extracted from the medicinal herb
Sinomenium acutum. SIN exhibits anti-inflammatory and
immune-regulatory effects (9,10),
and possesses notable therapeutic capacity in treating arthritis
(11,12). Studies in vivo have
indicated that SIN protects against several autoimmune and
inflammation-associated diseases (13,14).
In addition, SIN is able to attenuate 2,4,6-trinitrobenzene
sulphonic acid (TNBS)-induced colitis, an animal model that mimics
human CD (15,16). However, the therapeutic mechanism
remains unclear. In the current study, the therapeutic effects of
SIN were investigated in a dextran sulfate sodium (DSS)-induced
colitis model, which possesses similar fundamental clinical and
histological features to human UC (17).
In the current study, it was identified that SIN
alleviated DSS-induced colitis by producing antioxidant and
anti-inflammatory effects, partly via activating the Nrf2/NQO-1
pathway.
Materials and methods
Animals
A total of 40 6–8-week-old female C57BL/6 mice
(weight, 18–20 g) were purchased from Cavens Laboratory Animal Co.,
Ltd. (Changzhou, China). The certification number is SCXK (SU)
2011-0003. The mice were fed a standard laboratory diet, had access
to sterile water and were housed under controlled conditions
(23±2°C, 50±5% humidity and 12 h light/dark cycle). All animal
experiments were approved by the Ethics Committee of Changzhou No.
2 People's Hospital (Changzhou, China), following the ARRIVE
guidelines (18).
Reagents
SIN (purity >97%) was purchased from Aladdin
(Shanghai, China) and salicylazosulfapyridine (SASP) was obtained
from Shanghai Sine Tianping Pharmaceutical Co., Ltd. (Shanghai,
China). They were both dissolved in 0.9% NaCl solution. DSS
(molecular weight, 36,000–50,000 kDa) was purchased from MP
Biomedicals (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
reagent for superoxide dismutase (SOD) examination was obtained
from Nanjing Jiancheng Bioengineering Institute (Nanjing,
China).
Induction and assessment of
DSS-induced murine colitis model
A total of 40 mice were randomized into four equal
groups: Control group, DSS model group, DSS + SASP group and DSS +
SIN group. In the control group, colitis was not induced. The DSS
group mice were treated with 3% DSS in their drinking water for 7
days and then were given normal drinking water for 3 days for
recovery (19,20). SASP and SIN were administered by
gavage from day 1 to day 10 at doses of 400 and 100 mg/kg per day,
respectively. All mice were sacrificed on day 11. During the course
of the experiment, body weight, stool consistency and bleeding
scores of every mouse were observed to measure the disease activity
index (DAI) (21,22). The scoring system for the DAI is
presented in Table I. The rate of
body weight gain or loss in each mouse was calculated using the
following formula: Body weight change (%) = [(Weight change at day
X)-(Weight at Day 1)]/(Weight at Day 1) ×100.
 | Table I.DAI scoring system. |
Table I.
DAI scoring system.
| Score | Weight loss
(%) | Stool
consistency | Blood in stool |
|---|
| 0 | None | Normal | Negative |
| 1 | 1–5% | Normal | Negative |
| 2 | 6–10% | Loose stool | Hemoccult
positive |
| 3 | 11–15% | Loose stool | Hemoccult
positive |
| 4 | >15% | Diarrhea | Gross bleeding |
Histological analysis
After the mice were sacrificed, the colons were
removed and the colon length of each animal was measured. The
dissected colon tissue was washed with cold phosphate-buffered
saline (PBS). Two-thirds of the distal colon was stored at −80°C
for biochemical examination, and the rest was fixed in 4%
paraformaldehyde for 2 h at room temperature for histopathological
analysis. The colon sections were then embedded in paraffin, and
stained with hematoxylin and eosin for 5 min at room temperature,
according to standard protocols (23). Histological scoring was performed
as follows: 0, no signs of inflammation; 1, low leukocyte
infiltration; 2, moderate leukocyte infiltration; 3, high leukocyte
infiltration, moderate fibrosis, high vascular density, thickening
of the colon wall, moderate goblet cell loss and focal loss of
crypts; 4, transmural infiltrations, massive loss of goblet cell,
extensive fibrosis and diffuse loss of crypts.
SOD activity assessment
Colon tissue was homogenized in cold PBS. The
activity level of total superoxide dismutase was detected with
total superoxide dismutase assay kit (cat. no. A001-1-1) according
to the manufacturer's protocol (Nanjing Jiancheng Bioengineering
Institute). The values are expressed as U/mg protein.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the colon samples using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). cDNA was synthesized from RNA using a reverse transcriptase
kit (Takara Bio, Inc., Otsu, Japan). The reverse transcription
conditions were as follows: 37°C for 15 min, 85°C for 5 sec and
hold at 4°C. SYBR Green Master mix (Vazyme, Nanjing, China) and an
ABI 7500 system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) were used to conduct qPCR, in order to analyze gene
expression. The cycling conditions were as follows: Initial step at
95°C for 10 min, followed by 40 cycles at 95°C for 15 sec and 60°C
for 1 min. The primer sequences used in the present study are
presented in Table II. The mRNA
expression level for each target gene was normalized to the level
of GAPDH. Expression of target genes was analyzed by the
2−ΔΔCq method (24).
 | Table II.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table II.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Sequences
(5′-3′) |
|---|
| TNF-α |
|
| F |
CATCTTCTCAAAATTCGAGTGAC |
| R |
TGGGAGTAGACAAGGTACAACCC |
| IL-6 |
|
| F |
GCTGGTGACAACCACGGCCT |
| R |
AGCCTCGACTTGTGAAGTGGT |
| iNOS |
|
| F |
CCAACCTGCAGGTCTTCGATG |
| R |
GTCGATGCACAACTGGGTGAAC |
| HO-1 |
|
| F |
ATGTGGCCCTGGAGGAGGAGA |
| R |
CGCTGCATGGCTGGTGTGTAG |
| NQO-1 |
|
| F |
GGATTGGACCGAGCTGGAA |
| R |
AATTGCAGTGAAGATGAAGGCAAC |
| GAPDH |
|
| F |
AAGGTCGGAGTCAACGGATTT |
| R |
AGATGATGACCCTTTTGGCTC |
Protein extraction and western blot
analysis
Following the treatments, cytosolic and nuclear
proteins were isolated from colon samples using a Nuclear and
Cytoplasmic Protein Extraction kit (Sangon Biotech Co., Ltd.,
Shanghai, China) according to the manufacturer's protocol. The
protein concentration in the supernatants was determined using an
Enhanced BCA Protein Assay kit (Beyotime Institute of
Biotechnology, Nanjing, China). Extracts were stored at −70°C until
further experimentation.
An equal amount of protein (40 µg) was separated
with 12% SDS-PAGE. The proteins were then transferred to
polyvinylidene difluoride (PVDF) membranes (EMD Millipore,
Billerica, MA, USA) using a wet transfer system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Non-fat milk (5%) was used
to block the PVDF membranes for 2 h at 37°C. Proteins were detected
using specific primary antibodies against Nrf2 (cat. no. 12721T;
rabbit monoclonal; Cell Signaling Technology, Inc., Danvers, MA,
USA), NQO-1 (cat. no. ab80588; rabbit monoclonal; Abcam, Cambridge,
MA, USA), β-actin (cat. no. 479T; rabbit monoclonal; Cell Signaling
Technology, Inc.) and Histone H3 (cat. no. 4499T; rabbit
monoclonal; Cell Signaling Technology, Inc.) diluted 1:1,000
overnight at 4°C. Then, the membranes were incubated with goat
anti-rabbit IgG-horseradish peroxidase conjugated secondary
antibodies (cat. no. sc-2004; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) diluted 1:5,000 for 1.5 h at room temperature. All
antibodies were diluted in Primary Antibody Dilution Buffer
(Beyotime Institute of Biotechnology). Proteins were visualized
using enhanced chemiluminescent reagents (Thermo Fisher Scientific,
Inc.). The immunoblots were quantified using ImageJ software
version 1.8 (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All experimental results were expressed as the mean
± standard error of the mean of three independent experiments.
Statistical analysis was performed using Student's t-test or
one-way analysis of variance with Tukey's post-hoc test with
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
SIN restores DSS-induced colitis
Mice treated with 3% DSS developed severe colitis,
which led to body weight loss and high DAI score (body weight
change, stool consistency and the presence of blood in the stool)
in comparison with the control mice. SASP, an aminosalicylate, was
used as a positive control. SASP and SIN administration prevented
body weight loss and reduced the DAI score compared with the DSS
model group mice (Fig. 1A and B).
DSS-induced colonic shortening was also improved by SASP and SIN
treatment (Fig. 1C and D).
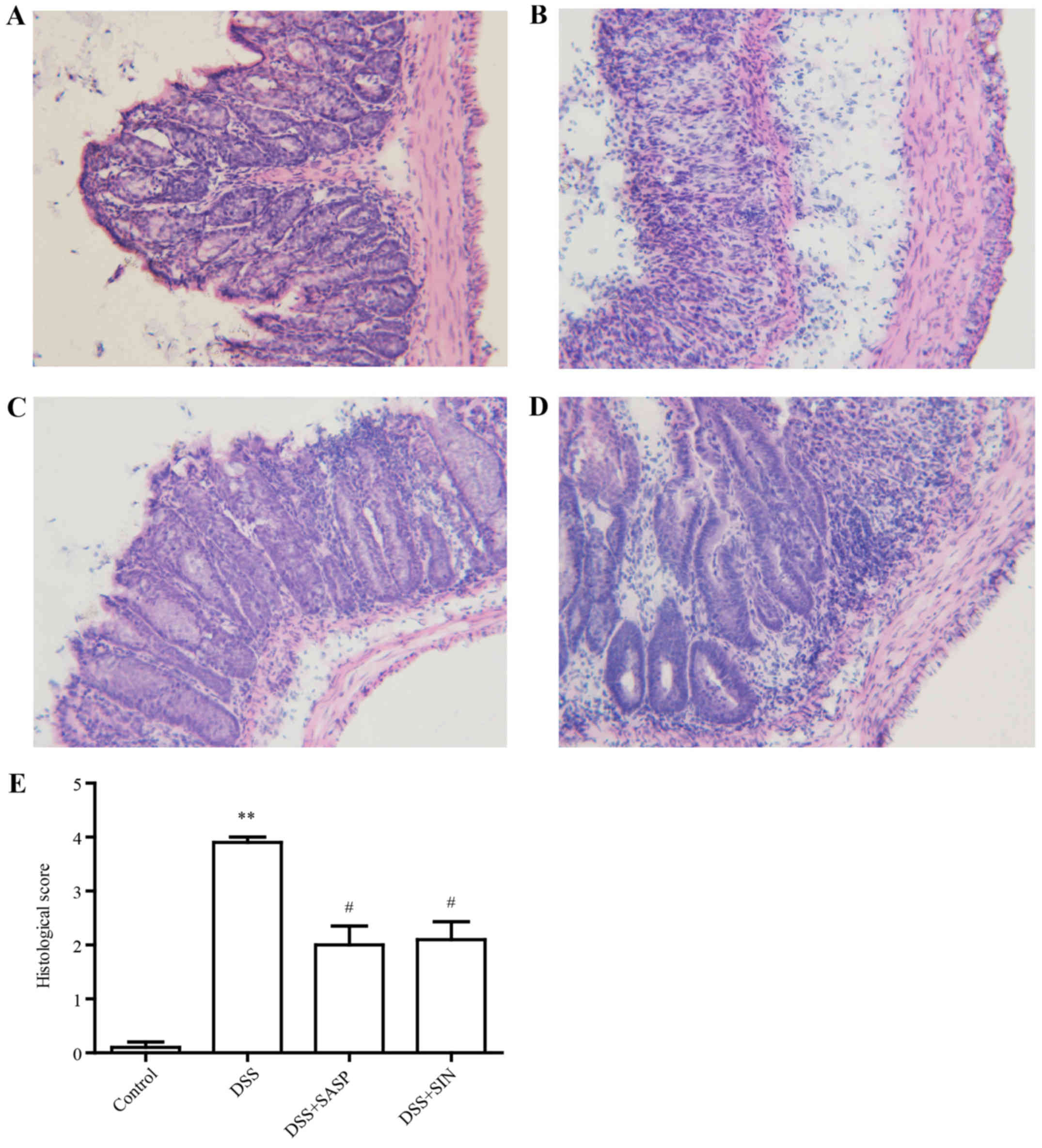
SIN attenuates colitis histological
damage
In the control group, the colons of mice exhibited
full structure without any obvious damage (Fig. 2A). However, infiltration of
inflammatory cells, defection of crypt structure and mucosal
ulceration were observed in the colons of DSS model group mice
(Fig. 2B). SASP and SIN treatment
attenuated this histological damage (Fig. 2C and D). Histological scores
indicated that DSS caused histological defects, while SASP and SIN
significantly attenuated these pathological changes (Fig. 2E).
SIN increases SOD activity
To evaluate the antioxidant effects of SIN in a
DSS-induced colitis model, the activity of SOD was analyzed using
the xanthine/xanthine oxidase method. As presented in Fig. 3, the SOD activity of the
DSS-induced colitis group was significantly decreased compared with
the control group (P<0.05). Conversely, mice treated with SASP
and SIN exhibited increased SOD activity compared with the DSS
model group.
SIN decreases mRNA levels of tumor
necrosis factor (TNF)-α, interleukin (IL)-6 and inducible nitric
oxide synthase (iNOS)
As presented in Fig. 4A
and B, the mRNA levels of TNF-α and IL-6 were greatly increased
in colonic tissues following treatment with DSS, whereas SIN
significantly suppressed this enhanced expression. DSS treatment
enhanced iNOS mRNA expression in the colon compared with the
control group (P<0.05), while SASP and SIN treatment
significantly inhibited the expression of iNOS induced by DSS
(Fig. 4C).
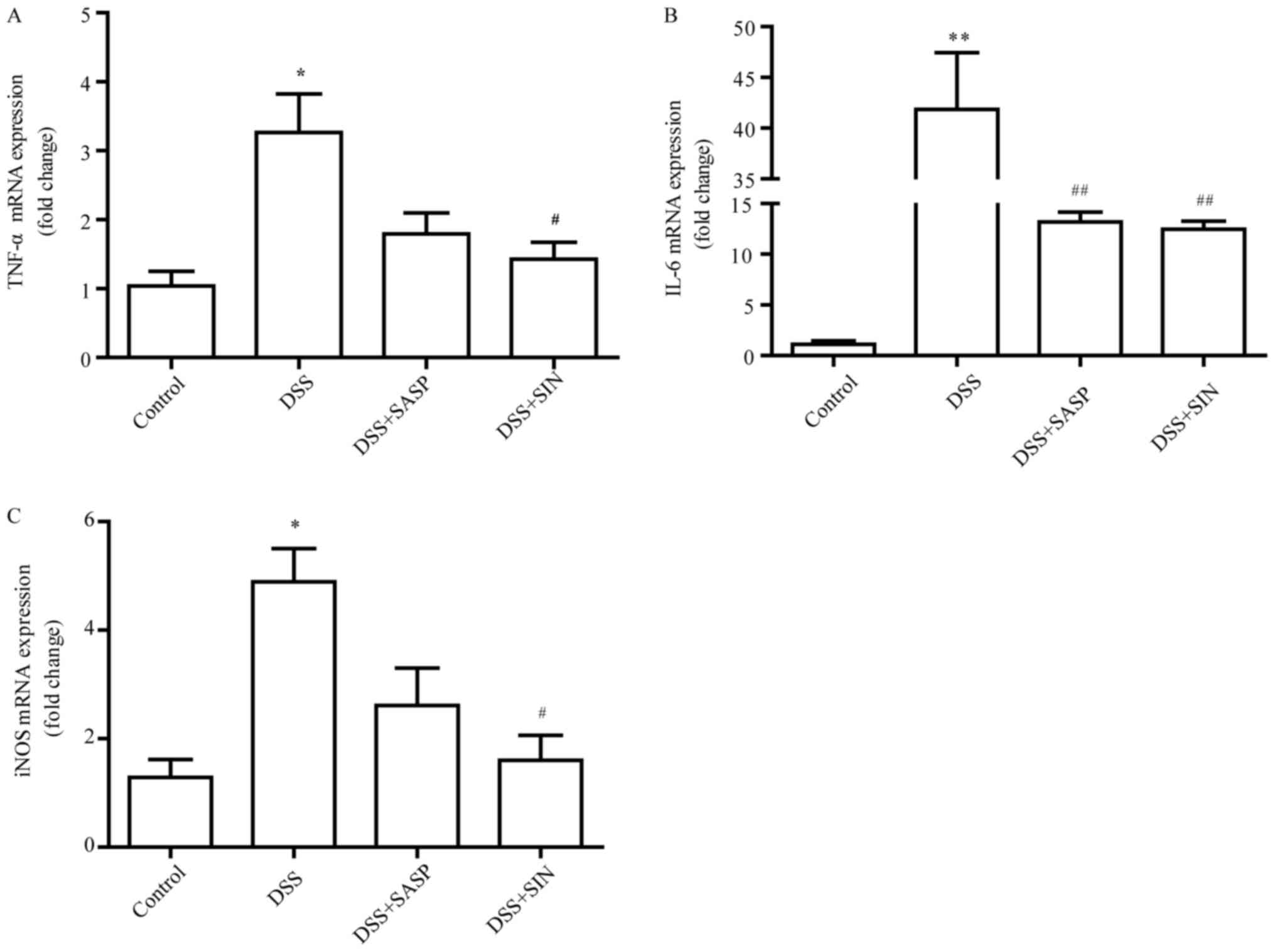 | Figure 4.SIN decreases the mRNA expression
levels of TNF-α, IL-6 and iNOS. Reverse transcription-quantitative
polymerase chain reaction was performed on gut homogenates to
detect (A) TNF-α, (B) IL-6 and (C) iNOS. Data are represented as
mean ± standard error of the mean. *P<0.05, **P<0.01 vs.
control group; #P<0.05, ##P<0.01 vs.
DSS group. DSS, dextran sulfate sodium; IL-6, interleukin-6; iNOS,
inducible nitric oxide synthase; SIN, sinomenine; SASP,
salicylazosulfapyridine; SOD, superoxide dismutase; TNF-α, tumor
necrosis factor-α. |
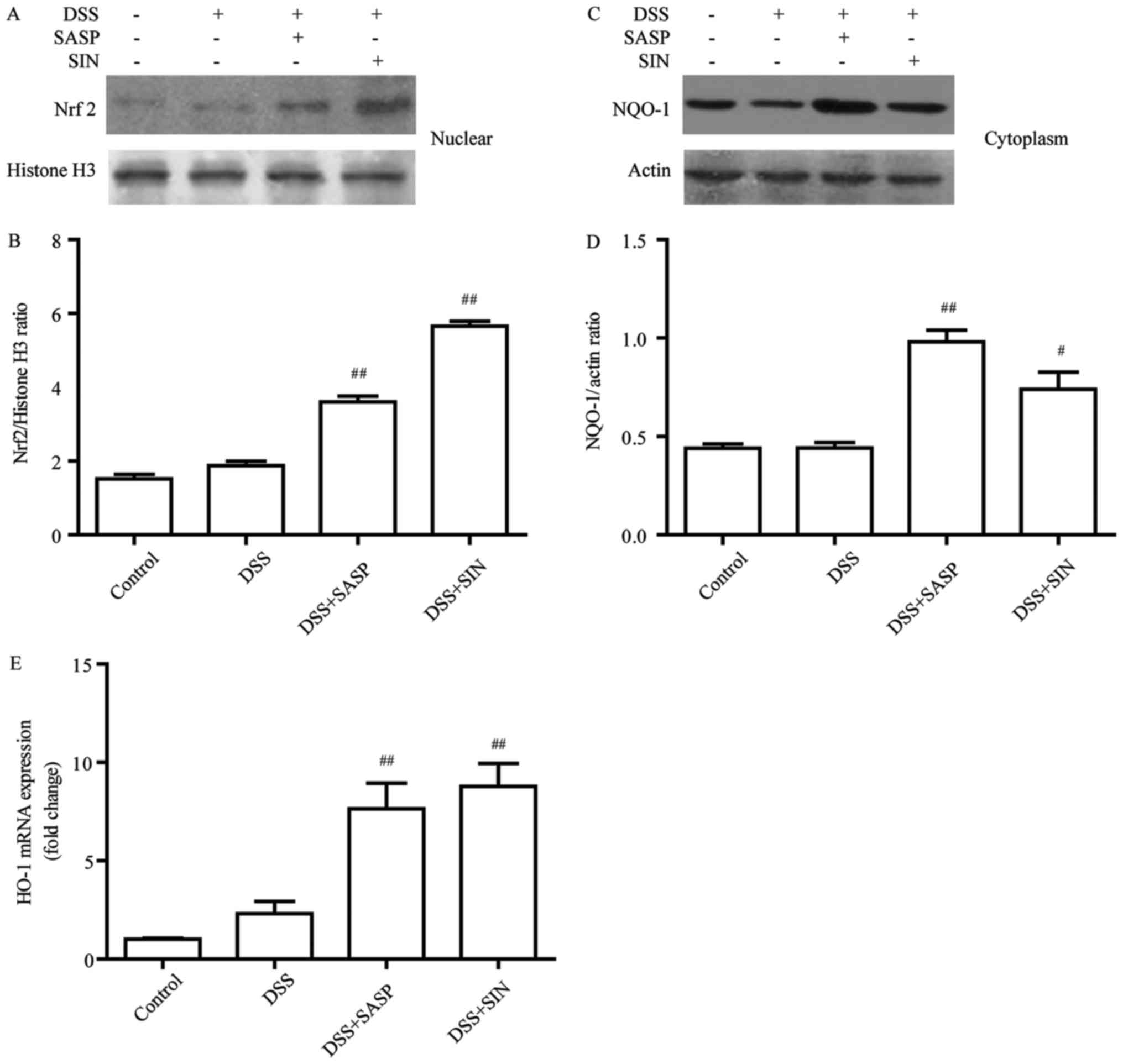
SIN induces the Nrf2/NQO-1 pathway in
DSS-induced colitis
Nrf2 protein level was evaluated in the DSS-induced
colitis model following treatment with SASP or SIN. As presented in
Fig. 5A and B, the nuclear
translocation of Nrf2 was significantly improved in the SASP and
SIN treatment groups compared with the DSS group. Furthermore, two
downstream targets of Nrf2 were examined: HO-1 and NQO-1. The
results indicated that the protein level of NQO-1 (Fig. 5C and D) and the mRNA level of HO-1
(Fig. 5E) were significantly
increased in SIN-treated mice compared with the DSS group.
Discussion
Ulcerative colitis has a serious negative impact on
human health, and is associated with colorectal cancer (25). In clinical practice,
aminosalicylates, corticosteroids, immunosuppressors and biological
agents are used to treat UC symptoms. These drugs are able to
regulate the immune and inflammatory response via specific targets;
however, they still have certain adverse effects (26). Therefore, it is important to
develop an effective drug for UC with few side effects. In the
current study, SASP, an aminosalicylate, was used as a positive
control. The effect and mechanism of SIN was investigated in the
treatment of DSS-induced colitis.
A previous study demonstrated that TNBS-induced
colitis is attenuated by SIN at doses of 30, 100 and 200 mg/kg by
gastric gavage (15). Yu et
al (16) reported that SIN
(100 or 200 mg/kg) administered orally in mice with TNBS-induced
colitis results in significant improvements. These findings suggest
that the dose and treatment of SIN used in the current study were
appropriate.
The current results indicated that DSS-induced colon
damage could be ameliorated by SIN treatment, according to the
evaluation of DAI, colon length and histological analysis. The DAI
in the DSS model group mice increased significantly, whereas SIN
administration decreased the DAI. Changes in colon length and
histological structure were also detected in the DSS model group
and the control group, and SIN attenuated these changes.
Oxidative stress is thought to be a key factor in
the development of UC (27). The
enhancement of reactive oxygen species and free radicals could play
a key role in the pathophysiology of UC. It could lead to
destruction of cell membrane integrity resulting from DNA damage,
protein oxidation and lipid peroxidation, and subsequent mucosal
inflammatory infiltration and ulceration formation (28,29).
A decrease of SOD activity may lead to redundant superoxide anions,
which usually generates other forms of carbon-, nitrogen- and
oxygen-centered radicals, and may aggravate the oxidative damage
induced by DSS (30). In the
current study, SOD activity was reduced in DSS group mice, while
SIN treatment markedly improved the SOD activity. This indicated
that SIN administration may counteract DSS-induced colon injury via
its antioxidant effect.
It was previously reported that activation of
excessive iNOS is correlated with gastrointestinal inflammation,
which could accelerate the development of IBD (31). Mouzaoui et al (32) identified that aminoguanidine and
curcumin could attenuate the colon damage in IBD by inhibiting iNOS
formation. The current results demonstrated that iNOS mRNA
expression in DSS group mice was increased, while administration of
SIN suppressed these levels.
Pro-inflammatory cytokines play a major role in UC
development and progression, particularly TNF-α. TNF-α exhibits a
pleiotropic effect in colonic mucosa by activating intracellular
signaling (33,34). IL-6 is also one of the crucial
cytokines in the pathogenesis of IBD (35). Reduced IL-6 could slow down the
development of UC and colitis-associated colon cancer (36). In the current study, RT-qPCR was
performed to evaluate the mRNA expression of TNF-α and IL-6 in the
colons of mice. DSS-induced colitis mice expressed increased levels
of TNF-α and IL-6, while SIN treatment decreased the mRNA levels.
This indicated that SIN may alleviate DSS-induced colitis by
suppressing the expression of TNF-α and IL-6 at the mRNA level.
Nrf2 signaling plays a crucial role in defending
against oxidative stress and inflammation reactions, which are both
associated with the occurrence of UC (37). HO-1, an Nrf2 target gene, is
activated by stimuli that induce cellular stress, and reduces
inflammatory cytokine secretion in numerous diseases, including
sepsis and LPS-stimulated macrophages (38,39).
Furthermore, HO-1 exerts cytoprotective effects by increasing
anti-oxidative capacity and inhibiting oxidative stress (40,41).
Numerous natural compounds isolated from plants could ameliorate
experimental colitis by activating the Nrf2 signaling pathway.
Wagner et al (42)
identified that pre-treatment of sulforaphane could reduce
DSS-induced colitis in mice by activating Nrf2 signaling and
subsequently inhibiting inflammatory mediators. The current results
indicated that SIN alleviated DSS-induced colitis via Nrf2
signaling. The mRNA levels of Nrf2 target gene HO-1 were elevated
in mice treated with SIN compared with colitis mice. Western
blotting indicated that SIN administration activated Nrf2 to induce
NQO-1 expression.
In conclusion, the present study demonstrated that
SIN treatment alleviated DSS-induced colitis by inhibiting
proinflammatory mediators in mice. In addition, SIN may activate
the Nrf2/NQO-1 signaling pathway to exert its antioxidant effect.
The current study provides evidence that maintaining a balance of
oxidative status is important for regulating inflammation in
colitis, and that SIN is a potential novel drug for treating UC in
patients.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81672799),
Changzhou Health and Family Planning Commission Project (grant nos.
ZD201606, QN201711) and Nanjing Medical University School Fund
(grant nos. 2016NJMUZD081, 2017NJMU043).
Availability of data and materials
All data generated and analyzed during the study are
available from the corresponding author on reasonable request.
Authors' contributions
YZ and LT designed the study and wrote the
manuscript. YZ, HL, JS and LC conducted the experiments and
performed the statistical analysis. YZ and CQ supervised the
experiments, analyzed the data and revised the manuscript. All
authors have read and approved the final manuscript.
Ethical approval and consent for
participation
Research involving animals was approved by the
Ethics Committee of the Affiliated Changzhou No. 2 People's
Hospital of Nanjing Medical University (Nanjing, China). All animal
protocols performed in this study were conducted strictly based on
the guidelines of the Jiangsu Committee on Animal Care.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
UC
|
ulcerative colitis
|
|
DSS
|
dextran sulfate sodium
|
|
SASP
|
salicylazosulfapyridine
|
|
SIN
|
sinomenine
|
|
DAI
|
disease activity index
|
|
SOD
|
superoxide dismutase
|
|
iNOS
|
inducible nitric oxide synthase
|
|
Nrf2
|
nuclear factor erythroid 2-related
factor 2
|
|
HO-1
|
heme oxygenase-1
|
|
NQO-1
|
NADP (H) quinone oxidoreductase 1
|
References
|
1
|
Abraham C and Cho JH: Inflammatory bowel
disease. N Engl J Med. 361:2066–2078. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Munkholm P: Review article: The incidence
and prevalence of colorectal cancer in inflammatory bowel disease.
Aliment Pharmacol Ther. 2 Suppl 18:1–5. 2003. View Article : Google Scholar
|
|
3
|
Kaistha A and Levine J: Inflammatory bowel
disease: The classic gastrointestinal autoimmune disease. Curr
Probl Pediatr Adolesc Health Care. 44:328–334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Danese S, Malesci A and Vetrano S:
Colitis-associated cancer: The dark side of inflammatory bowel
disease. Gut. 60:1609–1610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pandurangan AK, Mohebali N, Norhaizan ME
and Looi CY: Gallic acid attenuates dextran sulfate sodium-induced
experimental colitis in BALB/c mice. Drug Des Devel Ther.
9:3923–3934. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stachel I, Geismann C, Aden K, Deisinger
F, Rosenstiel P, Schreiber S, Sebens S, Arlt A and Schafer H:
Modulation of nuclear factor E2-related factor-2 (Nrf2) activation
by the stress response gene immediate early response-3 (IER3) in
colonic epithelial cells: A novel mechanism of cellular adaption to
inflammatory stress. J Biol Chem. 289:1917–1929. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uruno A and Motohashi H: The Keap1-Nrf2
system as an in vivo sensor for electrophiles. Nitric Oxide.
25:153–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim J, Cha YN and Surh YJ: A protective
role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in
inflammatory disorders. Mutat Res. 690:12–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng Y, Zhang J, Hou W, Wang D, Li F,
Zhang Y and Yuan F: Immunoregulatory effects of sinomenine on the
T-bet/GATA-3 ratio and Th1/Th2 cytokine balance in the treatment of
mesangial proliferative nephritis. Int Immunopharmacol. 9:894–899.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Q and Li XK: Immunosuppressive and
anti-inflammatory activities of sinomenine. Int Immunopharmacol.
11:373–376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tong B, Yu J, Wang T, Dou Y, Wu X, Kong L,
Dai Y and Xia Y: Sinomenine suppresses collagen-induced arthritis
by reciprocal modulation of regulatory T cells and Th17 cells in
gut-associated lymphoid tissues. Mol Immunol. 65:94–103. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mu H, Yao RB, Zhao LJ, Shen SY, Zhao ZM
and Cai H: Sinomenine decreases MyD88 expression and improves
inflammation-induced joint damage progression and symptoms in rat
adjuvant-induced arthritis. Inflammation. 36:1136–1144. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiong L and Yang L: Effects of alkaloid
sinomenine on levels of IFN-γ, IL-1β, TNF-α and IL-6 in a rat renal
allograft model. Immunotherapy. 4:785–791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Duan Z, Tian Y, Liu Z and Wang Q: A
novel perspective and approach to intestinal octreotide absorption:
Sinomenine-mediated reversible tight junction opening and its
molecular mechanism. Int J Mol Sci. 14:12873–12892. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng H, Xia B, Guo Q, Zhang L, Wang F,
Jiang L, Wang Z, Zhang Y and Li C: Sinomenine attenuates
2,4,6-trinitrobenzene sulfonic acid-induced colitis in mice. Int
Immunopharmacol. 7:604–611. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu Q, Zhu S, Zhou R, Yi F, Bing Y, Huang
S, Wang Z, Wang C and Xia B: Effects of sinomenine on the
expression of microRNA-155 in 2,4,6-trinitrobenzenesulfonic
acid-induced colitis in mice. PloS One. 8:e737572013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leonardi I, Nicholls F, Atrott K, Cee A,
Tewes B, Greinwald R, Rogler G and Frey-Wagner I: Oral
administration of dextran sodium sulphate induces a
caecum-localized colitis in rabbits. Int J Exp Pathol. 96:151–162.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kilkenny C, Browne WJ, Cuthill IC, Emerson
M and Altman DG: Improving bioscience research reporting: The
ARRIVE guidelines for reporting animal research. J Pharmacol
Pharmacother. 1:94–99. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bang B and Lichtenberger LM: Methods of
inducing inflammatory bowel disease in mice. Curr Protoc Pharmacol.
72:5.58.1–5.58.42. 2016. View Article : Google Scholar
|
|
20
|
Koboziev I, Karlsson F, Zhang S and
Grisham MB: Pharmacological intervention studies using mouse models
of the inflammatory bowel diseases: Translating preclinical data
into new drug therapies. Inflamm Bowel Dis. 17:1229–1245. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vong LB, Tomita T, Yoshitomi T, Matsui H
and Nagasaki Y: An orally administered redox nanoparticle that
accumulates in the colonic mucosa and reduces colitis in mice.
Gastroenterology. 143:1027–1036.e1023. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sann H, Erichsen Jv, Hessmann M, Pahl A
and Hoffmeyer A: Efficacy of drugs used in the treatment of IBD and
combinations thereof in acute DSS-induced colitis in mice. Life
Sci. 92:708–718. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feldman AT and Wolfe D: Tissue processing
and hematoxylin and eosin staining. Methods Mol Biol. 1180:31–43.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pandurangan AK and Esa NM: Signal
transducer and activator of transcription 3-a promising target in
colitis-associated cancer. Asian Pac J Cancer Prev. 15:551–560.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lowenberg M and D'Haens G: Novel targets
for inflammatory bowel disease therapeutics. Curr Gastroenterol
Rep. 15:3112013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu H and Li YR: Oxidative stress and
redox signaling mechanisms of inflammatory bowel disease: Updated
experimental and clinical evidence. Exp Biol Med (Maywood).
237:474–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang A, Keita AV, Phan V, McKay CM,
Schoultz I, Lee J, Murphy MP, Fernando M, Ronaghan N, Balce D, et
al: Targeting mitochondria-derived reactive oxygen species to
reduce epithelial barrier dysfunction and colitis. Am J Pathol.
184:2516–2527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mouzaoui S, Djerdjouri B, Makhezer N,
Kroviarski Y, El-Benna J and Dang PM: Tumor necrosis
factor-α-induced colitis increases NADPH oxidase 1 expression,
oxidative stress, and neutrophil recruitment in the colon:
Preventive effect of apocynin. Mediators Inflamm. 2014:3124842014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fernandes CG, da Rosa MS, Seminotti B,
Pierozan P, Martell RW, Lagranha VL, Busanello EN, Leipnitz G and
Wajner M: In vivo experimental evidence that the major metabolites
accumulating in 3-hydroxy-3-methylglutaryl-CoA lyase deficiency
induce oxidative stress in striatum of developing rats: A potential
pathophysiological mechanism of striatal damage in this disorder.
Mol Genet Metab. 109:144–153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goes AC, Pinto FM, Fernandes GC, Barbosa
JS, Correia ES, Ribeiro RA, Guimaraes SB, Lima Junior RC, Brito GA
and Rodrigues LV: Electroacupuncture ameliorates experimental
colitis induced by TNBS through activation of interleukin-10 and
inhibition of iNOS in mice. Acta Cir Bras. 29:787–793. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mouzaoui S, Rahim I and Djerdjouri B:
Aminoguanidine and curcumin attenuated tumor necrosis factor
(TNF)-α-induced oxidative stress, colitis and hepatotoxicity in
mice. Int Immunopharmacol. 12:302–311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park SY, Neupane GP, Lee SO, Lee JS, Kim
MY, Kim SY, Park BC, Park YJ and Kim JA: Protective effects of
pogostemon cablin bentham water extract on inflammatory cytokine
expression in TNBS-induced colitis in rats. Arch Pharm Res.
37:253–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Leppkes M, Roulis M, Neurath MF, Kollias G
and Becker C: Pleiotropic functions of TNF-α in the regulation of
the intestinal epithelial response to inflammation. Int Immunology.
26:509–515. 2014. View Article : Google Scholar
|
|
35
|
Chalaris A, Garbers C, Rabe B, Rose-John S
and Scheller J: The soluble Interleukin 6 receptor: Generation and
role in inflammation and cancer. Eur J Cell Biol. 90:484–494. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moriasi C, Subramaniam D, Awasthi S,
Ramalingam S and Anant S: Prevention of colitis-associated cancer:
Natural compounds that target the IL-6 soluble receptor. Anticancer
Agents Med Chem. 12:1221–1238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bryan HK, Olayanju A, Goldring CE and Park
BK: The Nrf2 cell defence pathway: Keap1-dependent and -independent
mechanisms of regulation. Biochem Pharmacol. 85:705–717. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Araujo JA, Zhang M and Yin F: Heme
oxygenase-1, oxidation, inflammation, and atherosclerosis. Front
Pharmacol. 3:1192012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Durante W: Protective role of heme
oxygenase-1 against inflammation in atherosclerosis. Front Biosci
(Landmark Ed). 16:2372–2388. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gao Z, Han Y, Hu Y, Wu X, Wang Y, Zhang X,
Fu J, Zou X, Zhang J, Chen X, et al: Targeting HO-1 by
epigallocatechin-3-gallate reduces contrast-induced renal injury
via anti-oxidative stress and anti-inflammation pathways. PloS One.
11:e01490322016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cheng HT, Yen CJ, Chang CC, Huang KT, Chen
KH, Zhang RY, Lee PY, Miaw SC, Huang JW, Chiang CK, et al: Ferritin
heavy chain mediates the protective effect of heme oxygenase-1
against oxidative stress. Biochim Biophys Acta. 1850:2506–2517.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wagner AE, Will O, Sturm C, Lipinski S,
Rosenstiel P and Rimbach G: DSS-induced acute colitis in C57BL/6
mice is mitigated by sulforaphane pre-treatment. J Nutr Biochem.
24:2085–2091. 2013. View Article : Google Scholar : PubMed/NCBI
|