Introduction
Occlusive atherothrombotic diseases occur in
different vascular territories such as in coronary, carotid and
peripheral arteries. The consequent diseases such as coronary heart
diseases (CHD), carotid disease (CD) and peripheral artery disease
(PAD) may be considered cardiovascular killers. In general,
cardiovascular diseases have mostly been investigated for
prevalence, frequency, pathophysiology, outcome and prevention, but
PAD is not sufficiently considered compared to other diseases
(1–3). There are discordant results on PAD
epidemiology but they derive from different study populations
(hospitalized, general) or from different diagnostic methods
(clinical, instrumental, discharge code). However, effective
epidemiological data are derived from the use of the ankle-brachial
index (ABI) to diagnose PAD (4–7). To
the best of our knowledge, the prevalence of PAD in advanced
countries ranges from 3 to 10% in individuals aged 40–70 years, and
10–20% in individuals over 70 years of age (8–14)
(Table I). The prevalence of PAD
is the same in men and women, whereas ABI is higher in women (10.6
vs. 4.3%) (15). Similarly, both
diabetics and regular smokers show a higher prevalence of PAD
(1,3,10–15).
The number of elderly people has tripled during the last 50 years
and therefore the number of patients with PAD has increased.
Secondly, PAD patients have been diagnosed more frequently in
hospital where there is greater awareness about managing them
according to guidelines for the secondary prevention of
cardiovascular events, improvement in physical performance,
clinical symptoms and improvement in the quality of life. Data
provided following a global estimation of PAD prevalence show that,
202 million individuals are affected by this occlusive artery
disease, but it is very interesting to note an increase of 23.5% in
PAD diagnoses from 2000 to 2010 (7). PAD has become a serious medical and
social issue. However, PAD is underrecognized although it may
easily be diagnosed by using a handle pocket Doppler to measure
ABI. PAD is also undertreated because its clinical signals (i.e.,
intermittent claudication) often do not appear in older individuals
or those leading a sedentary lifestyle (16–20).
 | Table I.Number of studies on the prevalence
of PAD in general and in hospitalized populations. |
Table I.
Number of studies on the prevalence
of PAD in general and in hospitalized populations.
| Author | Prevalence (%) | Subjects | Study
population | (Refs.) |
|---|
| Murabito et
al, 2002 | 3.9 (male) 3.3
(female) | 3,313 |
Population-based | (3) |
| Selvin et
al, 2004 | 4.3 | 2,174 | From National
Survey (NHANES) | (8) |
| Mostaza et
al, 2008 | a) 33.8 | 1,203 | Internal medicine
outpatients with: | (11) |
|
| b) 32.4 |
| a) previous
coronary event, |
|
|
| c) 53.9 |
| b) cerebrovascular
disease |
|
|
|
|
| c) disease in both
territories |
|
| Ramos et al,
2009 | 4.5 | 6,262 | Population-based
cross-sectional survey | (12) |
| Alzamora et
al, 2010 | 7.6 | 3,786 |
Population-based | (13) |
| Santo Signorelli
et al, 2010 | 2.3 | 3,412 | Population-based
from general physicians files | (19) |
Atherosclerosis and osteoporosis
Osteoporosis and atherosclerosis are two chronic
degenerative diseases whose incidence increases with age. At
present, mounting evidence indicates a relationship between
cardiovascular disease and osteoporosis, regardless of age. These
seem to have many common biochemical pathways and risk factors. In
addition, the mechanism of arterial calcification is similar to the
process of osteogenesis, involving various cells, proteins and
cytokines, which lead to tissue mineralization (21). Due to these strict
interconnections, the drugs used for osteoporosis treatment
(vitamin D, estradiol, and bisphosphonates) may interfere with
vessel wall processes. On the other hand, several of the drugs used
to treat cardiovascular diseases (statins, antihypertensives,
warfarin, and heparins) may affect bone tissue metabolism (22).
Previous studies have associated osteoporosis with
cardiovascular mortality (23,24),
aortic and coronary calcification (25,26),
carotid atherosclerosis (27), and
stroke (28) in men and women,
particularly the latter. There are little data available on the
relationship between osteoporosis and PAD.
Methods
A literature search was conducted to identify
studies published in English language journals since 1990
concerning the relationship between osteoporosis and PAD. The
MEDLINE electronic database was used to identify potential studies.
The MEDLINE search terms included peripheral artery disease and
osteoporosis or bone turnover markers, osteoprotegerin and
sclerostin.
Peripheral artery disease and osteoporosis:
Epidemiological data
The data associating PAD and osteoporosis are
conflicting. The osteodensitometric evaluation by Fehérvári et
al (29) found 37% of patients
with lower limb ischemia had osteopenia and 31% had osteoporosis,
with significantly more females than males having osteoporosis.
Most recently, Baldwin et al (30) found that osteopenia and
osteoporosis are independent risk factors for PAD in both males and
female. The data agree with previous results showing an increased
prevalence of PAD in osteoporotic postmenopausal women (31).
In a large prospective cohort of 3,998 Chinese men
and women (65–92 years of age) in Hong Kong, the ABI correlated
positively with hip BMD (correlation coefficient=0.27; P<0.001).
However, after adjustment for confounding factors, the correlation
became much weaker (correlation coefficient=0.03; P<0.05)
(32).
The Rotterdam Study (3,053 women and 2,215 men)
associated low femoral neck BMD and PAD in women only. By contrast,
lumbar spine BMD and PAD were not associated in either men or women
(33).
Pasqualini et al (34), reported that hypovitaminosis D and
increased bone turnover were risk factors for PAD and its severity.
However, the presence of PAD even if asymptomatic and diagnosed by
a lowered ABI could identify a population at risk of
osteoporosis.
Fehérvári et al (35) found a connection between the
severity of atherosclerosis and osteoporosis in patients with PAD,
specific to the site of the lesion. In patients with iliac disease,
a Bollinger angiographic score (BS, a method of PAD classification
according to the occlusive pattern) was associated with lumbar-BMD
and with femoral-BMD. These findings support the hypothesis that
reduced blood flow is the key factor in the inverse association of
BMD with atherosclerosis.
During an average 4-year follow-up, women with PAD
had a significantly higher rate of bone loss than women without PAD
(P=0.05). By contrast, PAD was not associated in men with
osteoporosis, but men with PAD had lower BMD at the femoral neck
than men without PAD (P=0.03). PAD was not associated with
osteoporotic fractures in either males or females (36).
Peripheral artery disease and osteoporosis:
Possible common pathogenic factors
Osteoprotegerin (OPG), a member of the tumor
necrosis factor receptor family, is involved in the process of bone
turnover and in the pathogenesis of osteoporosis and premature
calcification of the vascular system (37).
Few data are available associating serum OPG plasma
levels with PAD or its severity. Ziegler et al (38), reported that plasma OPG
concentrations were significantly higher in subjects with PAD
undergoing percutaneous transluminal angioplasty (PTA) because of
advanced clinical stages III–IV than in patients without ischemic
ulcerations. In addition, OPG correlated positively with BS of
disease, age and creatinine values and correlated negatively with
ABI.
Those data conflict with our results, which found
similar serum OPG and RANKL levels in PAD patients and controls. We
concluded there was no increased activation of the OPG-RANKL system
(39).
Recently, Demková et al (40), confirmed that OPG levels were
significantly higher in patients with PAD than patients without
PAD. Additionally, serum OPG levels were associated significantly
with PAD and its severity in patients with T2DM. Those data suggest
that OPG may be a biomarker for atherosclerosis in patients with
T2DM.
The role of OPG as a marker of arterial consequences
in diabetic patients particularly in PAD subjects has been
previously reported. Esteghamati et al (41) reported that in patients with T2DM
for >5 years without apparent diabetic foot ulcer, one standard
deviation increase in log-osteoprotegerin was associated with a
>2-fold increase in the risk of having PAD (odds ratio 2.26, 95%
confidence interval 1.50–3.40). In addition, in type 1 diabetes,
OPG levels were associated with the development of foot ulcer, even
after comprehensive adjustment (42).
OPG is not only important in diabetic patients. In
fact, O' Sullivan observed that PAD is associated with higher serum
OPG, regardless of the co-existence of DM (43).
In a systematic review, it was found that OPG is a
marker of atherosclerosis. OPG concentrations were associated with
stable coronary artery disease and its severity, acute coronary
syndrome, and cerebrovascular disease but no association was found
between PAD and OPG concentrations (44).
However, another recent review reported eight
studies showing correlations between OPG levels and PAD, its
progression and severity, whereas just one study did not find OPG
levels significantly higher. The authors concluded that the results
from clinical and experimental research on the role of vascular
calcification markers in PAD are controversial, although most
studies suggest a positive correlation (45).
Further studies are required to analyze the role of
OPG as a diagnostic marker for the severity of atherosclerosis
particularly in PAD patients.
Ye et al (46), suggested that a multi-marker
approach may improve the inter-individual prediction of variation
in ABI and it may be useful in predicting PAD. In particular, those
authors evaluated biomarkers including C-reactive protein,
interleukin-6, tumor necrosis factor receptor-II, lipoprotein(a),
N-terminal pro-brain natriuretic peptide, pro-atrial natriuretic
peptide, C-terminal pro-arginine vasopressin, osteoprotegerin, and
fibrinogen in African-Americans and non-Hispanic whites. It was
suggested that different factors could play a pathogenetic role for
osteoporosis and PAD, particularly data on hypovitaminosis D.
Hypovitaminosis D is frequent in patients with PAD.
Secondary hyperparathyroidism and osteomalacia as consequences may
contribute to bone pain and myalgias, and worsen clinical symptoms
of PAD such as intermittent claudication (47).
In the last decade, canonical Wnt/beta signaling has
been shown to play a significant role in the control of
osteoblastogenesis and bone formation. This signaling is finely
controlled via several soluble inhibitors such as sclerostin and
DKK-1. There is some evidence of a possible role for Wnt/beta
signaling in atherosclerosis, but data on PAD patients are
insufficient (48).
Sclerostin and Dkk-1 are two soluble inhibitors of
Wnt/beta signaling associated with acute ischemic stroke (49).
In an animal model, upregulating SOST (gene coding
for sclerostin) inhibits aortic aneurism and atherosclerosis
development with potentially important implications for treating
these vascular diseases (50).
In subjects with coronary artery disease who
underwent CABG (coronary artery bypass graft), serum sclerostin
levels were higher compared to controls (51).
In a previous study, we showed that, following
adjustment for confounders, sclerostin is an independent predictor
of arterial stiffness in the outpatient population (52). Sclerostin may serve as a useful
biomarker for early atherosclerosis in obese individuals without a
previous history of cardiometabolic disorders but this requires
further testing (53).
Morales-Santana et al (54) produced some interesting results on
sclerostin circulating levels in T2DM patients with atherosclerotic
lesions. Authors of that study found higher circulating sclerostin
levels, and a significant negative correlation between sclerostin
and other bone markers such as DKK-1 with intima-media thickness of
the carotid artery (CIMT) (55).
Based on those data, sclerostin may protect against arterial
consequences in T2DM, possibly by attenuating the upregulation of
β-catenin activity in vascular cells. Romosozumab is a monoclonal
antibody that binds to and inhibits sclerostin, increasing bone
formation and decreasing bone resorption. In a recent trial
conducted on post-menopausal women, serious adverse cardiovascular
events were observed more often with romosozumab than with
alendronate (56). The findings
support our hypothesis of a protective role for sclerostin in
atherosclerosis.
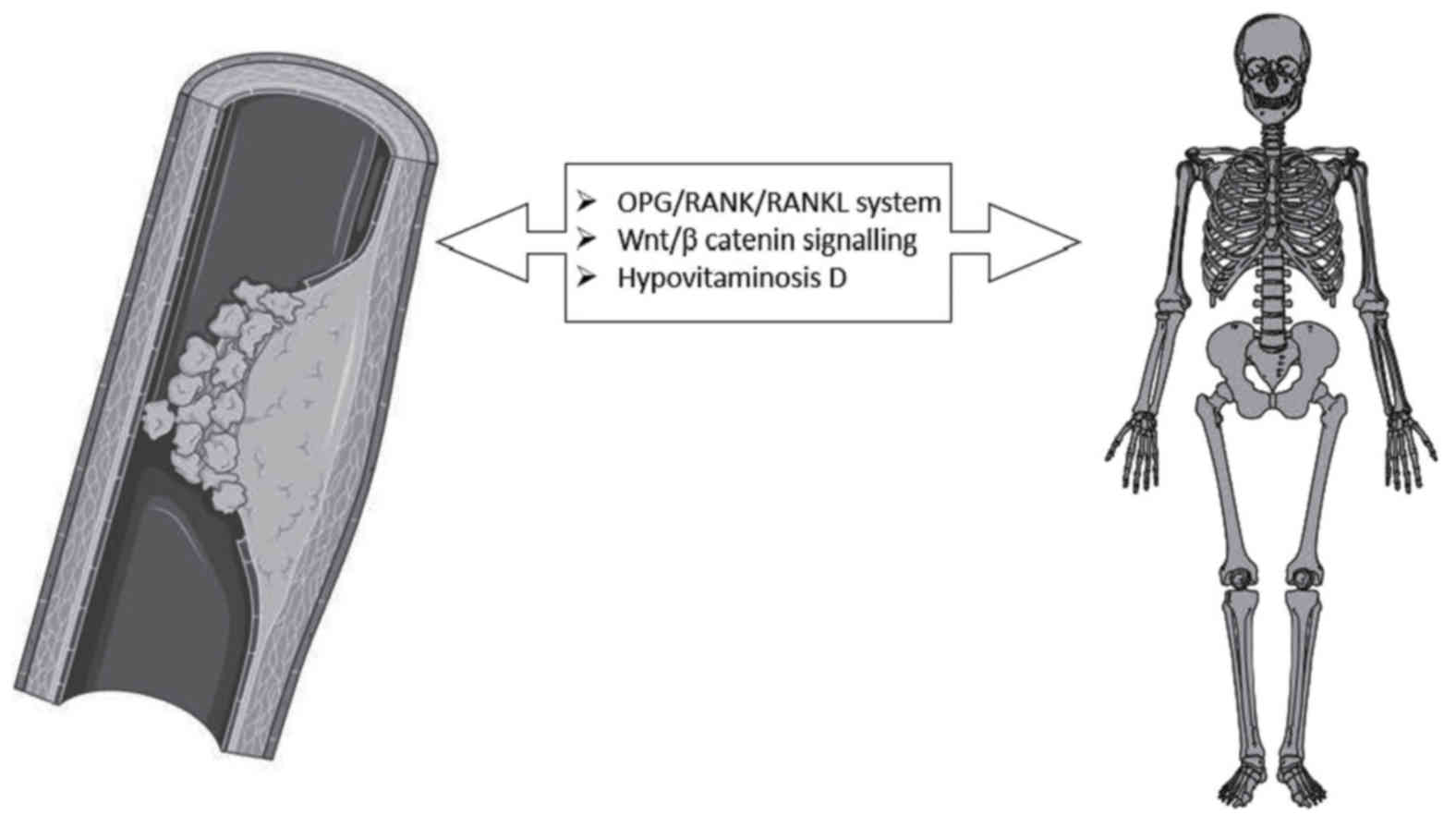
Conclusion
A large amount of data support, over an age-related
association between PAD and atherosclerosis, a possible sharing of
common pathogenetic links. In particular, the OPG/RANK/RANKL system
and Wnt/beta catenin signaling seem to be deeply involved in the
pathogenesis of bone alterations and atherosclerotic processes also
affecting the arteries of the lower extremities (Fig. 1). The complete comprehension of
these common pathways, through future studies, could be extremely
important in the designing of future innovative drugs able to
protect both arterial walls and bone.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
Authors contributed both in searching and in writing
the present review. AG, AX and RR were highly endorsed in
publications search from the literature database. SS and AG wrote
the review. AG, AX, RR, PC and SS revised it critically for
important intellectual content and approved the version to be
published. All authors read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hirsch AT, Haskal ZJ, Hertzer NR, Bakal
CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW,
Puschett JB, et al: ACC/AHA 2005 Practice Guidelines for the
management of patients with peripheral arterial disease (lower
extremity, renal, mesenteric, and abdominal aortic): A
collaborative report from the American association for vascular
surgery/society for vascular surgery, society for cardiovascular
angiography and interventions, society for vascular medicine and
biology, society of interventional radiology and the ACC/AHA task
force on practice guidelines (Writing Committee to Develop
Guidelines for the Management of Patients With Peripheral Arterial
Disease): Endorsed by the American association of cardiovascular
and pulmonary rehabilitation; national heart, lung, and blood
institute; society for vascular nursing; transatlantic
inter-society consensus; and vascular disease foundation.
Circulation. 113:e463–e654. 2006.PubMed/NCBI
|
|
2
|
Greenland P, Abrams J, Aurigemma GP, Bond
MG, Clark LT, Criqui MH, Crouse JR III, Friedman L, Fuster V,
Herrington DM, et al: Prevention conference V: Beyond secondary
prevention: Identifying the high-risk patient for primary
prevention: Noninvasive tests of atherosclerotic burden: Writing
Group III. Circulation. 101:E16–E22. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murabito JM, Evans JC, Nieto K, Larson MG,
Levy D and Wilson PW: Prevalence and clinical correlates of
peripheral arterial disease in the Framingham offspring study. Am
Heart J. 143:961–965. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Newman AB, Siscovick DS, Manolio TA, Polak
J, Fried LP, Borhani NO and Wolfson SK: Ankle-arm index as a marker
of atherosclerosis in the Cardiovascular health study.
Cardiovascular heart study (CHS) collaborative research group.
Circulation. 88:837–845. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Doobay AV and Anand SS: Sensitivity and
specificity of the ankle-brachial index to predict future
cardiovascular outcomes: A systematic review. Arterioscler Thromb
Vasc Biol. 25:1463–1469. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu D, Zou L, Xing Y, Hou L, Wei Y, Zhang
J, Qiao Y, Hu D, Xu Y, Li J and Ma Y: Diagnostic value of
ankle-brachial index in peripheral arterial disease: A
meta-analysis. Can J Cardiol. 29:492–498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fowkes FG, Rudan D, Rudan I, Aboyans V,
Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ,
Mensah GA and Criqui MH: Comparison of global estimates of
prevalence and risk factors for peripheral artery disease in 2000
and 2010: A systematic review and analysis. Lancet. 382:1329–1340.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Selvin E and Erlinger TP: Prevalence of
and risk factors for peripheral arterial disease in the United
States: Results from the national health and nutrition examination
survey, 1999–1999. Circulation. 110:738–743. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carbayo JA, Divisón JA, Escribano J,
López-Abril J, de Coca López E, Artigao LM, Martínez E, Sanchis C,
Massó J and Carrión L: Grupo de Enfermedades Vasculares de Albacete
(GEVA): Using anklebrachial index to detect peripheral arterial
disease: Prevalence and associated risk factors in a random
population sample. Nutr Metab Cardiovasc Dis. 17:41–49. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sigvant B, Wiberg-Hedman K, Bergqvist D,
Rolandsson O, Andersson B, Persson E and Wahlberg E: A
population-based study of peripheral arterial disease prevalence
with special focus on critical limb ischemia and sex differences. J
Vasc Surg. 45:1185–1191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mostaza JM, Manzano L, Suárez C, Cairols
M, Ferreira EM, Rovira E, Sánchez A, Suárez-Tembra MA, Estirado E,
Estrella Jde D, et al: Prevalence of asymptomatic peripheral artery
disease detected by the ankle-brachial index in patients with
cardiovascular disease. MERITO II study). Med Clin (Barc).
131:561–565. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramos R, Quesada M, Solanas P, Subirana I,
Sala J, Vila J, Masiá R, Cerezo C, Elosua R, Grau M, et al:
Prevalence of symptomatic and asymptomatic peripheral arterial
disease and the value of the ankle-brachial index to stratify
cardiovascular risk. Eur J Vasc Endovasc Surg. 38:305–311. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alzamora MT, ForeÂs R, Baena-DõÂez JM,
Pera G, Toran P, Sorribes M, Vicheto M, Reina MD, Sancho A,
Albaladejo C, et al: The peripheral arterial disease study
(PERART/ARTPER): Prevalence and risk factors in the general
population. BMC Public Health. 10:382010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aboyans V, Criqui MH, Abraham P, Allison
MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jönsson B, Lacroix P,
et al: Measurement and interpretation of the ankle-brachial index:
A scientific statement from the American heart association.
Circulation. 126:2890–2909. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fowkes FG, Rudan D, Rudan I, Aboyans V,
Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ,
Mensah GA and Criqui MH: Comparison of global estimates of
prevalence and risk factors for peripheral artery disease in 2000
and 2010: A systematic review and analysis. Lancet. 382:1329–1340.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wilkins JT, McDermott MM, Liu K, Chan C,
Criqui MH and Lloyd-Jones DM: Associations of noninvasive measures
of arterial compliance and ankle-brachial index: The multi-ethnic
study of atherosclerosis (MESA). Am J Hypertens. 25:535–541. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hiatt WR: Medical treatment of peripheral
arterial disease and claudication. N Engl J Med. 344:1608–1621.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Watson K, Watson BD and Pater KS:
Peripheral arterial disease: A review of disease awareness and
management. Am J Geriatr Pharmacother. 4:365–379. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Signorelli Santo S, Anzaldi M, Fiore V,
Catanzaro S, Simili M, Torrisi B and Neri S: Study on unrecognized
peripheral arterial disease (PAD) by ankle/brachial index and
arterial comorbidity in Catania, Sicily, Italy. Angiology.
61:524–529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Signorelli SS, Fiore V, Catanzaro S,
Simili M, Torrisi B and Anzaldi M: Prevalence of high
ankle-brachial index (ABI) in general population of Southern Italy,
risk factor profiles and systemic cardiovascular co-morbidity: An
epidemiological study. Arch Gerontol Geriatr. 53:55–59. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Danilevicius CF, Lopes JB and Pereira RM:
Bone metabolism and vascular calcification. Braz J Med Biol Res.
40:435–442. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karwowski W, Naumnik B, Szczepański M and
Myśliwiec M: The mechanism of vascular calcification-a systematic
review. Med Sci Monit. 18:RA1–RA11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sennerby U, Farahmand B, Ahlbom A,
Ljunghall S and Michaëlsson K: Cardiovascular diseases and future
risk of hip fracture in women. Osteoporos Int. 18:1355–1362. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marcovitz PA, Tran HH, Franklin BA,
O'Neill WW, Yerkey M, Boura J, Kleerekoper M and Dickinson CZ:
Usefulness of bone mineral density to predict significant coronary
artery disease. Am J Cardiol. 96:1059–1063. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kiel DP, Kauppila LI, Cupples LA, Hannan
MT, O'Donnell CJ and Wilson PW: Bone loss and the progression of
abdominal aortic calcification over a 25 year period: The
Framingham heart study. Calcif Tissue Int. 68:271–276. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barengolts EI, Berman M, Kukreja SC,
Kouznetsova T, Lin C and Chomka EV: Osteoporosis and coronary
atherosclerosis in asymptomatic postmenopausal women. Calcif Tissue
Int. 62:209–213. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jorgensen L, Joakimsen O, Mathiesen EB,
Ahmed L, Berntsen GK, Fønnebø V, Joakimsen R, Njølstad I, Schirmer
H and Jacobsen BK: Carotid plaque echogenicity and risk of
nonvertebral fractures in women: A longitudinal population-based
study. Calcif Tissue Int. 79:207–213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jørgensen L, Engstad T and Jacobsen BK:
Bone mineral density in acute stroke patients: Low bone mineral
density may predict first stroke in women. Stroke. 32:47–51. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fehérvári M, Krepuska M, Csobay-Novák C,
Lakatos P, Oláh Z, Acsády G and Szeberin Z: Prevalence of
osteoporosis in patients with severe peripheral artery disease. Orv
Hetil. 154:369–375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baldwin MJ, Policha A, Maldonado T,
Hiramoto JS, Honig S, Conte MS, Berger J and Rockman CB: Novel
association between bone mineral density scores and the prevalence
of peripheral artery disease in both sexes. Vasc Med. 22:13–20.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mangiafico RA, Russo E, Riccobene S,
Pennisi P, Mangiafico M, D'Amico F and Fiore CE: Increased
prevalence of peripheral arterial disease in osteoporotic
postmenopausal women. J Bone Miner Metab. 24:125–131. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wong SY, Kwok T, Woo J, Lynn H, Griffith
JF, Leung J, Tang YY and Leung PC: Bone mineral density and the
risk of peripheral arterial disease in men and women: Results from
Mr. and Ms Os, Hong Kong. Osteoporos Int. 16:1933–1938. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
van der Klift M, Pols HA, Hak AE, Witteman
JC, Hofman A and de Laet CE: Bone mineral density and the risk of
peripheral arterial disease: The rotterdam study. Calcif Tissue
Int. 70:443–449. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pasqualini L, Ministrini S, Macura A,
Marini E, Leli C, Siepi D, Lombardini R, Kararoudi MN, Scarponi AM,
Schillaci G, et al: Increased bone resorption: A possible
pathophysiological link between hypovitaminosis D and peripheral
arterial disease. Eur J Vasc Endovasc Surg. 52:352–359. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fehérvári M, Sarkadi H, Krepuska M,
Sótonyi P, Acsády G, Entz L, Lakatos P and Szeberin Z: Bone mineral
density is associated with site-specific atherosclerosis in
patients with severe peripheral artery disease. Calcif Tissue Int.
93:55–61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
von Mühlen D, Allison M, Jassal SK and
Barrett-Connor E: Peripheral arterial disease and osteoporosis in
older adults: The rancho bernardo study. Osteoporos Int.
20:2071–2018. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hsu H, Lacey DL, Dunstan CR, Solovyev I,
Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, et
al: Tumor necrosis factor receptor family member RANK mediates
osteoclast differentiation and activation induced by
osteoprotegerin ligand. Proc Natl Acad Sci USA. 96:3540–3545. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ziegler S, Kudlacek S, Luger A and Minar
E: Osteoprotegerin plasma concentrations correlate with severity of
peripheral artery disease. Atherosclerosis. 182:175–180. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pennisi P, Signorelli SS, Riccobene S,
Celotta G, Di Pino L, La Malfa T and Fiore CE: Low bone density and
abnormal bone turnover in patients with atherosclerosis of
peripheral vessels. Osteoporos Int. 15:389–395. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Demková K, Kozárová M, Malachovská Z,
Javorský M and Tkáč I: Osteoprotegerin concentration is associated
with the presence and severity of peripheral arterial disease in
type 2 diabetes mellitus. Vasa. 47:131–135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Esteghamati A, Aflatoonian M, Rad MV,
Mazaheri T, Mousavizadeh M, Nakhjavani M and Noshad S: Association
of osteoprotegerin with peripheral artery disease in patients with
type 2 diabetes. Arch Cardiovasc Dis. 108:412–419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zobel EH, von Scholten BJ, Lajer M, Jorsal
A, Tarnow L, Rasmussen LM, Holstein P, Parving HH, Hansen TW and
Rossing P: High osteoprotegerin is associated with development of
foot ulcer in type 1 diabetes. J Diabetes Complications.
30:1603–1608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
O'Sullivan EP, Ashley DT, Davenport C,
Kelly J, Devlin N, Crowley R, Leahy AL, Kelly CJ, Agha A, Thompson
CJ, et al: Osteoprotegerin is higher in peripheral arterial disease
regardless of glycaemic status. Thromb Res. 126:e423–e427. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hosbond SE, Poulsen TS, Diederichsen AC,
Nybo M, Rasmussen LM and Mickley H: Osteoprotegerin as a marker of
atherosclerosis: A systematic update. Scand Cardiovasc J.
46:203–211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kapetanios D, Karkos C, Giagtzidis I,
Papazoglou K, Kiroplastis K and Spyridis C: Vascular calcification
biomarkers and peripheral arterial disease. Int Angiol. 35:455–459.
2016.PubMed/NCBI
|
|
46
|
Ye Z, Ali Z, Klee GG, Mosley TH Jr and
Kullo IJ: Associations of candidate biomarkers of vascular disease
with the ankle-brachial index and peripheral arterial disease. Am J
Hypertens. 26:495–502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fahrleitner-Pammer A, Obernosterer A,
Pilger E, Dobnig H, Dimai HP, Leb G, Kudlacek S and
Obermayer-Pietsch BM: Hypovitaminosis D, impaired bone turnover and
low bone mass are common in patients with peripheral arterial
disease. Osteoporos Int. 16:319–324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mikhaylova L, Malmquist J and Nurminskaya
M: Regulation of in vitro vascular calcification by BMP4, VEGF and
Wnt3a. Calcif Tissue Int. 81:372–381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
He XW, Wang E, Bao YY, Wang F, Zhu M, Hu
XF and Jin XP: High serum levels of sclerostin and Dickkopf-1 are
associated with acute ischaemic stroke. Atherosclerosis. 253:22–28.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Krishna SM, Seto SW, Jose RJ, Li J, Morton
SK, Biros E, Wang Y, Nsengiyumva V, Lindeman JH, Loots GG, et al:
Wnt signaling pathway inhibitor sclerostin inhibits angiotensin
II-induced aortic aneurysm and atherosclerosis. Arterioscler Thromb
Vasc Biol. 37:553–566. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kim KM, Lim S, Moon JH, Jin H, Jung KY,
Shin CS, Park KS, Jang HC and Choi SH: Lower uncarboxylated
osteocalcin and higher sclerostin levels are significantly
associated with coronary artery disease. Bone. 83:178–183. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gaudio A, Fiore V, Rapisarda R, Sidoti MH,
Xourafa A, Catalano A, Tringali G, Zanoli L, Signorelli SS and
Fiore CE: Sclerostin is a possible candidate marker of arterial
stiffness: Results from a cohort study in Catania. Mol Med Rep.
15:3420–3424. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Popovic DS, Mitrovic M, Tomic-Naglic D,
Icin T, Bajkin I, Vukovic B, Benc D, Zivanovic Z, Kovacev-Zavisic B
and Stokic E: The Wnt/β-catenin signalling pathway inhibitor
sclerostin is a biomarker for early atherosclerosis in obesity.
Curr Neurovasc Res. 14:200–206. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Morales-Santana S, García-Fontana B,
García-Martín A, Rozas-Moreno P, García-Salcedo JA, Reyes-García R
and Muñoz-Torres M: Atherosclerotic disease in type 2 diabetes is
associated with an increase in sclerostin levels. Diabetes Care.
36:1667–1674. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gaudio A, Privitera F, Pulvirenti I,
Canzonieri E, Rapisarda R and Fiore CE: The relationship between
inhibitors of the Wnt signalling pathway (sclerostin and
Dickkopf-1) and carotid intima-media thickness in postmenopausal
women with type 2 diabetes mellitus. Diab Vasc Dis Res. 11:48–52.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Saag KG, Petersen J, Brandi ML, Karaplis
AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD and Grauer
A: Romosozumab or alendronate for fracture prevention in women with
osteoporosis. N Engl J Med. 377:1417–1427. 2017. View Article : Google Scholar : PubMed/NCBI
|