Introduction
Endometrial cancer (EC) is the fourth most prevalent
and aggressive type of gynecological cancer, which has become a
great threat against women's health (1,2).
Annually, >280,000 women are diagnosed with EC worldwide; EC
results in numerous cancer-associated mortalities (3). Unremitting efforts have been made to
develop effective methods for treating EC in the past decades.
Radical surgery may be the only effective therapeutic method for
early stage EC. At present, the incidence of EC is still rising due
to tumor recurrence and metastasis (4,5).
Therefore, investigating the molecular mechanisms of endometrial
carcinogenesis, screening out effective diagnostic biomarkers and
developing promising therapeutic targets are particularly important
for the treatment of EC.
MicroRNAs (miRNAs/miRs) are a member of the family
of noncoding RNAs and have a length of 21–23 nucleotides (6). Numerous reports have demonstrated
that miRNAs could regulate gene expression by directly associating
with the 3′-untranslated region of target mRNAs (7,8). By
modulating the protein expression levels of target genes, miRNAs
are widely involved in various biological processes and human
diseases (9). In addition,
accumulating evidence has indicated that miRNAs are essential
regulators in a variety of cancers by serving as oncogenes or tumor
suppressors (10). For instance,
miR-199a/b-3p suppresses the proliferation of gastric cancer cells
by regulating P21 activated kinase 4/mitogen activated protein
kinase/extracellular signal-regulated kinase signaling pathway
(11). Li et al (12) indicated that miR-34a directly
targeted high-mobility group box 1 and inhibited the proliferation,
migration and invasion of cancer cells in cutaneous squamous cell
carcinoma. In EC, Zhao et al (13) revealed that miR-126 inhibited the
migration and invasion of EC cells by targeting insulin receptor
substrate 1. In summary, the aforementioned findings highlighted
the importance of miRNAs in the progression of cancer, which
suggests that miRNAs may be promising biomarkers and therapeutic
targets in the treatment of cancers.
Recent studies have indicated that miR-494-3p could
induce lung carcinogenesis and regulate the proliferation,
invasion, migration and apoptosis of human glioblastoma cells
(14,15). On the contrary, it has been
suggested that miR-494-3p acts as a tumor suppressor in some
cancers, such as prostate (16)
and lung cancer (14). For
example, Shen et al (16)
have reported that miR-494-3p suppresses the proliferation,
invasion, and migration of prostate cancer; however, the role of
miR-494-3p remains unknown in EC. In the present study, it was
revealed that miR-494-3p was significantly upregulated in EC
tissues and was associated with the prognosis of patients with EC.
In addition, overexpression of miR-494-3p markedly promoted the
proliferation, migration and invasion of EC cells. Furthermore,
miR-494-3p directly targeted phosphatase and tensin homolog (PTEN)
and consequently regulated phosphoinositide 3-kinase (PI3K)/protein
kinase B (AKT) pathway in EC cells. Additionally, inhibition of the
PI3K/AKT pathway abolished miR-494-3p-mediated effects on HHUA and
JEC cells. In summary, the results of the present study revealed
the pivotal role of the miR-494-3p/PTEN/PI3K/AKT pathway in EC
progression, which may provide novel insight in the identification
of therapeutic targets for the treatment of EC.
Materials and methods
Patient samples
The present study was approved by the Human Studies
Committee at The Second Affiliated Hospital of Zhengzhou University
(Zhengzhou, China). Informed consent was obtained from each patient
prior to surgery. A total of 43 pairs of EC samples (all females;
age, 54±16 years) were obtained from patients; Patients that were
treated with chemotherapy or radiotherapy before surgery were
excluded. samples were histologically validated for type and grade.
These samples were collected from May 2012 to July 2016.
Cell lines and cell culture
Human EC cell lines HHUA and JEC were obtained from
the Cell Bank of Type Culture Collection of Chinese Academy of
Science (Shanghai, China) and cultured in DMEM (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) supplemented with 15% of fetal
bovine serum (FBS), 100 U/ml of penicillin and 100 µg/ml of
streptomycin (all from Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Cells were incubated at 37°C in a humidified
atmosphere of 5% CO2. To inhibit the PI3K/AKT signaling pathway, 50
µM LY294002 (cat. no. 9901; Cell Signaling Technology, Inc.,
Danvers, MA, USA) was added to the cultured cells for 24 or 48 h at
37°C according to the manufacturer's protocol.
Cell transfection
miR-494-3p mimics (5′-UGAAACAUACACGGGAAACCUC-3′),
inhibitors (5′-GAGGUUUCCCGUGUAUGUUUCA-3′) and negative controls
(NCs; 5′-ACAUCUGCGUAAGAUUCGAGUCUA-3′) were synthetized by
Invitrogen (Thermo Fisher Scientific, Inc.). For PTEN
overexpressioin, the coding sequence of PTEN was amplified by PCR
and constructed into the pcDNA3 vector (Invitrogen; Thermo Fisher
Scientific, Inc.) between EcoRI and XhoI. All
transfections were performed using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol, at a concentration of 50 nM for miRNAs and
1 µg for pcDNA3-PTEN. After 48 h at 37°C, the transfection
efficiency was validated using qRT-PCR as described below.
In vivo nude mouse xenograft
assay
All animal experiments were approved by the Ethics
Committee of The Second Affiliated Hospital of Zhengzhou
University. Female BALB/c nude mice (4 mice per group; weight,
20.00±1.92 g), 4–6-weeks-old were obtained from Beijing Vital River
Laboratories Animal Technology Co., Ltd. (Beijing, China) and were
routinely housed in light-(12 h dark/12 h light) and
temperature-controlled rooms (23°C). These mice were given free
access to sterile food and water during the experiment process.
HHUA cells (1×107) were transfected with miR-494-3p
inhibitors or controls resuspended in 200 µl FBS-free culture
medium and subcutaneously injected into the right flanks of mice.
miR-494-3p inhibitors or controls were injected into the formed
tumor tissues every three days for 42 days. The tumor volume and
weight were determined routinely following inoculation using direct
measurement and calculated using the formula (length ×
width2)/2. The mice were then sacrificed; tumor tissues
were obtained and tumor weights were determined.
Cell proliferation
Cell proliferation was examined using a Cell
Counting Kit-8 (CCK8) assay (R&D Systems, Inc., Minneapolis,
MN, USA) according to the manufacturer's protocols. Proliferation
was determined through measuring absorbance at 450 nm using an
ELx808 absorbance reader (BioTek Instruments, Inc., Winooski, VT,
USA). And a colony formation assay was also conducted. In brief,
1,000 HHUA or JEC cells were seeded into 6-well plates and cultured
for 14 days in DMEM medium at 37°C. Then the colonies were fixed
with paraformaldehyde for 30 min at 25°C and stained with 0.1%
crystal violet for 30 min at 25°C. Colony numbers were manually
counted.
Transwell assay
To examine cell migration, cells were plated onto a
24-well Transwell chamber (Corning Incorporated, Corning, NY, USA).
A total of 2×104 cells were diluted in serum-free medium at 24 h
post-transfection and subsequently inoculated onto the upper
chamber. A total of 600 µl Dulbecco's modified Eagles medium
(Sigma-Aldrich; Merck KGaA) with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) was added to the lower chamber at 37°C.
Non-migratory cells were removed using a cotton swab following
overnight incubation, while the migratory cells in the lower
chamber were fixed with paraformaldehyde for 30 min at 25°C and
stained with 0.1% crystal violet for 30 min at 25°C. A cell
invasion assay was performed in a similar manner, but Matrigel
(Collaborative Research, Bedford, MA, USA) was added to the upper
chamber. Finally, the number of migrated and invasive cells were
observed and counted using an optical microscope (magnification
×200, Nikon Corporation, Tokyo, Japan). Three random fields were
counted per group.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from tissues or cells was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocols. RNA (0.5 µg) was
reverse transcribed using a PrimeScript RT Kit (Takara Bio, Inc.,
Otsu, Japan) according to the manufacturer's protocols. Then, the
transcripts were analyzed on an ABI 7300 qPCR system (Applied
Biosystem; Thermo Fisher Scientific, Inc.) using specific primers
synthesized by Beijing Sunbiotech Co., Ltd. (Beijing, China) using
the TaqMan™ MicroRNA Assay kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) for miRNAs and the Fast SYBR™-Green Master Mix
(Applied Biosystems; Thermo Fisher Scientific Inc.) for mRNAs. The
thermocycling conditions were as follows: Denaturation at 95°C for
10 min; followed by 40 cycles of denaturation at 95°C for 15 sec
and elongation at 60°C for 1 min. Three repearts were performed.
Relative expression was calculated and normalized to endogenous
β-actin (ACTB) or U6. Relative expression levels were evaluated via
the 2−ΔΔCq method (17). Primer sequences were as follows:
miR-494-3p, forward, 5′-AACGAGACGACGACAGAC-3′ and reverse,
5′-TGAAACATACACGGGAAACCTC-3′; U6 forward, 5′-AACGAGACGACGACAGAC-3′
and reverse, 5′-GCAAATTCGTGAAGCGTTCCATA-3′; PTEN forward,
5′-TCCCAGACATGACAGCCATC-3′ and reverse,
5′-TGCTTTGAATCCAAAAACCTTACT-3′, and ACTB forward,
5′-CGGCGCCCTATAAAACCCA-3′ and reverse,
5′-GAGGCGTACAGGGATAGCAC-3′.
Western blotting
Total protein was isolated from cultured HHUA and
JEC cells using radioimmunoprecipitation assay buffer (Thermo
Fisher Scientific, Inc), and the supernatant was collected via
centrifugation at 13,282 × g for 10 min at 4°C. Protein
concentration was evaluated using a Pierce BCA Protein Assay kit
(Pierce; Thermo Fisher Scientific, Inc.). Subsequently, the
extracted protein was mixed with loading buffer and boiled at 100°C
for 5 min. Total protein (30 µg) was separated using 10% SDS-PAGE
gels and transferred onto polyvinylidene fluoride membranes (EMD
Milipore, Billerica, MA, USA). Subsequently, the membranes were
blocked with 5% (w/v) non-fat milk for 2 h at 25°C and incubated
with antibodies against PTEN (1:2,000; cat. no. 9188), AKT
(1:2,000; cat. no. 4691), phosphorylated-AKT (1:2,000; cat. no.
4060), PI3K (1:,2,000; cat. no. 4249), p-PI3K (1:2,000; cat. no.
4228), BCL2 (1:2,000; cat. no. 2872), caspase-3 (1:2,000; cat. no.
9664) or GAPDH (1:2,000; cat. no. 5174) (all from Cell Signaling
Technology, Inc.) for 2 h at 25°C. The membranes were then probed
with a horseradish peroxidase-conjugated secondary antibody
(1:5,000; cat. no. ab7090; Abcam, Cambridge, UK) at 25°C for 1 h,
and signals were visualized using an enhanced chemiluminescence kit
(Beyotime Institute of Biotechnology, Beijing, China) according to
manufacturer's protocol.
Luciferase reporter assay
The potential binding site for miR-494-3p in PTEN
3′-UTR region was predicted using the TargetScan7 tool (www.targetscan.org/vert_71/). Cells were seeded
on a 6-well-plate at a density of 1×106 cells/well and
transfected with 50 nM miR-494-3p mimics or negative controls, 100
ng pGL3-PTEN 3′-UTR-Wild type or Mutant vector and 1 ng pRL-TK
Renilla luciferase plasmid (Promega Corporation, Madison,
WI, USA) according to the manufacturer's protocols (Lipofectamine
RNAiMAX, Invitrogen; Thermo Fisher Scientific, Inc.). After 48 h,
luciferase assays were performed using the dual-luciferase reporter
assay system (Promega Corporation) according to the manufacturer's
protocols. Luminescent signals were quantified with a luminometer
(Glomax, Promega Corporation), and the value of firefly luciferase
was normalized to that of Renilla luciferase.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
(SPSS Inc., Chicago, IL, USA). The differences among groups, in at
least three separate experiments, were analyzed using a Student's
t-test or one-way analysis of variance followed by Dunnett's
multiple comparison test, as appropriate. The samples were divided
into miR-494-3p low and high expression groups according to the
median value of miR-494-3p. Then Kaplan-Meier survival analysis and
log-rank test were used for survival evaluation. Spearman's rank
correlation analysis was performed to analyze correlation between
miR-494-3p and PTEN expression levels. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-494-3p is upregulated in EC
tissues
To investigate the function of miR-494-3p in EC, 43
EC samples were employed and the expression of miR-494-3p was
determined by RT-qPCR in the present study. The results indicated
that miR-494-3p was significantly upregulated in EC tissues
compared with adjacent normal tissues (Fig. 1A). Then, these tissues were divided
into low- or high-expression subgroups according to miR-494-3p
expression, followed by Kaplan-Meier survival analysis. As
presented in Fig. 1B, increased
expression of miR-494-3p in patients with EC indicated poorer
prognosis.
Overexpression of miR-494-3p promotes
EC cell proliferation, migration and invasion
To further determine the effects of miR-494-3p on EC
cells, miR-494-3p was overexpressed in EC cell lines (HHUA and JEC;
Fig. 2A). Then, CCK8 and colony
formation assays were performed using HHUA and JEC cells
transfected with miR-494-3p mimics or controls. The results
revealed that overexpression of miR-494-3p significantly promoted
cellular proliferation and increased the number of the colonies
compared with the control group (Fig.
2B and C). In addition, the effects of miR-494-3p on cellular
apoptosis were evaluated by analysing the protein expression levels
of caspase-3 and B-cell lymphoma-2 (Bcl-2). The results revealed
that overexpression of miR-494-3p did not affect caspase-3 and
Bcl-2 expression (data not shown), indicating that apoptosis was
not affected by miR-494-3p. Tumor metastasis is one of the main
causes of tumor malignancy (18).
The effects of miR-494-3p on tumor cell metastasis were
investigated using a Transwell assay. As presented, overexpression
of miR-494-3p significantly promoted the migration and invasion of
HHUA and JEC cells compared with the control group (Fig. 2D and E). Furthermore, the effects
of miR-494-3p inhibition were evaluated by CCK8 and Transwell
assays. miR-494-3p expression levels were significantly inhibited
following transfection with miR-494-3p inhibitors (Fig. 2F). In addition, the results
demonstrated that miR-494-3p inhibitor significantly suppressed the
proliferation and invasion of HHUA and JEC cells compared with the
control (Fig. 2G and H).
Inhibition of miR-494-3p suppresses
tumor growth in vivo
To further investigate the physiological function of
miR-494-3p in vivo, a xenograft experiment was performed
using HHUA cells. miR-494-3p-silenced or control HHUA cells were
injected into nude mice. At indicative time points following
injection, the tumor volumes were measured; miR-494-3p knockdown
significantly delayed tumor growth in vivo compared with the
control (Fig. 3A). In addition,
the tumor weights were determined at the endpoints of experiments.
As presented in Fig. 3B,
miR-494-3p knockdown resulted in significantly reduced tumor weight
compared with the control.
miR-494-3p activates the PI3K/AKT
signaling pathway by targeting PTEN
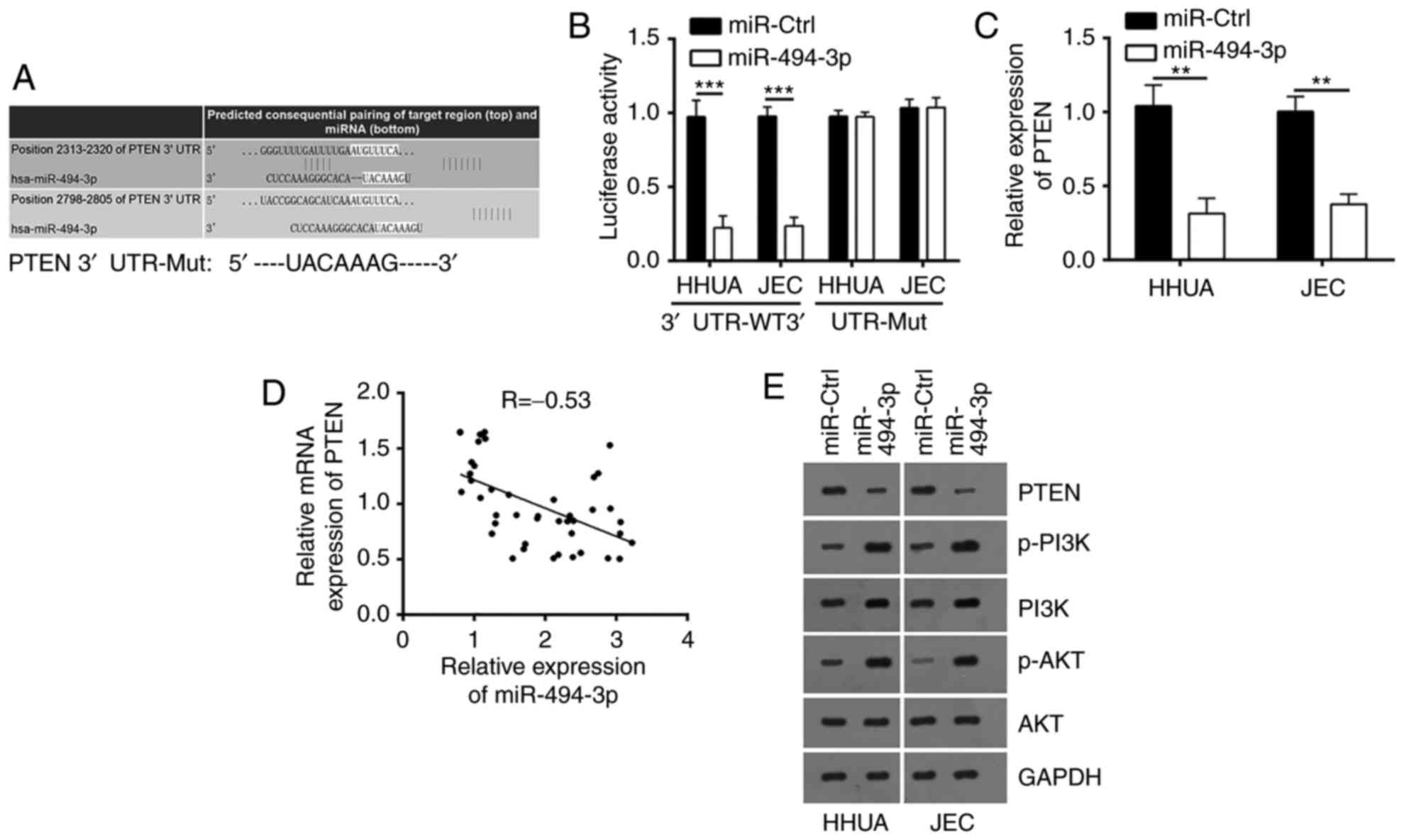
The miR-494-3p-regulated molecular mechanisms
associated with EC were investigated. According to bioinformatic
prediction, PTEN was identified to be a potential target gene of
miR-494-3p. There were two conserved potential binding sites in the
3′-UTR of PTEN mRNA (Fig. 4A);
then, luciferase reporter assays were conducted. As presented in
Fig. 4B, overexpression of
miR-494-3p significantly inhibited luciferase activity in HHUA and
JEC cells compared with the control. Furthermore, the present study
reported that overexpression of miR-494-3p significantly
downregulated the mRNA expression levels of PTEN in HHUA and JEC
cells compared with the control (Fig.
4C). Then, the expression levels of miR-494-3p and PTEN in EC
tissues were evaluated. The results of RT-qPCR indicated that there
was an inverse correlation between the expression levels of
miR-494-3p and PTEN in EC tissues (Fig. 4D). A recent study revealed that the
downstream signaling of PTEN comprised the PI3K/AKT pathway
(8), which was widely involved in
various human cancers, such as EC (19,20).
Furthermore, the effects of miR-494-3p on PTEN protein expression
and PI3K/AKT activation were evaluated using western blot analysis.
Overexpression of miR-494-3p notably downregulated the protein
expression levels of PTEN, and upregulated the phosphorylation of
PI3K and AKT (Fig. 4E). In
summary, the aforementioned data indicated that miR-494-3p
activated the PI3K/AKT pathway by inhibiting PTEN in EC cells.
Restoration of PTEN or inhibition of
the PI3K/AKT pathway abolishes miR-494-3p-mediated effects on EC
cells
To determine whether miR-494-3p affects EC cell
proliferation, migration and invasion via the PTEN/PI3K/AKT
pathway, the protein expression of PTEN was restored or the
PI3K/AKT pathway was inhibited using a specific inhibitor
(LY294002) in miR-494-3p-overexpressed HHUA and JEC cells (Fig. 5A). Then, CCK8 and colony formation
assays were performed to evaluate cellular proliferation. As
presented in Fig. 5B and C,
overexpression of miR-494-3p signficantly promoted cell
proliferation compared with the control; however, restoration of
PTEN or inhibition of the PI3K/AKT pathway abrogated the effects of
miR-494-3p overexpression on EC cells. Similarly, overexpression of
miR-494-3p significantly promoted cellular migration and invasion
compared with the control; however restoration of PTEN or
inhibition of the PI3K/AKT pathway in miR-494-3p-overexpressed HHUA
and JEC cells reversed these effects (Fig. 5D and E). In summary, the results in
the present study demonstrated that miR-494-3p promoted the
progression of EC via the PTEN/PI3K/AKT signaling pathway.
Discussion
MiRNAs can regulate the development and progression
of EC (21); however, the
functions of numerous miRNAs in EC have not been determined. In the
present study, the expression levels of miR-494-3p were
significantly upregulated in EC tissues compared with adjacent
normal tissues. Additionally, the expression of miR-494-3p was
positively associated with the poor prognosis of patients with EC.
Therefore, miR-494-3p may serve as an oncogene in EC, consequently
promoting tumor progression.
Numerous studies demonstrated that miR-494-3p was an
essential regulator in numerous types of cancer. For example, a
recent study indicated that miR-494-3p promoted the development of
lung cancer (14). Liu et
al (22) reported that miR-494
promoted cell proliferation, migration and invasion, and increased
sorafenib resistance in hepatocellular carcinoma by targeting PTEN.
Li et al (15) revealed
that miR-494-3p regulated cellular proliferation, invasion,
migration, and apoptosis via PTEN/AKT signaling in human
glioblastoma cells. Additionally, some studies have indicated that
miR-494 served as a tumor suppressor in certain types of cancer.
For instance, miR-494-3p could induce cellular senescence and
enhance radiosensitivity of oral squamous carcinoma cells (23). Shen et al (16) demonstrated that miRNA-494-3p
targeted C-X-C chemokine receptor type 4 (CXCR4) to suppress the
proliferation, invasion and migration of prostate cancer cells.
These reported contrary functions of miR-494-3p that may be due to
different target genes in various types of cancer. In the present
study, overexpression of miR-494-3p significantly promoted cellular
proliferation in EC in vitro and in vivo. In
addition, overexpressed miR-494-3p induced cellular migration and
invasion in vitro. The findings of the present study
indicated an oncogenic role of miR-494-3p in EC.
Furthermore, previous studies revealed that PTEN
could regulate a variety of biological processes, including the
cell cycle, apoptosis, migration and invasion by inhibiting the
PI3K/AKT signaling pathway (24,25).
PTEN mutations, deletions or silencing by promoter hypermethylation
often results in the tumorigenesis of several cancers, such as EC
(26). A previous study indicated
that PTEN was mutated in 83% of EC tissues (27). Downregulation of PTEN was
associated with activation of the PI3K/AKT pathway, consequently
promoting tumor development and progression (28). For example, E3 ubiquitin-protein
ligase regulated PTEN/PI3K/AKT signaling to promote the cell growth
and migration of hepatocellular carcinoma cells (29). Liu et al (30) reported that Sal-like protein 4
suppressed PTEN expression to promote glioma cell proliferation via
the PI3K/AKT pathway. Another study revealed that miRNA-1297
contributed to tumor growth of human breast cancer by targeting the
PTEN/PI3K/AKT signaling pathway (28). In addition, miRNA-92a promoted
epithelial-mesenchymal transition via the PTEN/PI3K/AKT pathway in
metastatic non-small cell lung cancer (18). In the present study, miR-494-3p
directly targeted PTEN and downregulated its expression.
Furthermore, restoration of PTEN and inhibition of the PI3K/AKT
pathway inhibited the proliferation, migration and invasion of EC
cells overexpressing miR-494-3p, which indicated that miR-494-3p
regulated the progression of EC in a PTEN/PI3K/AKT-dependent
manner.
In summary, the findings of the present study
revealed the essential role of miR-494-3p and its functional
mechanisms in EC. The results suggested that the
miR-494-3p/PTEN/PI3K/AKT axis may be a promising therapeutic target
for the treatment of EC.
Acknowledgements
Not applicable.
Funding
The present study was supported by Science and
Technology Department of Sichuan Province (grant no.
2018SZ0264).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LZ initiated, designed this work, analyzed and
interpreted the results. LZ wrote this manuscript. XW, TW, WZ and
XZ performed the experiments. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
For the use of human samples, the protocol for the
present study was approved by the Institutional Ethics Committee of
the Second Affiliated Hospital of Zhengzhou University (Zhengzhou,
China) and all enrolled patients signed a written informed consent
document. All animal experiments were approved by the Ethics
Committee of the Second Affiliated Hospital of Zhengzhou
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Banno K, Yanokura M, Iida M, Masuda K and
Aoki D: Carcinogenic mechanisms of endometrial cancer: Involvement
of genetics and epigenetics. J Obstet Gynaecol Res. 40:1957–1967.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yeramian A, Moreno-Bueno G, Dolcet X,
Catasus L, Abal M, Colas E, Reventos J, Palacios J, Prat J and
Matias-Guiu X: Endometrial carcinoma: Molecular alterations
involved in tumor development and progression. Oncogene.
32:403–413. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eritja N, Chen BJ, Rodriguez-Barrueco R,
Santacana M, Gatius S, Vidal A, Martí MD, Ponce J, Bergadà L,
Yeramian A, et al: Autophagy orchestrates adaptive responses to
targeted therapy in endometrial cancer. Autophagy. 13:608–624.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Colombo N, Creutzberg C, Amant F, Bosse T,
González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza
MR, et al: ESMO-ESGO-ESTRO consensus conference on endometrial
cancer: Diagnosis, treatment and follow-up. Ann Oncol. 27:16–41.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ramon LA, Braza-Boils A, Gilabert J,
Chirivella M, España F, Estellés A and Gilabert-Estellés J:
microRNAs related to angiogenesis are dysregulated in endometrioid
endometrial cancer. Hum Reprod. 27:3036–3045. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao C, Sun W, Zhang P, Ling S, Li Y, Zhao
D, Peng J, Wang A, Li Q, Song J, et al: miR-214 promotes
osteoclastogenesis by targeting Pten/PI3k/Akt pathway. RNA Biol.
12:343–353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Madhavan B, Yue SJ, Galli U, Rana S, Gross
W, Müller M, Giese NA, Kalthoff H, Becker T, Büchler MW and Zöller
M: Combined evaluation of a panel of protein and miRNA
serum-exosome biomarkers for pancreatic cancer diagnosis increases
sensitivity and specificity. Int J Cancer. 136:2616–2627. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Z, Yu X, Shen J and Jiang Y: MicroRNA
dysregulation in uveal melanoma: A new player enters the game.
Oncotarget. 6:4562–4568. 2015.PubMed/NCBI
|
|
11
|
Zeng B, Shi W and Tan G: miR-199a/b-3p
inhibits gastric cancer cell proliferation via down-regulating
PAK4/MEK/ERK signaling pathway. BMC Cancer. 18:342018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li S, Luo C, Zhou J and Zhang Y:
MicroRNA-34a directly targets high-mobility group box 1 and
inhibits the cancer cell proliferation, migration and invasion in
cutaneous squamous cell carcinoma. Exp Ther Med. 14:5611–5618.
2017.PubMed/NCBI
|
|
13
|
Zhao X, Zhu D, Lu C, Yan D, Li L and Chen
Z: MicroRNA-126 inhibits the migration and invasion of endometrial
cancer cells by targeting insulin receptor substrate 1. Oncol Lett.
11:1207–1212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Faversani A, Amatori S, Augello C, Colombo
F, Porretti L, Fanelli M, Ferrero S, Palleschi A, Pelicci PG,
Belloni E, et al: miR-494-3p is a novel tumor driver of lung
carcinogenesis. Oncotarget. 8:7231–7247. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li XT, Wang HZ, Wu ZW, Yang TQ, Zhao ZH,
Chen GL, Xie XS, Li B, Wei YX, Huang YL, et al: miR-494-3p
regulates cellular proliferation, invasion, migration and apoptosis
by PTEN/AKT signaling in human glioblastoma cells. Cell Mol
Neurobiol. 35:679–687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen PF, Chen XQ, Liao YC, Chen N, Zhou Q,
Wei Q, Li X, Wang J and Zeng H: MicroRNA-494-3p targets CXCR4 to
suppress the proliferation, invasion, and migration of prostate
cancer. Prostate. 74:756–767. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu CJ, Shan ZX, Hong J and Yang L:
MicroRNA-92a promotes epithelial-mesenchymal transition through
activation of PTEN/PI3K/AKT signaling pathway in non-small cell
lung cancer metastasis. Int J Oncol. 51:235–244. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun K, Wang S, He J, Xie Y, He Y, Wang Z
and Qin L: NCOA5 promotes proliferation, migration and invasion of
colorectal cancer cells via activation of PI3K/AKT pathway.
Oncotarget. 8:107932–107946. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang S, Wang M, Li Q and Zhu P: miR-101
reduces cell proliferation and invasion and enhances apoptosis in
endometrial cancer via regulating PI3K/Akt/mTOR. Cancer Biomark.
21:179–186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li L, Qu YW and Li YP: Over-expression of
miR-1271 inhibits endometrial cancer cells proliferation and
induces cell apoptosis by targeting CDK1. Eur Rev Med Pharmacol
Sci. 21:2816–2822. 2017.PubMed/NCBI
|
|
22
|
Liu K, Liu S, Zhang W, Jia B, Tan L, Jin Z
and Liu Y: miR-494 promotes cell proliferation, migration and
invasion, and increased sorafenib resistance in hepatocellular
carcinoma by targeting PTEN. Oncol Rep. 34:1003–1010. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weng JH, Yu CC, Lee YC, Lin CW, Chang WW
and Kuo YL: miR-494-3p induces cellular senescence and enhances
radiosensitivity in human oral squamous carcinoma cells. Int J Mol
Sci. 17:E10922016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiu HF, Li J, Clark LH, Jackson AL, Zhang
L, Guo H, Kilgore JE, Gehrig PA, Zhou C and Bae-Jump VL: JQ1
suppresses tumor growth via PTEN/PI3K/AKT pathway in endometrial
cancer. Oncotarget. 7:66809–66821. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Yang Y, Zhang Z, He Y, Liu Z, Yu
Y, Wu S, Cai B and Feng Y: Gankyrin plays an essential role in
estrogen-driven and GPR30-mediated endometrial carcinoma cell
proliferation via the PTEN/PI3K/AKT signaling pathway. Cancer Lett.
339:279–287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cancer Genome Atlas Research Network, ;
Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H,
Robertson AG, Pashtan I, Shen R, et al: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mutter GL, Lin MC, Fitzgerald JT, Kum JB,
Baak JP, Lees JA, Weng LP and Eng C: Altered PTEN expression as a
diagnostic marker for the earliest endometrial precancers. J Natl
Cancer Inst. 92:924–931. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu C, Liu ZK, Li X, Tang XJ, He JJ and Lu
SY: MicroRNA-1297 contributes to tumor growth of human breast
cancer by targeting PTEN/PI3K/AKT signaling. Oncol Rep.
38:2435–2443. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang ZJ, Zhu JJ, Yang XY and Biskup E:
NEDD4 promotes cell growth and migration via PTEN/PI3K/AKT
signaling in hepatocellular carcinoma. Oncol Lett. 14:2649–2656.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu CJ, Wu HB, Li YY, Shen L, Yu R, Yin H,
Sun T, Sun C, Zhou Y and Du Z: SALL4 suppresses PTEN expression to
promote glioma cell proliferation via PI3K/AKT signaling pathway. J
Neurooncol. 135:263–272. 2017. View Article : Google Scholar : PubMed/NCBI
|