Introduction
Diabetes occurs in ~8.5% of adults aged 18 years or
older, and resulted in 1.6 million mortalities in 2014 (1). Hyperglycemia may cause injury to the
peripheral nerves, and the renal and vascular systems (2). Diabetic retinopathy (DR), a
microvascular complication of diabetes, is cause of adult blindness
(3–5); visual impairment in DR is associated
with increased apoptosis of retinal cells, including pigment
epithelial cells, pericytes and endothelial cells (6–8).
Although DR can be treated by vitrectomy or laser photocoagulation,
these approaches are not satisfactory (9,10),
and novel and effective interventions are required to reduce
retinal injury in patients with DR.
High levels of glucose serve a key role in retinal
cell death. Several lines of evidence indicate that high glucose
induces overproduction of pro-inflammatory cytokines, such as tumor
necrosis factor (TNF)-α and interleukin (IL)-1β, which act as a
positive feedback mechanism to induce retinal cell apoptosis
(11–14). High concentrations of glucose
within retinal cells can induce oxidative stress by increasing
intracellular reactive oxygen species (ROS) production through the
mitochondrial electron transport chain (15–17).
Oxidative stress activates a cascade of several biochemical and
molecular events, which may ultimately lead to DR (18); therefore, anti-oxidants may
represent a promising therapy for the treatment of DR.
Curcumin (CUR), a natural phytochemical compound in
turmeric is reported to possess a variety of pharmacological
properties, including anti-oxidative (19–21),
anti-inflammatory (22–24) and anti-carcinogenic activities
(25–27). CUR has been proposed to prevent DR
by modulating antioxidant activities and several signaling pathways
(28,29). It remains unclear whether CUR
exerts its therapeutic effects in DR through its anti-inflammatory
properties, and to determine whether CUR was able to prevent
inflammatory injury in DR, its effect on retinal pigment epithelial
cells (RPECs) cultured in high levels of glucose were investigated.
Therefore, the present study aimed to investigate the potential
effects of CUR on RPECs, including the secretion of
pro-inflammatory cytokines, ROS production and the underlying
molecular mechanism, which may provide a theoretical basis for the
use of CUR as treatment strategy for DR.
Materials and methods
Regents
CUR (see Fig. 1 for
chemical structure) was obtained from BioBioPha Co., Ltd. (Kunming,
China). Glucose and mannitol were purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). RPMI-1640 medium with glucose
(5.6 mmol/l) was purchased from Gibco (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Fetal bovine serum (FBS) was purchased
from HyClone (GE Healthcare, Chicago, IL, USA). Penicillin and
streptomycin were purchased from Beijing Solarbio Science &
Technology Co., Ltd. (Beijing, China).
2′,7′-dichlorodihydrofluororescein diacetate (DCFH-DA), DAPI,
LY294002, rapamycin and N-acetylcysteine (NAC) were obtained from
Beyotime Institute of Biotechnology (Jiangsu, China). Cell Counting
Kit (CCK)-8 was purchased from Invitrogen (Thermo Fisher
Scientific, Inc.). AKT (catalog no. 9272), phosphorylated (p)-AKT
(catalog no. 9611) and p-mTOR (catalog no. 2971) antibodies were
purchased from Cell Signaling Technology Inc. (Danvers, MA, USA).
mTOR (catalog no. 66888-1-Ig) and β-actin (catalog no. 60008-1-Ig)
antibodies were obtained from ProteinTech Group, Inc. (Chicago, IL,
USA). Horseradish peroxidase (HRP)-conjugated goat anti-mouse
secondary antibodies (catalog no. TA130004) and HRP-conjugated goat
anti-rabbit secondary antibodies (catalog no. TA130023) were
purchased from the OriGene Technologies, Inc. (Beijing, China).
Cell culture
ARPE-19 human RPECs were obtained from the Type
Culture Collection of the Chinese Academy of Sciences (catalog no.
CRL-4000; Shanghai, China). RPECs were cultured in RPMI-1640 medium
supplemented with 10% FBS and 100 U/ml penicillin/streptomycin, and
maintained at 37°C in a saturated humidified atmosphere with 5%
CO2. Prior to experiments, RPECs were cultured in
RPMI-1640 medium with 1% FBS at 37°C for 12 h, and glucose was
added to the culture medium (30 mmol/l) for 0, 6, 12 and 24 h. To
exclude the effects of high osmolarity on RPECs, 24.4 mmol/l
mannitol was used as an equivalent osmolarity control to 30 mmol/l
glucose. For specific inhibitors or antioxidant treatments, RPECs
were incubated with LY294002 (1 µmol/l), rapamycin (10 µmol/l) or
NAC (1 mmol/l) for 1 h at 37°C before high glucose treatment; and
in CUR treatment experiments, RPECs were incubated with CUR for 1 h
at 37°C prior to high glucose treatment. The concentration of
LY294002 was selected based on our previous study (30), whereas the concentrations of
rapamycin and NAC were determined according to previously reported
methods (31).
CCK-8 assay
RPEC viability was measured using a CCK-8 method.
Briefly, RPECs were seeded in 96-well plates at a density of
1–1.5×104 cells/ml. After culturing at 37°C for 0, 6, 12
or 24 h, 10 µl CCK-8 solution was added and the cells were
incubated at 37°C for 4 h. Optical density (OD) was measured at 450
nm using a microplate reader (Molecular Devices, LLC, Sunnyvale,
CA, USA). This experiment was replicated three times.
ELISA
RPECs were seeded in 96-well plates at a density of
1–1.5×104 cells/ml and incubated for 12 h at 37°C.
Following incubation, the medium was collected and TNF-α (catalog
no. ab181421), IL-1β (catalog no. ab100562) and IL-6 (catalog no.
ab46027) content were measured by ELISA kits (Abcam, Cambridge,
UK), according to the manufacturer's protocols. This experiment was
replicated three times.
Detection of intracellular ROS
The intracellular ROS content of RPECs was examined
following incubation with cell-permeable DCFH-DA, which is
converted to fluorescent DCF in the presence of ROS. Briefly, cells
were seeded in a 6-well plate at a density of 1–1.5×107
cells/ml. Following treatment with 30 mmol/l glucose or 24.4 mmol/l
mannitol for 12 h, 10 µmol/l DCFH-DA and 1 µg/l DAPI in FBS-free
RPMI-1640 were added and the plates were incubated for 20 min at
37°C. Cells were washed using RPMI-1640 with 10% FBS, and images
were captured using a laser scanning confocal microscope (Olympus
Corporation, Tokyo, Japan). The fluorescence intensity was measured
in six random fields and analyzed using ImageJ software (version
1.4l; National Institutes of Health, Bethesda, MD, USA).
Western blot analysis
RPECs in the various treatments cultured on 6-well
plates were collected with pancreatin and lysed on ice for 30 min
in Western and IP Cell Lysis buffer (catalog no. P0013J; Beyotime
Institute of Biotechnology) supplemented with protease inhibitor
cocktail and phosphatase inhibitors (150 µl). Protein levels were
quantified using a Bicinchoninic Acid Protein Assay kit (catalog
no. P0009; Beyotime Institute of Biotechnology). Protein extracts
(50 µg) were separated by 10% SDS-PAGE and transferred onto
polyvinylidene difluoride membranes. Following blocking with 10%
skim milk overnight at 4°C, the membranes were incubated with
primary antibodies against p-AKT (1:500), p-mTOR (1:600) or control
β-actin (1:50,000) at 4°C for 12 h, washed three times with PBS,
followed by incubation with HRP-conjugated secondary antibodies
(1:2,000) for 2.5 h at room temperature. Each membrane was stripped
and re-probed for its corresponding total protein, AKT (1:1,000) or
mTOR (1:1,200). Protein bands were visualized using a
chemiluminescence detection system (Pierce; Thermo Fisher
Scientific, Inc.). Images were captured and analyzed using ImageJ
software (version 1.41; National Institutes of Health, Bethesda,
MD, USA); β-actin was used for normalization.
Statistical analysis
Statistical analysis was performed on GraphPad Prism
6.0 (GraphPad Software, Inc. La Jolla, CA, USA). All data are
presented as the mean ± standard error of the mean of three
independent experiments. Statistical analysis was performed by
one-way analysis of variance, followed by the Tukey-Kramer
post-test to determine any significant differences. P<0.05 was
considered to indicate a statistically significant difference.
Results
CUR ameliorates glucose-induced
toxicity in RPECs
Cells treated with glucose for 12 h exhibited a
significant reduction in viability in a concentration-dependent
manner, compared with untreated Control cells (Fig. 2A). Treatment with 30 mmol/l glucose
was considered high glucose group in subsequent experiments. To
examine the effects of high glucose on RPECs, cells treated with 30
mmol/l glucose was set as high glucose group (HG), and the control
equivalent osmolarity group was 24.4 mmol/l mannitol. Cells treated
high glucose for 24 h exhibited a reduction in viability in a
time-dependent manner (Fig. 2B).
However, cells treated with various concentrations of mannitol
(9.4–84.4 mmol/l) did not exhibit any effects on RPEC viability
(Fig. 2C). Cells treated with 24.4
mmol/l mannitol for 2 h 4, which has considered the equivalent
osmolarity to 30 mmol/l glucose, did not exhibit any effects on
viability (Fig. 2D). These results
suggested that the effect of high glucose on viability may not be
attributed to high osmolarity. Cells treated with CUR (0–20 µmol/l)
for 12 h did not exhibit any effects on RPECs viability (Fig. 2E); CUR also did not exhibit effects
on viability in cells treated for 0–24 h with CUR at a
concentration of 10 µmol/l (Fig.
2F). However, pretreatment with CUR (5 or 10 µmol/l) for 1 h
increased the cell viability of RPECs cultured with high glucose
(Fig. 2G). These data indicated
that CUR may serve a pro-survival role under the high glucose
condition by interfering with related signaling pathways, whereas
without high glucose stimulation the Control group exhibited no
obvious effects following CUR treatment.
High glucose treatment induces RPEC
secretion of IL-1β, IL-6 and TNF-α via the ROS/PI3K/AKT/mTOR
signaling pathway
Expression levels of IL-1β, IL-6 and TNF-α in the
culture medium were significantly higher in RPECs incubated in high
glucose conditions compared with the respective secretion levels in
the untreated Control cells (Fig.
3A-C, respectively); no significant differences in secretion
levels were observed in cells treated with mannitol, which
suggested that the high glucose-induced increase in IL-1β, IL-6 and
TNF-α secretion of may not mediated by high osmolarity. The results
from western blotting indicated that the protein expression levels
of p-AKT and p-mTOR were significantly higher in RPECs treated with
high glucose compared with the Control group (Fig. 3D and E, respectively), whereas
mannitol treatment exhibited no significant effects.
 | Figure 3.High glucose treatment increases the
secretion levels of IL-1β, IL-6 and TNF-α in RPECs. (A-C)
Expression levels of (A) IL-1β, (B) IL-6 and (C) TNF-α in the
culture medium of cells treated with either 30 mmol/l glucose or
24.4 mmol/l mannitol for 12 h were measured by ELISA. (D and E)
Expression levels of p-AKT, total AKT, p-mTOR and total mTOR in
cells treated with either 30 mmol/l glucose or 24.4 mmol/l mannitol
for 12 h as measured by western blot analysis. Data are presented
as the mean ± standard error of the mean; n=3; **P<0.01 vs.
control. CUR, curcumin; HG, high glucose; IL, interleukin; mTOR,
mammalian target of rapamycin; p, phosphorylated; PI3K,
phosphoinositide 3-kinase; RPEC, retinal pigment epithelial cell;
TNF, tumor necrosis factor. |
Cells treated with high glucose, but not mannitol,
exhibited significantly increased intracellular ROS formation in
RPECs (Fig. 4A). Pretreatment of
RPECs with the antioxidant NAC (1 mmol/l) for 1 h inhibited the
phosphorylation of AKT and mTOR (Fig.
4B and C). Furthermore, high glucose-induced increases in
secretion of IL-1β, IL-6 and TNF-α were significantly decreased by
pretreatment with NAC, PI3K inhibitor LY294002 (1 µmol/l) or the
mTOR inhibitor rapamycin (10 µmol/l) (Fig. 4D-F). Taken together, these data
suggested that high glucose exposure may have induced the secretion
of TNF-α, IL-6 and IL-1β via the ROS/PI3K/AKT/mTOR signaling
pathway in RPECs.
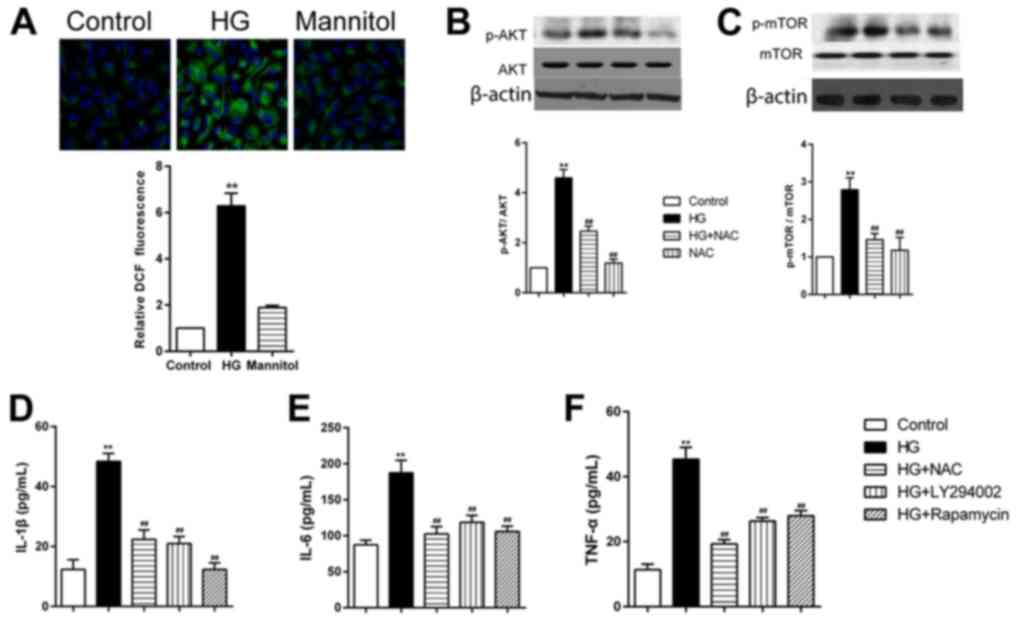 | Figure 4.HG treatment increases cytokine
secretion RPECs via the ROS/PI3K/AKT/mTOR signaling pathway. (A)
ROS formation in cells treated with high glucose or mannitol for 12
h was measured by 2′,7′-dichlorodihydrofluororescein diacetate
staining (green); nuclei were stained with DAPI (blue);
magnification, ×600. (B and C) Expression levels of (B) p-AKT and
total AKT, and (C) p-mTOR and total mTOR in cells treated with 30
mmol/l glucose and 1 mmol/l NAC, either alone or in combination,
were measured by western blot analysis. (D-F) Expression levels of
(D) IL-1β, (E) IL-6 and (F) TNF-α in the culture medium of cells
treated with 30 mmol/l glucose alone or in combination with 1
mmol/l NAC, 1 µmol/l LY294002 or 10 µmol/l rapamycin were measured
by ELISA. Data are presented as the mean ± standard error of the
mean; **P<0.01 vs. Control; ##P<0.01 vs. HG. HG,
high glucose; IL, interleukin; mTOR, mammalian target of rapamycin;
NAC, N-acetylcysteine; p, phosphorylated; PI3K, phosphoinositide
3-kinase; ROS, reactive oxygen species; RPEC, retinal pigment
epithelial cell; TNF, tumor necrosis factor. |
CUR inhibits the high glucose-induced
secretion of TNF-α, IL-6 and IL-1β through the ROS-AKT/mTOR
cascade
High glucose treatment increased secretion of TNF-α,
IL-6 and IL-1β in RPECs, which were significantly reduced in cells
pretreated with 10 µmol/l CUR for 12 h (Fig. 5A-C). In addition, pretreatment with
CUR significantly reduced the formation of intracellular ROS, in
high glucose co-treated RPECs (Fig.
5D). High glucose-induced upregulation of p-AKT and p-mTOR
expression levels were significantly reduces by pretreatment with
10 µmol/l CUR for 12 h (Fig. 5E and
F). These results suggested that the CUR may inhibit the high
glucose-induced secretion of TNF-α, IL-6 and IL-1β by interfering
with the ROS-AKT/mTOR cascade in RPECs.
 | Figure 5.CUR treatment reduces the high
glucose-induced secretion of IL-1β, IL-6 and TNF-α via the
ROS/PI3K/AKT/mTOR signaling pathway in RPECs. (A-C) Expression
levels of (A) IL-6, (B) IL-1β and (C) TNF-α in cells treated with
30 mmol/l glucose, 10 µmol/l CUR or their combination were measured
by ELISA. (D) ROS formation in cells treated with high glucose, 10
µmol/l CUR or their combination for 12 h was measured by
2′,7′-dichlorodihydrofluororescein diacetate staining (green);
nuclei were stained with DAPI (blue); magnification, ×600. (E and
F) Expression levels of (E) p-AKT and AKT, and (F) p-mTOR and mTOR
in cells treated with 30 mmol/l glucose, 10 µmol/l CUR or their
combination were measured by western blot analysis. **P<0.01 vs.
Control; ##P<0.01 vs. HG. CUR, curcumin; HG, high
glucose; IL, interleukin; mTOR, mammalian target of rapamycin; p,
phosphorylated; PI3K, phosphoinositide-3 kinase; ROS, reactive
oxygen species; RPEC, retinal pigment epithelial cell; TNF, tumor
necrosis factor. |
Discussion
Hyperglycemia is a primary factor that contributes
to retinal injury in the development of DR (32). RPECs serve many important functions
in the retina, including phagocytosis of photoreceptor outer
segments, isomerization of retinoids and various metabolic and
neurotrophic support functions (33). RPEC dysfunction contributes to the
pathogenesis of DR (34,35). Hyperglycemia induces inflammation
and oxidative stress in the retina, eventually leading to apoptosis
of RPECs (36,37). In addition, increased levels of
inflammatory cytokines serve an important role in the pathogenic
development of DR (38,39). It has been reported that high
concentrations of glucose increase production of inflammatory
cytokines in RPECs (40–42). Consistent with these reports,
results from the present study indicated that high glucose
treatment increased the secretion levels of TNF-α, IL-1β and IL-6
in RPECs. In addition, CUR co-treatment ameliorated these high
glucose-induced secretion levels via the ROS/PI3K/AKT/mTOR
signaling pathway. These results suggest that CUR may represent a
promising therapeutic agent for the treatment of DR.
In the present study, high glucose exposure induced
ROS production in RPECs. Previous studies reported that high levels
of ROS are observed in chronic human diseases, such as
atherosclerosis and other cardiovascular diseases (43–45).
The unbalanced ROS generation or ROS elimination results in the
presence of oxidative stress, which eventually leads to
inflammatory responses (46). In
hyperglycemic conditions, mitochondrial damage and endoplasmic
reticulum stress have been reported to trigger injury of many
retinal cells in the retina, and the activation of multiple
inflammatory pathways and oxidative damage to RPECs contributes to
the pathogenesis of DR (16,47).
In the present study, high glucose concentrations induced
intracellular ROS formation, whereas pretreatment with the
antioxidant NAC significantly inhibited high glucose-induced
secretion of IL-1β, IL-6 and TNF-α in RPECs. The results suggested
that ROS contributed to glucose-induced secretion of inflammatory
cytokines. The PI3K/AKT/mTOR signaling pathway is important in
inflammation, and PI3K/AKT/mTOR signaling activation promotes
expression of many pro-inflammatory cytokines (48,49).
In the present study, high glucose treatment activated the
PI3K/AKT/mTOR signaling pathway, which may have resulted in
increased secretion of TNF-α, IL-1β and IL-6 in RPECs, but this
requires further validation.
CUR, is a natural product of the rhizomes of
Curcuma longa, and is widely used as an anti-oxidant and
anti-inflammatory agent (50,51).
CUR is reported to delay development of DR by inhibiting vascular
endothelial growth factor and nuclear transcription factors
(28). In addition, CUR can
scavenge ROS, reduce degradation of anti-oxidant enzymes and reduce
lipid peroxidation (52). In the
present study, CUR treatment ameliorated the high glucose-induced
ROS formation and the secretion of pro-inflammatory cytokines in
RPECs. CUR was reported to exert its anticancer effects through the
PI3K/AKT/mTOR signaling pathway (53,54).
It was demonstrated in the present study that high glucose exposure
increased the secretion of pro-inflammatory cytokines via the
AKT/mTOR signaling pathway in RPECs, and CUR ameliorated these
effects via the PI3K/AKT/mTOR cascade. However, the in vivo
effects of CUR on DR remains to be further investigated.
In summary, high glucose exposure induced the
secretion of TNF-α, IL-1β and IL-6, in RPECs via the
ROS/PI3K/AKT/mTOR cascade. CUR inhibited the glucose-induced
inflammation via interfering with the ROS/PI3K/AKT/mTOR cascade in
RPECs. These findings confirmed the mechanism underlying the
inflammatory effect of glucose in RPECs and suggested that CUR may
represent a potential therapeutic strategy for glucose-induced
inflammation.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Science Foundation of China (grant no. 30973252).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article. The data sets used
and/or analyzed during the present study are available from the
corresponding author on reasonable request.
Authors' contributions
ZR and JM designed the present study. ZR analyzed
and interpreted the results, and drafted the manuscript. YZ and XW
performed experiments. JM edited the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chang LY, Lee AC and Sue W: Prevalence of
diabetic retinopathy at first presentation to the retinal screening
service in the greater Wellington region of New Zealand 2006–2015,
and implications for models of retinal screening. N Z Med J.
130:78–88. 2017.PubMed/NCBI
|
|
2
|
Maugh TH II: Diabetic retinopathy: New
ways to prevent blindness. Science. 192:539–540. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ahmed MS and Tahrani A: Diabetic
retinopathy in cystic fibrosis related diabetes. Diabetologia.
60:S472–S473. 2017.
|
|
4
|
Byberg S, Jorgensen ME, Larsen M,
Lundandersen H and Vistisen D: Risk score for diabetic retinopathy.
Diabetes. 66:A1612017.
|
|
5
|
Bajestani NS, Kamyad AV, Esfahani EN and
Zare A: Prediction of retinopathy in diabetic patients using type-2
fuzzy regression model. Eur J Oper Res. 264:859–869. 2018.
View Article : Google Scholar
|
|
6
|
Zvornicanin J and Zvorničanin E: Imaging
in diabetic retinopathy. Middle East Afr J Ophthalmol. 22:5312015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pasquel FJ, Hendrick AM, Ryan M, Cason E,
Ali MK and Narayan KM: Cost-effectiveness of different diabetic
retinopathy screening modalities. J Diabetes Sci Technol.
10:301–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jenkins AJ, Joglekar MV, Hardikar AA,
Keech AC, O'Neal DN and Januszewski AS: Biomarkers in diabetic
retinopathy. Rev Diabet Stud. 12:159–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hurley B: Therapeutic revolution in the
management of diabetic retinopathy. Can J Ophthalmol. 52 Suppl
1:S1–S2. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vaziri K, Schwartz SG, Relhan N, Kishor KS
and Flynn HW Jr: New therapeutic approaches in diabetic
retinopathy. Rev Diabet Stud. 12:196–210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang J and Kern TS: Inflammation in
diabetic retinopathy. Prog Retin Eye Res. 30:343–358. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rangasamy S, McGuire PG and Das A:
Diabetic retinopathy and inflammation: Novel therapeutic targets.
Middle East Afr J Ophthalmol. 19:52–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Joussen AM, Poulaki V, Le ML, Koizumi K,
Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B,
et al: A central role for inflammation in the pathogenesis of
diabetic retinopathy. FASEB J. 18:1450–1452. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abcouwer SF and Antonetti DA: A role for
systemic inflammation in diabetic retinopathy. Invest Ophthalmol
Vis Sci. 54:23842013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Behl T, Kaur I and Kotwani A: Implication
of oxidative stress in progression of diabetic retinopathy. Surv
Ophthalmol. 61:187–196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kowluru RA and Mishra M: Oxidative stress,
mitochondrial damage and diabetic retinopathy. Biochim Biophys
Acta. 1852:2474–2483. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kowluru RA, Kowluru A, Mishra M and Kumar
B: Oxidative stress and epigenetic modifications in the
pathogenesis of diabetic retinopathy. Prog Retin Eye Res. 48:40–61.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Calderon GD, Juarez OH, Hernandez GE,
Punzo SM and De la Cruz ZD: Oxidative stress and diabetic
retinopathy: Development and treatment. Eye (Lond). 31:1122–1130.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choudhury AK, Raja S, Mahapatra S,
Nagabhushanam K and Majeed M: Synthesis and evaluation of the
anti-oxidant capacity of curcumin glucuronides, the major curcumin
metabolites. Antioxidants (Basel). 4:750–767. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mandal MN, Patlolla JM, Zheng L, Agbaga
MP, Tran JT, Wicker L, Kasus-Jacobi A, Elliott MH, Rao CV and
Anderson RE: Curcumin protects retinal cells from light-and oxidant
stress-induced cell death. Free Radic Biol Med. 46:672–679. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie M, Fan D, Zhao Z, Li Z, Li G, Chen Y,
He X, Chen A, Li J, Lin X, et al: Nano-curcumin prepared via
supercritical: Improved anti-bacterial, anti-oxidant and
anti-cancer efficacy. Int J Pharm. 496:732–740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baghdasaryan A, Claudel T, Kosters A,
Gumhold J, Silbert D, Thüringer A, Leski K, Fickert P, Karpen SJ
and Trauner M: Curcumin improves sclerosing cholangitis in Mdr2-/-
mice by inhibition of cholangiocyte inflammatory response and
portal myofibroblast proliferation. Gut. 59:521–530. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bisht S, Khan MA, Bekhit M, Bai H, Cornish
T, Mizuma M, Rudek MA, Zhao M, Maitra A, Ray B, et al: A polymeric
nanoparticle formulation of curcumin (NanoCurc™) ameliorates
CCl4-induced hepatic injury and fibrosis through reduction of
pro-inflammatory cytokines and stellate cell activation. Lab
Invest. 91:1383–1395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fu Y, Gao R, Cao Y, Guo M, Wei Z, Zhou E,
Li Y, Yao M, Yang Z and Zhang N: Curcumin attenuates inflammatory
responses by suppressing TLR4-mediated NF-κB signaling pathway in
lipopolysaccharide-induced mastitis in mice. Int Immunopharmacol.
20:54–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Das L and Vinayak M: Anti-carcinogenic
action of curcumin by activation of antioxidant defence system and
inhibition of NF-κB signalling in lymphoma-bearing mice. Biosci
Rep. 32:161–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Heng MC: Curcumin targeted signaling
pathways: Basis for anti-photoaging and anti-carcinogenic therapy.
Int J Dermatol. 49:608–622. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Samuels TL, Pearson AC, Wells CW, Stoner
GD and Johnston N: Curcumin and anthocyanin inhibit pepsin-mediated
cell damage and carcinogenic changes in airway epithelial cells.
Ann Otol Rhinol Laryngol. 122:632–641. 2013.PubMed/NCBI
|
|
28
|
Aldebasi YH, Aly SM and Rahmani AH:
Therapeutic implications of curcumin in the prevention of diabetic
retinopathy via modulation of anti-oxidant activity and genetic
pathways. Int J Physiol Pathophysiol Pharmacol. 5:194–202.
2013.PubMed/NCBI
|
|
29
|
Steigerwalt R, Nebbioso M, Appendino G,
Belcaro G, Ciammaichella G, Cornelli U, Luzzi R, Togni S, Dugall M,
Cesarone MR, et al: Meriva®, a lecithinized curcumin
delivery system, in diabetic microangiopathy and retinopathy.
Panminerva Med. 54:11–16. 2012.PubMed/NCBI
|
|
30
|
Su Q, Wang Y, Yang X, Li XD, Qi YF, He XJ
and Wang YJ: Inhibition of endoplasmic reticulum stress apoptosis
by estrogen protects human umbilical vein endothelial cells through
the PI3 Kinase-Akt signaling pathway. J Cell Biochem.
118:4568–4574. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mao N, Cheng Y, Shi XL, Wang L, Wen J,
Zhang Q, Hu QD and Fan JM: Ginsenoside Rg1 protects mouse podocytes
from aldosterone-induced injury in vitro. Acta Pharmacol Sin.
35:513–522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ahsan H: Diabetic retinopathy-biomolecules
and multiple pathophysiology. Diabetes Metab Syndr. 9:51–54. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
White C, DiStefano T and Olabisi R: The
influence of substrate modulus on retinal pigment epithelial cells.
J Biomed Mater Res A. 105:1260–1266. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Okamura N, Ito Y, Shibata MA, Ikeda T and
Otsuki Y: Fas-mediated apoptosis in human lens epithelial cells of
cataracts associated with diabetic retinopathy. Med Electron
Microsc. 35:234–241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou W, Yu W, Xie W, Huang L, Xu Y and Li
X: The role of SLIT-ROBO signaling in proliferative diabetic
retinopathy and retinal pigment epithelial cells. Mol Vis.
17:1526–1536. 2011.PubMed/NCBI
|
|
36
|
Kim DI, Park MJ, Lim SK, Choi JH, Kim JC,
Han HJ, Kundu TK, Park JI, Yoon KC, Park SW, et al:
High-glucose-induced CARM1 expression regulates apoptosis of human
retinal pigment epithelial cells via histone 3 arginine 17
dimethylation: Role in diabetic retinopathy. Arch Biochem Biophys.
560:36–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Song MK, Roufogalis BD and Huang TH:
Reversal of the caspase-dependent apoptotic cytotoxicity pathway by
taurine from Lycium barbarum (Goji Berry) in human retinal pigment
epithelial cells: Potential benefit in diabetic retinopathy. Evid
Based Complement Alternat Med. 2012:3237842012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kaštelan S, Tomić M, Gverović Antunica A,
Salopek Rabatić J and Ljubić S: Inflammation and pharmacological
treatment in diabetic retinopathy. Mediators Inflamm.
2013:2131302013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
El-Asrar AM: Role of inflammation in the
pathogenesis of diabetic retinopathy. Middle East Afr J Ophthalmol.
19:70–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun J, Huang P, Liang J, Li J, Shen M, She
X, Feng Y, Luo X, Liu T and Sun X: Cooperation of Rel family
members in regulating Aβ1-40-mediated pro-inflammatory
cytokine secretion by retinal pigment epithelial cells. Cell Death
Dis. 8:e31152017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Singh M and Tyagi SC: Homocysteine
mediates transcriptional changes of the inflammatory pathway
signature genes in human retinal pigment epithelial cells. Int J
Ophthalmol. 10:696–704. 2017.PubMed/NCBI
|
|
42
|
Kutty RK, Samuel W, Duncan T, Postnikova
O, Jaworski C, Nagineni CN and Redmond TM: Proinflammatory cytokine
interferon-γ increases the expression of BANCR a long non-coding
RNA, in retinal pigment epithelial cells. Cytokine. 104:147–150.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shah MS and Brownlee M: Molecular and
cellular mechanisms of cardiovascular disorders in diabetes. Circ
Res. 118:1808–1829. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu T, Peng Y, Yan S, Li N, Chen Y and Lan
T: Andrographolide ameliorates atherosclerosis by suppressing
pro-inflammation and ROS generation-mediated foam cell formation.
Inflammation. 41:1681–1689. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang Y and Tabas I: Emerging roles of
mitochondria ROS in atherosclerotic lesions: Causation or
association? J Atheroscler Thromb. 21:381–390. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kanellakis P, Pomilio G, Walker C, Husband
A, Huang JL, Nestel P, Agrotis A and Bobik A: A novel antioxidant
3,7-dihydroxy-isoflav-3-ene (DHIF) inhibits neointimal hyperplasia
after vessel injury attenuating reactive oxygen species and nuclear
factor-kappaB signaling. Atherosclerosis. 204:66–72. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen W, Zhao M, Zhao S, Lu Q, Ni L, Zou C,
Lu L, Xu X, Guan H, Zheng Z and Qiu Q: Activation of the
TXNIP/NLRP3 inflammasome pathway contributes to inflammation in
diabetic retinopathy: A novel inhibitory effect of minocycline.
Inflamm Res. 66:157–166. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu X, Long L, Liu J, Zhang J, Wu T, Chen
X, Zhou B and Lv TZ: Gambogic acid suppresses inflammation in
rheumatoid arthritis rats via PI3K/Akt/mTOR signaling pathway. Mol
Med Rep. 16:7112–7118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Choi YH, Jin GY, Li LC and Yan GH:
Inhibition of protein kinase C delta attenuates allergic airway
inflammation through suppression of PI3K/Akt/mTOR/HIF-1 alpha/VEGF
pathway. PLoS One. 8:e817732013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Weber WM, Hunsaker LA, Abcouwer SF, Deck
LM and Vander Jagt DL: Anti-oxidant activities of curcumin and
related enones. Bioorg Med Chem. 13:3811–3820. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Edwards RL, Luis PB, Varuzza PV, Joseph
AI, Presley SH, Chaturvedi R and Schneider C: The anti-inflammatory
activity of curcumin is mediated by its oxidative metabolites. J
Biol Chem. 292:21243–21252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fazal Y, Fatima SN, Shahid SM and Mahboob
T: Effects of curcumin on angiotensin-converting enzyme gene
expression, oxidative stress and anti-oxidant status in
thioacetamide-induced hepatotoxicity. J Renin Angiotensin
Aldosterone Syst. 16:1046–1051. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tian B, Zhao Y, Liang T, Ye X, Li Z, Yan
D, Fu Q and Li Y: Curcumin inhibits urothelial tumor development by
suppressing IGF2 and IGF2-mediated PI3K/AKT/mTOR signaling pathway.
J Drug Target. 25:626–636. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cianciulli A, Calvello R, Porro C, Trotta
T, Salvatore R and Panaro MA: PI3k/Akt signalling pathway plays a
crucial role in the anti-inflammatory effects of curcumin in
LPS-activated microglia. Int Immunopharmacol. 36:282–290. 2016.
View Article : Google Scholar : PubMed/NCBI
|



















