Introduction
Lung cancer is one of the major causes of
cancer-related mortality, causing ~154,050 cases of mortality and
affecting over 234,000 new patients annually in the United States
(1,2). In developing countries, including
China, the incidence of lung cancer was predicted to further
increase in the near future due to aggregated air pollution
(3). Non-small cell lung cancer
(NSCLC) is the most common type of lung cancer, which accounts for
over 85% of cases (4). Despite
efforts made to improve treatment and prevention of NSCLC,
prognosis for patients with this disease remain poor because most
patients are diagnosed with existing distant metastasis, which is
not appropriate for radical surgical resection (5). At present, early diagnosis and
treatment are critical for the survival of patients with NSCLC.
The human genome transcribes a large set of
non-coding RNAs that are recognized as major players in both normal
physiological processes and pathological changes (6). Long non-coding (lnc)RNAs are a
subgroup of non-coding RNAs that are composed of over 200
nucleotides (7). It has been well
established that nearly all critical aspects of the onset,
development and progression of NSCLC require the involvement of
different lncRNAs (8,9). lncRNA AWPPH has been demonstrated to
serve as an oncogene in hepatocellular carcinoma and bladder cancer
(10,11); however, to the best of our
knowledge its involvement in NSCLC has not been reported. In
addition, the Wnt/β-catenin signaling pathway has pivotal roles in
tumor growth, and the modulation of Wnt/β-catenin signaling can in
some cases be achieved through the interaction with different
lncRNAs (12). In the present
study, a systematic investigation of the functionality of AWPPH in
NSCLC was carried out and the results revealed that AWPPH may
promote tumor growth of NSCLC via activation of the Wnt/β-catenin
signaling pathway.
Materials and methods
Subjects
The present study recruited 88 patients with NSCLC
who were diagnosed and treated at the Cangzhou Central Hospital
from January, 2013 to January, 2018. These patients included 56
males and 32 females, aged 23–71 years (mean age, 46±8.9 years).
Patients with other types of cancer, lung diseases and severe
diseases (such as severe infections) were excluded from the study.
Patients receiving treatment prior to admission, were also
excluded. The control group comprised 88 healthy volunteers. The
healthy volunteers included 58 males and 30 females, aged 26–70
years (mean age, 47±7.6 years). There were no significant
differences in age and gender between the patient and control
group.
The present study was approved by the Ethics
Committee of Cangzhou Central Hospital (Cangzhou, China), and all
participants provided written informed consent.
Specimen collection
Lung cancer tissue and healthy lung biopsies were
collected from patients with NSCLC and healthy controls,
respectively. Blood (~20 ml) was extracted from the elbow vein of
both patients and healthy controls. The blood was kept at room
temperature for 4 h, followed by centrifugation at 1,200 × g at
room temperature for 15 min to collect the serum. All samples were
stored in liquid nitrogen.
Cell lines and cell culture
The normal human lung tissue cell line WI-38, and
two human NSCLC cell lines NCI-H23 and NCI-H522 were purchased from
the American Type Culture Collection (ATCC; Manassas, VA, USA).
Cells were cultured in Eagle's minimal essential medium (EMEM;
ATCC) containing 10% fetal bovine serum (FBS; ATCC) according to
the manufacturer's protocol. The cells were collected during
logarithmic growth phase for subsequent experiments.
For activation of Wnt signaling, 10 ng/ml Wnt
agonist (catalog no. 853220-52-7; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) was added to the serum-free cell culture medium
and cells were incubated for 6 h at 37°C.
Construction of the AWPPH expression
vector and transfection
Full-length AWPPH cDNA was inserted into the
pIRES2-EGFP plasmid (Clontech Laboratories, Inc., Mountainview, CA,
USA) to establish the AWPPH expression vector. The three cell lines
were cultured overnight to reach 80–90% confluence, and 10 nM AWPPH
vector was transfected into 4×105 cells using
Lipofectamine® 2000 (cat. no. 11668-019; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). An empty vector
was used as the negative control. Non-transfected cells were used
as control cells. Cells were cultured in EMEM containing 10% FBS
for 48 h at 37°C prior to subsequent experimentation.
Cell proliferation assay
The three cell lines were harvested during the
logarithmic growth phase and were adjusted to a final cell density
of 4×104 cells/ml in cell culture medium. Then, 100 µl
cell suspension (4×103 cells) was transferred to each
well of a 96-well plate. Cells were cultured at 37°C and 5%
CO2. A total of 10 µl Cell Counting Kit-8 solution
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added to each
well following 24, 48, 72 and 96 h of cell culture. Then, the cells
were cultured for a further 4 h and optical desnity (OD) values at
450 nm were measured using a Fisherbrand™ accuSkan™ GO UV/Vis
microplate spectrophotometer (Thermo Fisher Scientific, Inc.).
In cases where cells were treated with a Wnt
inhibitor, 2.5 µM IWP-2 (Sigma-Aldrich; Merck KGaA) was added to
cells at the beginning of culture.
Cell apoptosis assay
Cells were adjusted to a final cell density of
4×104 cells/ml using medium containing 10 mM
tetraethylammonium (Sigma-Aldrich; Merck KGaA) to induce cell
apoptosis. Then, 10 ml cell suspension (4×105 cells) was
transferred to each well of a 6-well plate. Cells were cultured at
37°C for 24 h followed by digestion with 0.25% trypsin.
Subsequently, cells were stained with Annexin V-fluorescein
isothiocyanate (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) and propidium iodide (Sigma-Aldrich; Merck KGaA), followed
by the detection of apoptotic cells using flow cytometry. Cell
apoptosis was normalized to the control group using FCS Express 6
flow cytometry software (De Novo Software, Glendale, CA, USA).
In cases where cells were treated with a Wnt
inhibitor, 2.5 µM IWP-2 (Sigma-Aldrich; Merck KGaA) was added to
cells at the beginning of culture.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tumor tissues, healthy
lung tissues and serum samples using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.). Tumor and healthy
lung tissues were ground in liquid nitrogen prior to the addition
of TRIzol® reagent. RNA quality, reflected by the
A260/A280 ratio, was determined using a NanoDrop™ 2000
spectrophotometer (Thermo Fisher Scientific, Inc.). RNA samples
with an A260/A280 ratio between 1.8–2.0 were subjected to RT to
synthesize cDNA using SuperScript III Reverse Transcriptase kit
(Thermo Fisher Scientific, Inc.) using the following conditions:
55°C for 10 min and 75°C for 10 min. qPCR was performed using the
Applied Biosystems™ PowerUp™ SYBR™ Green Master Mix (Thermo Fisher
Scientific, Inc.). The following primers were used: AWPPH, forward,
5′-CTGGATGGTCGCTGCTTTTTA-3′ and reverse,
5′-AGGGGGATGAGTCGTGATTT-3′; β-actin, forward,
5′-GACCTCTATGCCAACACAGT-3′ and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′.
The thermocycling conditions were as follows: 95°C for 45 sec,
followed by 40 cycles of 95°C for 12 sec and 60°C for 40 sec.
Relative expression levels of AWPPH were normalized to endogenous
control β-actin using the 2−ΔΔCq method (13).
Western blotting
Total protein was extracted from cells using
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc.) and quantified using the bicinchoninic acid assay method.
Subsequently, 20 µg protein per lane was separated by SDS-PAGE
using 10% gels. Proteins were transferred onto polyvinylidene
difluoride membranes and blocked with 5% skimmed milk for 1 h at
room temperature. The membranes were then incubated with rabbit
anti-β-catenin (1:2,000; cat. no. ab32572; Abcam, Cambridge, UK)
and mouse anti-GAPDH (1:1,000; cat. no. ab8245; Abcam) primary
antibodies overnight at 4°C. The membranes were further incubated
with goat anti-rabbit immunoglobulin G-horseradish peroxidase
secondary antibody (1:1,000; MBS435036; MyBioSource, Inc., San
Diego, CA, USA) at room temperature for 4 h. Finally, ECL™ Blotting
Reagents GE Healthcare (Sigma-Aldrich; Merck KGaA) was added to
visualize the proteins and membranes were scanned by a myECL™
imager (Thermo Fisher Scientific, Inc.). Relative protein
expression levels of β-catenin were normalized to the endogenous
control GAPDH using ImageJ software version 1.6 (National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Experiments were performed in triplicate. SPSS
version 19.0 (IBM Corp., Armonk, NY, USA) was used for all
statistical analyses. The χ2 test was used to analyze
countable data. Measurement data are presented as the mean ±
standard deviation, and comparisons between two groups and multiple
groups were performed using the unpaired Student's t-test and
one-way analysis of variance followed by least significant
difference post hoc test, respectively. Receiver operating
characteristic (ROC) curve analysis was performed to evaluate the
diagnostic value of lncRNA AWPPH expression. Survival curves were
plotted using the Kaplan-Meier method and compared by log rank
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of lncRNA AWPPH in patients
with NSCLC and healthy controls
Expression levels of lncRNA AWPPH in the lung
tissues and serum samples of patients with NSCLC and healthy
controls were detected by RT-qPCR. As shown in Fig. 1, the expression levels of lncRNA
AWPPH in the lung tissues (Fig.
1A) and serum samples (Fig.
1B) of patients with NSCLC were significantly higher compared
with those in healthy controls. These data suggested that
upregulation of AWPPH may be involved in the pathogenesis of
NSCLC.
Diagnostic value of lncRNA AWPPH for
NSCLC
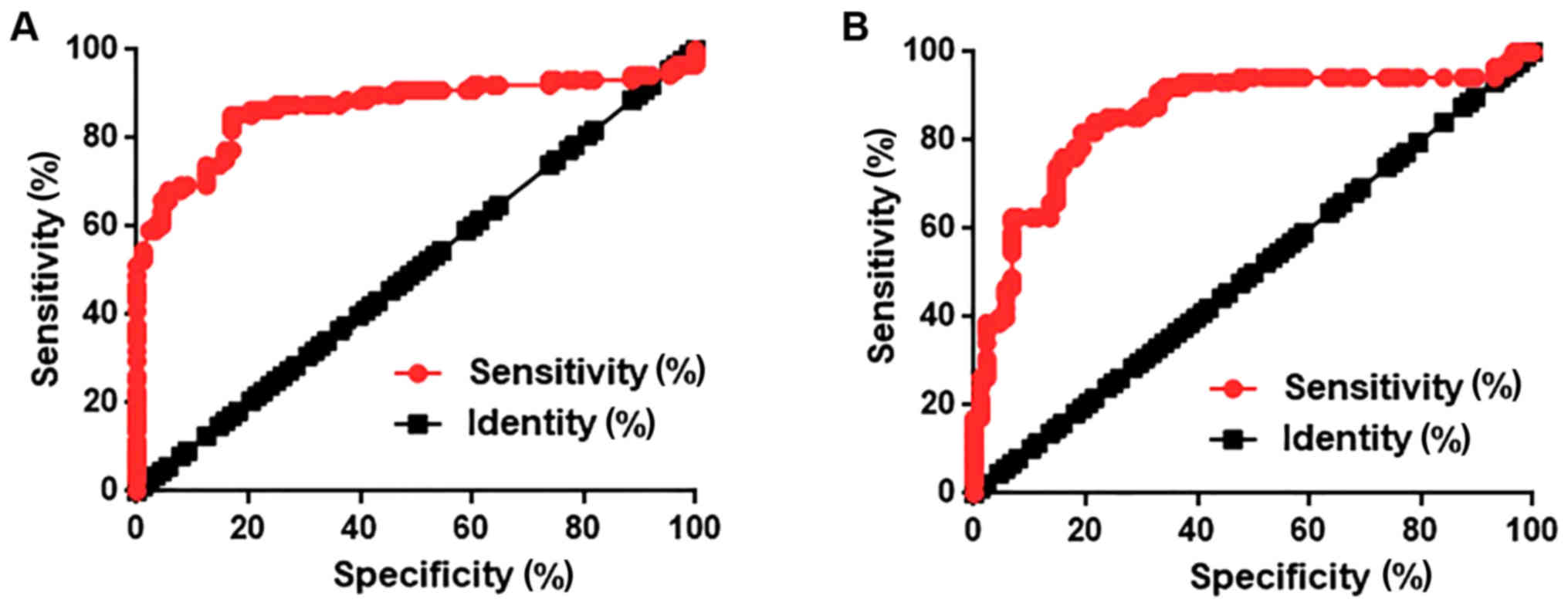
ROC curve analysis was performed to evaluate the
diagnostic value of lncRNA AWPPH expression in lung tissues and
serum for NSCLC. As shown in Fig.
2A, AWPPH expression levels in lung tissues could distinguish
patients with NSCLC from healthy controls with an area under the
curve (AUC) of 0.8686 and 95% confidence interval (CI) of
0.8102–0.9271 (P<0.0001). AWPPH expression levels in serum could
distinguish patients with NSCLC from healthy control with an AUC of
0.8569 and 95% CI of 0.7983–0.9156 (P<0.0001; Fig. 2B). These data suggested that lncRNA
AWPPH serves as a potential diagnostic marker for NSCLC.
Prognostic value of lncRNA AWPPH for
NSCLC
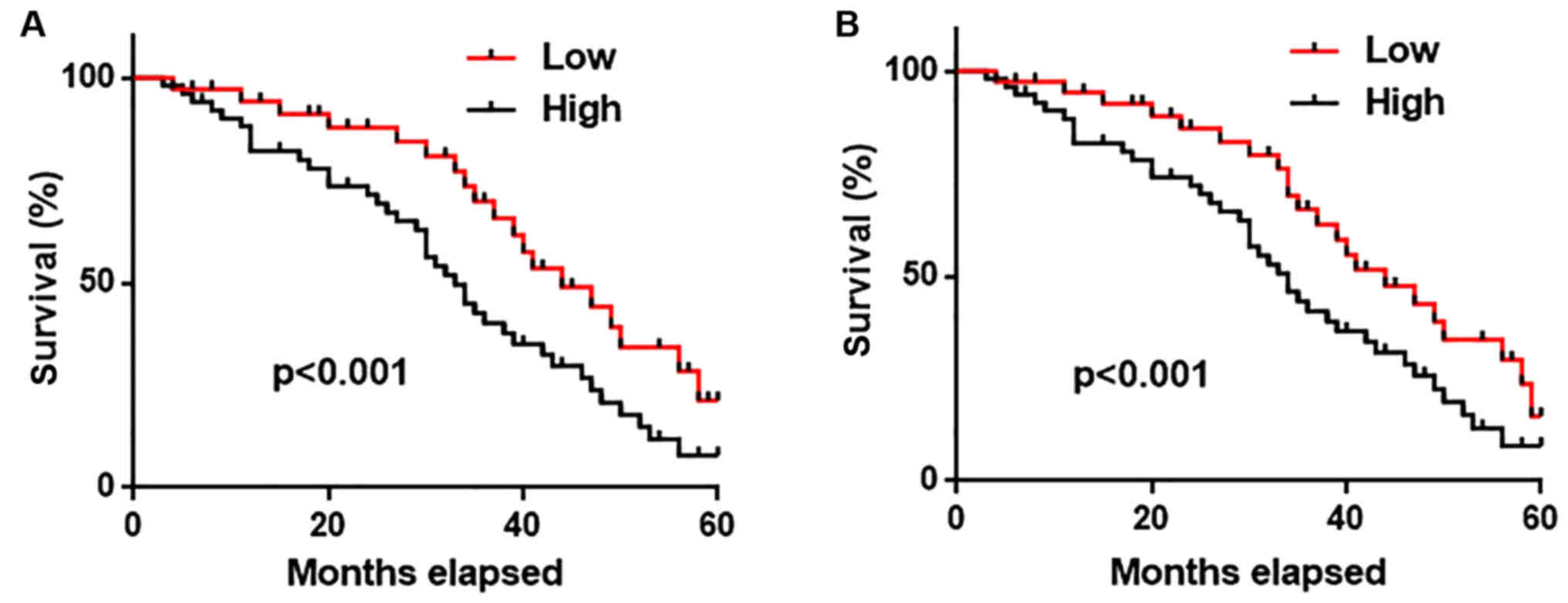
All the patients were followed-up for 5 years to
record their survival information. Patients were divided into high
(n=40) and low (n=40) expression groups, according to the median
expression level of AWPPH in lung tissue and serum. As shown in
Fig. 3, the overall survival rate
of patients with high expression levels of AWPPH in both lung
tissues (Fig. 3A) and serum
(Fig. 3B) was significantly lower
(P<0.001) compared with patients with low expression levels of
AWPPH. These data suggested that lung tissue and serum AWPPH may
serve as a biomarker for the prognosis of NSCLC.
Associations between expression levels
of lncRNA AWPPH in lung tissue and serum, and clinicopathological
data
According to the median expression level of lncRNA
AWPPH in lung tissue and serum, patients were divided into high and
low expression groups. Associations between expression levels of
lncRNA AWPPH in lung tissue and serum, and clinicopathological data
of patients were analyzed by χ2 test. As shown in
Tables I and II, there were no significant
associations between the expression levels of lncRNA AWPPH in lung
tissue and serum with the patients' sex, age, drinking habit as
well as distant tumor metastasis. However, the expression levels of
lncRNA AWPPH in lung tissue and serum showed significant
association with tumor size and smoking habit. Therefore, altered
expression of lncRNA AWPPH may be induced by lung cancer and
smoking.
 | Table I.Association between expression levels
of AWPPH in lung cancer tissue and clinicopathological data of
patients with non-small cell lung cancer. |
Table I.
Association between expression levels
of AWPPH in lung cancer tissue and clinicopathological data of
patients with non-small cell lung cancer.
| Variables | No. of patients | High-expression | Low-expression | χ2 | P-value |
|---|
| Sex |
|
|
| 0.79 | 0.382 |
| Male | 56 | 26 | 30 |
|
|
|
Female | 32 | 18 | 14 |
|
|
| Age |
|
|
| 0.73 | 0.391 |
| ≥45
years | 42 | 19 | 23 |
|
|
| <45
years | 46 | 25 | 21 |
|
|
| Primary tumor
diameter |
|
|
| 19.45 | <0.001 |
| >7
cm | 30 | 22 | 8 |
|
|
| 3–7
cm | 32 | 18 | 14 |
|
|
| <3
cm | 26 | 4 | 22 |
|
|
| Distant tumor
metastasis |
|
|
| 0.73 | 0.393 |
| Yes | 40 | 18 | 22 |
|
|
| No | 48 | 26 | 22 |
|
|
| Smoking |
|
|
| 5.86 | 0.024 |
| Yes | 55 | 33 | 22 |
|
|
| No | 33 | 11 | 22 |
|
|
| Drinking |
|
|
| 0.84 | 0.363 |
| Yes | 60 | 32 | 28 |
|
|
| No | 28 | 12 | 16 |
|
|
 | Table II.Association between serum levels of
AWPPH and clinicopathological data of patients with non-small cell
lung cancer. |
Table II.
Association between serum levels of
AWPPH and clinicopathological data of patients with non-small cell
lung cancer.
| Variables | No. of patients | High-expression | Low-expression | χ2 | P-value |
|---|
| Sex |
|
|
| 0.2 | 0.663 |
|
Male | 56 | 27 | 29 |
|
|
|
Female | 32 | 17 | 15 |
|
|
| Age |
|
|
| 0.73 | 0.390 |
| ≥45
years | 42 | 19 | 23 |
|
|
| <45
years | 46 | 25 | 21 |
|
|
| Primary tumor
diameter |
|
|
| 12.45 | 0.098 |
| >7
cm | 30 | 21 | 9 |
|
| 3–7
cm | 32 | 17 | 15 |
|
|
| <3
cm | 26 | 6 | 20 |
|
|
| Distant tumor
metastasis |
|
|
| 0.73 | 0.392 |
|
Yes | 40 | 18 | 22 |
|
|
| No | 48 | 26 | 22 |
|
|
| Smoking |
|
|
| 8.19 | 0.004 |
|
Yes | 55 | 34 | 21 |
|
| No | 33 | 10 | 23 |
|
|
| Drinking |
|
|
| 0.84 | 0.362 |
|
Yes | 60 | 32 | 28 |
|
|
| No | 28 | 12 | 16 |
|
|
Interaction between AWPPH and the
Wnt/β-catenin signaling pathway
The results in the present study indicated that
AWPPH may be involved in lung tumor growth. It is known that the
Wnt/β-catenin signaling pathway has a pivotal role in tumor growth
(12). Therefore, normal human
lung tissue cell line WI-38, and two human NSCLC cell lines NCI-H23
and NCI-H522 were employed to explore potential interactions
between AWPPH and the Wnt/β-catenin signaling pathway in
vitro. RT-qPCR revealed that AWPPH expression levels were
significantly higher in NCI-H23 and NCI-H522 cells compared with in
WI-38 cells (P<0.05; Fig. 4A).
Subsequently, the cells were transfected with the AWPPH expression
vector. As shown in Fig. 4B, AWPPH
overexpression was successfully achieved in all three cell lines.
AWPPH overexpression significantly upregulated the protein
expression levels of β-catenin in NCI-H23 and NCI-H522 cells
(P<0.05), but not in WI-38 cells (P>0.05; Fig. 4C). A Wnt agonist was used to
investigate the effects of the activation of Wnt/β-catenin
signaling pathway on AWPPH. However, treatment of cells with the
Wnt agonist demonstrated no significant effect on AWPPH expression
in all three cell lines (P>0.05; Fig. 4D). Therefore, AWPPH is likely an
upstream activator of Wnt/β-catenin signaling pathway.
Effect of AWPPH overexpression and Wnt
inhibition on cell proliferation and apoptosis
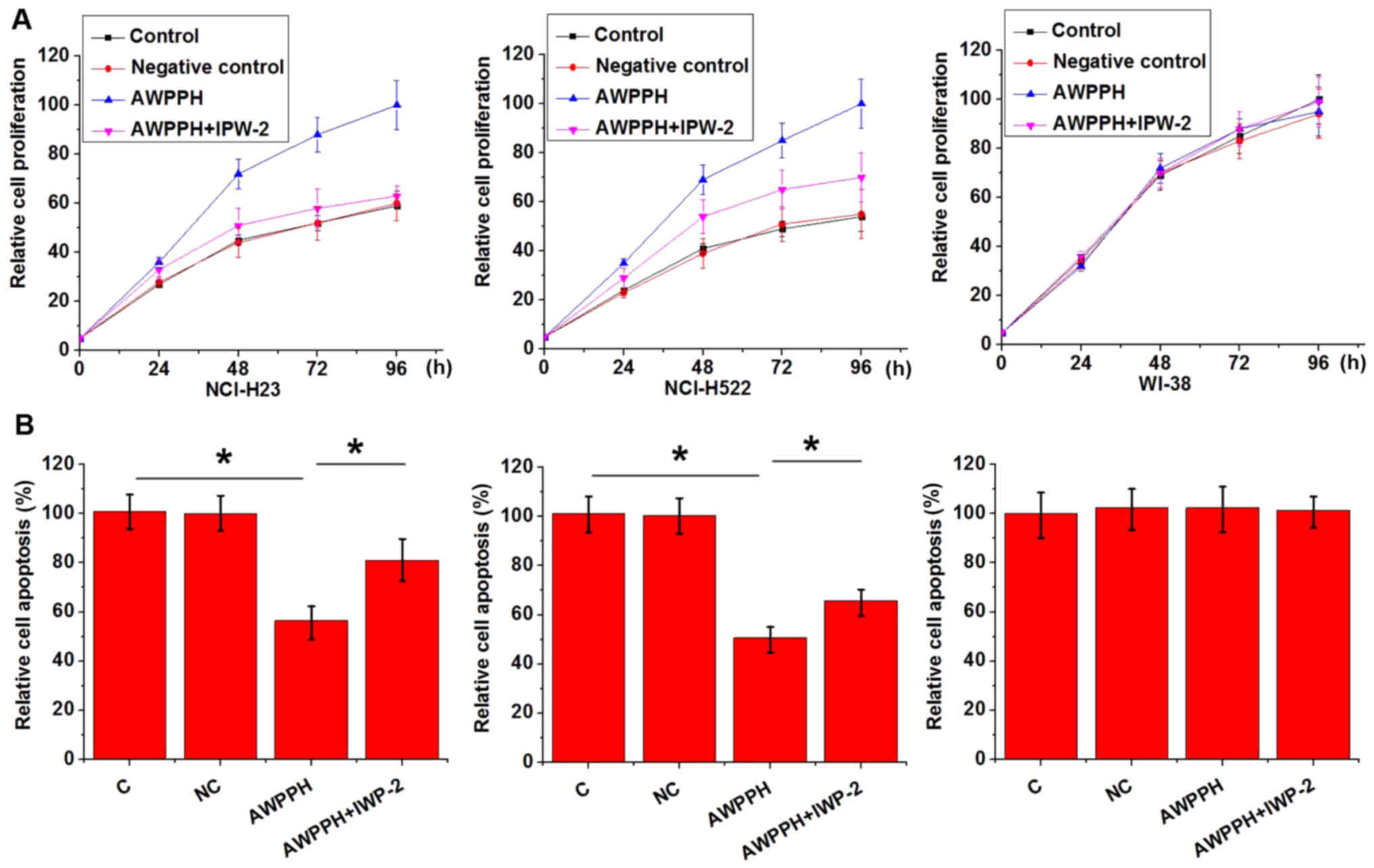
AWPPH expression vectors were transfected into
WI-38, NCI-H23 and NCI-H522 cells. As shown in Fig. 5, AWPPH overexpression promoted
proliferation (Fig. 5A), but
significantly inhibited apoptosis (Fig. 5B) of NCI-H23 and NCI-H522 cells.
Overexpression of AWPPH in WI-38 cells did not affect cell
proliferation or apoptosis. In addition, treatment with the Wnt
inhibitor IWP-2 reduced the effects caused by AWPPH overexpression
on cell proliferation and apoptosis. These data suggested that
AWPPH may promote proliferation and inhibit apoptosis of NSCLC
cells by activating the Wnt/β-catenin signaling pathway.
Discussion
lncRNA AWPPH has been demonstrated to be involved in
several types of cancer (10,11).
Zhao et al previously reported that AWPPH is highly
expressed in hepatocellular carcinoma tissue, and high expression
levels of AWPPH are closely correlated with advanced TNM stage,
microvascular invasion, encapsulation incomplete and Barcelona
Clinic Liver Cancer stage (10).
In another study, AWPPH was reported to be overexpressed in bladder
cancer, indicating its role as an oncogene in the disease (12). To the best of our knowledge, the
expression patterns of AWPPH in other diseases have not been
explored. In the present study, the expression levels of lncRNA
AWPPH in lung tissues and serum of patients with NSCLC and healthy
controls were detected. The results showed that lncRNA AWPPH
expression was upregulated in patients with NSCLC compared with in
healthy controls in both lung tissues and serum. These data
suggested that AWPPH may have an oncogenic role in NSCLC.
Early diagnosis and accurate prognosis is critical
for the survival of patients with NSCLC. Early diagnosis and
prediction of prognosis of diseases usually requires highly
sensitive markers. Development of human disease is usually
accompanied by changes in blood biomarkers. Therefore, monitoring
the levels of these markers in blood may provide guidance for the
treatment of diseases (14). In
the present study, ROC curve analysis revealed that expression
levels of AWPPH in lung tissues and serum could be used to
effectively distinguish NSCLC patients from healthy controls. In
addition, high expression levels of AWPPH in lung tissues and serum
were closely associated with poor survival after discharge. The
results suggested that AWPPH may serve as a sensitive diagnostic
and prognostic biomarker for NSCLC. Compared with an invasive lung
biopsy, a blood test is a non-invasive method that would be
preferred in the clinic. It is known that the expression of certain
lncRNAs can be affected by factors, including aging (15), smoking (16) and alcohol consumption (17). In the present study, no significant
associations were found between AWPPH expression levels and the
patients' sex, age and drinking habit. However, AWPPH expression
levels in lung tissues and serum were significantly associated with
the patients' smoking habit. Therefore, smoking should be
considered alongside other biomarkers in the diagnosis and
prognosis of NSCLC using AWPPH.
The present study also demonstrated that expression
levels of AWPPH in lung tissues and serum were significantly
associated with tumor size, but not tumor metastasis, indicating
the involvement of AWPPH in tumor growth. The Wnt/β-catenin
signaling pathway serves a pivotal role in the growth of different
types of cancer, including NSCLC (10,18),
and inhibition of the Wnt/β-catenin signaling pathway is considered
to be a therapeutic target for the inhibition of tumor growth
(19). It is known that the
Wnt/β-catenin signaling pathway can interact with lncRNAs to carry
out its biological function (20,21).
In the present study, transfection with the AWPPH expression vector
significantly upregulated the expression of β-catenin in two NSCLC
cell lines, while treatment with the Wnt agonist (or the activation
of Wnt signaling) did not produce a significant effect on AWPPH
expression. In addition, the Wnt inhibitor significantly reversed
the enhancing effects of AWPPH overexpression on cell proliferation
and its inhibitory effects on cell apoptosis. These data suggested
that AWPPH may promote the growth of NSCLC by serving as an
upstream activator of the Wnt/β-catenin signaling pathway. Notably,
AWPPH overexpression, and treatment with the Wnt inhibitor and
activator did not significantly effect the proliferation and
apoptosis of the normal human lung tissue cell line WI-38.
Therefore, AWPPH may be a potential target for the treatment of
NSCLC.
In conclusion, AWPPH was overexpressed in patients
with NSCLC. AWPPH expression may have diagnostic and prognostic
value for NSCLC. AWPPH may participate in the progression of NSCLC
by promoting proliferation and inhibiting apoptosis of NSCLC cells
through activation of the Wnt/β-catenin signaling pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZS and BS designed the experiments. ZS, JD and LZ
performed experiments. BS drafted the manuscript. ZS, JD and LZ
received and reviewed the manuscript. All authors approved the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Cangzhou Central Hospital (Cangzhou, China), and all
participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Martin EG, Kelly C and Richard BL: Genomic
profiling of advanced non-small cell lung cancer in community
settings: Gaps and opportunities. Clin Lung Cancer. 18:651–659.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sabari JK, Montecalvo J, Chen R, Dienstag
JA, Mrad C, Bergagnini I, Victoria Lai WC, Arbour KC, Shu CA,
Hellmann MD, et al: PD-L1 expression and response to immunotherapy
in patients with MET exon 14-altered non-small cell lung cancers
(NSCLC). J Clin Oncol. 35:8512. 2017. View Article : Google Scholar
|
|
3
|
Chen W, Zheng R, Zeng H, Zhang S and He J:
Annual report on status of cancer in China, 2011. Chin J Cancer
Res. 27:2–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nawaz K and Webster RM: The non-small-cell
lung cancer drug market. Nat Rev Drug Discov. 15:229–231. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ettinger DS, Akerley W, Borghaei H, Chang
AC, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Ganti AK,
Govindan R, et al: Non-small cell lung cancer. J Natl Compr Canc
Netw. 10:1236–1271. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mattick JS and Makunin IV: Non-coding RNA.
Hum Mol Genet. 15:17–29. 2006. View Article : Google Scholar
|
|
7
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Loewen G, Jayawickramarajah J, Zhuo Y and
Shan B: Functions of lncRNA HOTAIR in lung cancer. J Hematol Oncol.
7:902014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu X, Bao J, Wang Z, Zhang Z, Gu P, Tao F,
Cui D and Jiang W: The plasma lncRNA acting as fingerprint in
non-small-cell lung cancer. Tumor Biol. 37:3497–3504. 2016.
View Article : Google Scholar
|
|
10
|
Zhao X, Liu Y and Yu S: Long noncoding RNA
AWPPH promotes hepatocellular carcinoma progression through YBX1
and serves as a prognostic biomarker. Biochim Biophys Acta Mol
Basis Dis. 1863:1805–1816. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu F, Zhang X, Yu Q, Han G, Diao F, Wu C
and Zhang Y: LncRNA AWPPH inhibits SMAD4 via EZH2 to regulate
bladder cancer progression. J Cell Biochem. 119:4496–4505. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Postmus PE, Kerr KM, Oudkerk M, Senan S,
Waller DA, Vansteenkiste J, Escriu C and Peters S; ESMO Guidelies
Committee, : Early and locally advanced non-small-cell lung cancer
(NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 28 (Suppl_4):iv1–iv21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
O'brien PJ, Slaughter MR, Polley SR and
Kramer K: Advantages of glutamate dehydrogenase as a blood
biomarker of acute hepatic injury in rats. Lab Anim. 36:313–321.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Devaux Y, Zangrando J, Schroen B, Creemers
EE, Pedrazzini T, Chang CP, Dorn GW II, Thum T and Heymans S;
Cardiolinc network, : Long noncoding RNAs in cardiac development
and ageing. Nat Rev Cardiol. 12:415–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu L, Xu H, Luo F, Liu X, Lu X, Yang Q,
Xue J, Chen C, Shi L and Liu Q: Epigenetic silencing of miR-218 by
the lncRNA CCAT1, acting via BMI1, promotes an altered cell cycle
transition in the malignant transformation of HBE cells induced by
cigarette smoke extract. Toxicol Appl Pharmacol. 304:30–41. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mayfield RD: Emerging roles for ncRNAs in
alcohol use disorders. Alcohol. 60:31–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Uematsu K, He B, You L, Xu Z, McCormick F
and Jablons DM: Activation of the Wnt pathway in non-small cell
lung cancer: Evidence of disheveled overexpression. Oncogene.
22:7218–7221. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He BC, Gao JL, Zhang BQ, Luo Q, Shi Q, Kim
SH, Huang E, Gao Y, Yang K, Wagner ER, et al: Tetrandrine inhibits
Wnt/β-catenin signaling and suppresses tumor growth of human
colorectal cancer. Mol Pharmacol. 79:211–219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma Y, Yang Y, Wang F, Moyer MP, Wei Q,
Zhang P, Yang Z, Liu W, Zhang H, Chen N, et al: Long non-coding RNA
CCAL regulates colorectal cancer progression by activating
Wnt/β-catenin signalling pathway via suppression of activator
protein 2α. Gut. 65:1494–1504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Z, Zhao L and Wang Q: Overexpression of
long non-coding RNA HOTTIP increases chemoresistance of
osteosarcoma cell by activating the Wnt/β-catenin pathway. Am J
Transl Res. 8:2385–2393. 2016.PubMed/NCBI
|