Introduction
Adipose tissue has been recognized as a vital organ
within the endocrine system, as it produces a wide range of
hormones and cytokines, termed adipokines, which act in an
autocrine or paracrine manner to regulate metabolic functions,
inflammation, endothelial homeostasis and other processes (1). It is well established that obesity,
particularly the increased prevalence of excessive visceral
obesity, is closely associated with the rising incidence of
cardiovascular diseases and type 2 diabetes (2,3). One
of the potential causes for the increasing risk is that adipose
tissue dysfunction contributes directly and indirectly to vascular
disease through the dysregulation of endothelial homeostasis
(4). Recently, in an investigation
of the interplay between adipose tissue and vascular function,
attention was drawn to the regulation of endothelial homeostasis by
perivascular adipose tissue (PVAT) (5). PVAT directly surrounds all blood
vessels, thus factors secreted by adipocytes can easily target the
vasculature. Although PVAT has characteristics similar to brown
adipose tissue due to the generation of heat in the body (6), PVAT expands in obesity and diabetes,
and is particularly susceptible to inflammation and loss of brown
adipose tissue-like characteristics (7,8).
PVAT is different from subcutaneous adipose tissue and other
visceral adipose tissue, and is therefore considered to be a
distinct adipose tissue store, particularly regarding the
regulation of vessel function (9).
Exosomes are nanovesicles, released into the
extracellular space by various cell types, including adipose tissue
(10). A previous study reported
that exosomes contain substantial mRNAs, microRNAs and noncoding
RNAs (11), which serve vital
roles in regulating endothelial function, including migration,
proliferation, apoptosis and angiogenesis (12–14).
Furthermore, exosomes have an important role in paracrine
processes, such as the crosstalk between endothelial cells and
inflammatory cells (15). However,
whether exosomes from PVAT regulate endothelial function has not
been established to date.
Mangiferin is a xanthone glucoside present in
Anemarrhena asphodeloides Bunge that is widely used in
Chinese traditional medicine for the treatment of diabetes.
Mangiferin exerts anti-inflammatory and anti-oxidant effects
(16,17), and these actions have been reported
to be useful in the management of metabolic disorders (18,19).
In the present study, exosomes released from PVAT
were collected and used to stimulate endothelial cells in order to
investigate the functional link between the endothelium and PVAT.
The findings demonstrated that mangiferin regulated exosome release
from PVAT and ameliorated endothelial dysfunction, involving
nuclear factor-κB (NF-κB). The current study increases the
understanding on the effects of mangiferin in regulating adipose
and endothelial function, which may be beneficial for its potential
applications in the management of cardiovascular disorders
associated with obesity.
Materials and methods
Animals
A total of 40 male Sprague-Dawley rats (8 weeks old,
180–200 g) were obtained from the Laboratory Animal Center of
Nanjing Medical University (Nanjing, China). Animals were housed at
a constant temperature (22±1°C) in a 12:12-h light-dark cycle, with
ad libitum access to a standard diet and water. The rats
were cared for and treated in accordance with the Provisions and
General Recommendation of Chinese Experimental Animals
Administration Legislation. The present study received ethical
approval from the Animal Ethics Committee of Nanjing Medical
University.
Drugs and reagents
Mangiferin was purchased from Nanjing Zelang Medical
Technological Co., Ltd. (Nanjing, China) and palmitic acid (PA) was
from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). The
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
apoptosis detection kit (cat. no. KGA108) was obtained from Nanjing
KeyGen Biotech Co., Ltd. (Nanjing, China). Matrigel was purchased
from Corning, Inc. (Corning, NY, USA). Antibodies against
phosphorylated (p)-NF-κB p65 (Ser536; 1:1,000; cat. no. 3033),
NF-κB p65 (1:1,000; cat. no. 8242), NF-κB p50 (1:1,000; cat. no.
32360), p-NF-κB p50 (1:1,000; cat. no. 28849), cluster of
differentiation (CD)9 (1:1,000; cat. no. 9276), CD63 (1:1,000; cat.
no. 59479) and β-tubulin (1:1,000; cat. no. 2146) were obtained
from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Endothelial cell isolation and
culture
Rat arterial endothelial cells were isolated
according to a standard protocol as previously described (20). Briefly, the rats were anesthetized
with diethyl ether by inhalation prior to conducting the
experiments. After the rats were sacrificed by decapitation
following anesthesia, the arteries were excised and immersed in 75%
ethanol for 30 sec for disinfection. Next, the arteries were
digested with 0.25 collagenase type II for 10 min and 0.25% trypsin
for another 10 min at 37°C. Cells were then centrifuged at 300 × g
for 6 min at 4°C, resuspended in endothelial cell growth basal
medium-2 (EBM-2; cat. no. cc-4176; Lonza Group, Ltd., Basel,
Switzerland) with 10% fetal bovine serum (FBS; cat. no. 10100154,
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and plated
on laminin-coated dishes. Arterial endothelial cells at the third
passage were used for further analyses.
Preparation of PVAT-derived
exosomes
Rats were anesthetized with diethyl ether and
sacrificed by decapitation. PVAT samples were obtained from the rat
thoracic aorta, cut into small pieces, weighed to an equal size (50
mg) and rinsed using PBS. PVAT samples were cultured in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% exosome-free
FBS (cat. no. EXO-FBS500A-1; System Biosciences, Palo Alto, CA,
USA). According to our previous study (21), the PVAT samples were cultured with
PA (100 µM) in the presence or absence of mangiferin (0.1, 1 or 10
µM) for 2 h; samples cultured without PA or mangiferin were used as
the control group. Subsequent to washing with PBS, PVAT samples
were incubated in DMEM for a further 22 h. The supernatant was then
collected as the conditioned medium (CM), and larger particles were
removed by centrifugation at 10,000 × g for 5 min at 4°C and
filtration through a 0.22-µm filter. Exosomes in the PVAT CM were
separated using total exosome isolation reagent (cat. no. 4478359;
Invitrogen; Thermo Fisher Scientific, Inc.), and were observed by
transmission electron microscopy (JEM-2000EX; JEOL, Ltd., Tokyo,
Japan). Finally, exosomes derived from control, PA and PA +
mangiferin groups were added to endothelial cells.
Immunofluorescence staining
Endothelial cells were seeded in 24-well plates, and
then the cells were fixed with 4% paraformaldehyde for 30 min at
4°C. Triton X-100 (0.1%) was used to permeabilize endothelial cells
prior to washing with PBS for three times. Endothelial cells were
then blocked with 5% bovine serum albumin (BSA; Sangon Biotech Co.,
Ltd., Shanghai, China) for 20 min at room temperature. Cells were
incubated overnight at 4°C with the primary antibodies (anti-CD63,
anti-p65 and anti-p50) and then incubated at room temperature for 1
h with the secondary antibodies (Alexa Fluor®
594-conjugated donkey anti-rabbit secondary antibody; 1:100; cat.
no. 1869587, Invitrogen; Thermo Fisher Scientific, Inc.). The cells
were washed with PBS and the nuclei were stained with DAPI (~20
µg/ml; Thermo Fisher Scientific, Inc.) for 15 min at room
temperature.
Preparation of thoracic aorta and
assessment of endothelium-dependent relaxation
Rats were anesthetized with diethyl ether and
sacrificed by decapitation. The thoracic aorta was stripped,
immediately placed in 4°C Krebs-Henseleit (K-H) solution and
incubated with 95% O2 and 5% CO2. Following
careful removal of adherent tissues, four to eight samples of 3-mm
long rings were cut. Subsequently, the aortic samples were
suspended in an organ bath (SV-4; Chengdu Taimeng Software Co.,
Ltd., Chengdu, China) containing 10 ml K-H solution, and were
maintained at 37°C and pH 7.4, and continuously aerated with 95%
O2 and 5% CO2. The aortic segments were
exposed to 60 mM KCl to assess their functions following
progressive stretching to a basal tension of 2.0 g, and then
stabilized for ≥90 min. Exposure to 10 µM acetylcholine
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used to relax
the segment, which was pre-contracted with 1 µM phenylephrine
(Sigma-Aldrich; Merck KGaA). The integrity of the vascular
endothelium was confirmed, while the diastolic rate reached
>80%. The thoracic aorta was cultured with CM for 15 min in the
organ bath, and the tone change was recorded and expressed in terms
of tension.
Apoptosis analysis
Endothelial cells were incubated with exosomes
obtained from the different treatment groups for 48 h. Cells were
subsequently collected using 0.25% trypsin without EDTA. Following
three washes in PBS, cells were incubated with 500 µl binding
buffer containing 5 µl Annexin V/FITC and 5 µl PI (Nanjing KeyGen
Biotech Co., Ltd.) for 30 min in the dark. Subsequently, apoptosis
was measured using a BD FACSCalibur flow cytometer and data were
analyzed using BD CellQuest™ (both from BD Biosciences, Franklin,
Lakes, NJ, USA).
Transwell assay
Endothelial cells were incubated in EBM-2 at 37°C
for 48 with exosomes (10 µg/ml) obtained from the different PVAT
treatment groups. Cells were subsequently collected using 0.25%
trypsin for 1 min at 37°C without EDTA, seeded in the upper
chambers 6-well Transwell inserts with an 8-µm pore size at a
density of 2×104 cells/well in normal EBM-2, and
incubated at 37°C for 24 h; normal EBM-2 with 10% FBS was added to
the lower chambers. Non-migrated cells were gently removed with
cotton swabs from the upper chamber, whereas cells that had
migrated to the lower chamber were fixed using 4% paraformaldehyde
at 4°C for 24 h and stained with 0.1% crystal violet for 30 min at
room temperature. The filters were washed with distilled water, and
images were obtained using an inverted microscope (magnification,
×100; BX41; Olympus Corporation, Tokyo, Japan).
Tube formation assay
Matrigel was thawed at 4°C for 24 h and then
polymerized at 37°C for 1 h. Following incubation of endothelial
cells with exosomes derived from different PVAT groups for 48 h as
aforementioned, cells were collected using 0.25% trypsin and
resuspended to obtain a concentration of 2×105 cells/ml.
Cells were then seeded on Matrigel-coated dishes for 8 h. Tube
formation was examined using a microscope (magnification, ×200;
IX51; Olympus Corporation).
ELISA
Endothelial cells were incubated with exosomes from
different PVAT treatment groups for 48 h as aforementioned, and the
supernatants were subsequently collected. Then, the supernatants
were concentrated with ultrafiltration tubes (Amicon Ultra-3K;
Merck KGaA) by centrifugation at 3,000 × g for 30 min at 4°C. The
concentrations of interleukin-6 (IL-6; cat. no. PR6000B) and tumor
necrosis factor-α (TNF-α; cat. no. PRTA00) were then determined
using ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Endothelial cells were incubated with exosomes
obtained from PVAT treated with PA and mangiferin for 24 h as
aforementioned. Total RNA was extracted using TRIzol®
reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The extracted RNA was dissolved in diethyl
pyrocarbonate (DEPC)-treated water, and the RNA concentration was
determined by optical density measurement at 260 nm using a
spectrophotometer. RT was subsequently performed on RNA samples (2
µg) using the PrimeScript™ RT reagent (Takara Bio, Inc., Otsu,
Japan), following the manufacturer's protocol. cDNA (1 µl) mixed
with Ssofast™ EvaGreen® Supermix (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), DEPC-treated water, forward primer and
reverse primer (Sangon Biotech Co., Ltd.) to obtain a 20-µl
reaction mixture was used for qPCR (22). qPCR was conducted as follows: 95°C
for 30 sec, then 40 cycles of 95°C for 5 sec and 60°C for 30 sec.
The primers used in qPCR are presented in Table I. Expression was quantified using
the 2−ΔΔCq method and normalized to the endogenous
control β-actin (23).
 | Table I.Primers of TNF-α, IL-6, ARG1 and
β-actin. |
Table I.
Primers of TNF-α, IL-6, ARG1 and
β-actin.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| TNF-α |
GAGTAAGGGGATGCAGCTAAGA |
CAGTTTCAGGGCAAGAAGTACC |
| IL-6 |
TCTTGGGACTGATGTTGTTGAC |
GGGTGGTATCCTCTGTGAAGTC |
| ARG1 |
ATCATGGAAGTGAACCCACTC |
TCCAAAACAAGACAAGGTCAC |
| β-actin |
GACGTTGACATCCGTAAAGACC |
TGCTAGGAGCCAGGGCAGTA |
Detection of intracellular nitric
oxide (NO)
Cells were cultured in 6-well plates and treated
with exosomes from the various groups as aforementioned upon
reaching 70% confluence. The secretion of NO was determined 48 h
later using an NO test kit (cat. no. A013-2, Nanjing Jiancheng
Bioengineering Institute Co., Ltd., Nanjing, China) according to
the manufacturer's protocol.
Western blot analysis
Briefly, the total protein was extracted from the
samples using radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology, Shanghai, China). Protein
concentrations were quantified using a Bicinchoninic Acid Protein
Assay kit (Biosky Biotechnology, Nanjing, China) according to the
manufacturer's protocol. Next, protein samples (30 µg/lane) were
separated by SDS-PAGE on 10% gels and transferred to polyvinylidene
difluoride membranes (0.45 µm; EMD Millipore, Billerica, MA, USA).
Membranes were blocked for 2 h at room temperature with 1–5% BSA
(Sangon Biotech Co., Ltd.) in TBS-Tween-20 [containing 5 mmol/l
Tris-HCl (pH 7.6), 136 mmol/l NaCl and 0.05% Tween-20] and then
incubated with the aforementioned antibodies against p65, p-p65,
p50, p-p50, CD9, CD63 and tubulin overnight at 4°C. Subsequently,
the membranes were incubated with secondary antibody [horseradish
peroxidase-conjugated anti-rabbit immunoglobulin G (IgG; cat. no.
A0208) or anti-mouse IgG (cat. no. A0216); 1:5,000; Beyotime
Institute of Biotechnology] at 37°C for 2 h. Signals were detected
using enhanced chemiluminescence western detection reagents (Thermo
Fisher Scientific, Inc.) and quantified using Image-Lab version
5.2.1 software (Bio-Rad Laboratories, Inc.).
Statistical analysis
All values are presented as the mean ± standard
deviation. The statistical significance between mean values was
evaluated using one-way analysis of variance, followed by Tukey's
test. Statistical analyses were performed using SPSS software,
version 23.0 (IBM Corp., Armonk, NY, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Mangiferin promotes the release of
exosomes from PVAT
Initially, the effect of mangiferin on the release
of exosomes was determined. As demonstrated in Fig. 1A, a large number of exosomes were
present in the CM supernatant. Exosomes were extracted and
concentrated from PVAT CM following stimulation with PA and
mangiferin (0.1, 1 and 10 µM), or control. PA evidently inhibited
the release of exosomes from PVAT compared with the control group.
However, the number of PVAT-released exosomes was markedly
increased by mangiferin under PA-induced inflammation conditions in
a dose-dependent manner. Subsequently, exosomes were extracted from
CM using total exosome isolation reagents. A total of 10 µg
exosomes was selected in all groups to ensure that the same
concentration of exosomes was added to the endothelial cell
culture. As displayed in Fig. 1B,
the results revealed that the expression of CD9 and CD63 proteins,
which are specific markers of exosomes, was similar in all groups.
The uptake of exosomes by endothelial cells was similar when CM
that was obtained from PVAT stimulated with mangiferin and PA was
used, indicating that the intake of exosomes by endothelial cells
was not influenced by the pre-treatment with mangiferin or PA.
Immunofluorescence further demonstrated the uptake of PVAT-derived
exosomes by endothelial cells (Fig.
1C).
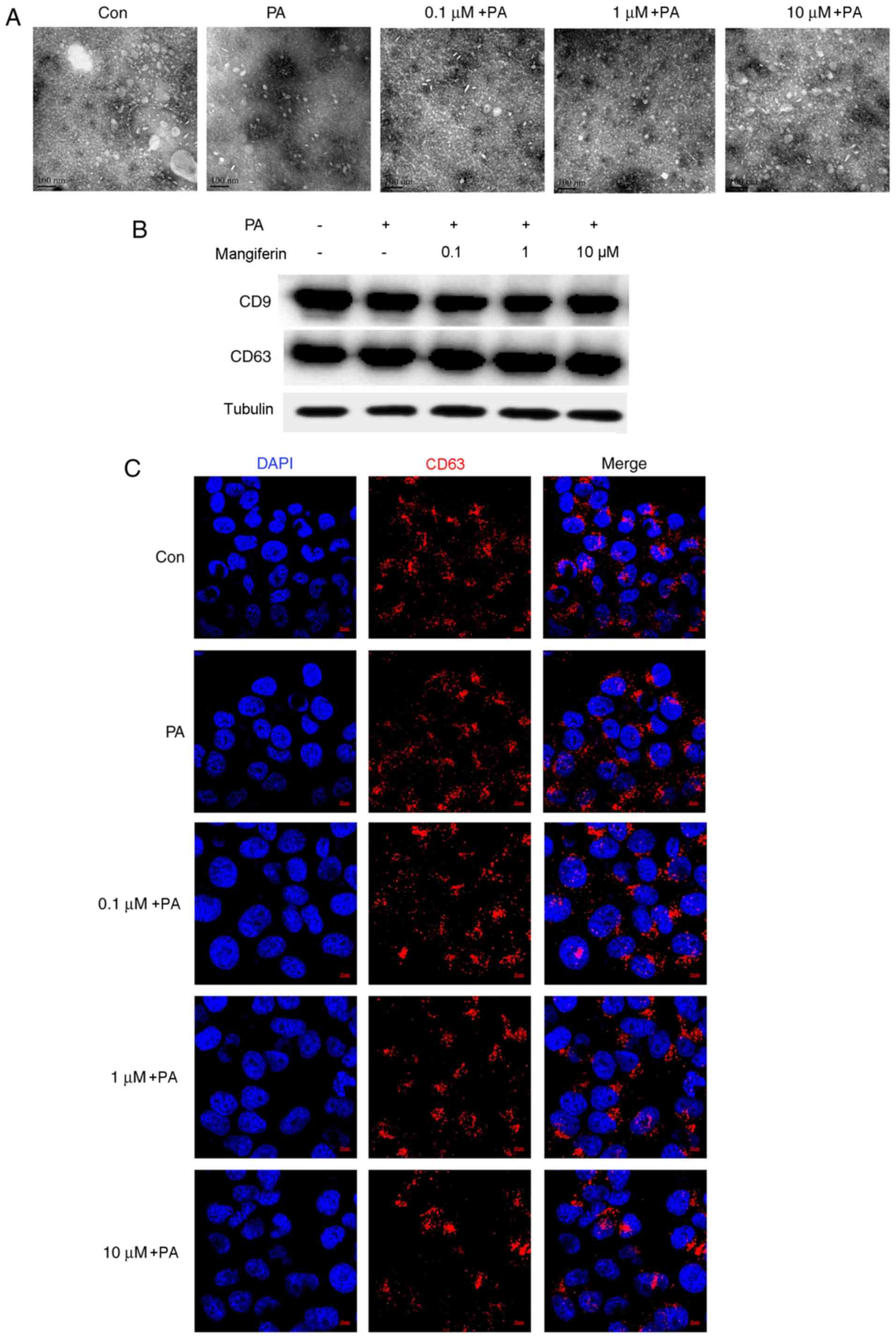 | Figure 1.Mangiferin promotes the release of
exosomes from PVAT. PVAT was collected from normal rats and
cultured with PA (100 µM) in the presence or absence of mangiferin
(0.1, 1 or 10 µM) for 2 h; samples cultured without PA and
mangiferin were used as control groups. Subsequent to washing with
PBS, PVAT was incubated in fresh DMEM for a further 22 h.
Conditioned medium was collected from all treatment groups, and
exosomes were extracted from this medium using a total exosome
isolation reagent. (A) Transmission electron microscopy was used to
observe the release of exosomes from PVAT with or without PA and
mangiferin stimulation (magnification, ×100,000). (B) CD9 and CD63
protein expression of different groups, examined by western blot
assay. (C) Exosomes were extracted from the supernatants of PVAT,
and then immunofluorescence was used to observe the ingestion of
exosomes in endothelial cells. (magnification, ×630). Results are
representative of three independent experiments. PVAT, perivascular
adipose tissue; PA, palmitic acid; Con, Control. |
Mangiferin-induced PVAT-derived
exosomes regulate endothelial function
Further experiments were performed to determine
whether exosomes from mangiferin-stimulated PVAT influence the
endothelial function. Preliminary results indicated that the effect
of exosomes on endothelial cells in vitro was not altered
following 24-h exposure of the cells to exosomes (data not shown).
Thus, 48-h exposure of endothelial cells to exosomes was conducted
in the current study in order to observe the regeneration and
apoptosis levels. Exosomes produced following mangiferin treatment
(1 and 10 µM) markedly promoted the tube formation ability of
endothelial cells compared with the PA-induced exosomes (Fig. 2A). In addition, the migration of
endothelial cells was inhibited by PA-induced exosomes, whereas
migration was clearly increased by mangiferin (10 µM)-induced
exosomes compared with PA-induced exosomes (Fig. 2B). The apoptosis level was also
determined in these treatment groups, and the data revealed that
mangiferin (1 and 10 µM)-induced exosomes significantly reduced the
apoptosis of endothelial cells compared with PA-induced exosomes
(Fig. 2C). Organ bath analysis
identified that PA-induced PVAT-derived exosomes significantly
stimulated the constriction of normal aortic rings compared with
the control, whereas mangiferin (1 and 10 µM)-induced exosomes
significantly promoted the relaxation of aortic rings compared with
PA-induced exosomes (Fig. 2D),
indicating that the relaxation of aortic rings was blocked by
exosomes produced from PA-stimulated PVAT. However, this effect was
reversed upon the use of exosomes produced by mangiferin-treated
PVAT, ameliorating the endothelial dysfunction caused by PA-induced
adipose tissue inflammation.
 | Figure 2.Exosomes from PVAT regulate
endothelial function. Endothelial cells were treated with exosomes
derived from PVAT, which was pre-treated with or without mangiferin
(0.1, 1 or 10 µM) and PA (100 µM) for 2 h, and then incubated
without mangiferin and PA for a further 22 h. (A) Angiogenesis was
detected by tube formation experiments (magnification, ×200). (B)
Endothelial cell migration was detected by conducting a transwell
assay (magnification, ×100). (C) Flow cytometry analysis was used
to evaluate the level of apoptosis. (D) Organ bath analysis of the
relaxation of normal aortic rings incubated with exosomes
pretreated with or without PA and mangiferin. Results are
representative of three independent experiments.
#P<0.05 vs. control group; *P<0.05 vs. PA group.
PVAT, perivascular adipose tissue; PA, palmitic acid; CM,
conditioned medium; DMEM, Dulbecco's modified Eagle's medium; K-H,
Krebs-Henseleit; Con, Control. |
Mangiferin-induced PVAT-derived
exosomes inhibit endothelial inflammation
Endothelial inflammation induced by PA treatment was
further analyzed using RT-qPCR and ELISA. The mRNA expression
levels of the inflammatory factors IL-6 and TNF-α were
significantly increased in endothelial cells treated with
PA-induced PVAT-derived exosomes (PA alone group) compared with the
control group (Fig. 3A); however,
these effects were significantly reversed in the cells incubated
with mangiferin-induced exosomes. In addition, the levels of ARG1
mRNA in endothelial cells were significantly upregulated upon
incubation with mangiferin-induced PVAT-derived exosomes compared
with PA-induced exosomes (Fig.
3A). As compared with the PA-stimulated alone group, exosomes
produced by mangiferin-stimulated PVAT (0.1, 1 and 10 µM)
significantly inhibited the release of IL-6 and TNF-α proteins
(Fig. 3B), and promoted NO
generation in endothelial cells (Fig.
3C). These results demonstrated that exosomes from
mangiferin-stimulated PVAT reduced endothelial inflammation and
increased endothelial relaxation.
 | Figure 3.Exosomes derived from PVAT inhibit
endothelial inflammation. Endothelial cells were treated with
exosomes from PVAT, which had been pre-treated with or without
mangiferin (0.1, 1 or 10 µM) and PA (100 µM) for 2 h, and then
incubated without mangiferin and PA for a further 22 h. (A)
Relative mRNA expression levels of IL-6, ARG1 and TNF-α were
detected by reverse transcription-quantitative polymerase chain
reaction. (B) Content of IL-6 and TNF-α in endothelial cells and
(C) NO released from endothelial cells were detected by ELISA.
Results are representative of six independent experiments.
#P<0.05 vs. control group; *P<0.05 vs. PA group.
PVAT, perivascular adipose tissue; PA, palmitic acid; IL-6,
interleukin-6; ARG1, arginase-1; TNF-α, tumor necrosis factor-α;
NO, nitric oxide. |
Mangiferin-induced PVAT-derived
exosomes block NF-κB signaling
As mangiferin-induced PVAT-derived exosomes
ameliorated endothelial inflammation, the mechanisms underlying the
effect of mangiferin-induced exosomes on endothelial cells were
investigated. Mangiferin (10 µM)-induced PVAT-derived exosomes
significantly inhibited p65 phosphorylation in endothelial cells
compared with the effect of PA-induced exosomes (Fig. 4A). Furthermore, mangiferin (10
µM)-induced PVAT-derived exosomes also markedly reduced p50
phosphorylation in endothelial cells (Fig. 4B). The translocation of p65 and p50
in endothelial cells treated with PVAT-derived exosomes was also
detected. PA-induced PVAT-derived exosomes evidently promoted the
translocation of p65 and p50, whereas the effects were reversed
when exosomes from mangiferin (10 µM)-treated PVAT were used
(Fig. 4C and D). These results
demonstrated that exosomes produced from mangiferin-treated PVAT
reduced the activation of NF-κB signaling.
Discussion
It is well established that adipose tissue
inflammation-induced cardiovascular diseases are associated with
diabetes and obesity. In particular, PVAT dysfunction serves a
vital role in endothelial injury, which may result in vascular
damage (24). Additionally, the
anatomical and functional features of PVAT d etermine its vital
role in the regulation of endothelial homeostasis (25). PVAT inflammation is known to be
involved in the regulation of endothelial function via inhibition
of AMP-activated protein kinase (AMPK) signaling, and mangiferin
has been reported to ameliorate endothelial injury by reducing PVAT
inflammation (26); however, the
major regulatory mechanisms are not clearly understood. The aim of
the current study was to research the potential mechanism by which
mangiferin regulates PVAT inflammation-induced endothelial
dysfunction.
In obesity, adipose tissue dysfunction commonly
causes increased release of free fatty acids (FFAs), which
elevating their level in circulation (27). Since PA is a major component of the
total serum FFAs, PVAT was stimulated with PA to induce endothelial
injury in the present study. As previous research identified that
PA-induced PVAT inflammation promotes endothelial dysfunction
(28), CM was collected following
treatment of PVAT with PA and mangiferin. Saez et al
(29) has reported that
extracellular vesicles (EVs), including exosomes (50–100 nm),
contribute to defective insulin sensitivity in the vasculature,
which has a vital role in cardiovascular complications (27). Furthermore, EVs are considered to
be important mediators of endocrine functions through cell to cell
communication. Exosomes can transfer Delta-like 4 (which is a Notch
ligand) between endothelial cells, resulting in inhibition of
angiogenesis through Notch signaling (30). Thus, in the current study, exosomes
were isolated from CM following stimulation of PVAT with PA, PA +
mangiferin and control groups. PA stimulation reduced the release
of exosomes; by contrast, mangiferin increased exosome release from
PVAT, even when combined with PA. Furthermore, it was confirmed
that the exosomes released from PVAT stimulated with PA and
mangiferin were taken up by endothelial cells, suggesting that
mangiferin stimulation of PVAT can have paracrine effects on
endothelial cells via exosomes.
Our previous studies have demonstrated that
mangiferin-containing polyphenols of Anemarrhena
asphodeloides inhibit inflammation in adipocytes, and
marginally in endothelial cells, via regulation of the AMPK pathway
(21,31); thus, it would be of interest to
determine whether mangiferin induces indirect effects on
endothelial cells. It has been reported that endothelial
dysfunction is associated with cell apoptosis (32). In addition, PA has previously been
demonstrated to induce inflammation and apoptosis in human
umbilical vein endothelial cells by regulating oxidative and
endoplasmic reticulum stress (33). Adipose-derived stem cells have been
investigated for their use as potential pro-angiogenic therapeutics
tools (34), suggesting that
angiogenesis is closely associated with adipose-derived factors.
Consistent with this, the present study observed that exosomes
derived from PA-stimulated PVAT induced endothelial dysfunction,
which was indicated by features including reduced cell
regeneration, reduced cell migration, increased cell apoptosis and
inhibition of vessel dilation. However, PVAT treatment with
mangiferin promoted the release of exosomes and further ameliorated
the PA-induced PVAT inflammation-associated endothelial injury.
PA evokes inflammation with dysregulation of
adipokine expression in PVAT, as demonstrated by enhanced NF-κB p65
phosphorylation, increased expression of pro-inflammatory
adipokines and reduced expression of ARG1 (35). The current study demonstrated that
exosomes produced by PA-stimulated PVAT induced the expression of
pro-inflammatory factors (IL-6 and TNF-α) in endothelial cells.
Furthermore, endothelial injury is known to be closely associated
with the activation of NF-κB signaling (36). The findings of the current study
revealed that PA-induced PVAT inflammation promoted NF-κB
activation in endothelial cells, as indicated by increased p65 and
p50 phosphorylation levels and protein translocation into the
nucleus. However, treatment of PVAT with mangiferin promoted the
release of exosomes and ameliorated the endothelial inflammation
caused by PA stimulation of PVAT.
In conclusion, it is recognized that adipose tissue
exerts profound effects on vascular function via secretion of
various bioactive molecules. In the current study, exosomes were
extracted from CM following the stimulation of PVAT with PA and
mangiferin. Endothelial cells were then incubated with the
PVAT-derived exosomes to determine the indirect effects of
mangiferin on the endothelial function via regulation of PVAT
function. The results revealed that PVAT-derived exosomes induced
endothelial dysfunction, and that exosomes derived from PVAT
treated with mangiferin promoted angiogenesis and migration, and
inhibited apoptosis in endothelial cells. Taken together,
mangiferin was demonstrated to ameliorate endothelial dysfunction
via exosomes derived from PA-stimulated PVAT.
Acknowledgements
Not applicable.
Funding
This study was funded by the Major Research Plan of
the National Natural Science Foundation of China (grant no.
91639115).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and FH designed the study and interpreted the
data. QZ wrote the manuscript and performed the experiments. JY and
BL analyzed the data and were involved in drafting the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This research was approved by the Animal Ethics
Committee of Nanjing Medical University (Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bassols J, Ortega FJ, Moreno-Navarrete JM,
Peral B, Ricart W and Fernández-Real JM: Study of the
proinflammatory role of human differentiated omental adipocytes. J
Cell Biochem. 107:1107–1117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldberg RB: Cytokine and cytokine-like
inflammation markers, endothelial dysfunction, and imbalanced
coagulation in development of diabetes and its complications. J
Clin Endocrinol Metab. 94:3171–3182. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mokdad AH, Ford ES, Bowman BA, Dietz WH,
Vinicor F, Bales VS and Marks JS: Prevalence of obesity, diabetes,
and obesity-related health risk factors. JAMA. 289:76–79. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun X, Lin J, Zhang Y, Kang S, Belkin N,
Wara AK, Icli B, Hamburg NM, Li D and Feinberg MW: MicroRNA-181b
improves glucose homeostasis and insulin sensitivity by regulating
endothelial function in white adipose tissue. Circ Res.
118:810–821. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schinzari F, Tesauro M and Cardillo C:
Endothelial and perivascular adipose tissue abnormalities in
obesity-related vascular dysfunction: Novel targets for treatment.
J Cardiovasc Pharmacol. 69:360–368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fitzgibbons TP, Kogan S, Aouadi M,
Hendricks GM, Straubhaar J and Czech MP: Similarity of mouse
perivascular and brown adipose tissues and their resistance to
diet-induced inflammation. Am J Physiol Heart Circ Physiol.
301:H1425–H1437. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Braunersreuther V, Mach F and Steffens S:
The specific role of chemokines in atherosclerosis. Thromb Haemost.
97:714–721. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Verhagen SN and Visseren FL: Perivascular
adipose tissue as a cause of atherosclerosis. Atherosclerosis.
214:3–10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brown NK, Zhou Z, Zhang J, Zeng R, Wu J,
Eitzman DT, Chen YE and Chang L: Perivascular adipose tissue in
vascular function and disease: A review of current research and
animal models. Arteriscler Thromb Vasc Biol. 34:1621–1630. 2014.
View Article : Google Scholar
|
|
10
|
Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang
H, Zhang Q, Guo C, Zhang L and Wang Q: Exosomes from
adipose-derived stem cells attenuate adipose inflammation and
obesity through polarizing M2 macrophages and beiging in white
adipose tissue. Diabetes. 67:235–247. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kowal J, Tkach M and Thery C: Biogenesis
and secretion of exosomes. Curr Opin Cell Biol. 29:116–125. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee HD, Kim YH and Kim DS: Exosomes
derived from human macrophages suppress endothelial cell migration
by controlling integrin trafficking. Eur J Immunol. 44:1156–1169.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu XM, Gao YB, Cui FQ and Zhang N:
Exosomes from high glucose-treated glomerular endothelial cells
activate mesangial cells to promote renal fibrosis. Biol Open.
5:484–491. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Castelli G, Parolini I, Cerio AM, D'Angiò
A, Pasquini L, Carollo M, Sargiacomo M, Testa U and Pelosi E:
Conditioned medium from human umbilical vein endothelial cells
markedly improves the proliferation and differentiation of
circulating endothelial progenitors. Blood Cells Mol Dis. 61:58–65.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raposo G and Stoorvogel W: Extracellular
vesicles: Exosomes, microvesicles, and friends. J Cell Biol.
200:373–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garrido G, González D, Lemus Y, García D,
Lodeiro L, Quintero G, Delporte C, Núñez-Sellés AJ and Delgado R:
In vivo and in vitro anti-inflammatory activity of Mangifera
indica L. extract (VIMANG). Pharmacol Res. 50:143–149. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sánchez GM, Re L, Giuliani A, Núñez-Sellés
AJ, Davison GP and León-Fernández OS: Protective effects of
Mangifera indica L. extract, mangiferin and selected
antioxidants against TPA-induced biomolecules oxidation and
peritoneal macrophage activation in mice. Pharmacol Res.
42:565–573. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo F, Huang C, Liao X, Wang Y, He Y, Feng
R, Li Y and Sun C: Beneficial effects of mangiferin on
hyperlipidemia in high-fat-fed hamsters. Mol Nutr Food Res.
55:1809–1818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Niu Y, Li S, Na L, Feng R, Liu L, Li Y and
Sun C: Mangiferin decreases plasma free fatty acids through
promoting its catabolism in liver by activation of AMPK. PLoS One.
7:e307822015. View Article : Google Scholar
|
|
20
|
Kevil CG and Bullard DC: In vitro culture
and characterization of gene targeted mouse endothelium. Acta
Physiol Scand. 173:151–157. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao Q, Sun Y, Ji Y, Xu L, Liu K, Liu B
and Huang F: Total polyphenol of Anemarrhena asphodeloides
ameliorates advanced glycation end products-induced endothelial
dysfunction by regulation of AMP-Kinase. J Diabetes. 6:304–315.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan DM, Yang X, Huang LM, Ouyang GJ, Yang
XX and Li M: Simultaneous detection of target CNVs and SNVs of
thalassemia by multiplex PCR and next-generation sequencing. Mol
Med Rep. Jan 24–2019.(Epub ahead of print) doi:
10.3892/mmr.2019.9896. View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang CN, Yang GH, Wang ZQ, Liu CW, Li TJ,
Lai ZC, Miao SY, Wang LF and Liu B: Role of perivascular adipose
tissue in nicotine-induced endothelial cell inflammatory responses.
Mol Med Rep. 14:5713–5718. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Horimatsu T, Patel AS, Prasad R, Reid LE,
Benson TW, Zarzour A, Ogbi M, Bruder do Nascimento T, Belin de
Chantemele E, Stansfield BK, et al: Remote effects of transplanted
prevascular adipose tissue on endothelial function and
atherosclerosis. Cardiovasc Drug Ther. 32:503–510. 2018. View Article : Google Scholar
|
|
26
|
Xu X, Chen Y, Song J, Hou F, Ma X, Liu B
and Huang F: Mangiferin suppresses endoplasmic reticulum stress in
perivascular adipose tissue and prevents insulin resistance in the
endothelium. Eur J Nutr. 57:1563–1575. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Larsson S and Voss U: Neuroprotective
effects of vitamin D on high fat diet- and palmitic acid-induced
enteric neuronal loss in mice. BMC Gastroenterol. 18:1752018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma Y, Li L, Shao Y, Bai X, Bai T and Huang
X: Methotrexate improves perivascular adipose tissue/endothelial
dysfunction via activation of AMPK/eNOS pathway. Mol Med Rep.
15:2353–2359. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saez T, Toledo F and Sobrevia L:
Extracellular vesicles and insulin resistance: A potential
interaction in vascular dysfunction. Curr Vasc Pharmacol. Oct
1–2018.(Epub ahead of print) doi:
10.2174/1570161116666181002095745. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sheldon H, Heikamp E, Turley H, Dragovic
R, Thomas P, Oon CE, Leek R, Edelmann M, Kessler B, Sainson RC, et
al: New mechanism for Notch signaling to endothelium at a distance
by Delta-like 4 incorporation into exosomes. Blood. 116:2385–2394.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao W, Wang M, Shao L, Liao M, Liu K,
Huang F and Liu B: The total phenolic fraction of Anemarrhena
asphodeloides inhibits inflammation and reduces insulin
resistance in adipocytes via regulation of AMP-kinase activity.
Planta Med. 80:146–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu Y, Chen Y, Li R, Liu Q, Wang N, Zhang
Y, Li B and Fang Z: Protective effects of Danzhi jiangtang
capsule on vascular endothelial damages induced by high-fat diet
and palmitic acid. Biomed Pharmacother. 107:1631–1640. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu JZ, Chai YL and Zhang YL: Effect of
rosuvastatin on high glucose-induced endoplasmic reticulum stress
in human umbilical vein endothelial cells. Genet Mol Res. Oct
17–2016.(Epub ahead of print). doi: 10.4238/gmr15048935. View Article : Google Scholar :
|
|
34
|
Souza LE, Beckenkamp LR, Sobral LM,
Fantacini DM, Melo FU, Borges JS, Leopoldino AM, Kashima S and
Covas DT: Pre-culture in endothelial growth medium enhances the
angiogenic properties of adipose-derived stem/stromal cells.
Angiogenesis. 21:15–22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun Y, Li J, Xiao N, Wang M, Kou J, Qi L,
Huang F, Liu B and Liu K: Pharmacological activation of AMPK
ameliorates perivascular adipose/endothelial dysfunction in a
manner interdependent on AMPK and SIRT1. Pharmacol Res. 89:19–28.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leng B, Tang F, Lu M, Zhang Z, Wang H and
Zhang Y: Astragaloside IV improves vascular endothelial dysfunction
by inhibiting the TLR4/NF-κB signaling pathway. Life Sci.
209:111–121. 2018. View Article : Google Scholar : PubMed/NCBI
|


















